Abstract
Despite the public health impact of childhood diarrhea caused by Cryptosporidium, effective drugs and vaccines against this parasite are unavailable. Efforts to identify vaccine targets have focused on critical externally exposed virulence factors expressed in the parasite’s invasive stages. However, no single surface antigen has yet been found that can elicit a significant protective immune response and it is likely that pooling multiple immune targets will be necessary. Discovery of surface proteins on Cryptosporidium sporozoites is therefore vital to this effort to develop a multi-antigenic vaccine. In this study we applied a novel single-domain camelid antibody (VHH) selection method to identify immunogenic proteins expressed on the surface of Cryptosporidium parvum sporozoites. By this approach, VHHs were identified that recognize two sporozoite surface-exposed antigens, the previously identified gp900 and an unrecognized immunogenic protein, Cp-P34. This Cp-P34 antigen, which contains multiple Membrane Occupation and Recognition Nexus (MORN) repeats, is found in excysted sporozoites as well as in the parasitès intracellular stages. Cp-P34 appears to accumulate inside the parasite and transiently appears on the surface of sporozoites to be shed in trails. Identical or nearly identical orthologs of Cp-P34 are found in the Cryptosporidium hominis and Cryptosporidium tyzzeri genomes. Except for the conserved MORN motifs, the Cp-P34 gene shares no significant homology with genes of other protozoans and thus appears to be unique to Cryptosporidium spp. Cp-P34 elicits immune responses in naturally exposed alpacas and warrants further investigation as a potential vaccine candidate.
Keywords: Cryptosporidium, Sporozoite, Surface antigen, MORN, VHH, P34, gp900
1. Introduction
Cryptosporidium is a leading cause of infant diarrhea in low- and middle-income countries around the world (Kotloff et al., 2012). This protozoan parasite is also a key opportunistic infection in immunocompromised individuals, including AIDS/HIV patients, causing debilitating and life-threatening illness (Flanigan et al., 1992; Schmidt et al., 2001; Agholi et al., 2013; Kiros et al., 2015). Despite the global burden and health impact, no effective drugs or vaccines are currently available for control of cryptosporidiosis. Immune-based approaches are studied to develop efficacious interventions to relieve disease burden in the most vulnerable populations. The development of a vaccine should be feasible based on the known protective adaptive immune response in previously infected individuals (Frost et al., 1997; Okhuysen et al., 1998b; Chappell et al., 1999; Frost et al., 2005) and the correlation between CD4+ T cell count and susceptibility of AIDS patients to cryptosporidiosis (Maggi et al., 2000; Rashmi and Kumar, 2013). Evidence also suggests that the humoral response directed against specific antigens may play a role in limiting the length and severity of the infection (Allison et al., 2011; Borad et al., 2012). In some instances, hyperimmune bovine colostrum has demonstrated alleviation of disease symptoms (Greenberg and Cello, 1996; Okhuysen et al., 1998a; Floren et al., 2006) and even resolution of infection (Tzipori et al., 1986; Nord et al., 1990; Ungar et al., 1990), however with variable results (Saxon and Weinstein, 1987). Along these lines, infants fed breast milk containing parasite-specific antibodies became protected from cryptosporidiosis (Korpe et al., 2013). Given the role of adaptive immunity in protection and clearance of a Cryptosporidium infection, identification of effective vaccine candidates is a realistic goal.
Infection with Cryptosporidium occurs in the duodenum of the host where invasive sporozoites excysted from the oocyst invade and establish intracellularly in the epithelial cells. While the unique localization within the cell protects the parasite from the host`s defense mechanisms, some virulence factors of sporozoites are exposed externally before invasion takes place and thus are accessible to the immune system. Upon excystation from the oocyst, the sporozoite propels itself to the host cell by an active form of movement called gliding motility while simultaneously secreting surface adhesive proteins from apical complex organelles named micronemes (Gubbels and Duraisingh, 2012). Gliding movement is powered by the actin-myosin system, termed the glideosome, which is highly conserved across the Apicomplexa (Wetzel et al., 2005; Baum et al., 2006). It is located between the plasma membrane and the inner membrane complex (IMC), a membranous structure found directly beneath the plasma membrane (Frenal et al., 2010). The micronemal proteins (MICs) are discharged through the membrane of the parasite in a cytoskeleton-dependent manner and are shed in trails behind the locomoting parasite, mediating the process of invasion (Chen et al., 2004; Wetzel et al., 2005; Baum et al., 2006). The current model of the apicomplexan glideosome indicates connectivity between motor function and protein secretion, as facilitated by the glideosome-associated connector linking the cytoskeleton to the surface adhesins (Jacot et al., 2016). A number of immunogenic, externally exposed proteins, including micronemal and other surface-resident proteins, have been described as important virulence factors (Singh et al., 2005; Preidis et al., 2007; Bedi and Mead, 2012) and have been studied as potential vaccine candidates (reviewed in Bouzid et al. (2013)).
In Cryptosporidium, several classes of proteins associated with the apical complex have been implicated in adhesion/invasion and motility, namely the mucin-like glycoproteins e.g., gp900, CSL, CpMuc4 and CpMuc5 (Barnes et al., 1998; Langer and Riggs, 1999; O’Connor et al., 2009), smaller secreted adhesins e.g., Cp12 and Cpa135 (Tosini et al., 2004; Yao et al., 2007) and thrombospondin-related adhesive proteins e.g., TRAP-C1 (Spano et al., 1998; Deng et al., 2002; Putignani et al., 2008). These MICs associate with the plasma membrane and then are shed from the surface in trails as they establish contact with the host surface and as the parasite glides forward. Another group of invasive proteins also transiently localize to the surface of sporozoites before being shed, however do not originate in micronemes e.g., p23, gp60/40/15 (Arrowood et al., 1991; Strong et al., 2000). Several proteins are rooted in the membrane using a transmembrane domain (e.g., gp900) or a GPI anchor (e.g., gp40) and their release from the surface requires proteolytic cleavage (Wanyiri et al., 2007; Li et al., 2016).
Some of the proteins listed above have been evaluated in the context of subunit vaccination, however no single antigen induced significant protective immunity i.e., p23 and gp60/40/15 (He et al., 2004; Ehigiator et al., 2007; Roche et al., 2013). Similarly, immunotherapeutic and immunoprophylactic approaches using antibodies raised against single antigens demonstrated only partial clearance or prevention against a Cryptosporidium infection i.e., gp60/40/15, CSL, p23 (Tilley et al., 1991; Enriquez and Riggs, 1998; Riggs et al., 1997, 2002). Incorporation of multiple antigens in vaccine design showed enhanced protection against infection in comparison to single antigen vaccines (Liu et al., 2010; Wang et al., 2010; Yu et al., 2010). Similarly, passive immunization with a combination of antibodies targeting multiple antigens has enhanced prevention from infection and reduction of clinical disease (Schaefer et al., 2000). These findings indicate that polyvalent formulations may enhance induction of protective immunity against cryptosporidiosis. The parasite appears to employ multiple, seemingly redundant adhesive molecules to maximize cell attachment and invasion, such that immunity against a single antigen may be easily compensated by the parasite. In this context, identification of novel surface antigens is pertinent to increasing the antigenic coverage and improving the design of a fully protective vaccine.
In this study we employed a VHH-display phage library of single-domain camelid antibodies (VHH) derived from an immunized alpaca and selected phage for binding to intact sporozoites as an approach for targeted identification of novel surface antigens. While several studies report on application of phage display in Cryptosporidium research (Chen et al., 2003; Yao et al., 2007; Pokorny et al., 2008; Boulter-Bitzer et al., 2009, 2010), the VHH-phage display technology has not yet been applied in the context of this parasite. The identification of novel surface molecules that mediate attachment and invasion of host cells is important to advance our understanding of Cryptosporidium infection.
2. Materials and methods
2.1. Parasite
Cryptosporidium parvum oocysts (Iowa isolate, isolated from infected calves) were purchased from Bunch Grass Farms (Deary, ID, USA). Cryptosporidium hominis, TU502 isolate, was originally derived from a Ugandan diarrheic child (Xu et al., 2004; Ifeonu et al., 2016) and has been maintained over a decade at Tufts University, USA, through numerous passages in gnotobiotic piglets (Akiyoshi et al., 2002). Cryptosporidium tyzzeri, a natural mouse strain derived from house mice (Mus musculus) from the Czech Republic (Kvac et al., 2013), was a gift from Martin Kváč (Biology Centre CAS, Czech Republic) and was serially propagated in C57BL/6 mice at Tufts University. For extraction of oocysts, fecal material and gut content from infected mice or piglets was purified using a Nycodenz® gradient, as described elsewhere (Widmer et al., 1998), and stored at 4°C.
2.1.1. Preparation of C. parvum lysate
Briefly, oocysts were bleached with 5% bleach for 7 min on ice and washed with PBS by centrifugation (18,000 g, 2 min). Oocysts were then excysted in 0.75% taurocholic acid (Sigma) suspension in PBS for 1 h at 37°C. Following centrifugation (18,000 g, 2 min), supernatant was collected and the pelleted sample consisting of sporozoites, unexcysted oocysts and oocyst shells was sonicated (Qsonica CL5, Qsonica Sonicators, CT, USA) with 30 cycles, 20 s each. The pelleted fraction was resuspended in previously collected supernatant and stored at −20°C. The concentration of the lysate was determined by measurement of O.D. using a Nanodrop instrument (ND-1000, NanoDrop Technologies, DE, USA).
2.1.2. Generation of sporozoite preparation for panning
Fifty μL of superparamagnetic beads conjugated with recombinant protein G (Invitrogen, CA, USA) were coated with the 5F10 monoclonal antibody (gift from Abhineet Sheoran, Tufts University), utilized here for its affinity to the oocyst wall as described earlier (Matos et al., 2019). Beads were incubated in 5F10 cell culture supernatant at 1:5 dilution for 30 min at room temperature with rotation, after which the sample was washed with PBS-0.02% Tween. Twenty million C. parvum oocysts were blocked by incubation in 10% fetal bovine serum (FBS) for 20 min at room temperature with rotation and washed with PBS by centrifugation (18,000 g, 2 min). Following the co-incubation of 5F10-coated beads with blocked parasite (30 min), beads were washed to remove unbound oocysts. Oocysts bound to magnetic beads were subjected to excystation in 0.75% taurocholic acid at 37°C for 1 h. Released sporozoites were collected by magnetic separation. Sporozoites were fixed without permeabilization in 4% paraformaldehyde at room temperature (20 min) and washed with PBS. Lack of permeabilization was confirmed by exclusion of propidium iodide (PI) at 10 μg/mL, as measured by flow cytometry.
2.2. Generation of VHHs
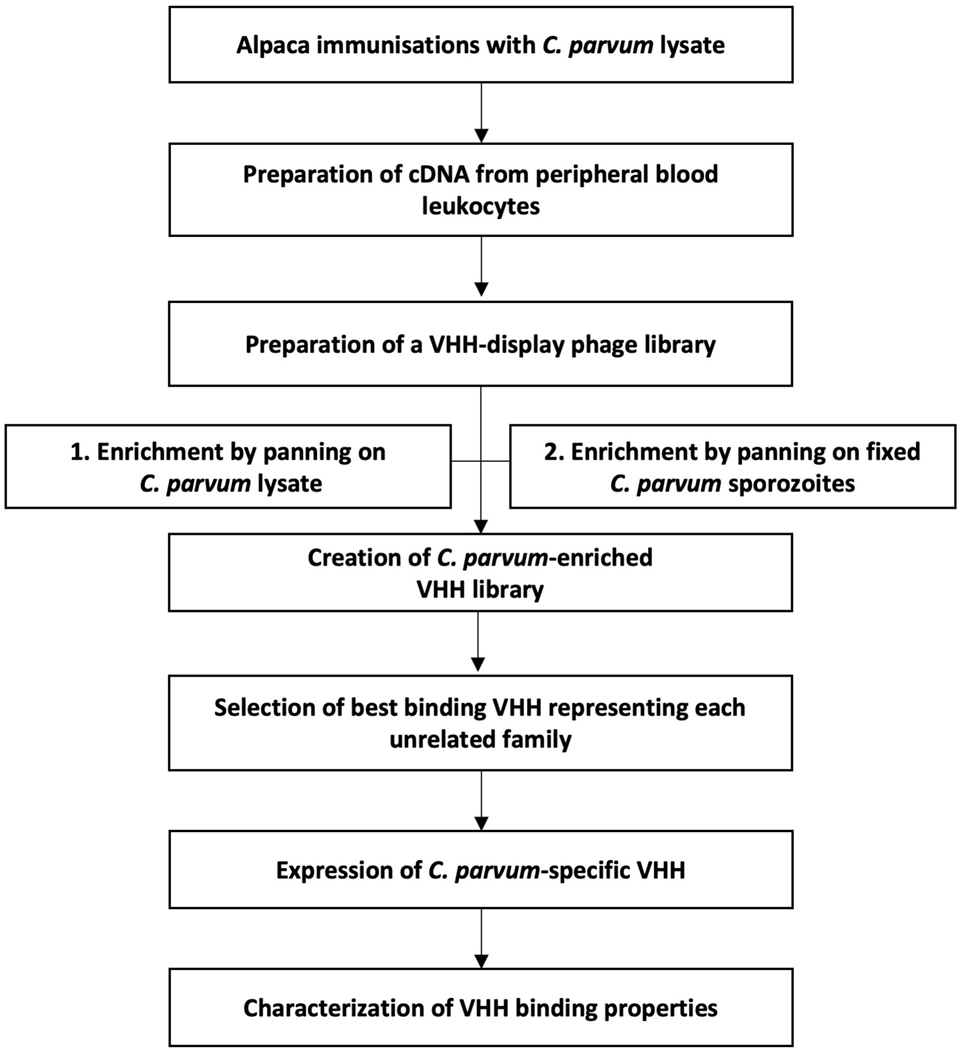
A simplified flowchart of procedures involved in generation and selection of Cryptosporidium-specific VHHs is shown in Fig. 1 and the details follow.
Fig. 1.
Simplified flowchart of the procedural steps involved in generation and selection of Cryptosporidium-specific single-domain camelid antibodies (VHHs) from alpaca.
2.2.1. Preparation of VHH-display phage library
A 2.5-years old male alpaca (Vicugna pacos) was immunized with a C. parvum lysate by seven consecutive subcutaneous injections at 3 week intervals. A single immunization contained 1 mg of antigen suspended in a preparation of CpG and alum adjuvant (Alhydrogel). Two weeks after the final boost, the whole blood was collected from the jugular vein for lymphocyte preparation and generation of a VHH-display phage library as described before (Tremblay et al., 2010; Moayeri et al., 2015). Briefly, RNA obtained from peripheral blood leukocytes was reverse-transcribed using both C2-specific and non-specific random primers to obtain a diverse cDNA pool. The VHH coding sequence was amplified by multiple PCRs using forward primers targeting VHH framework 1 sequence and reverse primers targeting a hinge region of the VHH, each introducing NotI and AscI restriction sites flanking the VHH coding region, respectively. Such prepared amplicon was then ligated upstream of an E-tag epitope sequence into the phagemid vector for M13 phage gene III display containing an ampicillin resistance cassette. The ligated DNA was transfected into high efficiency, electroporation-competent Escherichia coli TG1 cells. A VHH-display library (called JMG-2) of approximately 107 independent clones was selected using ampicillin. To create a VHH-displayed phage library, an amplified JMG-2 pool was infected with VCS-M13 helper phage and induced under kanamycin selection. The M13 phage liberated from TG1 cells, displaying functional VHH on their surface in fusion with gene III, was precipitated and stored as the VHH-display phage library (φ JMG-2).
2.2.2. Panning
The φ JMG-2 phage were selected for specific binding to C. parvum antigens in multiple cycles of panning. Panning and recovery of phage have been described previously (Maass et al., 2007; Tremblay et al., 2010; Mukherjee et al., 2012; Moayeri et al., 2015). Briefly, VHH-displaying phage were incubated with immobilized target antigen to select for strong VHH binders. In the effort to select for phage with high affinity to the target protein, several tactics were used to increase the stringency of panning in consecutive cycles, namely diminishing concentrations of target protein and/or phage and reduction of incubation times. Here, two panning strategies were performed using two different target antigens, parasite lysate and surface of C. parvum sporozoites, each panned with several rounds of increasing stringency. For panning on parasite extract, C. parvum lysate was coated onto Nunc Immunotubes at 20 μg/mL for both low and the following high stringency pan. For panning to select for VHHs binding to the sporozoite surface, non-permeabilized C. parvum sporozoites were obtained using 5F10-coated magnetic beads as described above. A total of 2×107 and 1×107 sporozoites in suspension were used for low and high stringency panning, respectively. Regardless of the method, during non-stringent panning the antigen was incubated with phage for 1 h at room temperature with rotation. To increase stringency, the amount of phage was reduced 100-fold and incubation time shortened to 10 min. Unbound and non-specifically bound phage were removed by 15 washes with PBS-0.1% Tween. To dissociate phage specifically bound, the sample was incubated with 0.2 M glycine-HCl for 10 min. Dissociated phage were neutralized with 1 M Tris-HCl (pH=9.1) and transduced into E. coli ER2738 by 15 min incubation at 37°C.
2.2.3. Selection of VHHs
Dilutions of transduced E. coli ER2738 were titrated on agar plates in the presence of ampicillin. Ninety-five single colonies from each panning were randomly selected for expression of VHHs and evaluation of their binding affinity to the target. Specifically, selected colonies were individually transferred to wells of a 96-well culture plate (Corning, NY, USA) for amplification. One well in each plate was occupied by an unseeded control. Expression was induced by overnight incubation in the presence of 1 mM isopropyl β- d-1-thiogalactopyranoside (IPTG) at 30°C. Collected culture supernatants containing expressed soluble VHHs were then screened for binding to C. parvum antigens. Culture supernatants were screened using a modified ELISA method and immunofluorescent microscopy as described below with the culture supernatants as the primary antibody at a 1:1 dilution in blocking solution. VHHs demonstrating significant binding to the target were further selected based on their unique clonal identity by DNA fingerprinting and analysis of sequence similarities in the complementarity-determining region 3 (CDR3). Briefly, DNA was obtained from selected E. coli ER2738 clones and amplified by PCR. DNA was then subjected to sequential digestion with BsaJI or BstNI restriction enzymes (New England Biolabs, MA, USA), each followed by agarose gel electrophoresis. Plasmids from clones producing the strongest binding signal within each group having identical DNA fingerprints were sequenced through the VHH coding regions (Genewiz, Boston, MA, USA) and aligned. Unrelated VHHs with unique CDR sequences and producing the strongest binding signals were selected for subcloning, expression and characterization.
2.2.4. Cloning
VHH-coding DNA was excised from the phagemid vector using NotI and AscI restriction enzymes (New England Biolabs) and ligated into the pET-32b(+) vector downstream from a thioredoxin (Trx) fusion partner and upstream from a peptide epitope tag for detection (E-tag (GAPVPYPDPLEPR) or myc tag (MEQKLISEEDL)). Where indicated, VHH monomers were dimerized, the two VHHs separated by a 15 amino acid long flexible spacer (GGGGS)3 (Wen et al., 2013). For this application, a gene encoding the dimer sequence was synthesized (GenScript Biotech, Piscataway, NJ, USA) and ligated into vectors between NotI and AscI cleavage sites, specifically pET32b(+) or pGEX. Dimer VHHs were subcloned between two flanking, in-frame, E-tag coding sequences. The ligation reactions were then transformed by heat shock into chemically competent, transformation-efficient E. coli TOP10 (Invitrogen), followed by sequencing to confirm appropriate in-frame ligation and no introduced mutations. Validated expression plasmids were then transformed into E. coli Rosetta-gami 2(DE3)pLac1 (MilliporeSigma, MA, USA). All VHHs expressed from the pET-32b(+) vectors contained an N-terminal Trx fusion partner, hexahistidine (6x His) sequence and a C-terminal epitope E-tag for detection. VHH dimers expressed from the pGEX vector contained a N-terminal GST tag in addition to an E-tag. Sequences of all VHH monomers and dimers employed in this study are listed in Supplementary Tables S1 and S2. All vectors used to clone VHH sequences were commercial vectors re-engineered with properly positioned NotI and AscI restriction sites and an E-tag or a myc tag sequence.
2.2.5. VHH expression and purification
Cytosolic expression of VHHs was induced by addition of 1 mM IPTG to bacterial cultures in mid-logarithmic growth (A600 = ~0.6) followed by overnight incubation in Luria-Bertani (LB) broth at 15°C and shaking at 250 rpm. VHHs expressed from pET-32b(+) and pGEX vectors were purified by nickel-agarose and glutathione-affinity chromatography, respectively. Briefly, VHHs were liberated from cytosol by lysis and then passed through a Ni-NTA agarose (Invitrogen) for pET-32b or glutathione-agarose (Sigma) for pGEX. Bound VHHs were then eluted using increasing concentrations of imidazole ranging from 10 mM to 250 mM for pET-32b or a 10 mM solution of reduced glutathione for pGEX. Eluted VHHs were dialyzed in PBS and stored at 4°C. VHH concentrations were assessed by measurement of O.D. using a Nanodrop instrument (ND-1000, NanoDrop Technologies) and by protein gels comparing band density with broad range SDS-PAGE standards (Bio-Rad, CA, USA). Longer term storage was in PBS at −20°C.
2.3. ELISA
ELISAs were used to titrate Cryptosporidium-specific IgGs in alpaca sera and to screen VHHs in culture supernatant for binding to C. parvum antigens. In both cases, 96-well MaxiSorp plates (Invitrogen) were coated with C. parvum lysate at 4 μg/mL and incubated at 4°C overnight. The following day, plates were washed and blocked with 4% milk-tris-buffered saline (TBS)-0.1% Tween solution for 2 h at 37°C. Plates were washed and alpaca sera in two-fold serial dilutions or culture supernatants in 1:1 dilution were added and incubated for 1 h. For alpaca sera ELISAs, sera from two juvenile alpacas with limited field exposure were used as putative near-negative control sera. For VHH screening, a control without culture supernatant was included. Results were normalized to ‘no serum’ or ‘no culture supernatant’ control. Plates were washed and incubated in secondary antibody dilutions for 1 h, anti-llama IgG horseradish peroxidase (HRP) antibody (Ab) (Bethyl Laboratories, TX, USA) at 1:5,000 dilution or anti-E-tag HRP Ab (Bethyl Laboratories) at 1:10,000 dilution, as appropriate. Plates were washed a final time and o-phenylenediamine (OPD) was added to each well for 20 min. The reaction was stopped with 1 M H2SO4 and absorbance was measured at 490 nm using a microplate reader.
2.4. Immunofluorescent staining techniques
2.4.1. Flow cytometry staining of non-permeabilized sporozoites
VHHs were evaluated for binding to the surface of C. parvum sporozoites by immunofluorescent staining and analyzed by flow cytometry. Pre-bleached (5% bleach, 7 min) oocysts were excysted in 0.75% taurocholic acid suspension in PBS for 1 h at 37°C. Samples were then washed with PBS by centrifugation (18,000 g, 2 min) to remove taurocholic acid. Pelleted parasites were fixed without permeabilization by incubation with 4% paraformaldehyde for 20 min and subsequently blocked with 10% FBS for 1 h, both with rotation at room temperature. Parasites were washed and probed with purified VHHs at 1 μg/mL and incubated for 1 h with rotation. Non-specific VHHs were used as negative controls. Results were adjusted to ‘no VHH’ control. Parasites were washed and incubated in secondary antibody dilutions for 1 h with rotation, anti-E-tag FITC at 1:100 dilution (Bethyl Laboratories). Intensity of fluorescence was measured by flow cytometry in the gated population of sporozoites. Lack of permeabilization was confirmed by exclusion of PI at 10 μg/mL in the presence of control permeabilized by heat inactivation (95°C, 10 min).
2.4.2. Fluorescent microscopy staining of permeabilized sporozoite
Here, purified and unpurified VHHs in culture supernatants were evaluated for binding to sporozoites. Pre-bleached Cryptosporidium oocysts were firstly excysted for 30 min at 37°C and then washed with PBS by centrifugation (18,000 g, 2 min) to remove taurocholic acid. Excysted parasites were then applied to a glass microscope slide and allowed to glide for 30 min at 37°C in a humidified container. In order to preserve the parasite and its shed trails in place, the sample was then air dried and fixed with 4% paraformaldehyde for 20 min. Parasites prepared in this manner were then blocked, washed and probed with primary and secondary antibodies in the same fashion as described above for analysis by flow cytometry. Slides were then dried and mounted with antifade medium. Fluorescing sporozoites were imaged under an epifluorescent microscope with a differential interference contrast (DIC) (Nikon Eclipse Ti-E microscope, Nikon Instruments Inc., NY, UAS). Permeabilization of sporozoites was confirmed by clear internal staining with PI at 10 μg/mL.
2.4.3. Fluorescent microscopy staining of non-permeabilized sporozoites
For techniques requiring non-permeabilized sporozoites, the parasite samples were kept wet at all times to prevent permeabilization by drying, even after fixation. This was achieved by capturing parasites on poly-l-lysine slides (Chromaview™) coated with 2E5 monoclonal Ab (mAb) (kindly provided by Dr. Abhineet Sheoran, Tufts University) which binds to 40, 300 and 900 kDa Cryptosporidium antigens (Zhang et al., 2009). Slides were coated with 2E5 at 20 μg/mL and incubated overnight at 4°C in a humidified container, after which they were washed with PBS. Pre-bleached oocysts were suspended in 0.75% taurocholic acid, transferred onto the 2E5-coated surface, and incubated for 1 h at 37°C under humidified conditions. The surface was then washed with PBS to remove oocyst debris, unexcysted oocysts and unbound sporozoites. Captured sporozoites were fixed with 4% paraformaldehyde at room temperature for 20 min and washed with PBS. Samples were blocked, stained and prepared for microscopy as described in section 2.4.2. A lack of permeabilization was confirmed by exclusion of PI at 10 μg/mL.
2.4.4. Cell infection assay and staining of intracellular stages
Monolayers of Madin-Darby bovine kidney epithelial (MDBK) cells (ATCC: CCL-22) were prepared in a 16 chamber glass slide system with removable wells (Thermo Fisher Scientific, MA, USA). A total of approximately 20,000 MDBK cells were seeded into each well and incubated for 2 days until confluent, defined as approximately 40,000 cells/well. Pre-bleached oocysts were incubated with 0.75% taurocholic acid for 15 min at 37°C to prompt excystation. Pre-excysted oocysts were then added onto confluent cell monolayers at a multiplicity of infection (MOI) of 1:1. To monitor the temporal expression of VHH targets during intracellular development, cell cultures were co-incubated with parasites for 2, 6, 24 and 48 h in order to capture development of different stages; namely trophozoites between two and 6 h p.i., immature meronts (type I) at 12 h, mature meronts (type II) at 24 h and sexual stages at 48 h (Current and Haynes, 1984; Mauzy et al., 2012; Wilke et al., 2018; Tandel et al., 2019). To terminate the infection, cultures were washed with PBS and permeabilized with 100% methanol for 10 min at room temperature. After fixation, cells were blocked with 10% FBS for 30 min and then incubated with FITC-labeled Vicia villlosa lectin (VVL) (Vector Laboratories, CA, USA) at 1 μg/mL concentration for 30 min to visualize intracellular stages of the parasite. For VHH detection, cells were subsequently probed with a myc-tagged VHH at 1 μg/mL, followed by the 9E10 anti-myc antibody (kindly provided by Dr. Jean Mukherjee, Tufts University) and anti-mouse IgG Alexa Fluor 568 conjugate (Invitrogen) at 1:500 dilution. Cell nuclei were counterstained with DAPI at 20 μg/mL. Same frame fluorescent micrographs were obtained under an epifluorescent microscope at constant exposure using FITC, TRITC and DAPI filters. Corresponding images were color-merged using ImageJ 1.48v software (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/, 1997–2018.).
2.4.5. Competition study
Competition experiments were designed to determine whether two unique VHHs compete for the same epitope. For this application, VHHs were expressed with different epitope tags, specifically myc and E-tag. Briefly, sporozoite samples were prepared on glass slides, fixed, dried and blocked as described above. Sporozoites were then probed simultaneously with two unique VHHs, each with a different tag, such that the concentration of a myc-tagged VHH exceeded that of an E-tagged VHH by 10-fold, and vice versa. Binding of an E-tagged VHH was detected with anti-E-tag FITC conjugate at 1:100 dilution. Binding of a myc-tagged VHH was detected by subsequent probing with the 9E10 anti-myc mAb at 10 μg/mL, followed by anti-mouse IgG Alexa Fluor 568 Ab at 1:500 dilution. Same frame fluorescent micrographs were obtained at constant exposure using FITC and TRITC filters (Nikon Instruments Inc). Corresponding images were color-merged using ImageJ 1.48v software (Rasband, 2018).
2.5. Western blotting
The western blotting technique was used to evaluate the binding specificities and affinities of alpaca sera and VHHs to C. parvum native proteins or recombinant proteins. Briefly, 1 μg of Cryptosporidium antigen or 0.1 μg of recombinant protein was denatured under non-reduced conditions in 1x lithium dodecyl sulfate (LDS) buffer at 70°C for 10 min. The sample was then loaded onto 4–12% Bis-Tris gel (Invitrogen) and electrophoresed in 1× 3-(N-morpholino)propanesulfonic acid (MOPS) buffer at 100 V for 10 min and then at 200 V for 40 min. The gel was transferred onto nitrocellulose membranes using a wet system (395 mA, 4 h) or semi-dry system (25 V, 30 min). Membranes were blocked with 4% milk-TBS with 0.1% Tween for 1 h and washed with TBS-0.1% Tween (TBS-T) before being blotted with primary antibody: VHH at 0.01–1 μg/mL or alpaca sera at 1:5,000 dilution for 1 h with rotation. A control serum from a juvenile alpaca with limited field exposure, or non-specific VHHs, were used as negative controls. Membranes were washed and incubated with secondary antibody dilutions at 1:5,000 for 1 h, anti-llama IgG HRP (Bethyl Laboratories) or anti-E-tag HRP, as appropriate. Western blot signals were detected using chemiluminescent substrate (GE Healthcare, IL, USA), imaged and analyzed using ChemiDoc system (Bio-Rad).
2.5.1. Deglycosylation
To determine whether VHHs bind to oligosaccharide or protein epitopes, O-linked and N-linked glycans were removed from the sample before western blot analysis. O-glycosylation was removed prior to blocking by incubation of membranes with 10 mM sodium periodate (Thermo Fisher Scientific) for 1 h and then with 50 mM sodium borohydride for 30 min, as described elsewhere (Sestak et al., 2002). N-glycosylation was removed from a sample before gel electrophoresis by treatment with PNGase F (New England Biolabs) for 1 h at 37°C following the manufacturer`s instructions. A glycosylation-insensitive 3A1 mAb (unpublished observation) was included as a control (gift from Dr. Abhineet Sheoran, Tufts University). Blotting and detection were performed as described above.
2.6. Cell infection assay
A total of 20,000 MDBK cells (ATCC: CCL-22) were seeded into each well of a 96-well culture plate (Corning). Cells were incubated in the CO2 incubator at 37°C in RPMI media containing 10% FBS (heat-inactivated at 56°C for 30 min), 2 mM L-glutamine and 100 IU/ml of penicillin/50 μg/ml of streptomycin sulfate solution for 2 days until confluent, defined as approximately 40,000 cells/well. Pre-bleached oocysts were incubated with 0.75% sodium taurocholate for 15 min at 37°C to prompt excystation. Pre-excysted oocysts were then added onto confluent cell monolayers at a MOI of 1:1 in the presence of uninfected controls. Cells were infected in the presence of VHHs at 100 μg/ml. Cells that were untreated with VHH, mock treated with a non-specific VHH, or treated with paromomycin at 2 mg/ml, served as controls. To terminate the infection, cultures were washed 24 h p.i. with PBS and permeabilized with 100% methanol for 10 min at room temperature. After fixation, cells were blocked with 10% FBS for 30 min and then incubated with FITC-labeled VVL (Vector Laboratories) at 1 μg/ml concentration for 30 min to visualize intracellular stages of the parasite. Monolayers were inspected under a fluorescent microscope (Nikon Instruments Inc.). Four images were taken of each well at 200x magnification using a FITC filter at 100 ms exposure. The number of fluorescing infection foci was then counted by ImageJ 1.48v particle analyzer (Rasband, 2018) using the following settings: triangle threshold at 15–255, size exclusion at 10–1,000 pixel2 and circularity at 0.1–1.0.
2.7. GST pull down of ~34 kDa protein
A VHH heterodimer consisting of JJS-A6 and JJS-A11 fused to GST (JJS-A6/A11-GST) was used in efforts to pull down the protein target from C. parvum lysate. Briefly, 50 μL of glutathione agarose bead slurry (Sigma-Aldrich, MO, USA) were blocked by incubation with 10% FBS-PBS-0.1% Triton for 1 h at room temperature with rotation and then washed with PBS. Beads were then co-incubated simultaneously with 10 μg of JJS-A6/A11-GST dimer and 150 μg of C. parvum lysate in a solution of PBS-0.1% Triton for 2 h at 4°C with end-over-end rotation. The beads were then washed six times with ice-cold PBS-1% Triton by centrifugation (550 g, 1 min). Subsequently, pelleted beads were denatured in 1x LDS buffer for 10 min at 70°C and electrophoresed through 4–12% Bis-Tris gel in 1x MOPS buffer at 100 V for 10 min, and then at 200 V for 40 min. The JJS-A6/A11-GST heterodimer preparation was also electrophoresed to later serve as a control for mass spectrometry. To visualize bands, the gel was stained with Coomassie dye by incubation with GelCode Blue (Thermo Fisher Scientific) for 1 h. Bands corresponding to an ~34 kDa protein were excised from the gel from each lane and placed in double distilled water (dd H2O).
2.8. Mass spectrometry
Gel plugs of the pulled down ~34 kDa protein were submitted for protein mass spectrometry analysis to the Taplin Mass Spectrometry Facility (Harvard Medical School, Boston, MA, USA) as ‘beads sample’ alongside the ‘dimer control’ sample (see above). For this analysis, briefly, samples were subjected to tryptic digestion and analyzed by microcapillary LC/MS-MS). The mass of peptide fragments yielded by the tryptic digest were determined and matched against the C. parvum genomic database. Six proteins also identified in ‘dimer control’ sample were considered to be a background and were excluded from the list of proteins identified from ‘beads sample’. Additionally, nine candidates were excluded from analysis due to their low abundance in the analyzed sample. The final list of potential candidates included two top-ranked proteins that were identified by numerous peptide matches and had the expected molecular size of approximately 34 kDa. The products of the two top-ranked proteins derive from C. parvum cgd1_1510 and cgd5_1640 genes.
2.9. Recombinant proteins
The Cp-P34 (product of C. parvum gene cgd5_1640 and annotated as ‘MORN repeat containing protein’) and the carboxyl terminus of Cp-gp900 extracellular domain 5 (product of C. parvum gene cgd7_4020 annotated as ‘Cryptosporidial mucin’) were obtained as synthetic, codon-optimized coding DNA (Genscript Biotech). The coding DNA was inserted into a modified version of the pSecTag2 expression vector (Invitrogen) that had been engineered to secrete encoded recombinant proteins fused at the amino terminus by an OLLAS-tag for product detection and both a strep-tag and a hexahistidine tag to facilitate product purification from conditioned media. The carboxyl-terminal domain 5 of Cp-gp900 was expressed as recombinant Cp-gp900-CT. The sequence was truncated by 85 amino acids to remove a hydrophobic region at the C-terminus in order to facilitate release from Chinese hamster ovary (CHO) cells into the medium. The full amino acid sequences encoded within the two vectors are shown in Supplementary Table S3. CHO-S cells (Invitrogen) were chemically transfected with the plasmid and incubated for 3 days in 5% CO2 at 37°C. Recombinant protein was then purified from the harvested culture supernatant by means of nickel-agarose chromatography. Eluted recombinant protein was dialyzed in PBS and stored at −20°C.
2.10. Cp-P34 sequence analysis
Orthologs of C. parvum ‘MORN repeat containing protein’ (product of cgd5_1640 gene) were identified using a protein BLAST search in the OrthoMCL DB 2.0 (www.orthomcl.org/orthomcl/). Alignment of MORN repeats from the N- to C-terminus was facilitated by identification of conserved repeats using the NovoPro repeat finder (www.novoprolabs.com). The phylogenetic tree was generated using MEGA X 10.1 software (Kumar et al., 2018), for which sequences were first aligned and trimmed using BioEdit (Hall, 1999). The sequence logo was generated using WebLogo (available at https://weblogo.berkeley.edu) (Crooks et al., 2004). The likelihood of O- and N-glycosylation sites was evaluated by NetOGlyc 4.0 software (available at www.cbs.dtu.dk/services/NetOGlyc/) (Steentoft et al., 2013) and the NetNGlyc 1.0 software (available at www.cbs.dtu.dk/services/NetNGlyc/), respectively.
2.11. Statistical analysis
Graphing and statistical analyses of data were done using GraphPad Prism software (v7.0c, GraphPad Software, Inc.).
2.12. Ethics statement
Cryptosporidium parasites used in this study were generated in animals in compliance with protocols No. G2017–107 and No. G2017–120. The VHH-library was generated and derived from an alpaca in accordance with the protocol No. G2019–142. All protocols describing animal use were approved by the Tufts University Institutional Animal Care Use Committee in accordance with the Guide for the Care and Use of Laboratory Animals of the National Research Council, USA.
3. Results
3.1. Generation and enrichment of VHHs specific to antigens on the surface of C. parvum sporozoites
An outline of procedures involved in generation and selection of Cryptosporidium-specific VHHs derived from an alpaca is shown in the Fig. 1. The immunizations with C. parvum lysate of an adult alpaca significantly boosted what was already a robust pre-existing anti-lysate serum titer and resulted in a final titer of ~105 (P<0.0001; two-way ANOVA) (Supplementary Fig. S1). Cryptosporidium is a ubiquitous pathogen in the farm setting, thus the existence of serum titers prior to experimental immunization with the Cryptosporidium lysate was expected. For that reason, we employed sera of two recently weaned juvenile alpacas with limited field exposure to serve as putatively ‘negative’ controls in our experiments with immune alpaca sera.
A VHH display phage library of nearly 107 independent clones was generated from peripheral blood leukocytes collected from the alpaca after the final immunization. We then utilized VHH-displayed phage from the library to select for Cryptosporidium-specific VHHs, employing multiple methods of panning, each with additional rounds of increased stringency to enrich for higher affinity VHHs. Initially we panned the library on a crude C. parvum lysate coated on plastic. Of 190 randomly selected clones obtained following the panning process, five unique lysate-binding VHHs were identified by ELISA and sequence analysis as positives with no apparent CDR homology (called JJ and JMP, Supplementary Fig. S2). Of the five VHHs, two were found to bind to a high molecular weight target localized to the sporozoite apical end (Supplementary Fig. S3). Both of these VHHs were later shown to recognize the previously identified gp900 antigen (see below).
To specifically enrich for VHH-displayed phage binding to sporozoite surface antigens, we performed additional panning in which we employed a pure population of whole fixed, non-permeabilized sporozoites as the target. Furthermore, we used immunofluorescent microscopy as a screening tool in an effort to identify new VHHs that bind to the sporozoite surface. Ninety-five VHH clones were randomly chosen following enrichment on whole sporozoites, and screened by ELISA and immunofluorescent microscopy. In addition to re-isolating VHHs to gp900, four unique VHHs were identified in this screen and each was later confirmed as a sporozoite surface binder (called JJS, Supplementary Fig. S2). The lack of CDR3 homologies within the unique VHHs identified in this study supports the concept that each derive from different B cell clonal origins (Supplementary Fig. S2). The amino acid sequences of all VHHs spanning frameworks 1–4 and CDRs 1–3 are included in SSupplementary Fig. S2.
3.2. Discovery of surface-exposed targets on C. parvum sporozoites bound by VHHs
3.2.1. Identification of Cp-P34 as a novel surface-exposed protein on C. parvum sporozoites
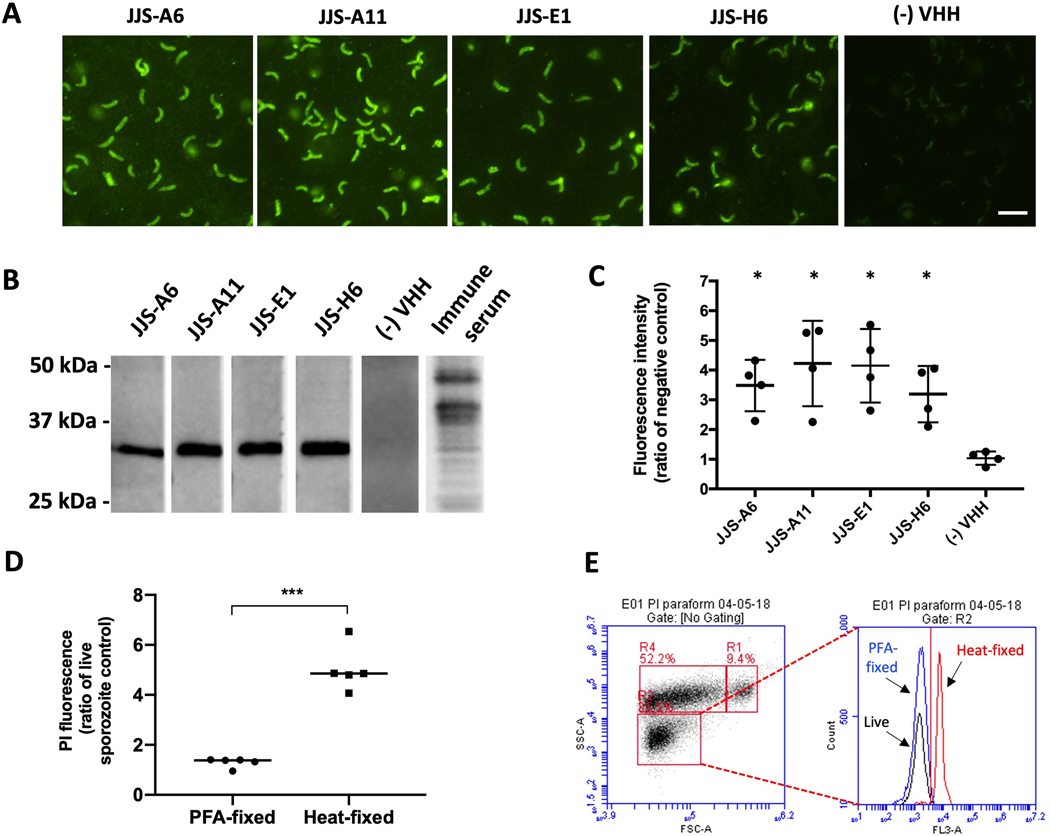
All unique VHHs generated from panning on whole parasites (JJS series) were found to bind targets exposed on the surface of sporozoites as assessed by the immunofluorescent technique. Fig. 2A shows micrographs visualizing localization of VHH binding to entire permeabilized sporozoites. The stained targets were present only on excysted sporozoites but not on sporozoites entrapped inside the oocyst, indicating their expression is triggered following excystation (Supplementary Fig. S3A). The results of flow cytometry with fixed, non-permeabilized sporozoites further confirmed that these VHHs bind epitopes exposed on the host-interactive surface of the parasite (Fig. 2C). The impermeability of the paraformaldehyde (PFA)-fixed sporozoites was confirmed by their exclusion of PI, in contrast to sporozoites permeabilized by heat treatment (Fig. 2D and 2E). All JJS series VHHs showed significantly stronger binding to sporozoites than a non-specific control VHH (P=0.02; Mann-Whitney test). To help inform the identification of VHH targets, we next performed western blot analysis in which non-reduced C. parvum lysate was separated and blotted with individual VHHs. All VHHs from the JJS series bound the same band of approximately 34 kDa, suggesting they all bind to the same protein (Fig. 2B). We thus assessed whether they bind overlapping epitopes on the same target by utilizing immunofluorescent methods to perform a VHH competition assay using VHHs expressed with different epitope tags, specifically myc and E-tag. To test for competition, we probed sporozoites simultaneously with two VHHs, each having a different tag, but with the concentration of a myc-tagged VHH exceeding that of the E-tagged VHH by 10-fold, or vice versa. Binding of VHHs was then analyzed by IFA with secondary antibodies conjugated with fluorescent tags, red for myc tag detection and green for E-tag. VHH competition for the same epitope was considered positive when the VHH present in excess substantially diminished binding of the less abundant VHH being detected by IFA. Controls were included in which each of the VHHs were tested at the same concentrations in the absence of competitor. Supplementary Fig. S4 provides examples of negative and positive competition outcomes between two VHHs. We found that JJS-A6 and JJS-E1 competed for the same epitope on the ~34 kDa protein (Supplementary Fig. S4C). Amongst the two competing VHHs, we chose the stronger competitor, namely JJS-A6, for further studies. Consistent with outcomes of our screening on plastic-coated sporozoite extracts, we were not able to detect significant binding of VHHs from the JJS series by ELISA. These VHHs were however capable of recognizing their targets by western blotting, even at sub-nanomolar concentrations (Fig. 3B).
Fig. 2.
Discovery of a ~34 kDa protein target exposed on the surface of Cryptosporidium parvum sporozoites bound by single-domain camelid antibodies (VHHs). (A) Fluorescent micrographs (600x) localizing FITC-labeled VHH monomers bound to permeabilized sporozoites. Scale bar = 5 μm. (B) An ~34 kDa protein band of separated C. parvum lysate bound by VHH monomers. The experiment was performed in the presence of a non-specific VHH as a negative control and immune alpaca serum as a positive control. (C) Detection of VHH monomers binding the surface of non-permeabilized sporozoites by flow cytometry corroborates findings from microscopy (Mann-Whitney test; *P=0.02). Data is adjusted to ‘no VHH’ control. Values indicate means and error bars indicate S.D. (n=4). (D) Sporozoites fixed with paraformaldehyde remain impermeable to propidium iodide (PI) in contrast to sporozoites fixed with heat as measured by flow cytometry (t-test, P<0.0001). Data points indicate the ratio of the PI signal generated by live sporozoites (n=5). PFA, paraformaldehyde.(E) A single replicate representative of flow cytometry output indicating the intensity of the PI fluorescence signal detected in FLA-3 channel for live, PFA-fixed and heat-permeabilized sporozoites. Sporozoites are pre-gated in excysted oocyst preparation, where R1 represents full oocysts, R2 sporozoites and R4 oocysts in different stages of excystation.
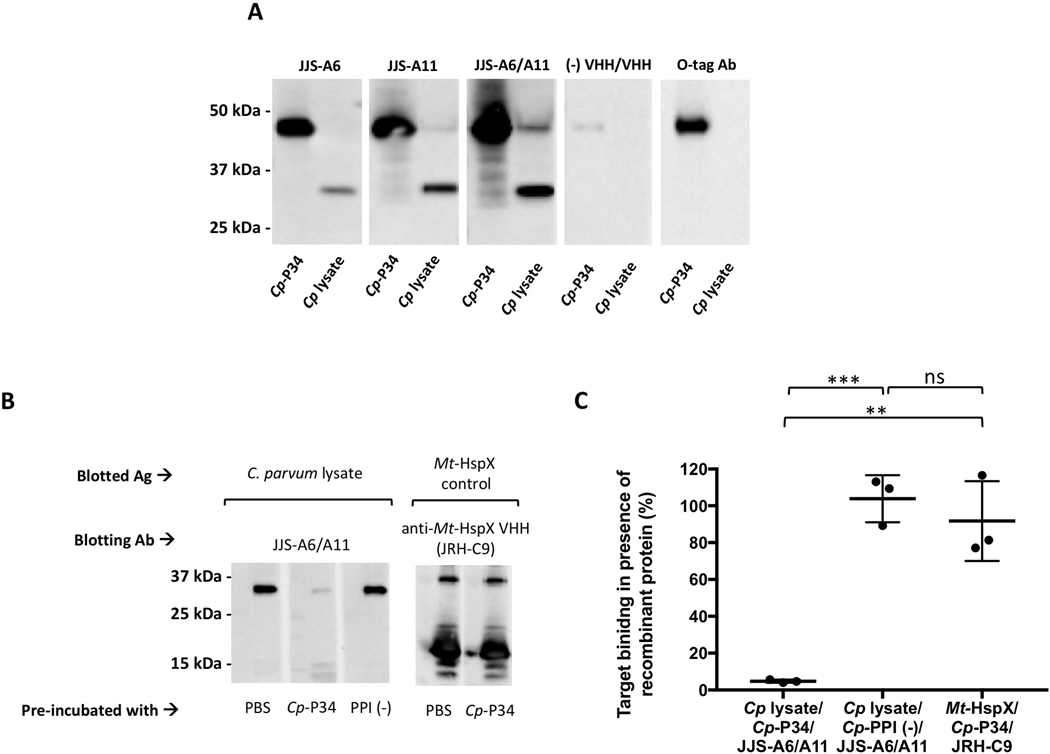
Fig. 3.
Identification of the ~34 kDa protein target as P34 of Cryptosporidium parvum (Cp-P34). (A) Recombinant Cp-P34 was resolved by SDS-PAGE in parallel with C. parvum lysate and blotted for recognition by the two JJS-A6 or JJS-A11 monomeric single-domain camelid antibodies (VHHs), the JJS-A6/A11 VHH dimer or a negative control VHH dimer control. The recombinant Cp-P34 is identified using an anti-OLLAS-tag horseradish peroxidase (HRP) conjugate. Ab, antibody (B) Cryptosporidium parvum lysate was separated and blotted with the mixture of a VHH at 0.01 μg/mL (0.2 nM for dimers and 0.3 nM for monomers) with recombinant protein at 0.25 μg/mL or PBS. A recombinant heat-shock protein of Mycobacterium tuberculosis (Mt-HspX) was included as a control antigen and blotted with an anti-HspX VHH monomer (JRH-C9). Additionally, recombinant peptidylprolyl isomerase of C. parvum (PPI) was included as a non-specific recombinant protein control. Ag, antigen (C) Summary of densitometry analysis from B indicating the percent of VHH binding in the presence of recombinant proteins as standardized to the PBS control. The binding of a JJS-A6/A11 heterodimer was blocked in the presence of recombinant Cp-P34 by 95.2±0.8% (t-test, **P=0.002, ***P=0.0002) but was unaffected in presence of the non-specific negative control protein Cp-PPI (t-test, P=0.44). Additional control indicates that JRH-C9 binding to Mt-HspX was unaffected by the presence of the recombinant Cp-P34 and maintained its binding at 91.7±21.6%. Values indicate means and error bars indicate S.D. (n=3). ns, not significant.
In an effort to highly enrich the target protein from C. parvum lysates, we engineered a VHH dimer consisting of non-competing VHH monomers targeting an ~34 kDa protein as a ‘pull-down’ reagent. Combining the two VHHs into a heterodimer permits the protein to bind two different epitopes on the target protein. It has been described before that linking multiple single-domain antibodies increases their valency and synergistically improves their binding capacity (Pluckthun and Pack, 1997; Moayeri et al., 2015; Barta et al., 2017). In this work, we created a heterodimer by linking JJS-A6 and JJS-A11 monomers separated by a commonly used 15-amino acid flexible spacer (GGGGS)3 (Supplementary Table S1) (Wen et al., 2013). The VHH heterodimer was expressed in fusion with either thioredoxin (Trx) partner or GST-tag. The Trx-fused heterodimer clearly bound to the same ~34 kDa protein target as confirmed by microscopy, flow cytometry and western blot analysis (Supplementary Fig. S5).
The GST-fused JJS-A6/A11 heterodimer was utilized to facilitate a pull-down of the ~34 kDa protein from C. parvum lysate using glutathione beads. This product was then subjected to LC/MS-MS analysis. The outcome of this analysis was matched against all putative transcripts from the C. parvum genome and is summarized in Supplementary Table S2. The analysis returned two strong candidates having an approximate predicted molecular size of 34 kDa and an abundant representation in the sample. These candidates are annotated in CryptoDB as: (i) the ‘signal peptide containing protein’ (product of cgd1_1510 gene), and (ii) the ‘MORN repeat containing protein’ (product of cgd5_1640 gene). While an effort was made to express both candidates as recombinant proteins in CHO cells, only the MORN-containing protein yielded significant product and it was called recombinant Cp-P34 protein.
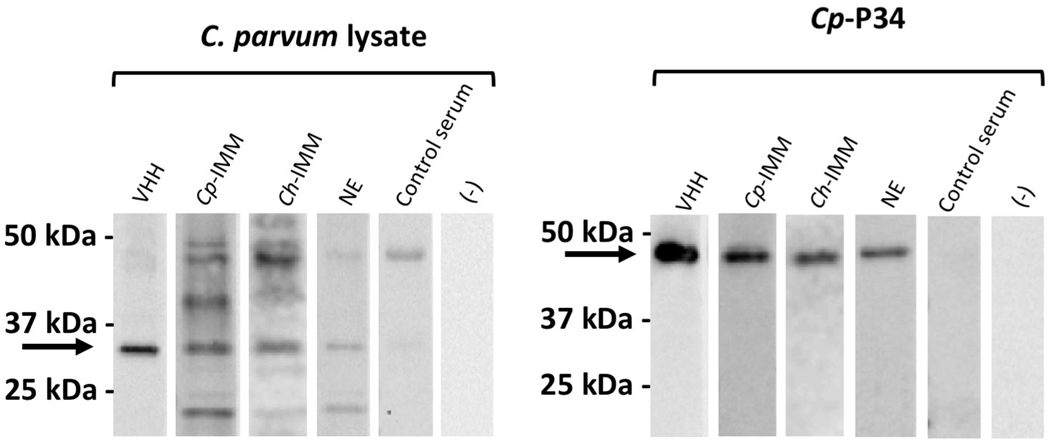
Specific recognition of both the recombinant Cp-P34 and native ~34 kDa proteins was clearly demonstrated by western blotting with the JJS-A6/A11 VHH heterodimer as well as the two monomer VHH components, each producing strong signals even at sub-nanomolar concentrations (Fig. 3A). The results strongly indicated that this protein is the primary target of the JJS series of VHHs. As an additional control, C. parvum lysate was probed with a mixture of JJS-A6/A11, +/− pre-incubation with recombinant Cp-P34, which showed that recombinant Cp-P34 substantially diminished the specific recognition of the native ~34 kDa C. parvum target (Fig. 3B). As expected, pre-incubation of the heterodimer with a negative control recombinant CHO-expressed protein of C. parvum (peptidylprolyl isomerase, Cp-PPI), did not affect the signal. An irrelevant recombinant protein, Mt-HspX, blotted with a VHH binding this protein (JRH-C9) served as an additional control. Lastly, we performed a densitometry analysis and reported the percent of VHH binding in the presence of recombinant protein compared with a PBS control (Fig. 3C). Binding of JJS-A6/A11 to the native target was reduced in the presence of Cp-P34 to 4.8±0.7% but was unchanged in the presence of Cp-PPI at 103.8±10.4% (P=0.0002; t-test), confirming its specificity for Cp-P34. The control VHH specific to the foreign Mt-HspX was unaffected by the presence of Cp-P34 (91.7±17.6%), as expected. The “signal peptide containing protein” co-pulled down with Cp-P34 is likely a binding partner. We consider the likelihood of cross-specificity due to shared epitopes between “signal peptide containing protein” and Cp-P34 to be highly unlikely due primarily to the complete lack of significant primary sequence homology.
3.2.2. Identification of sporozoite surface-binding VHHs recognizing Cp-gp900
Two camelid antibodies, JMP-F7 and JJ-D1, shown to bind a target localized to the anterior pole (Supplementary Fig. S3A), which is defined morphologically by a pointed end in contrast to the more rounded posterior end (Snelling et al., 2007). Both of these VHHs were found to recognize a high molecular weight target >250 kDa on western blots (Supplementary Fig. S3B). Additionally, JMP-F7 bound prominent trails left behind the gliding parasite (Fig. 4A). We hypothesized that the likeliest target of these VHHs was a previously reported invasive and highly immunogenic antigen, namely the large (~900 kDa) secretory glycoprotein (gp900) (Petersen et al., 1992, 1997; Barnes et al., 1998). Based on the mucin-like structure of gp900, we hypothesized that the C-terminal domain would be the most accessible and immunogenic region. To test this idea, we expressed a recombinant protein containing about 33 kDa of Cp-gp900 from the carboxyl-terminal region of domain 5, truncated by 85 amino acids to remove a hydrophobic region at the C-terminus (Cp-gp900-CT) (Supplementary Table S3). We then evaluated JMP-F7 and JJ-D1 binding to Cp-gp900-CT by western blot analysis and confirmed that both VHHs recognized Cp-gp900-CT (Supplementary Fig. S6A). We also demonstrated that pre-incubation with C. parvum lysate significantly reduced binding of either VHH to the Cp-gp900-CT while showing that the lysate had no impact on the binding of a control VHH to its target antigen (Supplementary Figs. S6B and C). Our observation of gp900 at the anterior pole of sporozoites is consistent with previous reports showing localization of gp900 to micronemes at the apical complex (Barnes et al., 1998). Additionally, competition studies indicate that JJ-D1 and JMP-F7 VHHs bind different epitopes on Cp-gp900 (Supplementary Fig. S4D).
Fig. 4.
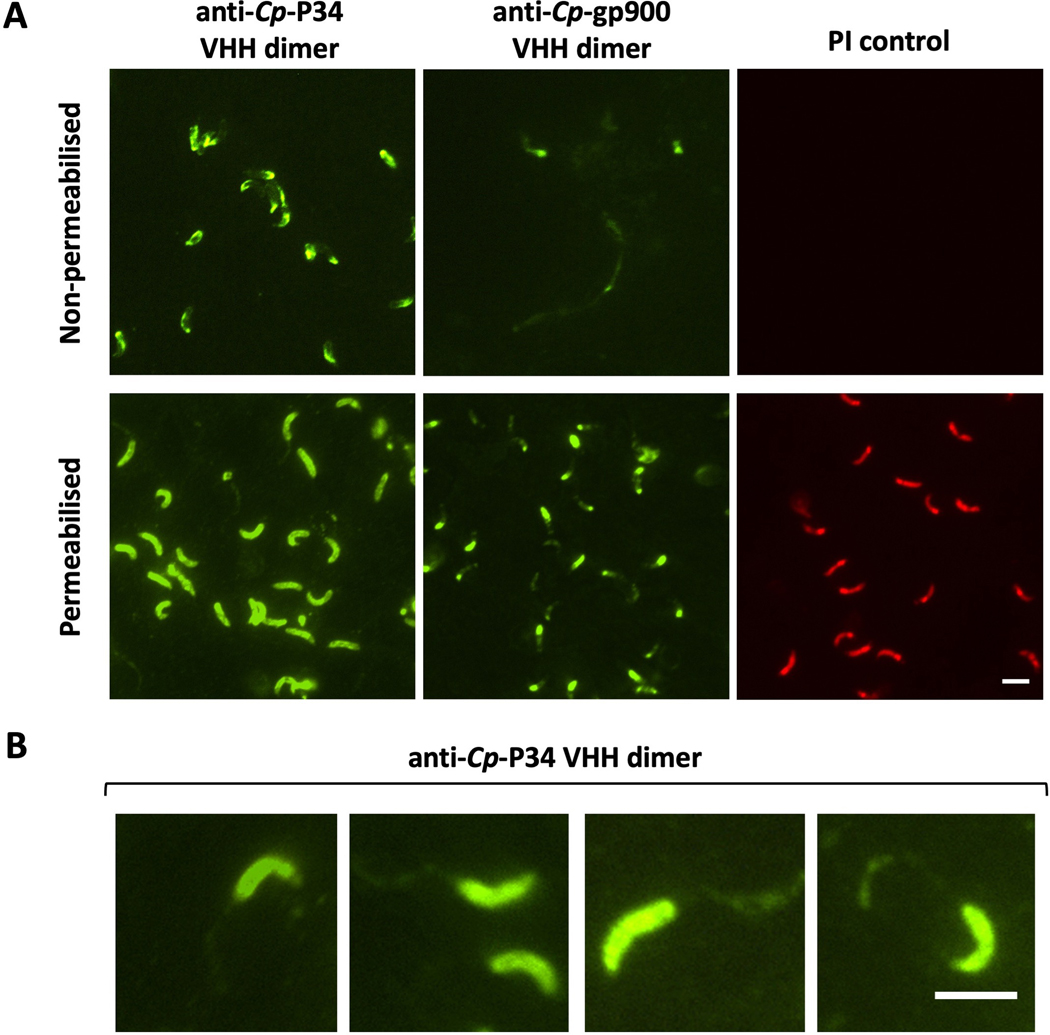
P34 of Cryptosporidium parvum (Cp-P34) appears on the sporozoite surface and is secreted in trails. (A) Fluorescent micrographs (600x) localize bound single-domain camelid antibody (VHH)dimers to non-permeabilized C. parvum sporozoites and sporozoites permeabilized by drying. Lack of permeabilization is confirmed by propidium iodide (PI) exclusion and by the reduced binding of anti-Cp-gp900 VHH dimer (JMP-F7/JMP-F7), which only binds Cp-gp900 accessible on the sporozoite surface or in trails. Scale bar = 5μm. (B) Anti-Cp-P34 VHH dimer (JJS-A6/JJS-A11) binding trails left behind gliding sporozoites. Scale bar = 5μm.
3.2.3. Discovery of VHHs binding other C. parvum protein targets
During our screening of VHHs to sporozoite proteins, we discovered several VHHs to additional unidentified sporozoite protein targets (Supplementary Fig. S7). Two VHHs (JMP-D4 and JJS-G3) were identified that bound targets shown by IFA to be localized at the anterior pole of sporozoites. Although these two VHHs produced a strong signal staining sporozoites by fluorescent microscopy (Supplementary Fig. S7A), they did not recognize a sporozoite target on western blots (Supplementary Fig. S7B). Most likely, these VHHs bind conformational epitopes which do not recognize their targets after loss of their quaternary structure following SDS-PAGE. Additionally, when panning on sporozoite lysate, we discovered two VHHs (JMP-E6 and JMP-F5) which bind unknown targets of molecular weight ranging from 130–250 kDa which are localized to multiple foci inside the sporozoites. It is however unlikely that these targets are transported through the membrane to the sporozoite surface as they produced no signal by flow cytometry after staining of sporozoites and are absent in shed trails (not shown).
3.3. Characterization of Cp-P34
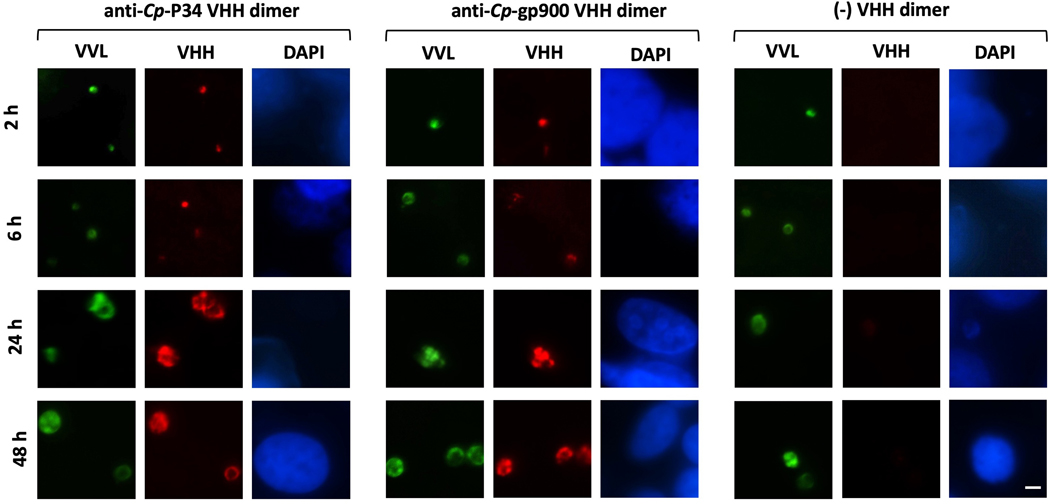
While Cp-P34 is shown to localize in the intracellular compartment of excysted and permeabilized sporozoites, experimentation with non-permeabilized sporozoites indicates that some of the protein also appears on the surface (Fig. 4A). Non-permeabilized sporozoites were captured onto poly-l-lysine slides, kept moist to prevent permeabilization due to dehydration and confirmed as intact by their exclusion of PI. As further evidence the captured sporozoite membranes remained intact, the anti-Cp-gp900 VHH was unable to bind intracellular gp900, which by contrast is well stained in permeabilized sporozoites. In non-permeabilized sporozoites, VHHs to Cp-gp900 only stained the extracellular trails left behind by sporozoites as they glide on the surface. On careful inspection of micrographs, we also observed staining of these trails by VHHs binding to Cp-P34 (Fig. 4B). While the expression of Cp-P34 is triggered by excystation, the protein remains detectable for at least 48 h during intracellular development, as does Cp-gp900 (Fig. 5).
Fig. 5.
Expression pattern of P34 (Cp-P34) and gp900 (Cp-gp900) of Cryptosporidium parvum during intracellular development in vitro. Fluorescent micrographs (600x) demonstrating Madin-Darby bovine kidney (MDBK) cells with intracellular stages of C. parvum over the course of infection- at 2, 6, 24 and 48 h p.i. Parasites are stained green with FITC-labeled Vicia villosa lectin (VVL); binding of the anti-Cp-P34 VHH dimer (JJS-A6/JJS-A11) and anti-Cp-gp900 VHH dimer (JMP-F7/JMP-F7) are detected in red with TRITC-labeled secondary antibody and the cell nucleus is counter-stained in blue with DAPI. A non-specific VHH dimer is included as a negative control. Scale bar = 5 μm.
Cp-P34 contains multiple MORN repeats at the N-terminus. MORN repeats are a common motif present in many proteins of eukaryotes and prokaryotes (Takeshima et al., 2000). The motif consists of 2–20 repeats of tyrosine, glutamic acid and glycine residues at the N-terminus which are generally conserved across species (Artz et al., 2011; Sajko et al., 2019). In fact, the primary structure of the Cp-P34 aligned from the N to C terminus indicates the presence of 11 MORN repeats at the N terminus with highly conserved glycines in position 3 and 13–15 with a rough consensus of YEGEYxxxGxKxGxGxYTxxNGxx (Supplementary Fig. S8).
Given that surface and secretory proteins of Cryptosporidium sporozoites are often heavily glycosylated (Liu et al., 2016; Haserick et al., 2017), we investigated whether Cp-P34 displays characteristics of glycoproteins such as the presence of N-linked and O-linked glycans. Firstly, N-linked polysaccharides were removed from C. parvum lysate by treatment with PNGase F before separation on SDS-PAGE gels. Glycosylated proteins should become reduced in molecular size as a result of enzymatic deglycosylation and thus migrate faster in gels. The native Cp-P34 was evaluated by western blot comparing deglycosylated and untreated C. parvum sporozoite lysates (Supplementary Fig. S9A). No band mobility shift was observed for the Cp-P34 band as recognized by the specific VHH heterodimer, indicating that the target is likely not a glycoprotein. In contrast, Cp-gp900 demonstrates a dramatic band mobility shift post-deglycosylation, which is consistent with its glycoprotein identity. Coincidentally, PNGase F is of approximately the same size as Cp-P34 and created a weak background signal in the blots that can be disregarded. Separately, O-linked polysaccharides were removed from C. parvum lysate by periodate oxidation after separation on the gel. Here recognition of targets by specific VHHs was evaluated to determine the O-glycosylation status of bound epitopes. Binding of Cp-P34 by the specific VHH dimer was retained following periodate treatment indicating that epitope binding is not dependent on glycosylation (Supplementary Fig. S9B). This is in contrast to Cp-gp900 for which VHH recognition was abolished as a result of O-glycan removal. Additionally, a glycosylation-resistant mAb (3A1) known to bind a protein epitope (unpublished observation) was included here as a control and demonstrated enhanced binding post-deglycosylation. Our findings with Cp-P34 are consistent with analysis of its amino acid sequence using glycosylation prediction software which indicates no putative N- and O-glycosylation sites to be present.
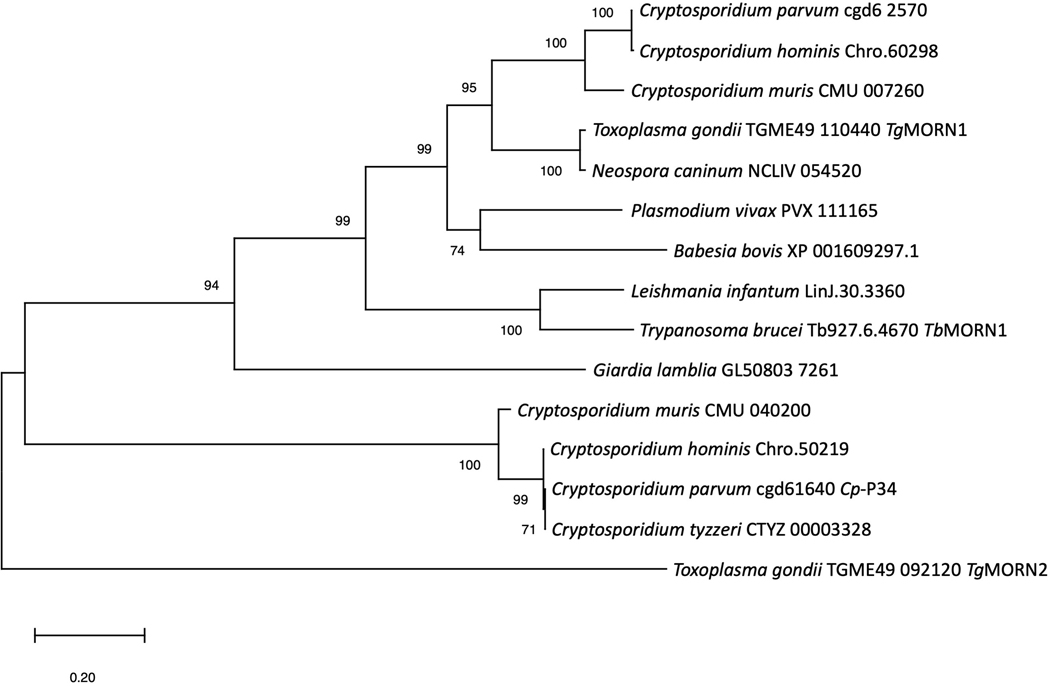
A BLASTp search of the OrthoMCL database with Cp-P34 identified 48 genes orthologous to gene cgd5_1640 with identities ranging from 30% to 100% as summarized in Supplementary Table S4. An identical gene was identified in C. tyzzeri on the same chromosome 5 (CTYZ_00003328). Near identical genes were found also in C. hominis (Chro.50219), and Cryptosporidium muris (CMU_040200) sharing 99% and 91% identity, respectively. This is consistent with our ability to detect Cp-P34 on sporozoites of C. hominis and C. tyzzeri using the specific VHH dimer (Supplementary Fig. S10). Also sharing similarities with Cp-P34 is a group of genes from protozoan species such as the well described TgMORN1 of Toxoplasma gondii (Gubbels et al., 2006; Ferguson et al., 2008; Lorestani et al., 2010) and TbMORN1 of Trypanosoma brucei (Morriswood and Schmidt, 2015), and some genes from distant organisms such as plants and primates, with identities ranging from 31–39%. On a closer look, these similarities are attributed to the highly conserved MORN repeat motif rather than overall gene sequence homology. In fact, phylogenetic analysis shows that Cp-P34 (cgd5_1640) and its close orthologs in other Cryptosporidium spp. (>90% identity) cluster independently, while other MORN-containing proteins, including another group from Cryptosporidium, cluster much more closely with the TgMORN1 than to Cp-P34 (Fig. 6). This suggests that the MORN-containing protein family identified as close orthologs of Cp-P34 is unique to Cryptosporidium and likely performs a new role that is quite different to that of TgMORN1 and its orthologs.
Fig. 6.
Maximum likelihood phylogenetic tree of selected orthologs of P34 of Cryptosporidium parvum (Cp-P34) orthologs. Cp-P34 coded by the cgd5_1640 gene and its close orthologs are unique and cluster away from the group of orthologs of TgMORN1 of Toxoplasma gondii and TbMORN1 of Trypanosoma brucei, including those of Cryptosporidium spp. The evolutionary history was inferred by using the Maximum Likelihood method with 500 bootstrap replications and JTT matrix-based model (Jones et al., 1992). The tree with the highest log likelihood (−6163.19) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Joining and BioNJ algorithms to a matrix of pairwise distances estimated using a JTT model, and then selecting the topology with superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. This analysis involved 15 amino acid sequences. There was a total of 383 positions in the final dataset. Evolutionary analyses were conducted in MEGA X (Kumar et al., 2018; Stecher et al., 2020).
During our studies we noted that Cp-P34 appeared to be an immunogenic Cryptosporidium protein as a result of natural pasture exposures based on the observed serum antibodies mounted against it by mature, grazing alpacas. For example, both native and recombinant Cp-P34 were recognized by the sera of alpacas used for VHH discovery following parenteral immunization with C. parvum and C. hominis lysate. However, the pre-immune serum, obtained prior to experimental immunization with C. parvum lysate, also showed binding to recombinant and native Cp-P34, presumably as a result of natural exposure at pasture (Fig. 7). We thus performed a serological survey of randomly selected adult grazing alpacas from local farms in the northeastern region of the USA, which found >50% seropositivity to Cp-P34, indicating that the natural field exposure to related Cryptosporidium spp. is often sufficient to elicit cross-specific anti-Cp-P34 antibody responses (Supplementary Fig. S11).
Fig. 7.
Recognition of P34 of Cryptosporidium parvum (Cp-P34) by immune alpaca sera. Immunoblots of separated C. parvum lysate proteins and recombinant Cp-P34, probed with the anti-Cp-P34 JJS-A6/A11 dimer (VHH) or various alpaca sera: from alpaca immunized with lysates of C. parvum (Cp-IMM) or Cryptosporidium hominis (Ch-IMM), alpaca following natural exposure (NE), a control serum from a juvenile alpaca, or ‘no serum’ negative control. The recombinant and native Cp-P34 are indicated by arrows.
Finally, we tested whether the anti-Cp-P34 VHHs inhibited C. parvum infection in a cell-based assay (Supplementary Fig. S12). None of the anti-Cp-P34 VHH agents, or the VHHs targeting gp900, proved capable of eliciting a significant level of parasite infection inhibition, while a paromomycin positive control was significantly protective.
4. Discussion
In this study, we identified VHH antibodies recognizing the surface-exposed proteins of C. parvum sporozoites by selection of VHH-displaying phage based on their affinity to intact sporozoites. Using these VHHs, we identified a novel sporozoite surface-exposed protein called Cp-P34. The VHHs were obtained from B cells of an alpaca immunized with a C. parvum sporozoite lysate. Multiple parenteral immunizations of the alpaca boosted pre-existing serum antibodies to sporozoite antigens that had likely resulted from natural field exposure to other Cryptosporidium spp. As a result, we may have selectively boosted responses to immunogenic, evolutionarily conserved sporozoite proteins.
To select for surface-specific VHHs, we employed two different strategies. Initial VHHs were identified by panning for phage binding to a parasite lysate. This approach did not lead to identification of VHHs recognizing novel proteins on the surface of intact parasites. We thus modified the panning strategy to bias the selection towards VHHs that bind the parasite surface. The surface of Cryptosporidium sporozoites is a dynamic medium on which many proteins important for host-cell invasion appear transiently and, as such, it is challenging to target this surface for antibody selection. For example, micronemal proteins often transit to the membrane where they establish temporary residence by means of transmembrane domain anchorage before being cleaved off and shed in trails as the parasite glides forward. The actin-myosin motor system mediates forward movement of the pellicle, which leaves behind tracks consisting of membranous and other secretory proteins. To select for VHHs recognizing this dynamic surface, we performed a second round of phage panning on fixed, non-permeabilized sporozoites. As a result of those selections, we successfully identified several different VHHs that bind intact sporozoites, some recognizing a previously described invasive protein gp900, and some binding a previously unknown target, Cp-P34, which is present both inside sporozoites and exposed on their surface. We also generated VHHs binding several other, yet unidentified, targets including one on the sporozoite surface. The success of our panning strategies seems to be validated by our re-isolation of several VHHs recognizing the same C. parvum antigens despite the use of two different panning strategies. The novel MORN repeat-containing protein we identified and named Cp-P34 in this report was, however, found only by panning on intact sporozoites.
MORN is a common interaction domain mediating protein to protein interactions and phospholipid binding (Ma et al., 2006; Im et al., 2007; Camacho et al., 2009). The function of several proteins containing MORN repeats has been previously studied in protozoans. The most studied is TgMORN1 of T. gondii which is required for replicative division of the parasite (Lorestani et al., 2010) and TbMORN1 of T. brucei which is essential for parasite survival by regulating the flow of molecules through the flagellar pocket (Morriswood and Schmidt, 2015; Sajko et al., 2019), both of which localize to the cytoskeleton in the inner membrane complex. Their orthologs in many species of apicomplexans are often annotated as phosphatidylinositol-4-phosphate 5-kinase and have a conserved role in asexual and sexual development (Gubbels et al., 2006; Ferguson et al., 2008; Lorestani et al., 2010). Orthologs of TgMORN1 and TbMORN1 in other protozoan parasites, including Cryptosporidium, share as much as 75% amino acid identity between each other. However, these two most commonly referenced orthologs in protozoans share only about 30% identity with Cp-P34, mostly attributed to the conserved MORN repeat motifs, and thus are not likely to be functional homologs. The Cp-P34 homologous group appears unique to Cryptosporidium and, except for the conserved MORN repeats, does not share significant amino acid sequence homology with any proteins yet identified in other species.
Surprisingly, the Cp-P34 sequence does not possess any predicted transmembrane domain, signal peptide or GPI anchor, thus it remains unclear how this protein appears on the surface of sporozoites and becomes shed in trails. The p23 (product of cgd4_3620 gene) is another example of an invasive protein which transiently occupies a parasite surface niche while lacking membrane association/secretion domains. We hypothesize that Cp-P34 deposits in the intracellular or intramembrane compartment and is transported to the surface where it is subsequently released in trails. The connectivity between motor function and protein secretion has been proposed in models of surface adhesin release in apicomplexans (Baum et al., 2006; Frenal et al., 2010; Jacot et al., 2016). The model of the apicomplexan glideosome also indicates linkage between cytoskeleton and surface adhesins facilitated by the glideosome-associated connector linkage (Jacot et al., 2016). Interactions of MORN-containing proteins with the cytoskeleton have been described before in other apicomplexans, e.g., TgMORN1 co-localizes to myosin C at the IMC of T. gondii (Gubbels et al., 2006). It is thus possible that Cp-P34 serves an inner membrane anchoring role by association with the cytoskeleton. Given that MORN repeats are interaction domains, it is possible that Cp-P34 utilizes the MORN motif to associate with the Cryptosporidium glideosome-associated proteins. Such protein complexes may be important to the mechanism by which Cp-P34 traffics to the sporozoite surface during gliding and help explain the presence of Cp-P34 in shed trails. One possible Cp-P34 binding partner might be the Cryptosporidium species-specific ‘signal peptide containing protein’ identified in our VHH heterodimer Cp-P34 pull-down study. Another possibility is that Cp-P34 associates with another protein, which provides anchorage in the plasma membrane and is liberated by the action of proteases similarly to how gp40 tethers to the membrane by association with gp15 (O’Connor et al., 2007). Regardless of the mechanism by which Cp-P34 arrives on the surface and is shed in trails, it seems to be an attractive target to investigate in the context of vaccination. While the function of Cp-P34 remains unknown, the fact that it is expressed at the sporozoite stage and released during gliding suggests it plays a role in the attachment and invasion processes. Other proteins involved in this process, such as gp900, have shown promise as vaccine targets. If it proves to elicit protective immunity, the presence of highly conserved Cp-P34 orthologs across a range of known Cryptosporidium pathogens would be advantageous. Additionally, the lack of glycosylation offers another benefit as it would simplify manufacture of the recombinant protein for vaccination. In one test of the target as a vaccine antigen, we were unable to block sporozoite infection in vitro with high concentrations of VHH proteins that bind to Cp-P34. While this finding was disappointing, it is important to recognize that camelid antibodies are epitope-specific and may not bind to sites that interfere with the normal functions of their protein targets. Future studies evaluating the anti-infective properties of polyclonal antisera to Cp-P34 will provide better insight into the possible role of this protein in the process of invasion and its eligibility as a vaccine candidate, alone or in polyvalent combinations with other invasive protein targets. Additionally, VHHs targeting non-neutralizing target epitopes may still be useful in other anti-infection strategies such as coupled with an immunotoxin partner or by promoting parasite aggregation or trapping to reduce motility and accelerate parasite elimination from the gastrointestinal tract.
Beyond other applications, panning of VHH-displayed phage libraries serves as a useful tool for discovery of novel targets. This project demonstrates the ability of the VHH-phage panning method to identify targets on the surface of protozoan pathogens. We show that antibody panning can be tailored to complex antigen mixtures such as a parasite lysate or a parasite surface, and that such methods can have value in the search for new vaccine candidates.
Supplementary Material
Supplementary Fig. S4. Epitope competition between single-domain camelid antibodies (VHHs). Two-color fluorescent micrographs (600x) of Cryptosporidium parvum sporozoites fixed to the slide and probed with two VHHs expressed with either an E-tag or a myc tag, such that the concentration of one exceeded that of the other by 10-fold. Myc-tagged VHHs were detected with TRITC-labeled antibody (red), while E-tagged VHHs were detected with FITC-labeled antibody (green). The images were merged using ImageJ 1.48v software (Rasband, 2018). (A, B, C) Binding of VHHs targeting sporozoitès ~34 kDa protein and (D) of >250 kDa protein. (Aa, Ba, Ca, Da) Binding of specific myc-tagged VHH in presence of a non-specific E-tagged VHH probed in excess which serves as a control. Scale bar = 5 μm.
Supplementary Fig. S12. Anti-Cryptosporidium single-domain camelid antibodies (VHHs) lack neutralization properties in vitro. Madin-Darby bovine kidney (MDBK) cells were infected with Cryptosporidium parvum oocysts in the presence of VHHs at 100 μg/ml for 24 h. Data points indicate reductions in the number of detected intracellular stages of the parasite in reference to the untreated control. Infection was performed in the presence of a mock treatment with a non-specific control VHH as a negative control and paromomycin as a positive control. None of the VHHs produced statistically significant neutralization in comparison to a control VHH (Mann-Whitney test, P>0.17). Paromomycin reduced infection by 69.0±8.2% (Mann-Whitney test, P=0.002). Values indicate means and error bars indicate S.D. (n=6 for monomers; n=7 for dimers).
Supplementary Fig. S1. Development of immune response against Cryptosporidium parvum in alpaca. An adult alpaca was repeatedly immunized with C. parvum sporozoite lysate and monitored for anti-lysate serum titers. The immune response was measured by ELISA using whole lysate of C. parvum as a coating antigen. Sera obtained before the first and after the last immunization are referred to as pre-immune and post-immune, respectively. Control serum derives from a juvenile alpaca with limited field exposure. Immunizations boost anti-lysate titers (*** P<0.0001; two-way ANOVA). Serum from a juvenile alpaca with limited exposure to the field was included as a control serum.
Supplementary Fig. S2. Alignment of amino acid sequences of 10 unique single-domain camelid antibodies (VHHs) specific to Cryptosporidium parvum. Alignment of the best representatives of all 10 unique VHH families are shown with their three complementarity-determining regions (CDRs) indicated to facilitate comparisons. Sequences were aligned using BioEdit (Hall, 1999).
Supplementary Fig. S3. Identification of >250 kDa protein at the anterior pole of Cryptosporidium parvum sporozoites. (A) Fluorescent micrographs (600x) demonstrate FITC-labeled single-domain camelid antibody (VHH) bound to permeabilized sporozoites. Individual and mergedfluorescent and bright field (BF) images obtained with differential interference contrast (DIC) localize the VHH signal to the anterior pole of sporozoites. Scale bar = 5 μm. (B) Western blot of a separated whole C. parvum lysate bound by VHHs in the presence of a non-specific VHH as a negative control and immune alpaca serum as a positive control (showing the repertoire of immunogenic protein bands within C. parvum lysate).
Supplementary Fig. S5. Single-domain camelid antibody (VHH) dimer binding the ~34 kDa surface-exposed protein of Cryptosporidium parvum. (A) Fluorescent micrographs (600x) demonstrate FITC-labeled VHH dimer bound to permeabilized sporozoites. Merged fluorescent and bright field (BF) images obtained with differential interference contrast (DIC) indicate bound VHH at the anterior pole of sporozoites. Black arrows indicate negatively stained intact oocysts. Scale bar = 5 μm. (B) Detection of VHH dimer binding to the surface of non-permeabilized sporozoites by flow cytometry corroborates findings from microscopy (Mann-Whitney test; *P=0.02). Data is adjusted to ‘no VHH’ control. Values indicate means and error bars indicate S.D. (n=4). (C) An ~34 kDa protein band of separated C. parvum lysate bound by the VHH dimer. The experiment was performed in presence of a non-specific VHH as a negative control and immune alpaca serum as a positive control (showing the repertoire of immunogenic protein bands within C. parvum lysate).
Supplementary Fig. S6. Identification of the >250 kDa protein as gp900 of Cryptosporidium parvum (Cp-gp900). (A) Recombinant carboxyl terminus of Cp-gp900 (Cp-gp900-CT) was resolved by SDS-PAGE and blotted with single-domain camelid antibodies (VHHs). The recombinant Cp-gp900-CT is identified with an anti-OLLAS-tag horseradish peroxidase (HRP) conjugate. (B) Recombinant Cp-gp900-CT was resolved by SDS-PAGE and each membrane strip was blotted with the mixture of VHH at 0.01 μg/mL and 10 μg/mL C. parvum lysate (Cp lysate) or PBS. A recombinant heat-shock protein of Mycobacterium tuberculosis (Mt-HspX) was included as a control antigen and blotted with an anti-HspX VHH (JRH-C9). (C) The summary of densitometry analysis from B indicates the percent of VHH binding in the presence of C. parvum antigen versus the PBS control. Binding of JJ-D1 and JMP-F7 was reduced in the presence of C. parvum native target by 89.5±3.8% (t-test, ***P=0.0002) and 88.8±2.9% (t-test, ***P=0.0002), respectively. Binding of the control JRH-C9 VHH was not significantly affected by the presence of C. parvum proteins. Values indicate means and error bars indicate S.D. (n=3).
Supplementary Fig. S7. Other Cryptosporidium parvum-specific single-domain camelid antibodies (VHHs) identified in this study. (A) Fluorescent micrographs (600x) localizing FITC-labeled VHH bound to permeabilized sporozoites. Merged fluorescent and bright field (BF) images obtained with differential interference contrast(DIC) indicate localization of bound VHH in green. Scale bar = 5 μm. (B) Western blot of a separated whole lysate of C. parvum bound by VHHs in the presence of a non-specific VHH as a negative control and immune alpaca serum as a positive control.
Supplementary Fig. S8. Alignment of conserved membrane occupation and recognition nexus (MORN) repeats in the P34 of Cryptosporidium parvum (Cp-P34) sequence. (A) Structure of Cp-P34 with individual MORN repeats shown in alignment from N to C terminus (left to right) and shaded according to amino conservation in occupied position. (B) Sequence logo indicates highly conserved MORN repeat motifs with a rough consensus of YEGEYxxGxKxGxGxYTxxNGxx. Sequence logo was generated using WebLogo (Crooks et al., 2004).
Supplementary Fig. S9. N- and O-deglycosylation of P34 of Cryptosporidium parvum (Cp-P34). (A) A whole lysate of C. parvum was separated after removal of N-glycans with PNGase F and probed with single-domain camelid antibody (VHH) dimers in parallel to the untreated control lysate. The experiment was performed in the presence of negative controls: non-specific VHH dimer, ‘no VHH dimer’ and vehicle controls, which indicate non-specific background signal. (B) A whole lysate of C. parvum or recombinant carboxyl terminus of gp900 of C. parvum (Cp-gp900-CT) was separated before removal of O-glycans with sodium periodate and probed with VHH dimers in parallel to the untreated control lysate. A deglycosylation-resistant 3A1 monoclonal antibody was included as a negative control. Small bands visible to the left of each blot are molecular marker bands.
Supplementary Fig. S10. Cross-species reactivity of single-domain camelid antibodies (VHHs) generated against P34 (Cp-P34) and gp900 (Cp-gp900) of Cryptosporidium parvum. Fluorescent micrographs (600x) indicate that homologous targets of anti-Cp-P34 VHH dimer (JJS-A6/A11) and anti-Cp-gp900 VHH dimer (JMP-F7/F7) are present in sporozoites of C. parvum, Cryptosporidium hominis and Cryptosporidium tyzzeri spp. Scale bar = 5 μm.
Supplementary Fig. S11. Serological screen of reactivity against P34 of Cryptosporidium parvum (Cp-P34) among adult alpacas. Immunoblots of the separated recombinant Cp-P34 were probed with alpaca sera at 1:500 dilution and detected using anti-llama horseradish peroxidase (HRP) conjugate. For each serum, a band corresponding to Cp-P34 was quantified by image densitometry and normalized to the background (an equal sized segment immediately below the Cp-P34 band). The bars indicate the ratio of the Cp-P34 signal to the background signal. Serum from an immune alpaca immunized with C. parvum lysate (Cp-IMM alpaca) served as a positive control. Sera from two juvenile alpacas with limited exposure to the field were included as control sera. The experiment was performed in the presence of a ‘no serum’ negative control.
Highlights.
A novel Cryptosporidium sporozoite surface antigen, Cp-P34, was discovered
Cp-P34 is the target of a camelid VHH antibody selected on fixed sporozoites
Non-permeabilized sporozoites are brightly stained by antibodies to Cp-P34
Cp-P34 deposits in sporozoite gliding trails, similarly to known virulence factors
Cp-P34 is a MORN-containing protein, apparently unique to Cryptosporidium spp.
Acknowledgements
This work was funded by the National Institutes of Health- Centers of Excellence for Translational Research (NIH-CETR), USA (grant U19AI109776) and internal funding from the Department of Infectious Diseases and Global Health at the Tufts University Cummings School of Veterinary Medicine, USA. The authors thank Dr. Abhineet Sheoran for generation of Cryptosporidium antigen for alpaca immunization and for gifting Cryptosporidium-specific monoclonal antibodies used in this study. The authors acknowledge Dr. Jean Mukherjee for immunizations, blood collections and care of the alpacas, Dr. Gillian Beamer for providing M. tuberculosis HspX and anti-MtHspX VHHs, Dr. Bruno Miranda Oliveira and Sarah Wilson for generation of C. tyzzeri oocysts, Donald Girouard, Melanie Ginese and Julia Dilo for generation of C. hominis oocysts, and Susan Chapman for excellent technical assistance. The authors also thank Ross Tomaino (Taplin Mass Spectrometry Facility, Department of Cell Biology, Harvard Medical School, USA) for conducting mass spectrometry analysis and matching identified peptides against the C. parvum genome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agholi M, Hatam GR, Motazedian MH, 2013. HIV/AIDS-Associated Opportunistic Protozoal Diarrhea. AIDS Res. and Hum. Retrov. 29, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi DE, Feng X, Buckholt MA, Widmer G, Tzipori S, 2002. Genetic analysis of a Cryptosporidium parvum human genotype 1 isolate passaged through different host species. Infect. Immun. 70, 5670–5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison GM, Rogers KA, Borad A, Ahmed S, Karim MM, Kane AV, Hibberd PL, Naumova EN, Calderwood SB, Ryan ET, Khan WA, Ward HD, 2011. Antibody responses to the immunodominant Cryptosporidium gp15 antigen and gp15 polymorphisms in a case-control study of cryptosporidiosis in children in Bangladesh. Am. J. Trop. Med. Hyg. 85, 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrowood MJ, Sterling CR, Healey MC, 1991. Immunofluorescent microscopical visualization of trails left by gliding Cryptosporidium parvum sporozoites. J. Parasitol. 77, 315–317. [PubMed] [Google Scholar]
- Artz JD, Wernimont AK, Allali-Hassani A, Zhao Y, Amani M, Lin YH, Senisterra G, Wasney GA, Fedorov O, King O, Roos A, Lunin VV, Qiu W, Finerty P Jr., Hutchinson A, Chau I, von Delft F, MacKenzie F, Lew J, Kozieradzki I, Vedadi M, Schapira M, Zhang C, Shokat K, Heightman T, Hui R, 2011. The Cryptosporidium parvum kinome. BMC Genomics 12, 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DA, Bonnin A, Huang JX, Gousset L, Wu J, Gut J, Doyle P, Dubremetz JF, Ward H, Petersen C, 1998. A novel multi-domain mucin-like glycoprotein of Cryptosporidium parvum mediates invasion. Mol. Biochem. Parasitol. 96, 93–110. [DOI] [PubMed] [Google Scholar]
- Barta ML, Shearer JP, Arizmendi O, Tremblay JM, Mehzabeen N, Zheng Q, Battaile KP, Lovell S, Tzipori S, Picking WD, Shoemaker CB, Picking WL, 2017. Single-domain antibodies pinpoint potential targets within Shigella invasion plasmid antigen D of the needle tip complex for inhibition of type III secretion. J. Biol. Chem. 292, 16677–16687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum J, Richard D, Healer J, Rug M, Krnajski Z, Gilberger TW, Green JL, Holder AA, Cowman AF, 2006. A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J. Biol. Chem. 281, 5197–5208. [DOI] [PubMed] [Google Scholar]
- Bedi B, Mead JR, 2012. Cryptosporidium parvum antigens induce mouse and human dendritic cells to generate Th1-enhancing cytokines. Parasite Immunol. 34, 473–485. [DOI] [PubMed] [Google Scholar]
- Borad AJ, Allison GM, Wang D, Ahmed S, Karim MM, Kane AV, Moy J, Hibberd PL, Ajjampur SS, Kang G, Calderwood SB, Ryan ET, Naumova E, Khan WA, Ward HD, 2012. Systemic antibody responses to the immunodominant p23 antigen and p23 polymorphisms in children with cryptosporidiosis in Bangladesh. Am. J. Trop. Med. Hyg. 86, 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter-Bitzer JI, Lee H, Trevors JT, 2009. Single-chain variable fragment antibodies selected by phage display against the sporozoite surface antigen P23 Of Cryptosporidium parvum. J. Parasitol. 95, 75–81. [DOI] [PubMed] [Google Scholar]
- Boulter-Bitzer JI, Lee H, Trevors JT, 2010. Single chain variable fragment antibodies selected by phage display against the sporozoite surface antigen S16 of Cryptosporidium parvum. Exp. Parasitol. 125, 124–129. [DOI] [PubMed] [Google Scholar]
- Bouzid M, Hunter PR, Chalmers RM, Tyler KM, 2013. Cryptosporidium Pathogenicity and Virulence. Clin. Microbiol. Rev. 26, 115–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho L, Smertenko AP, Perez-Gomez J, Hussey PJ, Moore I, 2009. Arabidopsis Rab-E GTPases exhibit a novel interaction with a plasma-membrane phosphatidylinositol-4-phosphate 5-kinase. J. Cell Sci. 122, 4383–4392. [DOI] [PubMed] [Google Scholar]
- Chappell CL, Okhuysen PC, Sterling CR, Wang C, Jakubowski W, Dupont HL, 1999. Infectivity of Cryptosporidium parvum in healthy adults with pre-existing anti-C. parvum serum immunoglobulin G. Am. J. Trop. Med. Hyg. 60, 157–164. [DOI] [PubMed] [Google Scholar]
- Chen L, Williams BR, Yang C-Y, Cevallos AM, Bhat N, Ward H, Sharon J, 2003. Polyclonal Fab phage display libraries with a high percentage of diverse clones to Cryptosporidium parvum glycoproteins. Int. J. Parasitol. 33, 281–291. [DOI] [PubMed] [Google Scholar]
- Chen XM, O’Hara SP, Huang BQ, Nelson JB, Lin JJ, Zhu G, Ward HD, LaRusso NF, 2004. Apical organelle discharge by Cryptosporidium parvum is temperature, cytoskeleton, and intracellular calcium dependent and required for host cell invasion. Infect. Immun. 72, 6806–6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE, 2004. WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Current WL, Haynes TB, 1984. Complete development of Cryptosporidium in cell culture. Science 224, 603–605. [DOI] [PubMed] [Google Scholar]
- Deng M, Templeton TJ, London NR, Bauer C, Schroeder AA, Abrahamsen MS, 2002. Cryptosporidium parvum genes containing thrombospondin type 1 domains. Infect. Immun. 70, 6987–6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehigiator HN, Romagnoli P, Priest JW, Secor WE, Mead JR, 2007. Induction of murine immune responses by DNA encoding a 23-kDa antigen of Cryptosporidium parvum. Parasitol. Res. 101, 943–950. [DOI] [PubMed] [Google Scholar]
- Enriquez FJ, Riggs MW, 1998. Role of immunoglobulin A monoclonal antibodies against P23 in controlling murine Cryptosporidium parvum infection. Infect. Immun. 66, 4469–4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DJ, Sahoo N, Pinches RA, Bumstead JM, Tomley FM, Gubbels MJ, 2008. MORN1 has a conserved role in asexual and sexual development across the apicomplexa. Eukaryot. Cell 7, 698–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanigan T, Whalen C, Turner J, Soave R, Toerner J, Havlir D, Kotler D, 1992. Cryptosporidium infection and CD4 counts. Ann. Intern. Med. 116, 840–842. [DOI] [PubMed] [Google Scholar]
- Floren CH, Chinenye S, Elfstrand L, Hagman C, Ihse I, 2006. ColoPlus, a new product based on bovine colostrum, alleviates HIV-associated diarrhoea. Scand. J. Gastroenterol. 41, 682–686. [DOI] [PubMed] [Google Scholar]
- Frenal K, Polonais V, Marq JB, Stratmann R, Limenitakis J, Soldati-Favre D, 2010. Functional dissection of the apicomplexan glideosome molecular architecture. Cell Host Microbe 8, 343–357. [DOI] [PubMed] [Google Scholar]
- Frost F, Craun GF, Calderon R, Hubbs SA, 1997. So Many Oocysts, So Few Outbreaks. J. Am. Water Works Ass. 89, 8–10. [Google Scholar]
- Frost FJ, Tollestrup K, Craun GF, Fairley CK, Sinclair MI, Kunde TR, 2005. Protective immunity associated with a strong serological response to a Cryptosporidium-specific antigen group, in HIV-infected individuals. J. Inf. Dis. 192, 618–621. [DOI] [PubMed] [Google Scholar]
- Greenberg PD, Cello JP, 1996. Treatment of severe diarrhea caused by Cryptosporidium parvum with oral bovine immunoglobulin concentrate in patients with AIDS. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13, 348–354. [DOI] [PubMed] [Google Scholar]
- Gubbels M-J, Duraisingh MT, 2012. Evolution of apicomplexan secretory organelles. Int. J. Parasitol. 42, 1071–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels MJ, Vaishnava S, Boot N, Dubremetz JF, Striepen B, 2006. A MORN-repeat protein is a dynamic component of the Toxoplasma gondii cell division apparatus. J. Cell Sci. 119, 2236–2245. [DOI] [PubMed] [Google Scholar]
- Hall TA, 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Widnows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98. [Google Scholar]
- Haserick JR, Klein JA, Costello CE, Samuelson J, 2017. Cryptosporidium parvum vaccine candidates are incompletely modified with O-linked-N-acetylgalactosamine or contain N-terminal N-myristate and S-palmitate. PLoS One 12, e0182395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Zhao B, Liu L, Zhou K, Qin X, Zhang Q, Li X, Zheng C, Duan M, 2004. The humoral and cellular immune responses in mice induced by DNA vaccine expressing the sporozoite surface protein of Cryptosporidium parvum. DNA Cell Biol. 23, 335–339. [DOI] [PubMed] [Google Scholar]
- Ifeonu OO, Chibucos MC, Orvis J, Su Q, Elwin K, Guo F, Zhang H, Xiao L, Sun M, Chalmers RM, Fraser CM, Zhu G, Kissinger JC, Widmer G, Silva JC, 2016. Annotated draft genome sequences of three species of Cryptosporidium: Cryptosporidium meleagridis isolate UKMEL1, C. baileyi isolate TAMU-09Q1 and C. hominis isolates TU502_2012 and UKH1. Pathog. Dis. 74, ftw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im YJ, Davis AJ, Perera IY, Johannes E, Allen NS, Boss WF, 2007. The N-terminal membrane occupation and recognition nexus domain of Arabidopsis phosphatidylinositol phosphate kinase 1 regulates enzyme activity. J. Biol. Chem. 282, 5443–5452. [DOI] [PubMed] [Google Scholar]
- Jacot D, Tosetti N, Pires I, Stock J, Graindorge A, Hung YF, Han H, Tewari R, Kursula I,Soldati-Favre D, 2016. An Apicomplexan actin-binding protein serves as a connector and lipid sensor to coordinate motility and invasion. Cell Host Microbe 20, 731–743. [DOI] [PubMed] [Google Scholar]
- Jones DT, Taylor WR, Thornton JM, 1992. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 8, 275–282. [DOI] [PubMed] [Google Scholar]
- Kiros H, Nibret E, Munshea A, Kerisew B, Adal M, 2015. Prevalence of intestinal protozoan infections among individuals living with HIV/AIDS at Felegehiwot Referral Hospital, Bahir Dar, Ethiopia. Int. J. Infect. Dis. 35, 80–86. [DOI] [PubMed] [Google Scholar]
- Korpe PS, Liu Y, Siddique A, Kabir M, Ralston K, Ma JZ, Haque R, Petri WA Jr., 2013. Breast milk parasite-specific antibodies and protection from amebiasis and cryptosporidiosis in Bangladeshi infants: a prospective cohort study. Clin. Infect. Dis. 56, 988–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff KL, Blackwelder WC, Nasrin D, Nataro JP, Farag TH, van Eijk A, Adegbola RA, Alonso PL, Breiman RF, Faruque ASG, Saha D, Sow SO, Sur D, Zaidi AKM, Biswas K, Panchalingam S, Clemens JD, Cohen D, Glass RI, Mintz ED, Sommerfelt H, Levine MM, 2012. The Global Enteric Multicenter Study (GEMS) of Diarrheal Disease in Infants and Young Children in Developing Countries: Epidemiologic and Clinical Methods of the Case/Control Study. Clin. Infect. Dis. 55, S232–S245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K, 2018. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 35, 1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvac M, McEvoy J, Loudova M, Stenger B, Sak B, Kvetonova D, Ditrich O, Raskova V, Moriarty E, Rost M, Macholan M, Pialek J, 2013. Coevolution of Cryptosporidium tyzzeri and the house mouse (Mus musculus). Int. J. Parasitol. 43, 805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer RC, Riggs MW, 1999. Cryptosporidium parvum apical complex glycoprotein CSL contains a sporozoite ligand for intestinal epithelial cells. Infect. Immun. 67, 5282–5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhang X, Gong P, Li J, 2016. Cryptosporidium parvum rhomboid1 has an activity in microneme protein CpGP900 cleavage. Parasit. Vectors 9, 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Zai D, Zhang D, Wei Q, Han G, Gao H, Huang B, 2010. Divalent Cp15–23 vaccine enhances immune responses and protection against Cryptosporidium parvum infection. Parasit. Immunol. 32, 335–344. [DOI] [PubMed] [Google Scholar]
- Liu S, Roellig DM, Guo Y, Li N, Frace MA, Tang K, Zhang L, Feng Y, Xiao L, 2016. Evolution of mitosome metabolism and invasion-related proteins in Cryptosporidium. BMC Genomics 17, 1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorestani A, Sheiner L, Yang K, Robertson SD, Sahoo N, Brooks CF, Ferguson DJ, Striepen B, Gubbels MJ, 2010. A Toxoplasma MORN1 null mutant undergoes repeated divisions but is defective in basal assembly, apicoplast division and cytokinesis. PLoS One 5, e12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Lou Y, Lin WH, Xue HW, 2006. MORN motifs in plant PIPKs are involved in the regulation of subcellular localization and phospholipid binding. Cell Res. 16, 466–478. [DOI] [PubMed] [Google Scholar]
- Maass DR, Sepulveda J, Pernthaner A, Shoemaker CB, 2007. Alpaca (Lama pacos) as a convenient source of recombinant camelid heavy chain antibodies (VHHs). J. Immunol. Methods 324, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi P, Larocca AM, Quarto M, Serio G, Brandonisio O, Angarano G, Pastore G, 2000. Effect of antiretroviral therapy on cryptosporidiosis and microsporidiosis in patients infected with human immunodeficiency virus type 1. Eur. J. Clin. Microbiol. Infect. Dis. 19, 213–217. [DOI] [PubMed] [Google Scholar]
- Matos LVS, McEvoy J, Tzipori S, Bresciani KDS, Widmer G, 2019. The transcriptome of Cryptosporidium oocysts and intracellular stages. Sci. Rep. 9, 7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauzy MJ, Enomoto S, Lancto CA, Abrahamsen MS, Rutherford MS, 2012. The Cryptosporidium parvum transcriptome during in vitro development. PLoS One 7, e31715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayeri M, Leysath CE, Tremblay JM, Vrentas C, Crown D, Leppla SH, Shoemaker CB, 2015. A heterodimer of a VHH (variable domains of camelid heavy chain-only) antibody that inhibits anthrax toxin cell binding linked to a VHH antibody that blocks oligomer formation is highly protective in an anthrax spore challenge model. J. Biol. Chem. 290, 6584–6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morriswood B, Schmidt K, 2015. A MORN Repeat Protein Facilitates Protein Entry into the Flagellar Pocket of Trypanosoma brucei. Eukaryot. Cell 14, 1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J, Tremblay JM, Leysath CE, Ofori K, Baldwin K, Feng X, Bedenice D, Webb RP, Wright PM, Smith LA, Tzipori S, Shoemaker CB, 2012. A novel strategy for development of recombinant antitoxin therapeutics tested in a mouse botulism model. PLoS One 7, e29941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord J, Ma P, DiJohn D, Tzipori S, Tacket CO, 1990. Treatment with bovine hyperimmune colostrum of cryptosporidial diarrhea in AIDS patients. AIDS 4, 581–584. [DOI] [PubMed] [Google Scholar]
- O’Connor RM, Burns PB, Ha-Ngoc T, Scarpato K, Khan W, Kang G, Ward H, 2009. Polymorphic mucin antigens CpMuc4 and CpMuc5 are integral to Cryptosporidium parvum infection in vitro. Eukaryot. Cell 8, 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor RM, Wanyiri JW, Cevallos AM, Priest JW, Ward HD, 2007. Cryptosporidium parvum glycoprotein gp40 localizes to the sporozoite surface by association with gp15. Mol. Biochem. Parasitol. 156, 80–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okhuysen PC, Chappell CL, Crabb J, Valdez LM, Douglass ET, DuPont HL, 1998a. Prophylactic effect of bovine anti-Cryptosporidium hyperimmune colostrum immunoglobulin in healthy volunteers challenged with Cryptosporidium parvum. Clin. Infect. Dis. 26, 1324–1329. [DOI] [PubMed] [Google Scholar]
- Okhuysen PC, Chappell CL, Sterling CR, Jakubowski W, DuPont HL, 1998b. Susceptibility and serologic response of healthy adults to reinfection with Cryptosporidium parvum. Infect. Immun. 66, 441–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluckthun A, Pack P, 1997. New protein engineering approaches to multivalent and bispecific antibody fragments. Immunotechnology 3, 83–105. [DOI] [PubMed] [Google Scholar]
- Petersen C, Barnes DA, Gousset L, 1997. Cryptosporidium parvum GP900, a unique invasion protein. J. Eukaryot. Microbiol. 44, 89–90. [DOI] [PubMed] [Google Scholar]
- Petersen C, Gut J, Doyle PS, Crabb JH, Nelson RG, Leech JH, 1992. Characterization of a > 900,000-M(r) Cryptosporidium parvum sporozoite glycoprotein recognized by protective hyperimmune bovine colostral immunoglobulin. Infect. Immun. 60, 5132–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorny NJ, Boulter-Bitzer JI, Hall JC, Trevors JT, Lee H, 2008. Inhibition of Cryptosporidium parvum infection of a mammalian cell culture by recombinant scFv antibodies. Anton. Leeuw. 94, 353–364. [DOI] [PubMed] [Google Scholar]
- Preidis GA, Wang HC, Lewis DE, Castellanos-Gonzalez A, Rogers KA, Graviss EA, Ward HD, White AC Jr., 2007. Seropositive human subjects produce interferon gamma after stimulation with recombinant Cryptosporidium hominis gp15. Am. J. Trop. Med. Hyg. 77, 583–585. [PubMed] [Google Scholar]
- Putignani L, Possenti A, Cherchi S, Pozio E, Crisanti A, Spano F, 2008. The thrombospondin-related protein CpMIC1 (CpTSP8) belongs to the repertoire of micronemal proteins of Cryptosporidium parvum. Mol. Biochem. Parasit. 157, 98–101. [DOI] [PubMed] [Google Scholar]
- Rashmi KSR, Kumar KLR, 2013. Intestinal Cryptosporidiosis and the Profile of the CD4 Counts in a Cohort of HIV Infected Patients. J. Clin. Diagn. Res. 7, 1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs MW, Schaefer DA, Kapil SJ, Barley-Maloney L, Perryman LE, 2002. Efficacy of monoclonal antibodies against defined antigens for passive immunotherapy of chronic gastrointestinal cryptosporidiosis. Antimicrob. Agents Chemother. 46, 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs MW, Stone AL, Yount PA, Langer RC, Arrowood MJ, Bentley DL, 1997. Protective monoclonal antibody defines a circumsporozoite-like glycoprotein exoantigen of Cryptosporidium parvum sporozoites and merozoites. J. Immunol. 158, 1787–1795. [PubMed] [Google Scholar]
- Roche JK, Rojo AL, Costa LB, Smeltz R, Manque P, Woehlbier U, Bartelt L, Galen J, Buck G, Guerrant RL, 2013. Intranasal vaccination in mice with an attenuated Salmonella enterica Serovar 908htr A expressing Cp15 of Cryptosporidium: impact of malnutrition with preservation of cytokine secretion. Vaccine 31, 912–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajko S, Grishkovskaya I, Kostan J, Graewert M, Setiawan K, Trübestein L, Niedermüller K, Gehin C, Sponga A, Puchinger M, Gavin A-C, Svergun D, Leonard T, Smith TK, Morriswood B, Djinovic-Carugo K, 2020. Structures of three MORN repeat proteins and a re-evaluation of the proposed lipid-binding properties of MORN repeats. Plos One 9, e0242677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon A, Weinstein W, 1987. Oral administration of bovine colostrum anti-cryptosporidia antibody fails to alter the course of human cryptosporidiosis. J. Parasitol. 73, 413–415. [PubMed] [Google Scholar]
- Schaefer DA, Auerbach-Dixon BA, Riggs MW, 2000. Characterization and formulation of multiple epitope-specific neutralizing monoclonal antibodies for passive immunization against cryptosporidiosis. Infect. Immun. 68, 2608–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W, Wahnschaffe U, Schafer M, Zippel T, Arvand M, Meyerhans A, Riecken EO, Ullrich R, 2001. Rapid increase of mucosal CD4 T cells followed by clearance of intestinal cryptosporidiosis in an AIDS patient receiving highly active antiretroviral therapy. Gastroenterology 120, 984–987. [DOI] [PubMed] [Google Scholar]
- Sestak K, Ward LA, Sheoran A, Feng X, Akiyoshi DE, Ward HD, Tzipori S, 2002. Variability among Cryptosporidium parvum genotype 1 and 2 immunodominant surface glycoproteins. Parasit. Immunol. 24, 213–219. [DOI] [PubMed] [Google Scholar]
- Singh I, Theodos C, Tzipori S, 2005. Recombinant proteins of Cryptosporidium parvum induce proliferation of mesenteric lymph node cells in infected mice. Infect. Immun. 73, 5245–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelling WJ, Lin Q, Moore JE, Millar BC, Tosini F, Pozio E, Dooley JS, Lowery CJ, 2007. Proteomics analysis and protein expression during sporozoite excystation of Cryptosporidium parvum (Coccidia, Apicomplexa). Mol Cell Proteomics 6, 346–355. [DOI] [PubMed] [Google Scholar]
- Spano F, Putignani L, Naitza S, Puri C, Wright S, Crisanti A, 1998. Molecular cloning and expression analysis of a Cryptosporidium parvum gene encoding a new member of the thrombospondin family. Mol. Biochem. Parasitol. 92, 147–162. [DOI] [PubMed] [Google Scholar]
- Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT, Lavrsen K, Dabelsteen S, Pedersen NB, Marcos-Silva L, Gupta R, Bennett EP, Mandel U, Brunak S, Wandall HH, Levery SB, Clausen H, 2013. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 32, 1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher G, Tamura K, and Kumar S, 2020. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. 37(4), 1237–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong WB, Gut J, Nelson RG, 2000. Cloning and sequence analysis of a highly polymorphic Cryptosporidium parvum gene encoding a 60-kilodalton glycoprotein and characterization of its 15- and 45-kilodalton zoite surface antigen products. Infect. Immun. 68, 4117–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K, 2000. Junctophilins: a novel family of junctional membrane complex proteins. Mol. Cell 6, 11–22. [DOI] [PubMed] [Google Scholar]
- Tandel J, English ED, Sateriale A, Gullicksrud JA, Beiting DP, Sullivan MC, Pinkston B, Striepen B, 2019. Life cycle progression and sexual development of the apicomplexan parasite Cryptosporidium parvum. Nat. Microbiol. 4, 2226–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley M, Upton SJ, Fayer R, Barta JR, Chrisp CE, Freed PS, Blagburn BL, Anderson BC, Barnard SM, 1991. Identification of a 15-kilodalton surface glycoprotein on sporozoites of Cryptosporidium parvum. Infect. Immun. 59, 1002–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini F, Agnoli A, Mele R, Gomez Morales MA, Pozio E, 2004. A new modular protein of Cryptosporidium parvum, with ricin B and LCCL domains, expressed in the sporozoite invasive stage. Mol. Biochem. Parasitol. 134, 137–147. [DOI] [PubMed] [Google Scholar]
- Tremblay JM, Kuo CL, Abeijon C, Sepulveda J, Oyler G, Hu X, Jin MM, Shoemaker CB, 2010. Camelid single domain antibodies (VHHs) as neuronal cell intrabody binding agents and inhibitors of Clostridium botulinum neurotoxin (BoNT) proteases. Toxicon 56, 990–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S, Roberton D, Chapman C, 1986. Remission of diarrhoea due to cryptosporidiosis in an immunodeficient child treated with hyperimmune bovine colostrum. Br. Med. J. (Clin. Res. Ed.) 293, 1276–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar BL, Ward DJ, Fayer R, Quinn CA, 1990. Cessation of Cryptosporidium-associated diarrhea in an acquired immunodeficiency syndrome patient after treatment with hyperimmune bovine colostrum. Gastroenterology 98, 486–489. [DOI] [PubMed] [Google Scholar]
- Wang C, Luo J, Amer S, Guo Y, Hu Y, Lu Y, Wang H, Duan M, He H, 2010. Multivalent DNA vaccine induces protective immune responses and enhanced resistance against Cryptosporidium parvum infection. Vaccine 29, 323–328. [DOI] [PubMed] [Google Scholar]
- Wanyiri JW, O’Connor R, Allison G, Kim K, Kane A, Qiu J, Plaut AG, Ward HD, 2007. Proteolytic processing of the Cryptosporidium glycoprotein gp40/15 by human furin and by a parasite-derived furin-like protease activity. Infect. Immun. 75, 184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D, Foley SF, Hronowski XL, Gu S, Meier W, 2013. Discovery and investigation of O-xylosylation in engineered proteins containing a (GGGGS)n linker. Anal. Chem. 85, 4805–4812. [DOI] [PubMed] [Google Scholar]
- Wetzel DM, Schmidt J, Kuhlenschmidt MS, Dubey JP, Sibley LD, 2005. Gliding motility leads to active cellular invasion by Cryptosporidium parvum sporozoites. Infect. Immun. 73, 5379–5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer G, Tzipori S, Fichtenbaum CJ, Griffiths JK, 1998. Genotypic and phenotypic characterization of Cryptosporidium parvum isolates from people with AIDS. J. Infect. Dis. 178, 834–840. [DOI] [PubMed] [Google Scholar]
- Wilke G, Ravindran S, Funkhouser-Jones L, Barks J, Wang Q, VanDussen KL, Stappenbeck TS, Kuhlenschmidt TB, Kuhlenschmidt MS, Sibley LD, 2018. Monoclonal Antibodies to Intracellular Stages of Cryptosporidium parvum Define Life Cycle Progression In Vitro. mSphere 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Widmer G, Wang YP, Ozaki LS, Alves JM, Serrano MG, Puiu D, Manque P, Akiyoshi D, Mackey AJ, Pearson WR, Dear PH, Bankier AT, Peterson DL, Abrahamsen MS, Kapur V, Tzipori S, Buck GA, 2004. The genome of Cryptosporidium hominis. Nature 432, 415–415. [DOI] [PubMed] [Google Scholar]
- Yao L, Yin J, Zhang X, Liu Q, Li J, Chen L, Zhao Y, Gong P, Liu C, 2007. Cryptosporidium parvum: identification of a new surface adhesion protein on sporozoite and oocyst by screening of a phage-display cDNA library. Exp. Parasitol. 115, 333–338. [DOI] [PubMed] [Google Scholar]
- Yu Q, Li J, Zhang X, Gong P, Zhang G, Li S, Wang H, 2010. Induction of immune responses in mice by a DNA vaccine encoding Cryptosporidium parvum Cp12 and Cp21 and its effect against homologous oocyst challenge. Vet. Parasitol. 172, 1. [DOI] [PubMed] [Google Scholar]
- Zhang L, Sheoran AS, Widmer G, 2009. Cryptosporidium parvum DNA replication in cell-free culture. J. Parasitol. 95, 1239–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S4. Epitope competition between single-domain camelid antibodies (VHHs). Two-color fluorescent micrographs (600x) of Cryptosporidium parvum sporozoites fixed to the slide and probed with two VHHs expressed with either an E-tag or a myc tag, such that the concentration of one exceeded that of the other by 10-fold. Myc-tagged VHHs were detected with TRITC-labeled antibody (red), while E-tagged VHHs were detected with FITC-labeled antibody (green). The images were merged using ImageJ 1.48v software (Rasband, 2018). (A, B, C) Binding of VHHs targeting sporozoitès ~34 kDa protein and (D) of >250 kDa protein. (Aa, Ba, Ca, Da) Binding of specific myc-tagged VHH in presence of a non-specific E-tagged VHH probed in excess which serves as a control. Scale bar = 5 μm.
Supplementary Fig. S12. Anti-Cryptosporidium single-domain camelid antibodies (VHHs) lack neutralization properties in vitro. Madin-Darby bovine kidney (MDBK) cells were infected with Cryptosporidium parvum oocysts in the presence of VHHs at 100 μg/ml for 24 h. Data points indicate reductions in the number of detected intracellular stages of the parasite in reference to the untreated control. Infection was performed in the presence of a mock treatment with a non-specific control VHH as a negative control and paromomycin as a positive control. None of the VHHs produced statistically significant neutralization in comparison to a control VHH (Mann-Whitney test, P>0.17). Paromomycin reduced infection by 69.0±8.2% (Mann-Whitney test, P=0.002). Values indicate means and error bars indicate S.D. (n=6 for monomers; n=7 for dimers).
Supplementary Fig. S1. Development of immune response against Cryptosporidium parvum in alpaca. An adult alpaca was repeatedly immunized with C. parvum sporozoite lysate and monitored for anti-lysate serum titers. The immune response was measured by ELISA using whole lysate of C. parvum as a coating antigen. Sera obtained before the first and after the last immunization are referred to as pre-immune and post-immune, respectively. Control serum derives from a juvenile alpaca with limited field exposure. Immunizations boost anti-lysate titers (*** P<0.0001; two-way ANOVA). Serum from a juvenile alpaca with limited exposure to the field was included as a control serum.
Supplementary Fig. S2. Alignment of amino acid sequences of 10 unique single-domain camelid antibodies (VHHs) specific to Cryptosporidium parvum. Alignment of the best representatives of all 10 unique VHH families are shown with their three complementarity-determining regions (CDRs) indicated to facilitate comparisons. Sequences were aligned using BioEdit (Hall, 1999).
Supplementary Fig. S3. Identification of >250 kDa protein at the anterior pole of Cryptosporidium parvum sporozoites. (A) Fluorescent micrographs (600x) demonstrate FITC-labeled single-domain camelid antibody (VHH) bound to permeabilized sporozoites. Individual and mergedfluorescent and bright field (BF) images obtained with differential interference contrast (DIC) localize the VHH signal to the anterior pole of sporozoites. Scale bar = 5 μm. (B) Western blot of a separated whole C. parvum lysate bound by VHHs in the presence of a non-specific VHH as a negative control and immune alpaca serum as a positive control (showing the repertoire of immunogenic protein bands within C. parvum lysate).
Supplementary Fig. S5. Single-domain camelid antibody (VHH) dimer binding the ~34 kDa surface-exposed protein of Cryptosporidium parvum. (A) Fluorescent micrographs (600x) demonstrate FITC-labeled VHH dimer bound to permeabilized sporozoites. Merged fluorescent and bright field (BF) images obtained with differential interference contrast (DIC) indicate bound VHH at the anterior pole of sporozoites. Black arrows indicate negatively stained intact oocysts. Scale bar = 5 μm. (B) Detection of VHH dimer binding to the surface of non-permeabilized sporozoites by flow cytometry corroborates findings from microscopy (Mann-Whitney test; *P=0.02). Data is adjusted to ‘no VHH’ control. Values indicate means and error bars indicate S.D. (n=4). (C) An ~34 kDa protein band of separated C. parvum lysate bound by the VHH dimer. The experiment was performed in presence of a non-specific VHH as a negative control and immune alpaca serum as a positive control (showing the repertoire of immunogenic protein bands within C. parvum lysate).
Supplementary Fig. S6. Identification of the >250 kDa protein as gp900 of Cryptosporidium parvum (Cp-gp900). (A) Recombinant carboxyl terminus of Cp-gp900 (Cp-gp900-CT) was resolved by SDS-PAGE and blotted with single-domain camelid antibodies (VHHs). The recombinant Cp-gp900-CT is identified with an anti-OLLAS-tag horseradish peroxidase (HRP) conjugate. (B) Recombinant Cp-gp900-CT was resolved by SDS-PAGE and each membrane strip was blotted with the mixture of VHH at 0.01 μg/mL and 10 μg/mL C. parvum lysate (Cp lysate) or PBS. A recombinant heat-shock protein of Mycobacterium tuberculosis (Mt-HspX) was included as a control antigen and blotted with an anti-HspX VHH (JRH-C9). (C) The summary of densitometry analysis from B indicates the percent of VHH binding in the presence of C. parvum antigen versus the PBS control. Binding of JJ-D1 and JMP-F7 was reduced in the presence of C. parvum native target by 89.5±3.8% (t-test, ***P=0.0002) and 88.8±2.9% (t-test, ***P=0.0002), respectively. Binding of the control JRH-C9 VHH was not significantly affected by the presence of C. parvum proteins. Values indicate means and error bars indicate S.D. (n=3).
Supplementary Fig. S7. Other Cryptosporidium parvum-specific single-domain camelid antibodies (VHHs) identified in this study. (A) Fluorescent micrographs (600x) localizing FITC-labeled VHH bound to permeabilized sporozoites. Merged fluorescent and bright field (BF) images obtained with differential interference contrast(DIC) indicate localization of bound VHH in green. Scale bar = 5 μm. (B) Western blot of a separated whole lysate of C. parvum bound by VHHs in the presence of a non-specific VHH as a negative control and immune alpaca serum as a positive control.
Supplementary Fig. S8. Alignment of conserved membrane occupation and recognition nexus (MORN) repeats in the P34 of Cryptosporidium parvum (Cp-P34) sequence. (A) Structure of Cp-P34 with individual MORN repeats shown in alignment from N to C terminus (left to right) and shaded according to amino conservation in occupied position. (B) Sequence logo indicates highly conserved MORN repeat motifs with a rough consensus of YEGEYxxGxKxGxGxYTxxNGxx. Sequence logo was generated using WebLogo (Crooks et al., 2004).
Supplementary Fig. S9. N- and O-deglycosylation of P34 of Cryptosporidium parvum (Cp-P34). (A) A whole lysate of C. parvum was separated after removal of N-glycans with PNGase F and probed with single-domain camelid antibody (VHH) dimers in parallel to the untreated control lysate. The experiment was performed in the presence of negative controls: non-specific VHH dimer, ‘no VHH dimer’ and vehicle controls, which indicate non-specific background signal. (B) A whole lysate of C. parvum or recombinant carboxyl terminus of gp900 of C. parvum (Cp-gp900-CT) was separated before removal of O-glycans with sodium periodate and probed with VHH dimers in parallel to the untreated control lysate. A deglycosylation-resistant 3A1 monoclonal antibody was included as a negative control. Small bands visible to the left of each blot are molecular marker bands.
Supplementary Fig. S10. Cross-species reactivity of single-domain camelid antibodies (VHHs) generated against P34 (Cp-P34) and gp900 (Cp-gp900) of Cryptosporidium parvum. Fluorescent micrographs (600x) indicate that homologous targets of anti-Cp-P34 VHH dimer (JJS-A6/A11) and anti-Cp-gp900 VHH dimer (JMP-F7/F7) are present in sporozoites of C. parvum, Cryptosporidium hominis and Cryptosporidium tyzzeri spp. Scale bar = 5 μm.
Supplementary Fig. S11. Serological screen of reactivity against P34 of Cryptosporidium parvum (Cp-P34) among adult alpacas. Immunoblots of the separated recombinant Cp-P34 were probed with alpaca sera at 1:500 dilution and detected using anti-llama horseradish peroxidase (HRP) conjugate. For each serum, a band corresponding to Cp-P34 was quantified by image densitometry and normalized to the background (an equal sized segment immediately below the Cp-P34 band). The bars indicate the ratio of the Cp-P34 signal to the background signal. Serum from an immune alpaca immunized with C. parvum lysate (Cp-IMM alpaca) served as a positive control. Sera from two juvenile alpacas with limited exposure to the field were included as control sera. The experiment was performed in the presence of a ‘no serum’ negative control.