Abstract
Purpose of review:
The coronavirus disease 2019 (COVID-19) pandemic has led to almost 3,000,000 deaths across 139 million people infected worldwide. Involvement of the pulmonary vasculature is considered a major driving force for morbidity and mortality. We set out to summarize current knowledge on acute manifestations of pulmonary vascular disease (PVD) resulting from COVID-19 and prioritize long-term complications that may impact pulmonary hypertension (PH).
Recent findings:
Acute COVID-19 infection can result in widespread involvement of the pulmonary vasculature, myocardial injury, evidence of persistent lung disease, and venous thromboembolism. Post COVID-19 survivors frequently report ongoing symptoms and may be at risk for the spectrum of PH, including group 1 pulmonary arterial hypertension, group 2 PH due to left heart disease, group 3 PH due to lung disease and/or hypoxia, and group 4 chronic thromboembolic PH.
Summary:
The impact of COVID-19 on the pulmonary vasculature is central to determining disease severity. While the long-term PVD manifestations of COVID-19 are currently uncertain, optimizing the care of risk factors for PH and monitoring for the development of PVD will be critical to reducing long-term morbidity and improving the health of survivors.
Keywords: Pulmonary hypertension, pulmonary vascular disease, pulmonary embolism, COVID-19
Introduction
Coronavirus disease 2019 (COVID-19) pandemic has led to almost 3,000,000 deaths across 139 million people infected worldwide (1). COVID-19 manifests with a range of presentations from mild fevers and shortness of breath to severe respiratory failure with multisystem complications, including thrombosis (2). Among those with symptoms, approximately 5% require hospitalization in the intensive care unit (ICU) (2) with survivors at risk for significant long-term morbidity (3). The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes COVID-19 and has a predilection for involvement of the pulmonary vasculature, considered a major driving force for morbidity and mortality in COVID-19.
In the first year of the pandemic, clinical trials grew exponentially for drug therapy (4**) with the eventual development of multiple vaccines to prevent severe infection (5). While the future impact remains uncertain with the distinct possibility of the virus becoming endemic (6), emerging research is focused on the after-effects of COVID-19, including defining optimal care parameters for long-term sequelae. We now summarize current knowledge on acute manifestations of pulmonary vascular disease (PVD) resulting from COVID-19 and prioritize related long-term complications that may impact the field.
Acute COVID-19 and the pulmonary vasculature
Binding of the viral spike protein to the angiotensin-converting enzyme 2 (ACE2) receptor allows entry of SARS-CoV-2 into bronchial epithelial cells, type I and II alveolar cells, and possibly, capillary endothelial cells (ECs) (2, 7, 8). Virus replication results in additional virions with rapid increases in viral load (7). COVID-19 activates both the innate and adaptive immune system with subsequent inflammatory response (9, 10, 11).
Within the vasculature, evidence for direct infection of ECs remains unclear (12, 13). Severe infection leads to EC dysfunction with resulting inflammation, immune cell and platelet recruitment, altered vascular integrity, and activation of the coagulation cascade (2, 12, 14, 15, 16, 17, 18, 19). Progressive microvascular dysfunction is likely related to both direct infection and from a resulting cytokine storm, characterized by elevated inflammatory markers (20). These cytokines induce lung vascular leak, deranged coagulation, and vasoconstriction in a destructive cycle that results in hypoxia (21, 22). The pulmonary vasculopathy that follows is defined by perfusion impairments and increased physiological dead space (23).
Disruption of EC homeostasis and activation of neutrophils and platelets tips the blood:vessel interface toward thrombosis. Laboratory findings of altered coagulation are common and associated with outcomes including prolonged prothrombin time, thrombocytopenia, elevated D-dimer, changes in fibrinogen, and presence of antiphospholipid antibodies (2, 20, 24, 25, 26, 27). Activation results in neutrophil cell death in the form of extracellular trap formation (NETs) resulting in further coagulation and fibrin deposition that traps platelets and other leukocytes resulting in occlusive thrombi (28, 29, 30, 31). “Rogue” prothrombotic autoantibodies triggered by COVID-19 may also contribute to a self-amplifying loop of endothelial injury, inflammation, and thrombosis (11, 19, 32).
Pathology involving EC dysfunction, hypoxemic vasoconstriction, and coagulation abnormalities suggest increased vulnerability to the development of PVD including pulmonary hypertension (PH) and pulmonary embolism (PE). Evidence of right ventricular (RV) enlargement and dysfunction is well-documented with COVID-19 (33, 34, 35). Among 200 non-ICU patients hospitalized for COVID-19, echocardiographic evidence of PH and RV dysfunction was present in 12% and 15% of patients, respectively. Patients with PH, defined as a systolic PA pressure (PAP) >35 mmHg, had a higher rate of ICU admission and death (36). An additional association between RV dysfunction and mortality has been observed (37, 38). Findings in the acute setting suggest the involvement of both the right and left ventricles (LV). In a multicenter cohort of 305 patients, echocardiographic changes included regional LV wall motion abnormalities in 24%, global LV dysfunction in 18%, and grade II or III diastolic dysfunction in 13% (35). Beyond echo, an enlarged main PA diameter on chest computerized tomography (CT), suggestive of PH, was associated with a 50% increased adjusted mortality among almost 1500 patients with COVID-19 (39). Understanding of invasive hemodynamic profiles of patients with PH and COVID remains limited. Among 21 patients requiring mechanical ventilation for COVID-19, PH, defined as a mean PAP ≥25 mmHg, was present in 76% without an increase in pulmonary vascular resistance (40). Cumulatively, these data suggest that the presence of PH is associated with worse short-term outcomes.
The incidence of venous thromboembolism (VTE) and PE varies with the severity of illness. In a large multicenter study (n=1,114), symptomatic VTE occurred in 2% of patients admitted not requiring ICU level care and in 27% of patients admitted to the ICU (41). A meta-analysis of 18,093 patients from 49 studies reported VTE and PE had a pooled VTE incidence of 17.0% (95% CI, 13.4–20.9). The incidence of PE alone was 7.1% (95% CI, 5.3–9.1) with risk for both small artery occlusive thrombosis and large-vessel PE (42*). An autopsy study of 38 patients found evidence of platelet-fibrin thrombi in small PAs in 87% of patients (43).
Given the high incidence for thrombosis, appropriate prophylaxis is critical. Recommendations for the optimal dosing and duration of prophylaxis to prevent VTE differ between societies, are evolving, and will likely change as additional studies are reported (44, 45, 46, 47, 48, 49). Initial concerns prompted VTE prophylaxis with dosing stratified by VTE risk for patients admitted to the hospital. Societies differ on their recommendations for using (49) or not using (50) intermediate or therapeutic anticoagulation in patients without documented or suspected VTE. This area is actively being shaped by clinical trials including the REMAP-CAP, ACTIV-4a, ATTACC trial. Interim analyses of the moderate state patients (e.g., hospitalized but not requiring critical care) suggests benefit from therapeutic anticoagulation (51) while there was no benefit of higher dosing in critically ill patients (52). It will be critical to stay up-to-date on evolving best practices through living documents such as the recommendation from the American Society of Hematology (50). Post-hospitalization prophylaxis should be considered on an individual basis if patients continue to exhibit risk factors for VTE (48). Patients diagnosed with VTE/PE associated with hospitalization for COVID-19 should be treated for a minimum of 3 months with therapeutic anticoagulation (48).
Post-COVID-19 and the pulmonary vasculature
The long-term impact of COVID-19 on the pulmonary vasculature remains uncertain. Early reports of survivors suggest that COVID-19 impacts long-term cardiopulmonary health. In similar disease processes like ARDS, failure to repair the pulmonary vascular and alveolar injury that occurs has been hypothesized to be a driver of long-term disabilities (53). As such, providing optimal care for survivors requires an understanding of current reports of the emerging symptoms, the therapeutic approaches, and the best practices for monitoring.
With intermediate-term follow-up of COVID-19 survivors now reported, patterns in symptoms, functional capacity, and lung function are emerging. Despite general improvement, the incidence of on-going symptoms varies with the population studied and the severity of the initial presentation (54, 55). In a combined cohort of 112 hospitalized and 2001 non-hospitalized patients, dyspnea was present among 71% of patients at a mean follow-up of 79 days compared to 90% at the onset of symptoms. Fatigue remained among 87% of patients, slightly decreased from 95% at presentation (56). Among a cohort of 145 patients of whom 75% were hospitalized, symptoms continued to improve over time from 60 to 100 days; however, 36% continued to experience dyspnea (57**).
The presence of dyspnea post-COVID appears to be cardiopulmonary in origin, likely also correlated with disease severity. Reports of pulmonary function tests (PFTs) suggest common patterns. A meta-analysis with 380 patients reporting PFTs post-COVID found a reduced DLCO among 39% (95% CI 24%−56%), restrictive pattern PFTS among 15% (9%−22%), and an obstructive pattern among 7% (4%−11%) of survivors when PFTs performed between a month after symptoms to 3 months post discharge (58). The pattern of PFT abnormalities continues to persist though improve with longer follow up. In a multicenter observational study of 145 patients, impaired lung function was found among 42% and 36% of participants at ~60 and 100 days after diagnosis, respectively, with a reduction in FVC and/or FEV1 among 22% and impaired DLCO among 21% of patients at 100 days with improvements between visits. Abnormal CT findings most commonly including ground class opacities, consolidation, and reticulation were present in 77% of patients at 60 days and in 63% at 100 days, again with improvement between visits (57**). Similar patterns continue out to six months (59).
Follow-up evidence of echocardiographic changes are less well-described. There was no echocardiographic evidence of PH or LV dysfunction among 33 survivors not requiring mechanical ventilation followed up 6 weeks after discharge (60). Evidence of PH by echocardiogram has been estimated out to 100 days among 145 COVID-19 patients. While signs of PH only occurred in 10% of patients, they persisted between visits at 60 and 100 days (57**). A single health system study of 91 patients without a history of cardiovascular disease with an echo performed 60 days after discharge similarly found evidence of PH in one-in-thirteen patients with RV dysfunction occurring in one-in-ten (61). LV dysfunction at 100 days was rare (<3%) though over half of patients exhibited evidence of diastolic dysfunction (57**).
Intersection of COVID-19 and risk of PH
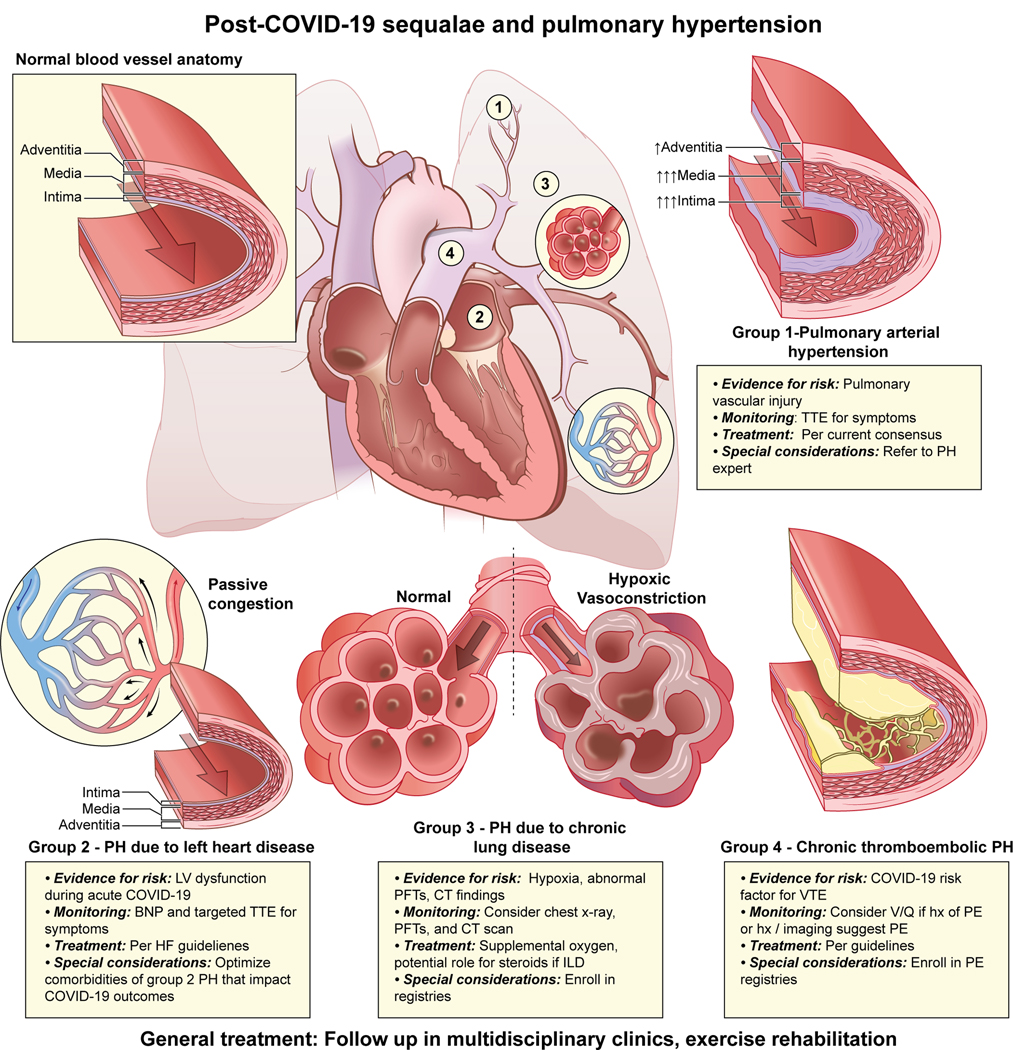
Widespread involvement of the pulmonary vasculature, myocardial injury, evidence of persistent lung disease, and incidence of PE suggests COVID survivors are at risk for a spectrum of PH, including group 1 PAH, group 2 PH due to left heart disease, group 3 PH due to lung disease and/or hypoxia, and group 4 PH due to PA obstructions (chronic thromboembolic PH or CTEPH) (Figure 1) (62). With a greater understanding of the clinical trajectory of COVID survivors, guidelines for monitoring for the development of chronic post-COVID conditions have been considered (63). Given the cardiopulmonary manifestations of COVID, surveillance for the development of PH should be considered in post-COVID patients as enough time may not yet have passed to observe the spectrum of PVD complications.
Figure 1. Post-COVID-19 sequalae and pulmonary hypertension.
COVID-19 survivors may be at risk for the long-term development of PH. Evidence for risk, recommendations for monitoring, treatment options, and special considerations for Groups 1–4 are described.
Group 1 PAH.
Several findings suggest an increased risk for developing PAH including: 1) the risk associated with other viral infections, 2) the presence of thickened pulmonary vasculature on autopsy (64), 3) low ACE2 activity (65), 4) upregulation of angiotensin II (66), and 5) EC dysfunction that shares common mechanistic manifestations with PAH. Moreover, overlap of the previously reported roles of auto-immunity in PAH and during COVID-19 have not been studied. Despite these observations, there is a paucity of rigorous evidence for increased risk of developing new group 1 PAH post-COVID. Currently, there is no consensus on the use of echo for the screening of any cardiopulmonary disease, including PAH (67). The early post-COVID follow-up supports that primary respiratory processes appear to be more common, and the utility of routine echo screening is likely low. As such, regular follow-up with targeted screening with an echocardiogram for patients with signs and symptoms of PAH will be critical. This is in agreement with current consensus PH recommendations (68)(69). Notably, throughout the pandemic, there has been an interest in the use of PAH-specific therapies for the treatment of COVID-19 infection (70, 71). For example, prior work suggests that phosphodiesterase-5 inhibitors such as sildenafil can lower PAPs, despite a mixed impact on outcomes, depending on the etiology of respiratory failure (70). Given the limited evidence for use among patients without pre-existing precapillary PH, these therapies should generally be avoided outside the setting of clinical trials. Studies utilizing PAH-specific therapies to treat severe COVID-19 infection including, inhaled nitric oxide and inhaled epoprostenol, are on-going (72). Patients who were started on off-label PAH therapies during acute COVID-19 infection should be referred for evaluation at a center with expertise in PH if the medications were continued at discharge to ensure appropriateness of use.
Group 2 PH due to left heart disease.
Given a high prevalence of myocardial injury in acute COVID-19 (35), there is the potential for long-term LV dysfunction that could predispose to the development of post-capillary PH. Echocardiographic follow-up out to 100 days suggests high rates of diastolic dysfunction on echo (55% of patients) with rare LV dysfunction (57**). To date, much of the focus on myocardial recovery has focused on athletes involved in competitive athletics (73). While prior HF is associated with poor outcomes in the acute setting (74, 75), early reports of longer-term follow-up do not suggest high rates of new HF following infection that would predispose to group 2 PH with a significant pulmonary vasculopathy. Similar to group 1, regular follow-up with monitoring of signs and symptoms and enrollment in registries will be critical to understanding the long-term impact on the LV.
Group 3 PH due to lung disease and/or hypoxia.
The long-term respiratory sequelae seen among post-COVID patients suggests that routine monitoring of pulmonary status with imaging and lung function tests could play a role. Routine pulse oximetry should be performed at all follow-up encounters to monitor for hypoxia. An American Thoracic Society and European Respiratory Society task force was split on whether or not routine testing including chest CT and PFTs to establish a new baseline should be performed at 30–60 days among all patients absent symptoms (67). Informed by symptoms, routine imaging including a CT scan and PFTs are being adopted in some post-COVID-19 recovery clinics (63). Recognizing that early diagnosis will facilitate identification of severe lung disease at the earliest stage to allow engagement with specialty care as soon as possible, a chest x-ray performed 12 weeks after discharge for patients who had abnormal imaging at presentation should be considered to determine the need for follow up CT imaging (76). PFTs should also be considered for patients with symptoms. This will facilitate the involvement of specialists who can monitor for and treat chronic lung disease that could predispose to PVD.
With the widespread involvement of the lung parenchyma and the high incidence of persistent decrements in lung function after infection, treatment options to prevent long-term sequela are being evaluated (Table 1). Treatment of hypoxia with supplemental oxygen per established guidelines is critical to reducing further vasoconstriction that could lead to the development of Group 3 PH. The role of anti-fibrotic and anti-inflammatory therapies to prevent long-term complications is uncertain (77). A single-center observational study suggests potential benefits for treating patients with steroids if interstitial lung disease is present on imaging at follow-up. In this small study, treatment resulted in significant symptomatic and radiological improvement (78*). The potential to develop chronic lung disease appears to present the greatest risk of long-term complications to the pulmonary vasculature from COVID-19 infection. As such, there is a critical need for prospective observational registries and enrollment of patients in clinical trials whenever possible to determine best practices and develop evidence-based treatments, including the potential role of PAH-specific therapies should ILD result in PH (79).
Table 1.
Potential therapies for treatment of post-COVID-19 cardiopulmonary manifestations
| Therapy | WHO PH group(s) | Mechanism | ClinicalTrials.gov Identifier |
|---|---|---|---|
| Rehabilitation/resistance training | Groups 2 & 3 | Exercise |
NCT04595773, NCT04836351, NCT04841759, NCT04718506, NCT04841759, NCT04634318, NCT04797871 |
| Inspiratory Muscle Training | Group 3 | Exercise | NCT04811859 |
| Hyperbaric Oxygen | Group 3 | Hyperoxia and hyperbaric pressure |
NCT04842448, NCT04647656 |
| LYT-100 (deupirfenidone) | Group 3 | Anti-inflammatory and anti-fibrotic | NCT04652518 |
| Leronlimab | Group 3 | *CCR5 monoclonal antibody | NCT04678830 |
| Prednisone | Group 3 | Anti-inflammatory |
NCT04551781, NCT04534478 |
| Prednisolone | Group 3 | Anti-inflammatory | NCT04657484 |
| Pirfenidone | Group 3 | Anti-inflammatory and anti-fibrotic | NCT04607928 |
| Nintedanib | Group 3 | Anti-inflammatory and anti-fibrotic | NCT04541680 |
| Omni-Biotic Pro Vi 5 | Group 3 | Gut microbiome impacts gut-lung axis | NCT04813718 |
| Nebulized Platelet Lysate | Group 3 | Immune regulation | NCT04487691 |
| Colchicine | Group 3 | Anti-fibrotic | NCT04818489 |
| AffloVest | Group 3 | Frequency chest wall oscillation | NCT04654481 |
| Autologous monocytes (MON002) | Group 3 | Anti-fibrotic | NCT04805086 |
CCR5- C chemokine receptor type 5
Summary of trials aimed at the treatment of post-COVID syndrome from ClinicalTrials.gov that have the potential to impact the development of pulmonary hypertension.
Group 4 CTEPH.
Patients diagnosed with PE in the setting of COVID-19 require treatment and follow-up per established consensus recommendations (80). The purpose of the follow-up includes monitoring of symptoms, determination of appropriate duration of anticoagulation, need for additional testing, and diagnosis of sequelae of PE including group 4 PH (80, 81). The long-term sequela of PE in the setting COVID-19 is currently uncertain. Perfusion imaging with a ventilation-perfusion scan to evaluate for chronic thromboembolic disease should be considered at 3 months in survivors who had PE, if initial evaluation including history, chest x-ray, and symptom-guided echocardiogram is suggestive and may be considered in patients without a known PE history after initial evaluation for symptoms (82). Close follow-up and enrollment in registries such as the PERT Consortium recently created a COVID-19/PE registry will be critical in understanding the long-term impact on the pulmonary vasculature (81). For patients without a PE diagnosed during acute COVID, prior COVID-19 should be considered a risk factor for VTE. Many patients ultimately diagnosed with CTEPH don’t have a history of an acute PE (83). As such, CTEPH should be considered in the evaluation of long-term symptoms post COVID while awaiting longer follow-up to better understand risk.
Post-COVID-19: Long term monitoring and treatment
In particular, multidisciplinary post-COVID-19 survivor clinics with regular follow-up are being developed to care for both the persistent symptoms and long-term complications, and are a critical step in the response to COVID-19 (3, 63). One such proposed clinic recommends an initial follow up visit with additional visits at 3, 6, and 12 months or more frequently as needed to optimize recovery (63). Although there is a paucity of evidence-based therapies to facilitate recovery, several trials are underway to study the efficacy of therapies (Table 1). There is likely benefit from rehabilitation in the form of either cardiac or pulmonary or exercise (clinical trials on-going) rehabilitation post COVID-19 for patients who experience cardiopulmonary manifestations of COVID. Initiation and treatment plans, including exercise rehabilitation, should be individualized based on the severity of infection with the goals of improving symptoms, physical function and quality of life (84, 85). Adopting current algorithms used to identify participants based on accepted indications for either chronic lung diseases or cardiac conditions will facilitate enrollment while trials are underway (86).
Conclusion
The impact of COVID-19 on the pulmonary vasculature is central to determining the disease severity of acute COVID-19 and will likely play a role in the recovery from COVID-19 or the development of new chronic illnesses. As more people become infected and survive, an understanding of the potential long-term impacts and monitoring for the development of PVD will be critical to reduce the complications and improve the health of survivors.
Key points:
Acute COVID-19 infection can result in widespread involvement of the pulmonary vasculature, myocardial injury, evidence of persistent lung disease, and venous thromboembolism.
Post-COVID-19 patients frequently have persistent symptoms and abnormalities on cardiopulmonary testing that have the potential to predispose to the development of group 1 pulmonary arterial hypertension, group 2 PH due to left heart disease, group 3 PH due to lung disease and/or hypoxia, and group 4 chronic thromboembolic PH.
Optimizing the care of risk factors for PH and monitoring for the development of pulmonary vascular disease will be critical to reducing the long-term impact of COVID-19 infection and improving the health of survivors.
Illustration acknowledgement:
Erina He (National Institutes of Health)
Financial support and sponsorship A.A. Desai was supported by NIH NHLBI R01136603. Y. Kanthi was supported by the Intramural Research Program of the NIH and NHLBI, Lasker Foundation, Frankel Cardiovascular Center COVID Ignitor Award and the A. Alfred Taubman Medical Research Institute.
Footnotes
Conflicts of interest: T.M. Cascino has received research funding from Janssen Pharmaceuticals.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/?gclid=Cj0KCQiA-OeBBhDiARIsADyBcE4Pw7bgZo_JexQ8R-PJNi73nfXe4VZG_jKk4p5C6zPSCUA3L1Wt_DIaAuJBEALw_wcB. Accessed February 27. [
- 2.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. Jama. 2020;324(8):782–93. [DOI] [PubMed] [Google Scholar]
- 3.Prescott HC, Girard TD. Recovery From Severe COVID-19: Leveraging the Lessons of Survival From Sepsis. Jama. 2020;324(8):739–40. [DOI] [PubMed] [Google Scholar]
- 4. Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. Bmj. 2020;370:m2980. ** Living systematic review of therapies for COVID-19.
- 5.COVID-19 Vaccines. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines.AccessedL February 27.
- 6.Phillips N. The coronavirus is here to stay - here’s what that means. Nature. 2021;590(7846):382–4. [DOI] [PubMed] [Google Scholar]
- 7.Potus F, Mai V, Lebret M, Malenfant S, Breton-Gagnon E, Lajoie AC, et al. Novel insights on the pulmonary vascular consequences of COVID-19. American journal of physiology Lung cellular and molecular physiology. 2020;319(2):L277–l88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi H, Zuo Y, Yalavarthi S, Gockman K, Zuo M, Madison JA, et al. Neutrophil calprotectin identifies severe pulmonary disease in COVID-19. Journal of leukocyte biology. 2021;109(1):67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuo Y, Kanthi Y, Knight JS, Kim AHJ. The interplay between neutrophils, complement, and microthrombi in COVID-19. Best practice & research Clinical rheumatology. 2021:101661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuo Y, Estes SK, Ali RA, Gandhi AA, Yalavarthi S, Shi H, et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Science translational medicine. 2020;12(570). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts KA, Colley L, Agbaedeng TA, Ellison-Hughes GM, Ross MD. Vascular Manifestations of COVID-19 - Thromboembolism and Microvascular Dysfunction. Front Cardiovasc Med. 2020;7:598400-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet (London, England). 2020;395(10234):1417–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. The Lancet Haematology. 2020;7(8):e575–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pine AB, Meizlish ML, Goshua G, Chang CH, Zhang H, Bishai J, et al. Circulating markers of angiogenesis and endotheliopathy in COVID-19. Pulm Circ. 2020;10(4):245894020966547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brosnahan SB, Jonkman AH, Kugler MC, Munger JS, Kaufman DA. COVID-19 and Respiratory System Disorders: Current Knowledge, Future Clinical and Translational Research Questions. Arterioscler Thromb Vasc Biol. 2020;40(11):2586–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huertas A, Montani D, Savale L, Pichon J, Tu L, Parent F, et al. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur Respir J. 2020;56(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi H, Zuo Y, Gandhi AA, Sule G, Yalavarthi S, Gockman K, et al. Endothelial cell-activating antibodies in COVID-19. medRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karmouty-Quintana H, Thandavarayan RA, Keller SP, Sahay S, Pandit LM, Akkanti B. Emerging Mechanisms of Pulmonary Vasoconstriction in SARS-CoV-2-Induced Acute Respiratory Distress Syndrome (ARDS) and Potential Therapeutic Targets. International journal of molecular sciences. 2020;21(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nature reviews Immunology. 2020;20(7):389–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel BV, Arachchillage DJ, Ridge CA, Bianchi P, Doyle JF, Garfield B, et al. Pulmonary Angiopathy in Severe COVID-19: Physiologic, Imaging, and Hematologic Observations. Am J Respir Crit Care Med. 2020;202(5):690–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. The New England journal of medicine. 2020;382(18):1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of thrombosis and haemostasis : JTH. 2020;18(4):844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borghi MO, Beltagy A, Garrafa E, Curreli D, Cecchini G, Bodio C, et al. Anti-Phospholipid Antibodies in COVID-19 Are Different From Those Detectable in the Anti-Phospholipid Syndrome. Frontiers in immunology. 2020;11:584241-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, et al. Neutrophil extracellular traps in COVID-19. JCI insight. 2020;5(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veras FP, Pontelli MC, Silva CM, Toller-Kawahisa JE, de Lima M, Nascimento DC, et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. The Journal of experimental medicine. 2020;217(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radermecker C, Detrembleur N, Guiot J, Cavalier E, Henket M, d’Emal C, et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. The Journal of experimental medicine. 2020;217(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Althaus K, Marini I, Zlamal J, Pelzl L, Singh A, Häberle H, et al. Antibody-induced procoagulant platelets in severe COVID-19 infection. Blood. 2021;137(8):1061–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bleakley C, Singh S, Garfield B, Morosin M, Surkova E, Mandalia MS, et al. Right ventricular dysfunction in critically ill COVID-19 ARDS. Int J Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahmoud-Elsayed HM, Moody WE, Bradlow WM, Khan-Kheil AM, Senior J, Hudsmith LE, et al. Echocardiographic Findings in Patients With COVID-19 Pneumonia. The Canadian journal of cardiology. 2020;36(8):1203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giustino G, Croft LB, Stefanini GG, Bragato R, Silbiger JJ, Vicenzi M, et al. Characterization of Myocardial Injury in Patients With COVID-19. J Am Coll Cardiol. 2020;76(18):2043–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pagnesi M, Baldetti L, Beneduce A, Calvo F, Gramegna M, Pazzanese V, et al. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19. Heart. 2020;106(17):1324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moody WE, Mahmoud-Elsayed HM, Senior J, Gul U, Khan-Kheil AM, Horne S, et al. Impact of Right Ventricular Dysfunction on Mortality in Patients Hospitalized with COVID-19 according to Race. CJC open. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Argulian E, Sud K, Vogel B, Bohra C, Garg VP, Talebi S, et al. Right Ventricular Dilation in Hospitalized Patients With COVID-19 Infection. JACC: Cardiovascular Imaging. 2020;13(11):2459–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esposito A, Palmisano A, Toselli M, Vignale D, Cereda A, Rancoita PMV, et al. Chest CT-derived pulmonary artery enlargement at the admission predicts overall survival in COVID-19 patients: insight from 1461 consecutive patients in Italy. Eur Radiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caravita S, Baratto C, Di Marco F, Calabrese A, Balestrieri G, Russo F, et al. Haemodynamic characteristics of COVID-19 patients with acute respiratory distress syndrome requiring mechanical ventilation. An invasive assessment using right heart catheterization. Eur J Heart Fail. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piazza G, Campia U, Hurwitz S, Snyder JE, Rizzo SM, Pfeferman MB, et al. Registry of Arterial and Venous Thromboembolic Complications in Patients With COVID-19. J Am Coll Cardiol. 2020;76(18):2060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jiménez D, García-Sanchez A, Rali P, Muriel A, Bikdeli B, Ruiz-Artacho P, et al. Incidence of VTE and Bleeding Among Hospitalized Patients With Coronavirus Disease 2019: A Systematic Review and Meta-analysis. Chest. 2020:S0012–3692(20)35146–1. * VTE is a frequent complication of COVID-19 infection. Manuscript gives best estimates of risk of VTE and bleeding among COVID-19 patients.
- 43.Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. The Lancet Infectious diseases. 2020;20(10):1135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.COVID-19 rapid guideline: reducing the risk of venous thromboembolism in over 16s with COVID-19. https://www.nice.org.uk/guidance/ng186.Accessed March 2. [PubMed]
- 45.Spyropoulos AC, Levy JH, Ageno W, Connors JM, Hunt BJ, Iba T, et al. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. Journal of thrombosis and haemostasis : JTH. 2020;18(8):1859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moores LK, Tritschler T, Brosnahan S, Carrier M, Collen JF, Doerschug K, et al. Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019: CHEST Guideline and Expert Panel Report. Chest. 2020;158(3):1143–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(23):2950–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barnes GD, Burnett A, Allen A, Blumenstein M, Clark NP, Cuker A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. Journal of thrombosis and thrombolysis. 2020;50(1):72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.https://www.brit-thoracic.org.uk/document-library/quality-improvement/covid-19/bts-guidance-on-venous-thromboembolic-disease-in-patients-with-covid-19/.Accessed March2, 2021. BGoVTDipwC-.
- 50.Cuker A, Tseng EK, Nieuwlaat R, Angchaisuksiri P, Blair C, Dane K, et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood advances. 2021;5(3):872–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Interim Presentation. https://www.attacc.org/presentations.Accessed May 3.
- 52.Zarychanski R. Therapeutic Anticoagulation in Critically Ill Patients with Covid-19 – Preliminary Report. medRxiv. 2021:2021.03.10.21252749. [Google Scholar]
- 53.Burnham EL, Janssen WJ, Riches DWH, Moss M, Downey GP. The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. The European respiratory journal. 2014;43(1):276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of Long-COVID: analysis of COVID cases and their symptoms collected by the Covid Symptoms Study App. medRxiv. 2020:2020.10.19.20214494. [Google Scholar]
- 55.Carfì A, Bernabei R, Landi F, Gemelli Against C-P-ACSG. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020;324(6):603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goërtz YMJ, Van Herck M, Delbressine JM, Vaes AW, Meys R, Machado FVC, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ open research. 2020;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sonnweber T, Sahanic S, Pizzini A, Luger A, Schwabl C, Sonnweber B, et al. Cardiopulmonary recovery after COVID-19 - an observational prospective multi-center trial. Eur Respir J. 2020. ** Post-COVID-19 patients frequently have persistent symptoms and abnormalities on cardiopulmonary testing out to a 100 days post-infection that improve over time.
- 58.Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, Solis-Navarro L, Burgos F, Puppo H, et al. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daher A, Balfanz P, Cornelissen C, Müller A, Bergs I, Marx N, et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir Med. 2020;174:106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tudoran C, Tudoran M, Lazureanu VE, Marinescu AR, Pop GN, Pescariu AS, et al. Evidence of Pulmonary Hypertension after SARS-CoV-2 Infection in Subjects without Previous Significant Cardiovascular Pathology. Journal of clinical medicine. 2021;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lutchmansingh DD, Knauert MP, Antin-Ozerkis DE, Chupp G, Cohn L, Dela Cruz CS, et al. A Clinic Blueprint for Post-Coronavirus Disease 2019 RECOVERY: Learning From the Past, Looking to the Future. Chest. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki YJ, Nikolaienko SI, Shults NV, Gychka SG. COVID-19 patients may become predisposed to pulmonary arterial hypertension. Medical hypotheses. 2021;147:110483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hemnes AR, Rathinasabapathy A, Austin EA, Brittain EL, Carrier EJ, Chen X, et al. A potential therapeutic role for angiotensin-converting enzyme 2 in human pulmonary arterial hypertension. Eur Respir J. 2018;51(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kümpers P, Nickel N, Lukasz A, Golpon H, Westerkamp V, Olsson KM, et al. Circulating angiopoietins in idiopathic pulmonary arterial hypertension. Eur Heart J. 2010;31(18):2291–300. [DOI] [PubMed] [Google Scholar]
- 67.Bai C, Chotirmall SH, Rello J, Alba GA, Ginns LC, Krishnan JA, et al. Updated guidance on the management of COVID-19: from an American Thoracic Society/European Respiratory Society coordinated International Task Force (29 July 2020). European Respiratory Review. 2020;29(157):200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frost A, Badesch D, Gibbs JSR, Gopalan D, Khanna D, Manes A, et al. Diagnosis of pulmonary hypertension. The European respiratory journal. 2019;53(1):1801904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Desai AA, Machado RF. Diagnostic and therapeutic algorithm for pulmonary arterial hypertension. Pulm Circ. 2011;1(1):122–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reinert JP, Reinert NJ. The Role of Phosphodiesterase-5 Inhibitors in COVID-19: An Exploration of Literature From Similar Pathologies. Journal of intensive care medicine. 2021;36(1):3–8. [DOI] [PubMed] [Google Scholar]
- 71.Sahay S, Farber HW. Management of hospitalized patients with pulmonary arterial hypertension and COVID-19 infection. Pulm Circ. 2020;10(3):2045894020933480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Franco V, Bradley EA, Badagliacca R, Sabanayagam A, Rajpal S, Lastinger LT, et al. Pulmonary vasodilators: beyond the bounds of pulmonary arterial hypertension therapy in COVID-19. Pulm Circ. 2020;10(4):2045894020970369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Phelan D, Kim JH, Elliott MD, Wasfy MM, Cremer P, Johri AM, et al. Screening of Potential Cardiac Involvement in Competitive Athletes Recovering From COVID-19: An Expert Consensus Statement. JACC Cardiovascular imaging. 2020;13(12):2635–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alvarez-Garcia J, Lee S, Gupta A, Cagliostro M, Joshi AA, Rivas-Lasarte M, et al. Prognostic Impact of Prior Heart Failure in Patients Hospitalized With COVID-19. J Am Coll Cardiol. 2020;76(20):2334–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhatt AS, Jering KS, Vaduganathan M, Claggett BL, Cunningham JW, Rosenthal N, et al. Clinical Outcomes in Patients With Heart Failure Hospitalized With COVID-19. JACC Heart Fail. 2021;9(1):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.George PM, Barratt SL, Condliffe R, Desai SR, Devaraj A, Forrest I, et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75(11):1009–16. [DOI] [PubMed] [Google Scholar]
- 77.Bangash MN, Owen A, Alderman JE, Chotalia M, Patel JM, Parekh D. COVID-19 recovery: potential treatments for post-intensive care syndrome. The Lancet Respiratory medicine. 2020;8(11):1071–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Myall KJ, Mukherjee B, Castanheira AM, Lam JL, Benedetti G, Mak SM, et al. Persistent Post-COVID-19 Inflammatory Interstitial Lung Disease: An Observational Study of Corticosteroid Treatment. Annals of the American Thoracic Society. 2021. *Early study suggests a potential role for steroids in the treatment of post-COVID-19 ILD.
- 79.Waxman A, Restrepo-Jaramillo R, Thenappan T, Ravichandran A, Engel P, Bajwa A, et al. Inhaled Treprostinil in Pulmonary Hypertension Due to Interstitial Lung Disease. New England Journal of Medicine. 2021;384(4):325–34. [DOI] [PubMed] [Google Scholar]
- 80.Rivera-Lebron B, McDaniel M, Ahrar K, Alrifai A, Dudzinski DM, Fanola C, et al. Diagnosis, Treatment and Follow Up of Acute Pulmonary Embolism: Consensus Practice from the PERT Consortium. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2019;25:1076029619853037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosovsky RP, Grodzin C, Channick R, Davis GA, Giri JS, Horowitz J, et al. Diagnosis and Treatment of Pulmonary Embolism During the Coronavirus Disease 2019 Pandemic: A Position Paper From the National PERT Consortium. Chest. 2020;158(6):2590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dhawan RT, Gopalan D, Howard L, Vicente A, Park M, Manalan K, et al. Beyond the clot: perfusion imaging of the pulmonary vasculature after COVID-19. The Lancet Respiratory medicine. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim NH, Delcroix M, Jais X, Madani MM, Matsubara H, Mayer E, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Demeco A, Marotta N, Barletta M, Pino I, Marinaro C, Petraroli A, et al. Rehabilitation of patients post-COVID-19 infection: a literature review. The Journal of international medical research. 2020;48(8):300060520948382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barker-Davies RM, O’Sullivan O, Senaratne KPP, Baker P, Cranley M, Dharm-Datta S, et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med. 2020;54(16):949–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Polastri M, Nava S, Clini E, Vitacca M, Gosselink R. COVID-19 and pulmonary rehabilitation: preparing for phase three. European Respiratory Journal. 2020;55(6):2001822. [DOI] [PMC free article] [PubMed] [Google Scholar]