Abstract
We report two patients with Multisystem Inflammatory Syndrome in Children (MIS-C) with evidence of hyponatremia on admission. Despite fluid resuscitation and resolution of dehydration, the hyponatremia worsened. Serum and urine studies were evaluated and demonstrated evidence of syndrome of inappropriate antidiuretic hormone (SIADH). Fluid restriction and anti-inflammatory therapy were initiated with resolution of hyponatremia.
Keywords: MIS-C, SIADH, COVID-19, Hyponatremia
Introduction
MIS-C is a delayed hyperinflammatory state that presents weeks after acute SARS-CoV-2 infection in children and adolescents.1 The CDC reports 2060 cases and 30 related deaths, as of February 2021.2 Understanding of the pathophysiology is actively evolving, and treatment options are being studied.3 We present two cases of children who met the CDC definition for MIS-C1 and had progressive hyponatremia despite correction of dehydration. The syndrome of inappropriate antidiuretic hormone (SIADH) has been associated with SARS-CoV-2 acute infection in adults, but the etiology is unknown4,5. Hypercytokinemia activating the non-osmotic secretion of ADH and damage to the lung tissue stimulating ADH secretion via hypoxic pulmonary hypoconstriction have been postulated.4 To our knowledge, SIADH has not been previously described as a complication of MIS-C.
Case 1
A 7-year-old previously healthy, Hispanic female presented with three days of fever, abdominal pain and non-bloody, non-bilious emesis. The abdominal pain was initially diffuse but transitioned to the right lower quadrant (RLQ). She had decreased oral intake and urine output and became increasingly fatigued on the day of admission. On review of systems, she had tactile fevers but no coughing, diarrhea, or rashes. Family members tested positive for SARS-CoV-2 infection about five weeks prior to presentation, but the patient had a negative PCR test for the virus at that time.
On admission, the patient was febrile (39.4 C), tachycardic (130 bpm), but normotensive. She had red, dry cracked lips, bilateral conjunctival injection with perilimbal sparing, and diffuse abdominal tenderness with no rebound or guarding. The initial presentation raised concern for appendicitis, but neither abdominal ultrasound nor the MRI showed evidence of inflammation. Laboratory findings demonstrated thrombocytopenia, bandemia, hyponatremia, and elevated inflammatory markers including CRP, ferritin, and D-dimer (see figure 1). Her nasal swab for SARS-CoV-2 PCR was negative but her SARS-CoV-2 IgG for the nucleocapsid was positive. An echocardiogram showed normal left ventricular ejection fraction and normal Z scores for the coronary arteries. She was treated with intravenous immunoglobulin (IVIG) and methylprednisolone. Despite isotonic fluid administration (20cc/kg) and IVIG (2g/kg), her serum sodium continued to decline with a nadir of 125 meq/L on day 3 of admission (Figure). Although she had improvement in hydration status and tachycardia, she had a urine osmolality of 700 mOsm/kg H2O, urine sodium of 45 mmol/L, and plasma osmolality of 269 mOsm/kg H2O, with decreased urine output. These studies were consistent with SIADH.
Figure 1:
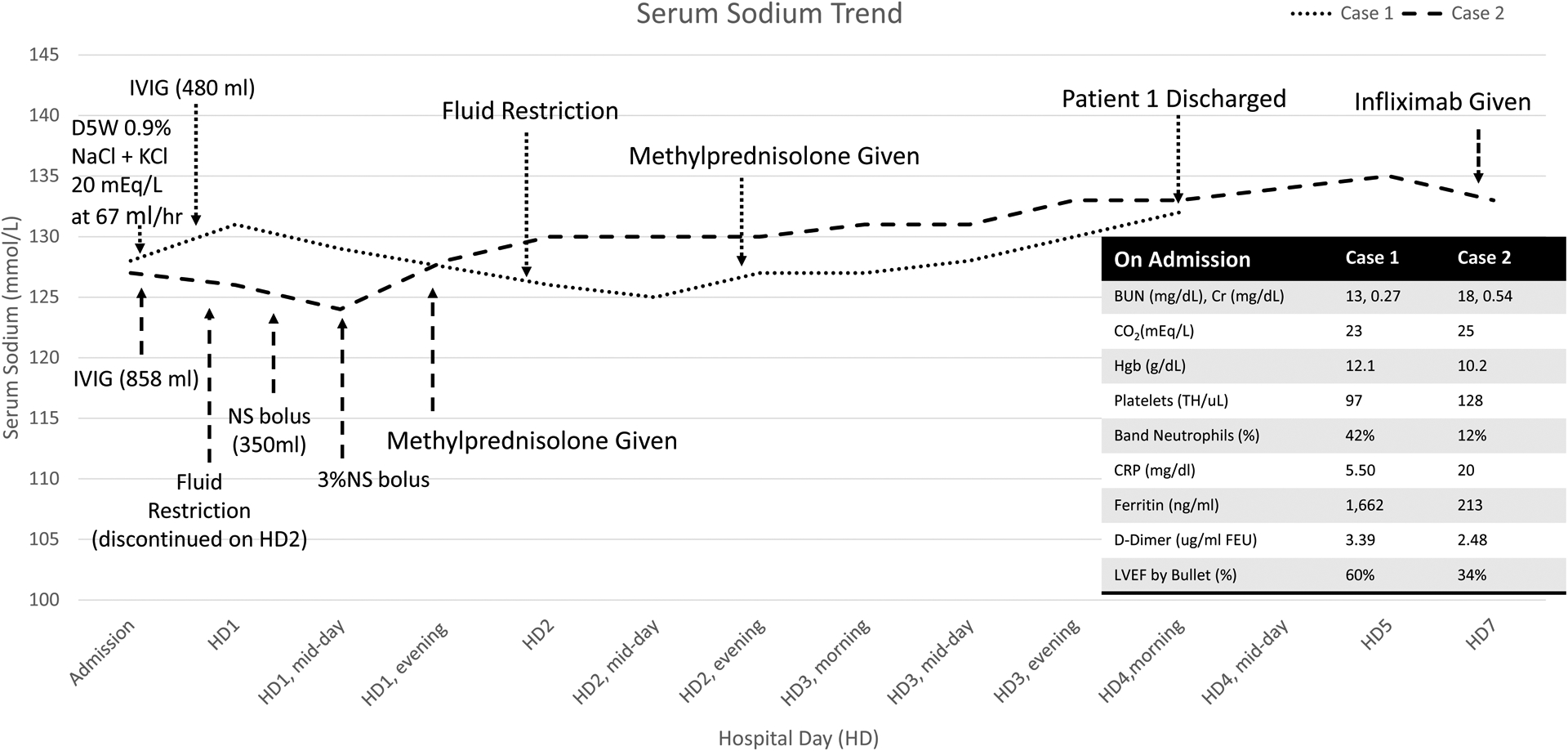
Sodium levels overall increased throughout the hospital course after fluid restriction and steroids for case 1 (blue) and case 2 (orange).
D5W- dextrose 5% in water; NaCl- sodium chloride; KCl- potassium chloride, IVIG- intravenous immunoglobulin; NS Bolus- normal saline bolus; 3% NS – 3% sodium chloride infusion; BUN- blood urea nitrogen; Cr- Creatinine; Hgb- hemoglobin; CRP- C-reactive protein; LVEF- left ventricular ejection fractions
Fluid restriction to 50% of maintenance volume was initiated with a concomitant increase in serum sodium to 132 meq/L. After IVIG and initiation of steroid therapy, the patient had resolution of conjunctival injection, mucosal erythema, and vomiting, along with improvement in inflammatory markers. She was discharged on hospital day 4 on a volume restriction to 75% of maintenance fluid intake with complete normalization of serum sodium to 139 mmol/L on the day after discharge.
Case 2
A 12-year-old Caucasian girl with a history of autism presented with four days of crampy abdominal pain, fevers and an urticarial rash on the arms, abdomen and knees. She developed transient redness of palms and soles along with bilateral conjunctival injection. Family members were SARS-CoV-2 PCR positive seven weeks prior to presentation, although the patient was PCR negative at that time.
On examination, the patient was febrile (38C), tachycardic (132bpm), normotensive, and had mild conjunctival injection with perilimbic sparing and a diffuse macular rash. Initial laboratory values were suggestive of MIS-C, and were notable for positive IgG antibody to the SARS-CoV-2 nucleocapsid, hyponatremia, hypoalbuminemia, mild normocytic anemia, and elevated inflammatory markers (see figure 1). The patient was diagnosed with MIS-C, and was treated with IVIG (2g/kg). Clinically she was euvolemic with a plasma osmolality of 274 mOsm/kg, urine sodium of 26, and urine osmolality of 884 mOsm/kg which supported the diagnosis of SIADH. She was placed on fluid restriction with 50% maintenance fluids. Her serum sodium level was serially measured and reached a nadir of 124 mmol/L on hospital day 2 (see figure 1).
On hospital day 2, the patient developed hypotension with evidence of worsening cardiac function, with rapidly increasing cardiac markers, and required transfer to the pediatric intensive care unit for further management. Left ventricular ejection fraction assessed by echocardiogram was as low as 34%. She was oliguric and thus fluid restriction was discontinued. Treatment with methylprednisolone was initiated in addition to epinephrine and milrinone to maintain peripheral vascular resistance and end-organ perfusion. Inotropic support was discontinued when mean arterial pressures were maintained at normal levels. She had clinical and laboratory evidence of improvement, but due to recurrent fevers, she received infliximab (10mg/kg). Her fever resolved and she was discharged with improved inflammatory markers and cardiac function. Her serum sodium was normal at 135mmol/L.
Discussion
Hyponatremia has been described as a typical laboratory abnormality in MIS-C.3 While SIADH has previously been associated with acute COVID-19 infection, hyponatremia in patients with MIS-C has been ascribed to dehydration with excess losses of sodium.4 Evaluation of both patients revealed hypotonic hyponatremia with elevated urine sodium and osmolality, which is consistent with SIADH.
Other causes of hypotonic hyponatremia associated with natriuresis and highly concentrated urine such as hypothyroidism were ruled out, since both patients’ thyroid studies were normal. Adrenal insufficiency and adrenal crisis were considered in both cases but neither of the patients had other features such as hyperkalemia or hypoglycemia. Drug-induced SIADH was also considered but unlikely since neither patient had any home medications on admission. Previous literature describes several mechanisms of ADH secretion, including non-osmotic baroreceptor-mediated response, osmoreceptor, and stress induced ADH secretion. Both of the patients with MIS-C demonstrated hypotonic hyponatremia, and therefore decreased intravascular osmolality may have led to osmoreceptor activation and ADH release.4 It has been shown that the inflammatory cytokine, IL-6, which is elevated in MIS-C patients, can directly stimulate non-osmotic ADH secretion, leading to SIADH in the setting of stress6,7 This is similar to the mechanism postulated for patients with Kawasaki Disease who may develop SIADH. Additionally, Case 2 developed left ventricular failure with reduced ejection fraction on her echocardiogram. The resultant decrease in left atrial stretch could also increase ADH secretion, thus providing a third possible SIADH mechanism.8 The first line treatment of SIADH involves fluid restriction.9 Other treatments to consider include hypertonic saline, oral sodium repletion, or loop diuretic if the response to fluid restriction is inadequate.
Conclusion
Our two patients had dramatically different severities of illness, and clinical courses varied dramatically. Therefore, hyponatremia did not correlate with severity of MIS-C, or predict the onset of shock. However, in both cases, hyponatremia resolved rapidly with fluid restriction and with treatment of the underlying inflammation. Methylprednisolone was used in both cases to treat inflammation. However, because it has minimal mineralocorticoid activity, it was unlikely to be responsible for the increase in serum sodium.10 As such, hyponatremia appears to be a transient and reversible phenomenon that can be associated with MIS-C. Just as organ systems involvement varies with MIS-C, so too does associated hyponatremia and likely SIADH. Fluid resuscitation should be initiated judiciously in patients presenting with evidence of dehydration (i.e., elevated urine specific gravity, dry mucous membranes, tachycardia) with careful monitoring of vascular leak and third-spacing of fluid. Fluid administration can be made even more complicated when there is also cardiac involvement. IVIG administration is a valuable adjunct to maintaining oncotic pressure in the vascular space. If, however, serum sodium continues to fall as euvolemia is restored, urine and plasma osmolality and urine sodium, along with morning cortisol levels should be measured to assess for hypotonic hyponatremia. Cortisol levels would be useful to rule out adrenal crisis in the setting of acute inflammation. If SIADH is confirmed, fluid restriction should be instituted for management of the hyponatremia. Further studies are needed to correlate SIADH in MIS-C patients with specific cytokines or other mediators of inflammation.
Acknowledgments
The work supported in part by the National Institutes of Health IR61HD105590 to JCB and 3R01HL140898-03S1 to AHT and JCB
References
- 1.“Information for Healthcare Providers about Multisystem Inflammatory Syndrome in Children (MIS-C).” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 5 February. 2021, www.cdc.gov/mis-c/hcp/. [Google Scholar]
- 2.“Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States .” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 8 February. 2021, www.cdc.gov/mis-c/cases/index.html. [Google Scholar]
- 3.Nakra NA, Blumberg DA, Herrera-Guerra A, Lakshminrusimha S. Multi-System Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management. Children (Basel). 2020July1;7(7):69. doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yousaf Z, Al-Shokri SD, Al-Soub H, Mohamed MFH. COVID-19-associated SIADH: a clue in the times of pandemic! Am J Physiol Endocrinol Metab. 2020June1;318(6):E882–E885. doi: 10.1152/ajpendo.00178.2020. Epub 2020 May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saleh AO, Al-Shokri SD, Ahmed AO, Musa AE, Mohamed MF. Urinary Retention and Severe Hyponatremia: An Unusual Presentation of COVID-19. Eur J Case Rep Intern Med. 2020September11;7(10):001905. doi: 10.12890/2020_001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park Se Jin, and Shin Jae Il. “Inflammation and hyponatremia: an underrecognized condition?.” Korean journal of pediatrics vol. 56,12 (2013): 519–22. doi: 10.3345/kjp.2013.56.12.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panigrahy Neha, Policarpio Joseph, and Ramanathan Rahul. ‘Multisystem Inflammatory Syndrome in Children and SARS-CoV-2: A Scoping Review’. 1January. 2020. : 301 – 316. [DOI] [PubMed] [Google Scholar]
- 8.Koizumi K, Yamashita H. Influence of atrial stretch receptors on hypothalamic neurosecretory neurons. J Physiol 285: 341–358, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant P, Ayuk J, Bouloux PM, Cohen M, Cranston I, Murray RD, Rees A, Thatcher N, Grossman A. The diagnosis and management of inpatient hyponatraemia and SIADH. Eur J Clin Invest. 2015August;45(8):888–94. doi: 10.1111/eci.12465. Epub 2015 Jun 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuel Sophie, et al. “Pharmacologic Characteristics of Corticosteroids.” Journal of Neurocritical Care, vol. 10, no. 2, 2017, pp. 53–59., doi: 10.18700/jnc.170035. [DOI] [Google Scholar]


