Abstract
Alterations in olfactory functions are proposed to be early biomarkers for neurodegeneration. Many neurodegenerative diseases are age-related, including two of the most common, Parkinson’s disease (PD) and Alzheimer’s disease (AD). The establishment of biomarkers that promote early risk identification is critical for the implementation of early treatment to postpone or avert pathological development. Olfactory dysfunction (OD) is seen in 90% of early-stage PD patients and 85% of patients with early-stage AD, which makes it an attractive biomarker for early diagnosis of these diseases. Here, we systematically review widely applied smelling tests available for humans and some animal models and the relationships between OD and normal aging, PD, AD, and other conditions. The utility of OD as a biomarker for neurodegenerative disease diagnosis and future research directions are also discussed.
Keywords: aging, olfactory dysfunction, Alzheimer’s disease, Parkinson’s disease, neurodegeneration
1. Introduction
The ability to smell, olfaction, refers to a chemosensory process during which volatile molecules are detected by specialized sensory cells. These cells, called olfactory sensory neurons (OSNs), express protein receptors which bind to a specific odorant substrate. After binding, a signalling cascade occurs and the stimulus is converted into an electrical signal, which is transmitted to the central olfactory system for further processing. The olfactory system is critical for shaping behaviour, facilitating communication, and survival by obtaining environmental information from odorants. Important information processed by the olfactory system includes identification of food, mates, toxins, predators, and impending danger (Branigan and Tadi, 2020). The specific functions, organizational, and genetic complexity of olfactory systems vary drastically between species. In species which rely heavily on information gathered using the sense of smell, olfactory receptor (OR) genes are more abundant than in those which are less reliant, as shown in Fig. 1. For example, there are around 1,130 intact OR genes in mice, 1207 in rats, 811 in dogs while only 380 in chimpanzees and 396 in humans (Niimura et al., 2014). The number of total OR genes is low in fruit flies (Drosophila melanogaster), which depend a lot on olfaction, but the intact OR genes significantly outnumber the OR pseudogenes (Nozawa and Nei, 2007), while in human there are similar numbers of intact OR genes and OR pseudogenes. The evolutionary importance of olfactory function underscores anatomical differences between the olfactory system of rodents and humans, specifically that rodents possess an accessory olfactory bulb and a “Vomeronasal” organ (Oboti and Peretto, 2014). In addition, the percentage of olfactory bulb volume to the total brain volume is much higher in rodents than humans, as illustrated in Fig. 1.
Fig. 1.
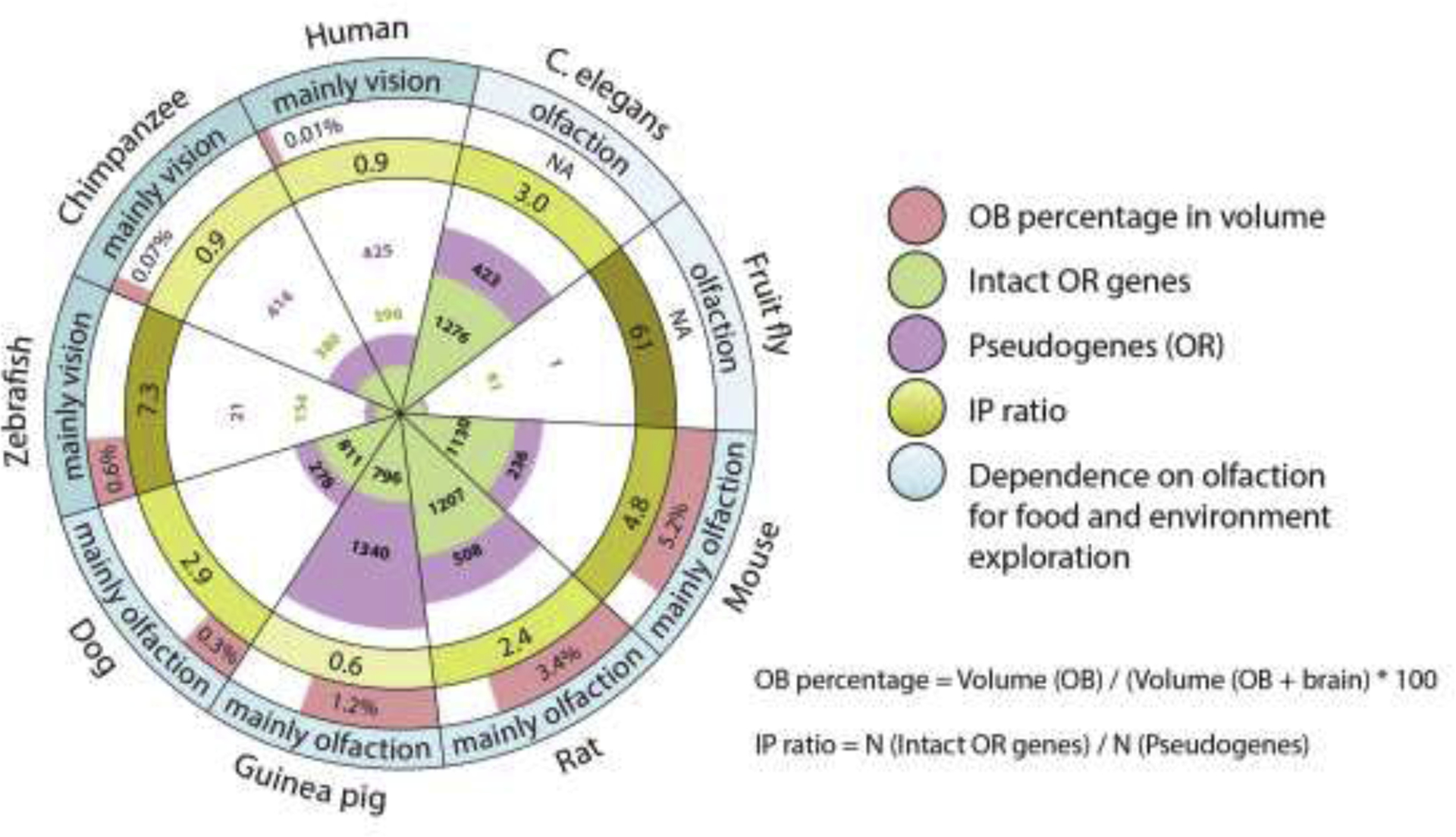
Numbers of OR genes and relative volume of OB to brain in species with different dependence on olfaction. IP ratio is calculated by dividing the number of intact OR genes to the number of OR pseudogenes in C.elegans (Robertson and Thomas, 2006), fruit fly (Nozawa and Nei, 2007), zebrafish (Niimura, 2009), mouse, rat, guinea pig, dog, chimpanzee, and human (Niimura et al., 2014). OB percentage in volume is calculated by dividing the volume of OB to the total volume of OB and the rest of the brain in mouse (Ma et al., 2008), rat (Welniak-Kaminska et al., 2019), guinea pig (Mullins et al., 2020; Srinivasan and Stevens, 2019), dog (Kavoi and Jameela, 2011), zebrafish (Paskin et al., 2011; Ullmann et al., 2015), chimpanzee (Laska, 2015) and human (Kavoi and Jameela, 2011).
For humans, smell processing in the olfactory bulb (OB) is associated with experiencing emotions and memories through direct connection with the limbic system and cerebral cortex (Branigan and Tadi, 2020). Olfaction, along with the functions it serves, declines with normal aging. Unfortunately, olfactory dysfunction, compared to dysfunction in other sensory systems, is often not quickly recognized (Kondo et al., 2020). Statistically, less than a quarter of individuals with olfactory dysfunction (OD) are aware of their problem until tested (Doty, 2017), highlighting the necessity of olfactory tests, especially for the elders. OD may be an indicator of health and pathological conditions. In older adults, olfaction, but not visual or hearing impairment, is associated with increased mortality (Seubert et al., 2017). Mental health issues including anxiety, depression, and other negative emotions are also associated with loss of smell (Croy et al., 2014). In the case of the pandemic caused by Coronavirus Disease 2019 (COVID-19), OD is an important symptom reported by about 67% of those affected (Chung et al., 2020). Notably, OD is associated with a variety of neurodegenerative diseases in humans, including Parkinson’s disease (PD), Alzheimer’s disease (AD), Lewy body (LB) disease, Huntington’s disease, and others (Damm et al., 2014). Some PD patients have impaired sense of smelling several years before motor symptoms appear and studies have revealed early lesions in olfactory structures (Braak et al., 2003). One of the possible explanations for the involvement of OD in different health conditions is that the olfactory system is exposed to the external environment. Therefore, it has a high chance for direct contact with pathogens and toxins, making it a suitable starting point for pathology. The inability to detect odors also increases the risk of pathogen and toxin penetration into the brain through the olfactory system due to reduced awareness of harmful elements in the environment (Rey et al., 2018).
OD is proposed to be an important clinical indication for underlying pathology, and is a potential marker for disease progression (Branigan and Tadi, 2020). The establishment of olfactory testing as a non-invasive, inexpensive, and early screening method for neurodegenerative diseases associated with smelling defects has gained growing research interest in recent years. In the present review, we provide a systematic overview of smelling tests available for humans as well as olfaction assessments for use in animal models. We then critically evaluate the current literature on OD and its involvement in normal aging and neurodegenerative diseases as well as its role as an early biomarker for neurodegenerative disease.
2. The olfactory system in humans and some animal models
In humans, gaseous or volatile odorant molecules enter the nasal passages and encounter olfactory receptors on the primary dendritic cilia of OSNs, which are embedded on the olfactory epithelium (Jenkins et al., 2009). Each OSN expresses G-protein coupled receptors (GPCRs) that bind to specific odorant molecule(s). The axons of the OSNs synapse to glomerular cells, or glomeruli, which form the outermost layer of the OB (Nagayama et al., 2014). Sensory cells expressing the same GPCR converge on and activate the same glomeruli (Doty, 1995; Mori, 2009). In this way, glomeruli form the functional interface between odor detection and neural processing. Deeper within the OB are the external plexiform layer and mitral cell layer, in descending order (Nagayama et al., 2014). The external plexiform layer is populated by external tufted cells, while the mitral cell layer contains mitral cells. Both cell types project their primary dendrites to the glomerular layer. External tufted cells send their axons to the anterior olfactory cortex and mitral cells send their axons throughout the entire olfactory cortex (Igarashi et al., 2012). The most internal layer of the olfactory bulb is the granule cell layer. Granule cells lack axons, but their dendrites extend into the mitral cell layer and external plexiform layers where they form reciprocal synapses with lateral dendrites (Strowbridge, 2010), as shown in Fig. 2A. Glutamate released from a mitral or tufted cell dendrite can excite granule cell spines, triggering recurrent inhibition back onto the principal cell in an inhibitory feedback loop. Thus, granular cells play an important role in discriminatory latency in difficult smelling tasks so that closely-related scents can be differentiated (Abraham et al., 2010).
Fig.2.
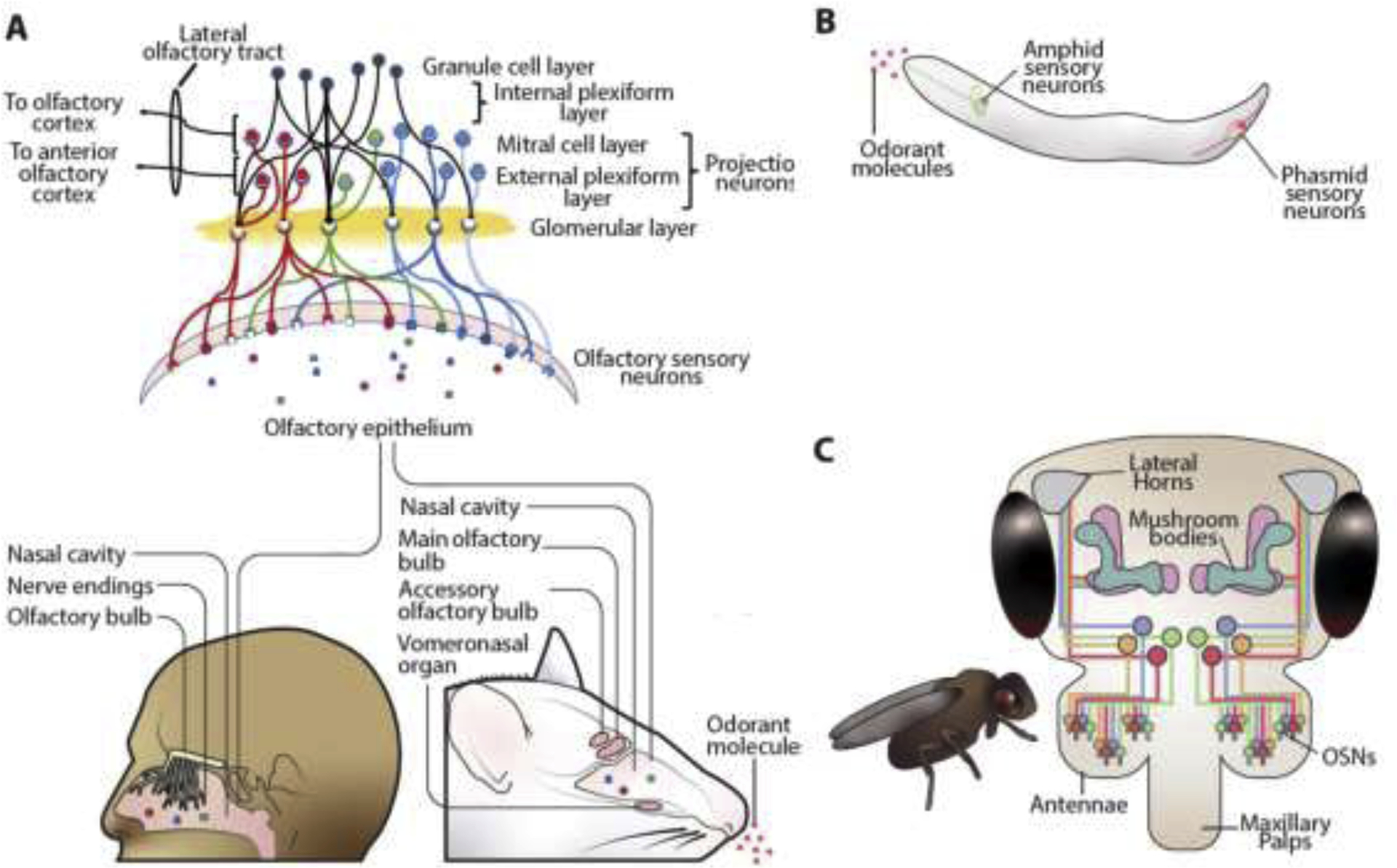
The olfactory circuits in different organisms. (A) Olfactory system in humans and mice. Olfactory sensory neurons (OSNs) detect odorant molecules from olfactory epithelium, and project their signals to neurons in the glomerular layer, where axons then synapse with neurons in the mitral cell and external plexiform layers. Mitral cell axons transmit to the entire olfactory cortex, while external plexiform layers axons are confined to the anterior olfactory cortex. Mice have an accessory olfactory bulb and vomeronasal system. (B) The olfactory system in C. elegans. C. elegans possess four chemosensory organs or sensilla: the amphid, the phasmid, and the inner and outer labia (not shown). C. elegans chemosensory neurons are sensitive to multiple odorants due to expression of multiple chemoreceptors in each cell. Receptors of the same cell may propagate their signal intracellularly via different pathways, permitting a wide range of responses to odors that are detected by the same neuron. (C) Olfactory system in fruit flies. They have OSNs like those in humans and mice but in fewer numbers. OSNs expressing the same olfactory receptors send their signals to specific glomeruli of the antennal lobe where it synapses with the dendrites of specific second order projection neurons (not shown). The projection neurons in turn connect to the mushroom bodies, which are involved in olfactory learning and memory, and the lateral horns, which primarily route information instructing innate behaviours.
Mice, C. elegans (Caenorhabditis elegans) and fruit flies are commonly used animal models for olfactory-related behavioural tests. Interestingly, mice share a similar structure and function of the OB with humans. The primary difference is that their olfactory system is more sophisticated, with additional structures called the accessory olfactory bulb and vomeronasal organ (Oboti and Peretto, 2014) (Fig. 2A). C. elegans sense their environment through GPCRs on the ciliated ends of sensory neurons (Hart and Chao, 2010). These neurons are located at four chemosensory organs, including the amphid, inner and outer labial at the head and phasmid at the tail (Fig. 2B). Unlike vertebral OSNs, C. elegans are constrained by the small population of chemosensory neurons that detect a broad range of chemical inputs, and so a single ciliated neuron may express many different GPCRs (Bargmann, 2006). To make up for this, many of the GPCRs are capable of triggering a receptor-specific signalling cascade (Ardiel and Rankin, 2010). Habituation, defined below, to an odorant detected by one OSN does not habituate the cell to other odorants detected by that same OSN (Colbert and Bargmann, 1995). Thus, detection, discrimination, and habituation to different odorants may all occur within a single sensory neuron via distinct molecular pathways (L’Etoile and Bargmann, 2000). In this way, C. elegans is capable of performing and integrating many rudimentary olfactory and cognitive functions despite their small number of neurons. Fruit flies possess two sets of sensilla which are decorated with sensory hairs, the antennae and the maxillary palps (Fig. 2C). There are two main types of molecular receptors in olfactory reception in fruit flies, the odorant receptors and the inotropic receptors, which are respectively expressed in basiconic & trichoid sensilla and coeloconic sensilla (Gomez-Diaz et al., 2018). The olfactory system of fruit flies bears close resemblance to that of vertebrates (Wilson, 2013) and they can recognize and discriminate hundreds of discrete odorants (Vosshall, 2000). The similarity of the olfactory system in fruit flies with those of vertebrates has made them an attractive model for the study of olfaction.
3. Measures of olfactory functions
3.1. Four major aspects of olfactory evaluation
Carefully designed behavioural tests are vital tools in characterizing OD. Many of the tests below were selected for their widespread use as reliable assays for basic olfactory functions shared by humans and most model organisms. The tests assess odor identification, discrimination, sensitivity, and habituation. Olfactory functions are regulated by different regions of the brain which are also relevant to aging, PD and AD (Fig. 3).
Fig.3.
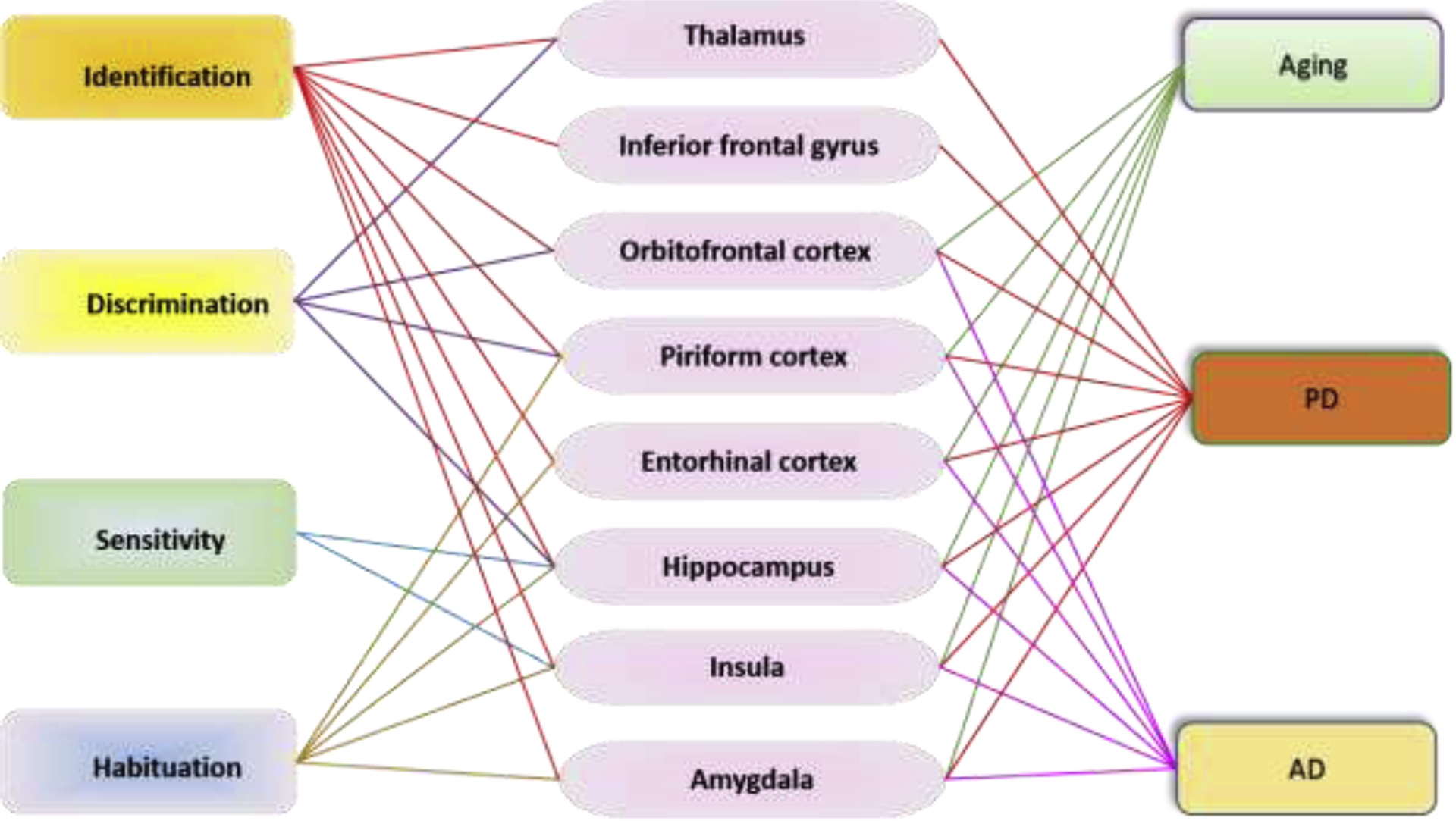
The interconnection between different olfaction aspects and brain regions and the involvement of these brain region in aging, PD, and AD in human and mammalian animal models. Among the mentioned brain regions involved in olfaction, all are related with PD (Braak et al., 1994; Criaud et al., 2016; Henderson et al., 2000; Jia et al., 2019; Kobayakawa et al., 2017; Liu et al., 2019; Sancandi et al., 2018; Terada et al., 2018) and a majority (orbitofrontal cortex, piriform cortex, entorhinal cortex, hippocampus, insula, and amygdala) are related with AD (DeTure and Dickson, 2019; Saiz-Sanchez et al., 2015) and aging (Churchwell and Yurgelun-Todd, 2013; Gocel and Larson, 2013; Reagh et al., 2018; Resnick et al., 2007; St Jacques et al., 2010).
Odor identification is the detection and recall of a previous smell associated with an individual’s knowledge or experience (Murphy, 2019). The key brain regions in humans and mammalian animal models involved in odor identification are the entorhinal cortex, hippocampus, insula, orbitofrontal cortex, inferior frontal gyrus, piriform cortex, thalamus, and amygdala (Kjelvik et al., 2012; Merrick et al., 2014; Wu et al., 2019). Odor identification is affected by sensitivity to different aspects of the odorant, integration of these aspects to define its qualities, and further integration with previous experiences to provide identity and significance. In humans, this is indicated by recognition and correct naming of an odor presented during the test. For animal models, this relies on a specific behavioural response on exposure, such as travel towards an attractive odorant, or away from a repellent one. Odor identification has been found to be a central deficit in early stages of AD and PD (Kjelvik et al., 2012), as well as in normal aging (Seubert et al., 2017).
Odor discrimination is the ability to distinguish between two or more odors (Hummel et al., 1997). It relies heavily on the hippocampus, piriform cortex, orbitofrontal cortex, and thalamus (Martin et al., 2007; Merrick et al., 2014; Tanabe et al., 1975; Wilson, 2009). Like odor identification, odor discrimination is tied closely to cognitive abilities and is thought to be developed through experiencing odors (Hedner et al., 2010). Essentially, an odor must be learned before it can be discriminated against other smells (Wilson, 2009). Similar to odor identification, odor discrimination declines with age (Hummel et al., 1997).
Odor sensitivity/threshold is the ability to detect an odor at a given concentration wherein the lowest detectable concentration is considered the threshold (Trimmer and Mainland, 2017). The brain regions involved in odor sensitivity include insula and hippocampus (Wabnegger et al., 2019). Odor sensitivity varies in individuals depending on the odorant qualities, pleasantness, and familiarity. Individual variation is also due in part to differences in the number of OSNs specific to that odorant. Sensitivity decreases with age, although the mechanism of this decline is not well understood. OSN sensitivity remains relatively consistent through age, as does axonal density and convergence on glomeruli (Lee et al., 2009; Richard et al., 2010). As such, peripheral changes of the olfactory system do not appear to provide sufficient explanation for changes in odor sensitivity with age.
Odor habituation is defined as the temporary decreased sensitivity to an odor on repeated or extended exposures to the same odor or odor mixture (Pellegrino et al., 2017). The brain regions involved in odor habituation include piriform, entorhinal cortex, amygdala, hippocampus, and anterior insula (Poellinger et al., 2001). In animal models, this is expressed as a decreased or slower behavioural response not resulting from motor or sensory fatigue. For humans, it is experienced as perceived lessening of odor intensity over time. Different features affect the rate of habituation: for example, pleasing odors habituate at a slower rate than unpleasant odors (Jacob et al., 2003). These two odor types activate different regions of the brain and unpleasant odors are more trigeminal (Purves et al., 2001). Additionally, depending on the odorant, habituation to one odorant may generalize to other odorants as well (Pellegrino et al., 2017). Habituation that occurs in OSNs or glomerular neurons is termed peripheral habituation, while central habituation is regulated by the primary olfactory cortex, which receives direct afference from the glomerular layer. Central habituation allows for more rapid detection of odors against a background (Kadohisa and Wilson, 2006). Habituation occurs more rapidly in older individuals, recovers more slowly, and is impaired in individuals with mild cognitive impairment or AD (Murphy, 2019).
3.2. Olfactory tests in humans
Olfactory function in humans is associated with experiences and odor environments (Maresh et al., 2008). Different odor experiences mediate odor perceptions that differ geographically and culturally (Herz, 2009; Majid et al., 2018). Here, we provide a brief overview of major smelling tests to characterize aspects of human olfaction (Table 1).
Table 1.
Common olfactory tests for humans.
| Test | Testing aspects | Description | Advantages | Drawbacks |
|---|---|---|---|---|
| UPSIT | Identification |
|
|
|
| B-SIT | Identification |
|
|
|
| SDOIT | Identification |
|
|
|
| BAST-24 | Identification |
|
|
|
| Sniffin’ Sticks | Identification |
|
|
|
| Threshold sensitivity |
|
|||
| Discrimination |
|
Odor identification tests
The University of Pennsylvania Smell Identification Test (UPSIT) is a classical and highly reliable test to measure odor identification ability (Eibenstein et al., 2005). This test is commercially available and can be self-administered at home. The test-retest reliability coefficient exceeds 0.9 (Doty et al., 1989). It is currently a worldwide standard test for olfactory function, and according to the information from the manufacturer, it has been administered to nearly 1,000,000 people worldwide.
The Brief Smell Identification Test (BSIT) is a shortened version of UPSIT. Unlike the UPSIT, which contains 40 odors, BSIT test only consists of 12 odors. The low number of odors limits the analytical power of this test but shortens the time commitment required and has been found to be sufficient for detecting OD (Menon et al., 2013).
The San Diego Odor Identification Test (SDOIT) is like the BSIT. It is relatively short and simple to administer, however, it cannot be self-administered. Notably, it can be adapted for young children (Murphy et al., 1994). Eight common household smells are presented to the subject in a randomized order, for 45 seconds each. Impairment is judged by identifying fewer than 6 odors. The SDOIT and BSIT are reported to have very similar and reliable classification of impairment or abnormal status, but they are limited in distinguishing OD when deficits are not robust (Krantz et al., 2009).
The sniffin’ sticks test is validated in several European countries, where it is most commonly employed (Rumeau et al., 2016). It can be reused and tests for threshold, discrimination, and identification. Though more time consuming, it offers greater flexibility than the UPSIT in terms of what olfactory domains it can measure. Results are interpreted based on normal values normalized on gender and age (Rumeau et al., 2016). Along with the sniffin’ sticks test, the UPSIT and its variations are the most popularly employed tests in clinical examinations and in aging research.
The Barcelona Smell test-24 (BAST-24) is commonly used in Spain (Cardesin et al., 2006; Marino-Sanchez et al., 2020). It is administered in a similar manner to the sniffin’ sticks odor identification test. The odors used in the test focuses on Spanish cultural norms, making it less reliable outside of Spain (Doty, 2007). The extensive length also limits its accessibility for studies with large sample-sizes.
Odor discrimination test
Sniffin’ sticks can also be applied to measure discrimination. Subjects are blindfolded. They are presented with three sticks, two of which contain the same odor, and one that does not. The odorants should be selected to be of similar intensity and hedonic tone: for example, pairing apple scent with an orange scent. Healthy individuals should be able to discriminate greater than 75% of the odors (Hummel et al., 1997).
Sensitivity test
Sniffin’ sticks can be adapted to measure threshold sensitivity. The subject is blindfolded, and sticks are filled with a series of concentrations of odorant, or solvent. The subject is presented with three sticks at a time, one of which contains the odorant at a particular concentration and the other two containing only solvent. The subject then selects which stick contains the odorant. The concentration of odorant increases at each presentation until the subject correctly detects the odorant on two consecutive trials. Odor habituation is rarely tested in humans.
Modification of olfactory tests and development of new methods
The olfactory assessments described above are common and widely applied in epidemiological studies. However, modifications of these tests have been made. To reduce the time of tests in the clinical routine, Hummel et al. used a 3-item odor identification test that can discriminate between anosmic, hyposmic or normosmic subjects with a specificity of 96% (Hummel et al., 2010). Additionally, it has been argued that human sensitivity to a single odorant varies significantly in people since prior familiarity with a specific odorant impacts smell test performance and may bias results (Hsieh et al., 2017). Thus, new tests for olfactory sensitivity and olfactory resolution, using mixtures of odorants to create unfamiliar smells were developed (Hsieh et al., 2017). With COVID-19 infections known to affect smelling, fast and reliable psychophysical tests for large scale screening are needed. Hyposmia in COVID-19 patients was mainly diagnosed through subjective reports obtained from questionnaires/surveys or interviews (Marchese-Ragona et al., 2020). However, self-reported sense of smell is known to be significantly discrepant with results of psychophysical tests and may lead to confusing and inconclusive results (Marchese-Ragona et al., 2020). Based on the need for fast screening during the pandemic, a novel device was designed to produce a precise level of smell digitally controlled through a mobile app. The selected smell can be remotely triggered and programmed to give standard or customised smell tests through this app for rapid screening (Prasanna Gandhi, 2020).
3.3. Olfactory tests in mice
Olfactory tests in mice rely primarily on the innate curiosity of the mouse to novel stimulations, or on their motivation to forage for food (Hånell and Marklund, 2014). Odor identification is achieved with the initial exposure to the scent; subsequent sniffing is used to provide additional information regarding location, spatial intensity, and dynamics of the odorant (Wachowiak, 2010). The behavioural tests described below are among the most employed in olfactory evaluation in mice.
Odor identification test
The buried food test is widely used to assess general olfactory ability and integration, as completion depends on how well the mouse detects and locates food buried beneath a layer of bedding (Machado et al., 2018; Zou et al., 2015). To provide motivation, mice are deprived of food in advance of the experiment, and fed a very small portion of the food to be used in the test (Zou et al., 2015). On the day of the experiment, the mice are placed in plain cages with bedding. They are allowed an hour to become familiarized with the cage, then moved to another cage with a small portion of food (<2 grams) buried beneath the bedding. The latency to initial digging and to finding the food is recorded. Olfactory function is assessed by these latencies wherein better function is associated with shorter time.
Odor discrimination test
To investigate how well mice discriminate between two odors, they are presented with the same scent three consecutive times (Sundberg et al., 1982; Zou et al., 2015). On the fourth time, a novel scent is presented. At each presentation, the time the mouse spends smelling the scent is recorded. As evidence of habituation, mice will spend the most time investigating at the first presentation, and less time on subsequent presentations of the same odor. If the mouse can discriminate between the first and second odor, it will spend significantly more time smelling the fourth stick presented than the third. This test allows for great flexibility in pairing odorants based on their structure and their significance to the mouse. For example, pairing structurally similar odorants presents a more challenging task than more diverse odorants. The test may also test for sensitivity to social scents such as urine samples collected from other mice.
Odor sensitivity test
The odor detection threshold test is designed to determine the sensitivity of the rodent to gradually increasing concentrations of an odorant (Zou et al., 2015). The mouse is presented with cotton-tipped sticks that have been dipped in either a diluted odorant or vehicle. The concentration of the odorant is gradually increased until the mouse indicates that it can detect it. If the mouse cannot detect the odorant, it will spend roughly equal time smelling the vehicle or the odorant stick. The mouse is scored positive for smelling the odorant when the proportion of time spent smelling the odorant is significantly greater than that on the vehicle stick.
3.4. Olfactory tests in C. elegans
The unique anatomy of the worm necessitates a very different approach to how behaviour tests are structured and evaluated. It is important to note that these assays rely on movement, and so locomotion assays should be performed in conjunction. Odor recognition is expressed as normal chemotaxis: in the presence of a chemoattractant or aversive chemical stimulation, C. elegans will preferentially travel to its source or in the opposite direction, respectively (Miller et al., 2005; Ward, 1973). C. elegans movement in response to either attractants or repellents is a commonly used metric in olfactory assays. A definitive guide to these and more behavioural tests can be found in Wormbook (Hart, 2006) under “Behavior”. Below we summarize the most employed behavioural assays that characterize chemosensation.
Odor detection and discrimination test
Odor discrimination test in C. elegans is defined by the absence of cross-saturation between two odorants. A high uniform concentration of odor will diminish chemotaxis to the point source while it may not affect the chemotaxis to other odors (L’Etoile and Bargmann, 2000). In the assay, a plate of agar is prepared with a uniform concentration of chemoattractant (Colbert and Bargmann, 1995). At one end of the dish (marked with an X), a small volume of a different chemoattractant is placed along with an anaesthetic such as sodium azide, with an equal volume of diluent and anaesthetic on the opposite side of the dish. Before testing, worms are kept on a plate with the same uniform chemoattractant as the testing plate for habituation. For testing, worms are washed then placed at the center of the plate and allowed to disperse over time. The number of worms at the X are counted and compared against the total worms deposited on the plate half an hour later. For odor detection, agar that is free of odorant is used in dish preparation. Chemotaxis towards the attractant indicates the ability to detect the odorant. Interestingly, one study (Hirotsu et al., 2015) showed that wild type C. elegans can sense odors from human urine and display discriminative chemotaxis toward the urine from cancer patients (attractive) and normal control (evasive). By applying a Nematode Scent Detection Test, the research group reported a 95.8% diagnostic accuracy and 95% specificity based on 24 samples from cancer patients and 218 control samples (Hirotsu et al., 2015).
Odor habituation test
Habituation can be measured in C. elegans by a reduced response or a longer inter-stimulus interval on exposure to a habituated chemorepellent (Hart, 2006; Troemel et al., 1995). This may be accomplished by placing a sizable droplet of chemorepellent in a dish containing worms to be tested. The worms are allowed to swim in the droplet for a duration before they are removed, washed, and re-exposed to the chemorepellent via the drop test or “smell-on-a-stick” test.
Habituation with this method may be altered by adjusting the duration or dilution. Alternatively, repeated trials of the drop test may be used to habituate the worm. The latency to and severity of the response to each drop test or “Smell-on-a-stick” trial are recorded. Habituation is indicated by a longer inter-stimulus interval or decreased backwards movement with repeated trials. It is important that this decreased response is differentiated from toxic effects of the repellent.
The drop test chemorepellent assay is a test used to probe detection of a repellent and integration between the amphid and phasmid sensilla (Hilliard et al., 2002). The amphid and the phasmid are both chemosensitive organs by which C. elegans monitor their chemical environment. The amphid is by far the best characterized sensillum, but the phasmid plays an important role in integrating odor response. Worms are washed in a low salt solution and placed on an unseeded agar dish. A glass pipette pulled by hand on a flame delivers a small drop containing either diluent or repellent close to the tail of the worm. The drop envelops the animal by capillary action and the worm soon reverses direction. The “dry” drop test is used to isolate stimulus of the amphid neurons without stimulating the phasmid neurons to provide a behavioural control to the drop test (Hilliard et al., 2002). To conduct the “dry” drop test, a drop of odorant is presented just ahead of the animal rather than to its tail. The “smell-on-a-stick” test allows gentle and temporary exposure to chemorepellents (Hart, 2006). Without touching the animal, an eyelash brush dipped in repellent is presented just ahead of the animal. Upon detection, the worm should back up.
3.5. Olfactory tests in fruit flies
Behaviour tests for fruit flies rely on chemoattractant and repellent behaviour as a mean of measuring response in a manner like those in C. elegans. Fruit flies can detect a wide range of odorants, and typically are attracted to sources of food (i.e. vinegar or fruit) and avoid toxins (benzaldehyde, 1-octen-3-ol, geosmin, and phenol) (Verschut et al., 2019). Their bimodal method of travel also complicates behavioural analysis, and so some tests constrain the fly to walking (Faucher et al., 2006).
Odor detection and discrimination test
To measure discrimination, a group of flies are placed within the center of a set of tubes and three vials arranged to form a T-maze (Xia and Tully, 2007). Odors are delivered to specific arms as vapors bubble through mineral oil. Preference and discrimination are measured bythe fraction of flies that navigate to each arm. Spontaneous odor detection is performed by introducing unscented vapor in one of the arms, while the other arm presents odorant (Xia and Tully, 2007). Depending on the type of odorant, chemotaxis towards or away from the scented arm indicates detection. Spontaneous odor identity discrimination tests are performed by perusing both arms with a saturated background odor (Xia and Tully, 2007). One of the arms contains a mixture of background odor and some other odorant, typically chemoattractant. Discrimination of that odor against the background results in a majority of flies occupying that arm. Sensitivity may be probed by adjusting the concentration of the second odor. Spontaneous intensity discrimination is performed by detecting the choice of flies between different concentrations of the same odorant, typically a chemorepellent such as benzaldehyde or 4-methyl cyclohexanol (Xia and Tully, 2007). One arm contains a higher concentration, while the other is an order of magnitude less in concentration. Avoidance of the higher concentration tube indicates functioning discrimination.
Odor habituation test
Olfactory startle tracks changes in movement speed associated with sudden exposure to ethanol, which functions as a repellent odorant (Twick et al., 2014) (Cho et al., 2004). The flies’ movement may be tracked by a video apparatus and software. The test should be carried out in a low ceiling container to prevent flight. Air flow is split between two streams, one of which is bubbled through distilled water to provide humidity, and the other through ethanol. These two streams are combined before entering the chamber at a constant flow rate. The fly is given time to acclimate to the chamber and humidified air flow. Pulses of odorant are delivered by turning on and off the air flow through the ethanol stream at 30 second intervals. Initial exposure to ethanol fumes prompts rapid movement. As the fly habituates, this movement will lessen. Habituation to this stimulus relies heavily on function of the mushroom body. Additionally, habituation of this sort is generalized: habituation to ethanol will result in habituation to ethyl acetate and isoamyl alcohol as well. Olfactory jump reflex habituation is tested in a similar manner to olfactory startle, but at a much more rapid rate of exposure: 4 seconds of odorant exposure given at intervals of 0.25–20 minutes (Asztalos et al., 2007). Additionally, the test is carried out in a high-ceilinged tube that limits horizontal travel. The test measures the percentage of flies that jump at repeated exposure to air that has been bubbled through mineral oil in which benzaldehyde has been dissolved. Habituation occurs more rapidly with a higher concentration of benzaldehyde, ruling out adaptation responsible for decreased jump-response.
4. Olfactory dysfunction
4.1. Olfaction declines with aging
Olfactory dysfunction (OD) can range from hyposmia, with a decreased ability to smell, to anosmia in which smell is fully compromised (Daramola and Becker, 2015). In some cases, impaired olfaction may also manifest as phantosmia or parosmia, in which perception of odors is produced without a stimulus, or a distorted perception of an odor is produced in the presence of a stimulus, respectively (Scangas and Bleier, 2017). The cause of OD is multifactorial. Aging, diseases, genetic factors, lifestyle, nutrition, injury history, medical procedures, exposure to viruses, and occupation likely all have a relevant role (Daramola and Becker, 2015). As mentioned before, the loss of sense of smell has a profound negative impact on the overall quality of life (Daramola and Becker, 2015). In fact, 17–30% of the patients with OD have symptoms of depression (Daramola and Becker, 2015). Olfactory function declines with aging as the prevalence of OD increased from around 10% at age of 60 to around 60% at age of 90 (Fig. 4). Smelling ability as assessed by TDI (threshold- discrimination-identification) score decreases an average of 1 point every 5 years of age (Schlosser et al., 2020). Without OD at baseline, new OD cases rose 14.2% over 6 years based on a follow up study with 1004 individuals (mean age ~67.5 years with range 60.1–90.8 years) (Palmquist et al., 2020). Given this epidemiological evidence, the association between nonpathological aging and OD is clear.
Fig.4.

Prevalence of OD in healthy adults at and over 60 years of age. Prevalence rates obtained from three studies (Rawal et al., 2014; Schubert et al., 2012; Seubert et al., 2017) which utilized odor identification as reflective of general olfactory function in the older adult population.
Interestingly, men are more susceptible to OD with aging than women (Murphy et al., 2002). One study found the prevalence of OD in women is around 9% at age 60 and 29% at age 78, while in men it was reported to be 15% at age 60 and 49% at age 78 (Seubert et al., 2017). However, there seems to be no difference in the prevalence between women and men at age 90, where it is about 66% (Seubert et al., 2017). In general, females perform better in odor tasks than males (Sorokowski et al., 2019). Analysis based on post-mortem OB from men and women showed that despite similar weight, the number of total cells, neurons, and non-neurons in the OB of males is lower than in females (Oliveira-Pinto et al., 2014). Sexually dimorphic sensory processing in the peripheral olfactory system is proposed to be one of the factors that contributes to differences in olfactory function between males and females. Indeed, there was a higher number of odor-evoked glomeruli and faster odor-evoked OSN output in female mice than male mice (Kass et al., 2017). Interestingly, circulating sex hormones were shown to affect these responses, as gonadectomized females exhibited slower OSN responses in fewer glomeruli than control females, whereas gonadectomized males showed faster OSN responses in more glomeruli than control males for similar odorants (Kass et al., 2017). Gonadal hormonal differences appear to facilitate odor detection and discrimination in female mice, but impair it in males (Kass et al., 2017). Consistently, OD may develop during menopause in women (Kass et al., 2017) and gonadal hormone replacement therapy can positively affect odor memory and discrimination in postmenopausal women (Doty et al., 2015). Lifestyle choices, like smoking, alcohol consumption, and overweight are each related to OD (Palmquist et al., 2020) and may also cause some differences since women generally lead a more healthy lifestyle than men (Chang et al., 2019).
While it has been well documented that olfactory function declines with age and a variety of features are identified to be involved in this age-associated dysfunction, the exact underlying cellular and molecular mechanisms remain unclear. Neurogenic changes in the OB and olfactory epithelium are identified as prominent contributors to age-associated OD. The lifespan of olfactory receptor cells is 30–60 days (Sultan-Styne et al., 2009). Damaged olfactory receptor cells are replaced by new cells differentiating from basal stem cells. However, not all receptor cells may be replaced across a lifespan, contributing to the diminished sense of smell with age (Suzukawa et al., 2011). OSNs reproduce mitotically throughout the lifespan in humans and rodents, however these neurogenic processes slow down with age and pathogenic exposure further contributes to the degeneration over time (Doty and Kamath, 2014; Mobley et al., 2014). OB interneurons originate as neuroblasts in the subventricular zone and navigate to the olfactory bulb where they differentiate into periglomerular cells (PGs) and granule cells and integrate into synaptic networks (Mobley et al., 2014). In mice, a reduction in the number and diversity of olfactory neurons caused by cell cycle arrest and decline in subventricular neurogenesis have both been reported as contributors to olfactory loss during normal aging (Seo et al., 2018). Interestingly, in spite of decreased neurogenesis, granule cell density in the OB does not change significantly with age (Richard et al., 2010). This is thought to reflect a decrease in turnover but an increase in lifespan of granule cells in the OB of aged animals than in young ones, which potentially leads to less flexible reorganization of neural connections in response to a novel odor (Kondo et al., 2020). Neuronal loss may be not ubiquitous during aging, on the contrary, age-related loss of synapses is layer-specific in mice as synaptic density decreases in the glomerular layer, but not in the external plexiform layer, and thus OD might be better explained by an imbalance in OB circuitry (Richard et al., 2010).
Chronic inflammation developed with aging has also been implicated in many age-associated chronic diseases and in OD. Chronic nasal inflammation induced by repeated intranasal administration of lipopolysaccharide (LPS) induced OB atrophy and impaired OSN regeneration in male mice (Hasegawa-Ishii et al., 2019). Interestingly, the OB recovered from atrophy after a non-treatment period while OSN regeneration still remained incomplete (Hasegawa-Ishii et al., 2019). Clinically, the proinflammatory cytokine, IL-6, is significantly elevated in plasma and nasal mucus of hyposmia patients when compared with the normal controls (Henkin et al., 2013). Importantly, inflammation and neurogenesis are interconnected in OB. The adult OB hosts a large population of microglial cells which are innate immune cells of the brain. Microglial cells are activated in response to various environmental stimuli, and transform into an amoeboid-like morphology and perform phagocytosis of apoptotic cells during neurogenesis (Seo et al., 2018). They proliferate rapidly and get activated after deafferentation of the OB and a concomitant reduction of OB neurogenesis is induced (Lazarini et al., 2012). Beyond changes of olfactory neurogenesis and inflammation, differences in odorant receptor expression and synaptic organization are also reported to potentially contribute to OD during aging (Mobley et al., 2014).
Impaired odor identification in older humans is associated with a decrease in global cognition and a decline in episodic memory (Attems et al., 2015; Park et al., 2021). Notably, compared with age-related hyposmia, hyposmia with dementia has a higher odor threshold, indicating lower smelling sensitivity (Suzuki et al., 2021). A magnetic resonance imaging (MRI) study found that odor identification scores were correlated with right amygdalar volume as well as bilateral perirhinal and entorhinal cortex grey matter volume (Segura et al., 2013). In nonpathological aging, deficits in central and peripheral OD are both observed (Kondo et al., 2020). However, central olfactory impairments are prominent and likely underlie the majority of olfactory changes observed in older adults (Kjelvik et al., 2012; Murphy, 2019; Xu et al., 2020). Olfaction assessments are useful tools for delineating this dysfunction due to their neuroanatomical specificity and can be utilized in longitudinal studies to track the progression of this dysfunction in pathological and nonpathological contexts.
In summary, olfaction decreases considerably after the 7th decade of life. OD is observed in 7.5–11% of presumed healthy 60 year olds and increases to a prevalence of 35% by age 78 (Schubert et al., 2012; Seubert et al., 2017). Males are more affected than females. Both the peripheral and central olfactory nervous systems may get involved in age-related OD with physiological degeneration and pathologies related with age. The factors which may contribute to age-related OD includes abnormalities of the olfactory epithelium, neurogenic changes in the OB, imbalanced OB circuitry, decline in subventricular neurogenesis and inflammation.
4.2. Olfactory dysfunction in neurodegenerative diseases
The number of people worldwide with dementia is estimated to be around 65.7 million in 2030 (Prince et al., 2013). By 2050, the number of people living with Alzheimer’s dementia may grow to 13.8 million in the United States alone (Gaugler et al., 2019). OD is associated with a variety of neurodegenerative diseases as reviewed in (Marin et al., 2018) and aggregations of pathological proteins usually affect olfactory regions prior to other regions. Here, we review the association of OD with PD and AD, the most common neurodegenerative diseases in humans.
Olfactory dysfunction precedes the onset of motor symptoms in PD
PD is a neurodegenerative disease characterized by progressive and preferential loss of dopaminergic neurons in the substantia nigra. In 2016, 6.1 million individuals were diagnosed with PD globally (E Ray Dorsey, 2018). Though therapies are available to ameliorate certain symptoms, none of them can slow or halt the disease, and the clinical benefits fade as the disease progresses (Regensburger et al., 2014). Clinical diagnosis of PD are based on symptoms of motor bradykinesia, resting tremors, rigidity, and postural instability (Regensburger et al., 2014). The presence of α-synuclein-containing Lewy bodies in the substantia nigra by post-mortem examination confirms the diagnosis.
The conversion from prodromal PD to PD is marked by the transition from non-motor symptoms to clinically diagnosable motor symptom presentation, at which time approximately 60% of the nigrostriatal neurons of the substantia nigra have already degenerated (Becker et al., 2002). Those considered to be in the prodromal PD stage exhibit deficits in odor identification, discrimination, and detection threshold compared to healthy controls (Berg et al., 2013; Mahlknecht et al., 2015; Ross et al., 2008). In addition, conversion risk is increased in prodromal PD subjects that demonstrate impaired odor identification, significantly reduced combination of threshold-discrimination-identification (TDI) score, and/or idiopathic REM sleep behavior disorder (RBD) diagnosis (Mahlknecht et al., 2015; Ross et al., 2008). A 2-factor assessment consisting of an odor identification and dopamine transporter (DAT) imaging predicted a 67% conversion rate from prodromal PD to PD within 4 years (Jennings et al., 2017). OD is found in 90% of early-stage PD patients (Doty, 2012) and it precedes the onset of motor symptoms by many years. Consistently, the UPSIT score of PD patients was significantly lower than that of normal controls, as shown in Fig. 5A. Normosmic PD patients have less severe motor deficits and clinical manifestations and are less prone to cognitive impairment when compared to hyposmic PD patients (Bohnen et al., 2010; He et al., 2020; Lee et al., 2015). Compared to healthy controls, the effect size of odor identification, discrimination and threshold performance are all above 0.8, which is considered a large effect size (Fig. 5B). Odor identification is more frequently impaired than odor discrimination (Boesveldt et al., 2008) and it has higher sensitivity and specificity than odor discrimination tests in distinguishing PD patients from the controls (Boesveldt et al., 2008). Interestingly, smoking significantly decreases the risk of PD and current PD smokers exhibit less attenuated olfactory function than non-smoking PD patients who never smoke or smoked in the past (Sharer et al., 2015). There are potential sex differences in olfaction among PD patients as males have significantly more pronounced deficits in olfaction than females (Liu et al., 2015).
Fig. 5.
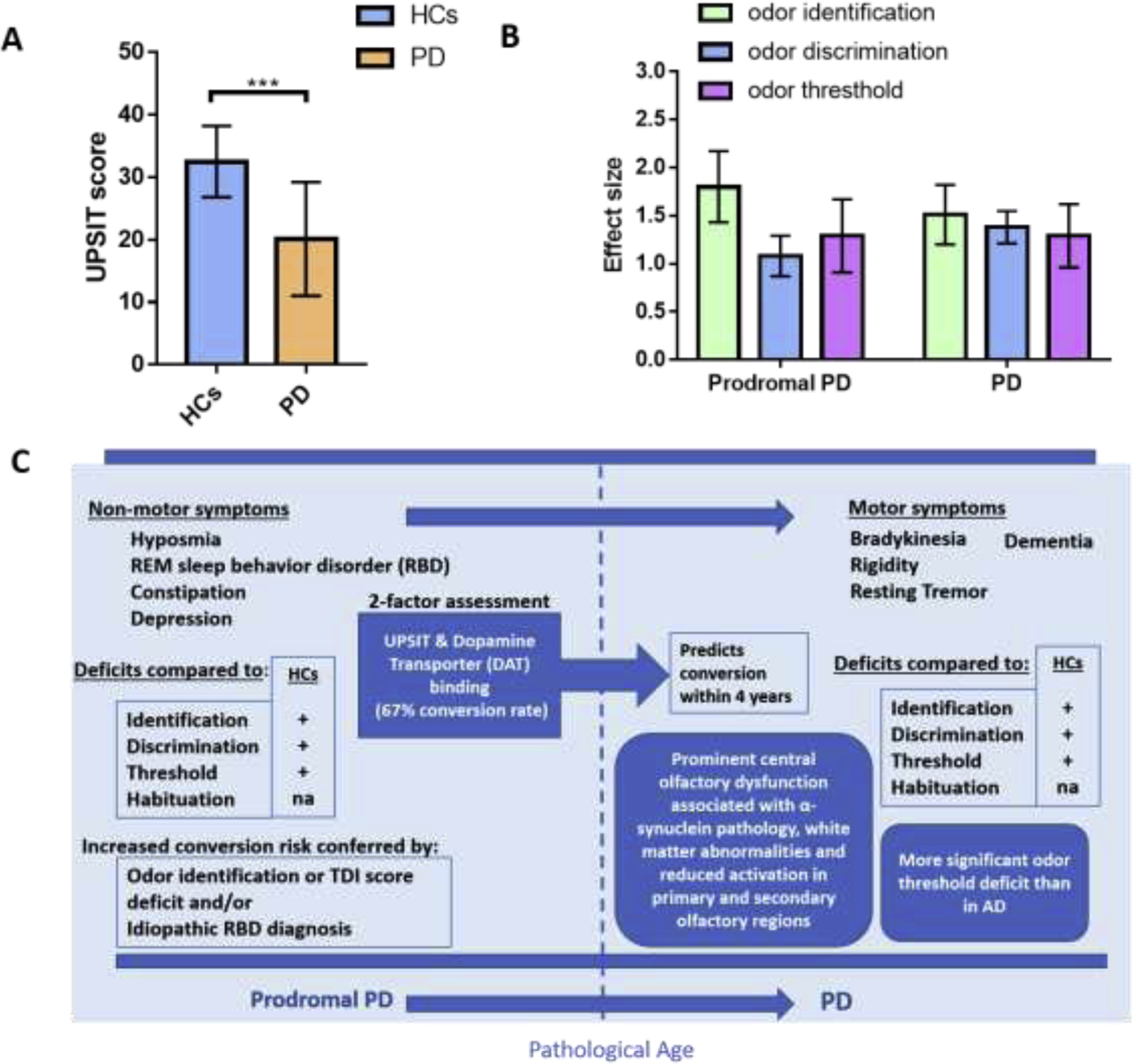
OD in PD. (A) Comparison of odor identification ability between healthy control (HC) and PD patients. Graph plotted based on (Chou and Bohnen, 2009). NC (n=44): 59.6±10.8 years; PD (n=44): 59.3±10.1years. (B) Effect sizes for different olfactory domains in prodromal PD and PD (error bars indicate 95% confidence interval). Prior convention has classified effect sizes as small (d=0.2), medium (d=0.5) or large (d≥0.8). Graph plotted based on 2 meta-analyses (Lyu et al., 2021; Rahayel et al., 2012). (C) Infographic profiles of OD in prodromal PD and PD patients.
The exact relationship between OD and PD has not been fully elucidated although olfactory decline shows strong correlation with progressive deposition of Lewy bodies (Wilson et al., 2011) and elevation of alpha-synuclein (α-syn) in cerebrospinal fluid from PD patients (Guo et al., 2020). It is proposed that the OB may be one of the earliest affected regions in PD patients. As PD progresses, pathology spreads from the olfactory bulb, the anterior olfactory nucleus, and the lower brainstem to other regions of the brain (Johnson et al., 2020). The formation of Lewy bodies composed of misfolded α-syn in OB is typical in early PD, and it predicts the presence of Lewy-type alpha-synucleinopathy in other brain regions (Beach et al., 2009). Administration of human mutant α-syn into the OB of rats by an adeno-associated virus infection caused broad pathological changes in the brain regions associated with PD (Niu et al., 2018). Additionally, olfactory impairment as tested by an odor discrimination task was detected in the rats three weeks after virus injection when overexpression of human mutant α-syn was detected in the OB (Niu et al., 2018). Interestingly, impairment of motor ability and muscular coordination with decreased number of tyrosine hydroxylase positive cells and fibres in the substantia nigra were observed at later time points post-injection (Niu et al., 2018). This indicates a possibility of α-syn transfer from the OB to other brain regions and initiation of certain pathological phenotypes similar to PD (Niu et al., 2018). In a more recent study, the authors bilaterally injected α-syn preformed fibrils into the OB of wild type mice and found the presence of α-syn in the anterior olfactory nucleus and piriform cortex at six months after injection (Johnson et al., 2020). Interestingly, only females injected with fibrils exhibited reduced odor detection sensitivity at one-, three- and six-months after injection as monitored by odor-evoked sniffing behaviour in a plethysmograph (Johnson et al., 2020). Further investigation is necessary to understand the role of sex difference in this animal model study.
Interestingly, the presence of α-syn in the OB can be induced by chronic inflammation in mice (Niu et al., 2020). Intranasal infusion of LPS induced microglia activation, inflammatory cytokine expression and abnormally phosphorylated α-syn in the OB, substantia nigra and striatum in an IL-1R1-dependent manner (Niu et al., 2020). Mice exhibited PD-like behaviour and reduced odor discrimination ability after 6 weeks treatment (Niu et al., 2020). Both PD-like behaviour and OD in mice that received the LPS treatment was reversed or attenuated in IL-1R1-deficient mice, as well as in mice that received Minocycline, which inhibits microglial activation (Niu et al., 2020). It is hypothesized that environmental toxins, which cannot move across the blood-brain barrier may induce inflammatory activation of the olfactory mucosa and pathology in OB, then pathology spreads to other brain regions (Niu et al., 2020).
In conclusion, OD is among the earliest non-motor features of PD, preceding the onset of motor symptoms by years and may predict conversion from prodromal PD to PD (Fig. 5C). It remains unclear whether OD is associated with disease duration and severity (Sasaki and Horie, 2020). Odor identification is more frequently impaired than odor discrimination and is the most practical olfactory factor in differentiating PD patients (Zhao et al., 2020). OBs are one of the earliest affected brain regions and as diseases progresses, pathology spreads from the OB, the anterior olfactory nucleus, and the lower brainstem to other regions of the brain. This is supported in animal studies in which the administration of α-syn in the OB can induce the presence of α-syn in other brain regions and elicit pathological and behavioural changes similar to PD (Johnson et al., 2020; Niu et al., 2018).
Olfactory dysfunction confers risk of AD
AD is the most common cause of dementia, resulting in a great economic and psychological burden for the affected family, and society in general. The specific hallmarks of AD are the formation of neurofibrillary tangles due to hyperphosphorylation of Tau protein (p-tau) and the deposition of amyloid plaques. Currently, there are no cures available for AD. Accessible and feasible prognostic screening methods are important if individuals are to receive prompt treatment to slow down progression of the disease.
AD patients perform poorly on olfactory tasks (Buchsbaum et al., 1991), and it has been proposed that a simple smelling test could help identify patients who are at higher risks of accelerated decline in global cognition with the first 7 years from diagnosis (Gjerde et al., 2018). Indeed, in a 3.5 year follow-up study on new cases of mild cognitive impairment (MCI) among 1,430 cognitively normal participants showed that decreasing olfactory identification is associated with an increased risk of amnestic MCI and that the B-SIT test could predict progression from amnestic MCI to AD (Roberts et al., 2016). As shown in Fig. 6A, the UPSIT score of AD patients was significantly lower than that of normal controls, and this score worsens with the progression from MCI to AD (Vasavada et al., 2017). In addition to odor identification, odor discrimination, and habituation are also affected in AD compared to MCI, however, results from studies looking at the odor threshold between these two groups are less conclusive (Jung et al., 2019b; Murphy, 2019; Rahayel et al., 2012). The effects sizes of odor identification, discrimination, and threshold in MCI and AD are shown in Fig. 6B. Olfactory habituation was found to be significantly impaired in AD patients compared to healthy controls and MCI patients, however, it was unclear whether the MCI and control group demonstrated a clear group difference (Zhang et al., 2019). The infographic profiles of olfactory dysfunction in MCI and AD are shown in Fig. 5C.
Fig. 6.
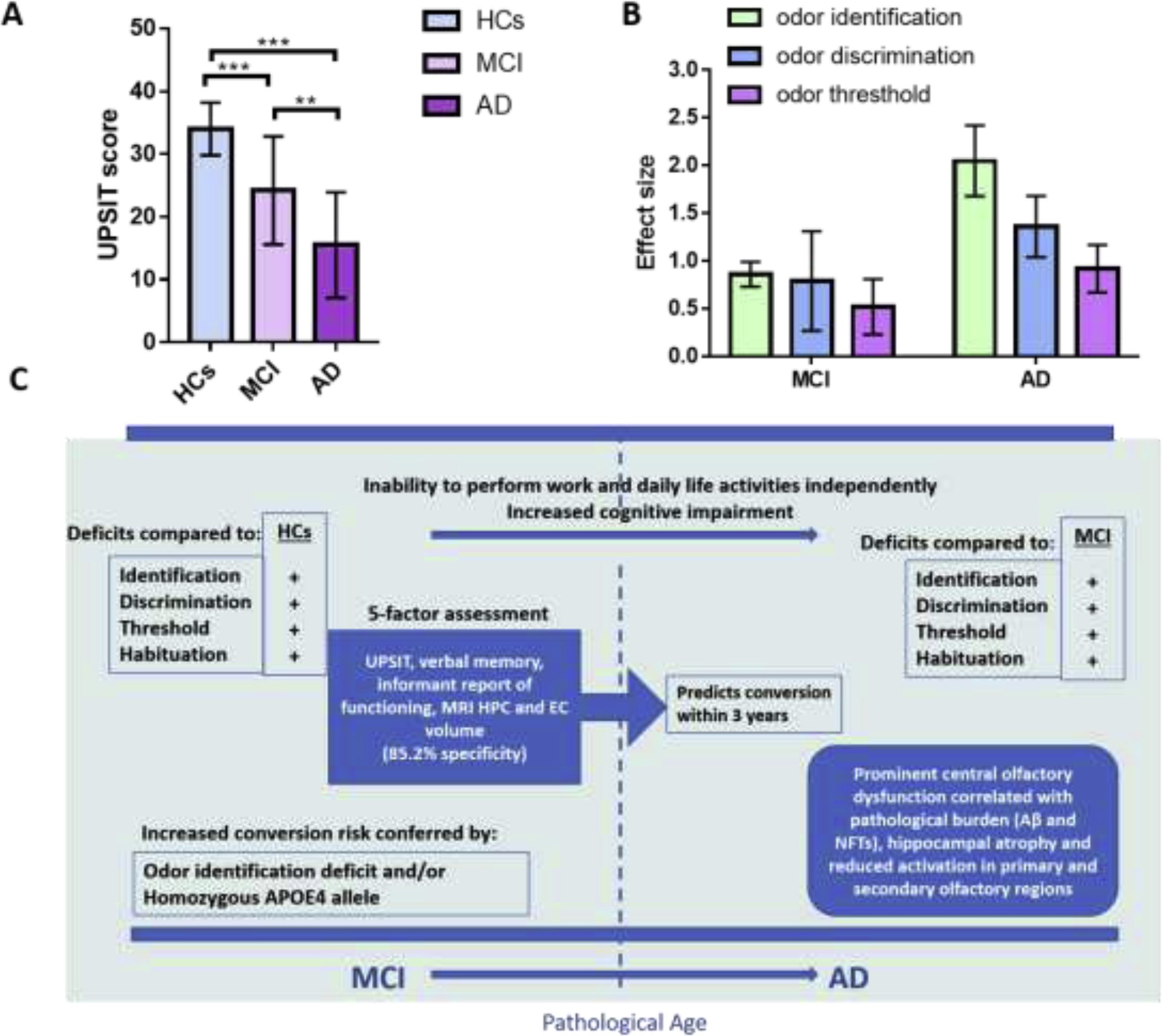
OD in AD. (A) Comparison of odor identification ability among NC, MCI, and AD patients. Graph plotted based on Vasavada et al. (Vasavada et al., 2017). NC (n=27): 69.5±10.4, MCI (n=21): 73.2±9.0, AD (n=15): 71.9±11.9. Mean ± SD. ** P < 0.01, *** P < 0.001. (B) Effect sizes for different olfactory domain deficits in prodromal MCI and AD (error bars indicate 95% confidence interval). Prior convention has classified effect sizes as small (d=0.2), medium (d=0.5) or large (d≥0.8). Graph plotted based on 2 meta-analyses (Rahayel et al., 2012; Roalf et al., 2017). (C) Profiles of OD of MCI and AD patients. HPC: hippocampus; EC: entorhinal cortex.
The risk of AD is known to be increased by multiple factors, including amyloid beta, excessive levels of P-tau protein in neurofibrillary tangles (NFTs) (Alonso et al., 1997) and apolipoprotein-E (APOE) genetic risk variants (Genin et al., 2011). These risk factors are also associated with OD. P-tau is observed in the olfactory bulb of AD patients and a p-tau transgenic mouse model (P301S) which exhibits AD-like features (Li et al., 2019). The olfactory system of P301S mice had early tau pathology, progressive neurodegeneration in the piriform cortex, and functional olfactory deficits (Yang et al., 2016). The observed olfactory deficit was associated with mitral cell dysfunction in the olfactory bulb of P301S tau transgenic mice (Li et al., 2019). The authors speculated that mitral cells could be used as a putative target for the treatment of p-tau-induced early OD in AD (Li et al., 2019). The APOE4 allele is the most powerful genetic predictor of familial AD. Risk can increase from 20% to 90% with increasing numbers of APOE4 alleles in families with late onset AD (Corder et al., 1993). It is interesting to note that odor identification ability in populations with the APOE4 alleles decline more rapidly with age than in adults without APOE4, and can be detected before any differences could be observed in tests for odor threshold, picture identification, or Dementia Rating Scale (Calhoun-Haney and Murphy, 2005). People with ApoE alleles and olfactory impairment have a particularly high risk for cognitive decline (Calhoun-Haney and Murphy, 2005; Misiak et al., 2017b). Interestingly, a recent review proposed a mechanism stating that aging and/or stress induces neuronal expression of APOE in γ-aminobutyric acid (GABA)-expressing interneurons in the hippocampus (Najm et al., 2019). These GABAergic interneurons may be selectively vulnerable to APOE4, through a tau dependent mechanism causing neurotoxicity (Najm et al., 2019). In turn, GABAergic interneuron loss causes hyperexcitability and dysregulation of neural networks in the hippocampus and cortex, leading to cognitive deficits.
In preclinical AD, the first region to show significant presence of NFTs in Braak’s staging is the entorhinal cortex (Alafuzoff et al., 2008; Braak et al., 2006). This region plays a role in contextualizing olfactory memory and is a processing center for olfactory information prior to the hippocampus (Hyman et al., 1991; Li et al., 2017). A higher tau-pathology in the entorhinal cortex correlated with a lower volume in this region, and a steeper decline in memory performance tests (Ziontz et al., 2019). Braak stage II is associated with tauopathy spreading to the hippocampus CA1 region, which receives direct olfactory input from the entorhinal cortex for associative learning, followed by further involvement of the neocortical regions (Alafuzoff et al., 2008; Braak et al., 2006; Li et al., 2017). It was suggested that the lesioning of the entorhinal cortex characteristic of Braak stage I promotes compensatory hyperactivation of adjacent brain areas involved in olfactory processing, which are the hippocampus and the olfactory bulb, which in turn contributes to degeneration of these regions (Murphy, 2019). Kovacs et al. examined 15 cases of AD and 15 control cases for β-amyloid deposition and NFTs formation, and the results showed that NFTs and β-amyloid deposits were present in every layer of OB in AD (Kovács et al., 1999). Interestingly, NFTs were also found in 87% of the control cases while β-amyloid deposits were comparatively rare and no classical plaques were found in the control (Kovács et al., 1999). It was proposed that declining olfaction function during aging may be the result of early AD-type pathology in the olfactory stems when dementia has not yet developed (Kovács et al., 1999). Decreased volumes of many brain regions in cognitively normal individuals strongly correlates with likelihood and severity of impaired odor identification scores measured via the B-SIT test (Vassilaki et al., 2017). Many of these regions are also significantly affected by tau pathology reflected in Braak’s NFT staging in AD (Vassilaki et al., 2017).
In conclusion, AD patients have poorer performance in olfaction tests than the aged matched normal controls and olfactory function worsens with the progression from MCI to AD. Odor identification was the most affected domain in MCI (Roalf et al., 2017) and AD (Rahayel et al., 2012; Silva et al., 2018). The risk factors of AD, including amyloid beta deposition, excessive levels of P-tau protein in NFTs and genetic risk variants of APOE are all associated with OD. Compared with PD, in which the OB is proposed to be one of the earliest affect regions, the initial stages of tau pathology in AD suggest that olfactory impairment begins with higher brain processing centers. The first region to show degradation is the entorhinal cortex, which may then induce compensatory hyperactivation of adjacent brain regions in olfactory processing, such as the hippocampus and the OB, causing degeneration of these regions and OD (Murphy, 2019).
4.3. Olfactory dysfunction induced by COVID-19
Temporary loss of smell is one of earliest prodromal symptoms in COVID-19 patients and can better predict the disease than other symptoms. High viral load has been detected in nasal swabs of both symptomatic and asymptomatic patients, indicating the possible role of nasal epithelium as an initial infection site and a key reservoir for viral spread (Sungnak et al., 2020). SARS-CoV-2, the coronavirus causing the COVID-19 syndrome, infects cells through interactions between its spike protein and angiotensin-converting enzyme 2 (ACE2) expressed on the target cells. Interestingly, single cell RNA sequencing analysis showed that ACE2 is not expressed in olfactory sensory neurons, but in non-neuronal cells in the OE and OB which provide metabolic and structural support to olfactory sensory neurons (Brann et al., 2020). This indicates that non-neuronal cell types may be the infection target in COVID-19, instead of the sensory neurons. This may explain why anosmia is temporary in COVID-19 patients and the majority of patients recover faster than it usually takes to recover from anosmia induced by other viral infections, which directly cause damage to olfactory sensory neurons (Brann et al., 2020). However, another study showed that SARS-CoV-2 can cross the neural-mucosal interface in the olfactory mucosa and enter the nervous system via axonal transport (Meinhardt et al., 2021). The SARS Cov-2 virus in the CNS resulted in local CNS responses mediated by HLA-DR+ microglia and inflammatory mediators were found in cerebrospinal fluid (Meinhardt et al., 2021). Interestingly, IL-6 levels in COVID-19 patients are correlated with olfactory and gustatory disorders, highlighting a possible role of IL-6 in the pathogenesis of chemosensitivity disorders (Cazzolla et al., 2020).
4.4. Olfactory dysfunction induced by other factors.
OD is affected by many other factors besides aging and neurodegenerative diseases, such as genetic background, gender, environment, and other diseases, etc. For example, APOE is an important gene associated with olfactory function, as mentioned earlier. Additionally, PTEN-alpha, one of the isoforms of phosphatase and TENsin homolog deleted on chromosome 10 (PTEN), has been recently identified to have a new role in maintaining mitral cells, regulating endocytosis in OB neurons, and controlling olfactory behaviours in mice (Yuan et al., 2019) along with its previously known roles in maintenance of mitochondrial structure, energy metabolism (Liang et al., 2019), modulation of hippocampal long-term potentiation and processes of learning and memory (Wang et al., 2019). Expression of PTEN-alpha is much higher in olfactory bulb than other regions of mouse brain, and PTEN-alpha-deficient mice exhibit OD and a decrease in mitral cell number in OB (Yuan et al., 2019). DNA repair gene deficiencies may also impede olfactory performance. NEIL1 knockout on a C57BL/6J background and Polymerase beta (3xTg Polβ) heterozygous mice on an Alzheimer’s disease background (3xTg) both demonstrate impaired olfactory performance relative to littermate controls (Misiak et al., 2017a; Navarro et al., 2020). The DNA glycosylase NEIL1 is more highly expressed in the OB than other brain regions and a complete loss of NEIL1 in mice greatly impaired their performance in the buried food test (Canugovi et al., 2015). Likewise, depletion of Polβ in an AD animal model left the mice with more severe OD. Loss of Polβ leads to greater degeneration of the OB neurons and significantly fewer newly generated neurons (Misiak et al., 2017a). Combined, these results suggest that DNA repair mechanisms may also be important for olfactory performance.
In addition to these factors, cigarette smoking has been shown to greatly affect olfactory function and the impairment persists as long as 15 years after quitting, which is consistent with a vascular mechanism of impairment (Siegel et al., 2019). A number of diseases, including allergic rhinitis (Liang et al., 2019), frontal lobe epilepsy (Mercado-Gomez et al., 2018), cervical dystonia (Marek et al., 2018), and virus infections (Sungnak et al., 2020) are also associated with OD.
5. Olfactory dysfunction as an early biomarker for neurodegenerative diseases
Hyposmia precedes motor symptoms for PD patients by several years (Haehner et al., 2011), and is highly prevalent in patients experiencing MCI, the transition state between normal cognition and full blown AD (Jung et al., 2019a; Masurkar and Devanand, 2014). Evaluation of olfactory function as a non-invasive and inexpensive way to predict neurodegenerative disease and diagnosis is appealing as early diagnosis of the disease is critical for the implementation of interventions when the brain is still relatively normal in terms of pathology. Simple olfactory tests are effective in detecting OD in the two most common neurodegenerative diseases (Morley et al., 2018; Velayudhan et al., 2015) and may help differentiate patients from the normal controls and patients with certain similar features but different pathological causes (Duff et al., 2002). However, despite the great advantages of non-invasion and low cost, the application of OD as biomarkers for neurodegenerative diseases is still challenging due to the fact that OD is shared among aging and multiple age-related neurodegenerative disorders, including AD, PD, Lewy Body Dementia, and Huntington’s disease. Thus, olfactory tests alone may be not be specific enough to identify specific diseases (Mesholam et al., 1998).
To address this problem, different strategies may be applied. First of all, the combination of olfactory tests with other tests for disease specific phenotypes can provide a more accurate disease prediction and diagnosis. For example, five biomarkers combined with odor identification, cognitive testing, genotyping, MRI scans for hippocampal and entorhinal cortex volumes gave 90% specificity in predicting whether an individual with MCI would eventually develop into AD (Devanand et al., 2008). Recently, a methodology combining olfaction tests with the coherency results of the electroencephalography recording, which measures the functional connectivity of different brain regions by the synchrony of oscillations was developed for AD (Sedghizadeh et al., 2020). This method required a smaller number of measurements when compared with previous combinations and can be applied to longitudinal monitoring of patients in the progression of disease. For PD, we have already mentioned the 2-factor assessment method combining odor identification and dopamine transporter (DAT) imaging for predicting prodromal PD development to PD (Jennings et al., 2017), however, other different combinations are also proposed. For example, a combination of hyposmia and substania nigra hyperechogenicity, which are two important risk markers of PD, can improve diagnostic specificity in discriminating PD patients from patients with essential tremors in a Chinese study (Chen et al., 2012). The combination of response to acute levodopa challenge with olfaction tests improved the diagnosis sensitivity for early PD patients who have subtle motor features (Terroba Chambi et al., 2017).
The method of combination may sometimes be challenging in cases when neuropathological features are not significant, especially at the very early stage of disease. The strategy of establishing disease-specific OD features may then be necessary to distinguish age-related and neurodegenerative disease-related OD. This can be established based on that hyposmia induced by different factors can produce different patterns of olfactory loss. For example, a study investigating the involvement of orthonasal (nose) and retronasal (oral cavity) OD in PD-related and non-Parkinsonian OD (postviral, post-traumatic, sinunasal, idiopathic and other factors induced OD) showed that patients with non-Parkinsonian OD had significantly better orthonasal scores than PD patients and orthonasal and retronasal scores were significantly correlated while no such correlation was observed in PD patients (Aubry-Lafontaine et al., 2020). Although OD can be induced by aging, PD or AD, the olfaction domains may be affected to different degrees. For age-related OD, odor threshold declined most dramatically when compared to odor discrimination and odor identification (Hummel et al., 2007). PD patients performed relatively well in odor thresholds, but poorly in odor identification and discrimination. In contrast, patients with postinfectious and posttraumatic hyposmia did well in odor threshold and discrimination but poorly in odor identification (Whitcroft et al., 2017). For OD in MCI (Roalf et al., 2017) and AD (Rahayel et al., 2012; Silva et al., 2018), odor identification was reportedly the most affected domain. However, most current studies are based on a small number of samples and inconsistent results may be reported due to differences of methodology and sample population with different cultural backgrounds. Importantly, most people do not conduct olfaction tests until they develop certain pathological features, therefore it is challenging to characterize the features of OD as an early biomarker for neurodegenerative diseases without retrospective data available. We propose that aged people should undergo a smelling test once a year to keep a longitudinal record which would pick up any sharp declines, which will not only contribute to a retrospective review for early PD or AD diagnosis, but also help establish a database for research.
Lastly, the establishment of disease-specific odor tests may be helpful. Woodward et al. examined whether there were differences among a normal aging population and AD patients in recognizing specific odors (Woodward et al., 2018). Their study showed that disease-and age-associated odorants clustered separately in aging and AD. Ten AD-associated odors and ten aging associated odors were selected for in the screening of 841 participants (234 healthy control, 192 amnestic MCI, 415 AD), with results showing that the Ten AD-associated odors test better distinguished the healthy controls from AD subjects and predicted the conversion from amnestic MCI to AD (Woodward et al., 2018). A more recent study showed that only two odors, grape and chocolate, can discriminate between the healthy participants and mild AD patients with good accuracy (Sedghizadeh et al., 2020). In PD, selective olfactory deficits account for 70% to 90% of patients (Double et al., 2003). Three odors, banana, licorice, and dill pickle, were the most frequently misidentified odors in PD patients when compared to age- and gender-matched controls and tests based on these three odors showed stronger correlations with nigrostriatal dopamine denervation than the conventional UPSIT (Bohnen et al., 2008). In another study, gasoline, banana, pineapple, smoke, and cinnamon were reported to be specifically more difficult to detect by PD patients (Double et al., 2003). An abbreviated 3-stick test comprised of anise, licorice and banana was able to detect OD in PD and iRBD patients with high levels of accuracy and could be used in busy clinical environments (Lo et al., 2021). These studies indicate the feasibility of developing more disease specific odor tests for more accurate diagnosis. However, we still lack consistency to define the list of disease specific odors and the mechanisms for selective odor detection deficits in AD and PD are still not understood. Whether there are more specific odors associated with certain diseases and how disease specific odor deficits are related with cultural backgrounds need further study.
In a short, the application of OD as an early biomarker for neurodegenerative diseases is promising, however, more efforts are required to improve the specificity to differentiate OD induced by normal physiological aging and different neurodegenerative diseases.
6. Concluding Remarks
The sense of smell significantly affects different aspects of people’s lives, from the enjoyment of food flavor to recognizing warning signs of environmental hazards. Sadly, most people are unaware of their smelling problems until proper tests are performed. 52% of PD patients overrated their sense of smell and only 27% correctly identified themselves as being hyposmic (Leonhardt et al., 2019). Similarly, only 6% of AD patients complained of a decline in olfaction during the early stage of the disease, while over 80% of AD patients actually demonstrated a significant impairment of olfactory function (Zou et al., 2016). This is partially attributed to the deficits in metacognitive knowledge, called anosognosia, which is common in neurodegenerative diseases (Leonhardt et al., 2019). Consistent and adequate evaluation of olfactory function in the elderly population is important for early detection of smelling impairment. Although impairment may not necessarily indicate the onset of neurodegenerative diseases, it certainly indicates increased risk.
Tests of olfactory function are cost effective and simple to perform at the bedside without the need for specific training or sophisticated equipment. Although smelling tests can effectively differentiate AD or PD from normal controls, it remains challenging to identify whether a healthy individual with OD will develop dementia in the future. This is confounded by the fact that olfaction declines concomitantly with normal and pathological aging. The identification and fine tuning of more disease-specific smelling tests and use of olfactory assessments in combination with other biomarkers are warranted in future research.
Based on the great advantages of smelling tests that are commercially available, brief, easy, sensitive, accurate and convenient, individuals could keep a longitudinal record of their olfactory function by conducting smelling tests once a year. In this way, they would not only be able to compare their olfactory function with age and gender-controlled populations but also track olfaction changes and notice a potential dramatic declines soon as possible. Physicians could then review olfactory records retrospectively if patients develop any neurodegenerative diseases later. Collection and analysis of these longitudinal records based on large populations will provide invaluable information and contribute to improving the accuracy and specificity of OD in neurodegenerative disease diagnosis.
Highlights.
The prevalence of olfactory dysfunction (OD) is high in neurodegenerative diseases, especially in Parkinson’s disease (PD) and Alzheimer’s disease (AD).
Olfactory bulbs are one of the earliest affected brain regions in PD and OD is among the earliest non-motor features in PD patients.
Risk factors of AD are associated with OD and olfactory impairment begins in the higher brain processing centers in AD patients.
Olfaction tests for human and animal models focus on odor identification, discrimination, sensitivity and habituation.
Less than a quarter of individuals with OD are aware of smelling problems, highlighting the necessity for olfactory tests.
Individual longitudinal records of olfactory function are necessary and may predict future cognitive dysfunction.
Acknowledgements
The authors acknowledge the valuable work of the many investigators whose published articles they were unable to cite owing to space limitations. They thank Dr. Luka Culig, Dr. Xixia Chu and Dr. Mansoor Akbar Ali for critical reading of the manuscript. They thank Lauren Brick for improving the figures in the paper. This research was supported by the Intramural Research Program of the NIH, the National Institute on Aging (V.A.B.) and by an NIA AD grant, AG000790.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Abraham NM, Egger V, Shimshek DR, Renden R, Fukunaga I, Sprengel R, Seeburg PH, Klugmann M, Margrie TW, Schaefer AT, Kuner T, 2010. Synaptic Inhibition in the Olfactory Bulb Accelerates Odor Discrimination in Mice. Neuron 65, 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alafuzoff I, Arzberger T, Al-Sarraj S, Bodi I, Bogdanovic N, Braak H, Bugiani O, Del-Tredici K, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Ince P, Kamphorst W, King A, Korkolopoulou P, Kovacs GG, Larionov S, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Seilhean D, Tagliavini F, Stadelmann C, Streichenberger N, Thal DR, Wharton SB, Kretzschmar H, 2008. Staging of neurofibrillary pathology in Alzheimer’s disease: a study of the BrainNet Europe Consortium. Brain Pathol 18, 484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K, 1997. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci U S A 94, 298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardiel EL, Rankin CH, 2010. An elegant mind: Learning and memory in Caenorhabditis elegans. Learning & Memory 17, 191–201. [DOI] [PubMed] [Google Scholar]
- Asztalos Z, Arora N, Tully T, 2007. Olfactory jump reflex habituation in Drosophila and effects of classical conditioning mutations. Journal of neurogenetics 21, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attems J, Walker L, Jellinger KA, 2015. Olfaction and Aging: A Mini-Review. Gerontology 61, 485–490. [DOI] [PubMed] [Google Scholar]
- Aubry-Lafontaine E, Tremblay C, Durand-Martel P, Dupre N, Frasnelli J, 2020. Orthonasal, but not Retronasal Olfaction Is Specifically Impaired in Parkinson’s Disease. Chem Senses 45, 401–406. [DOI] [PubMed] [Google Scholar]
- Bargmann C, 2006. Chemosensation in C. elegans. WormBook. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, White CL 3rd, Hladik CL, Sabbagh MN, Connor DJ, Shill HA, Sue LI, Sasse J, Bachalakuri J, Henry-Watson J, Akiyama H, Adler CH, Arizona Parkinson’s Disease C, 2009. Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta neuropathologica 117, 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker G, Muller A, Braune S, Buttner T, Benecke R, Greulich W, Klein W, Mark G, Rieke J, Thumler R, 2002. Early diagnosis of Parkinson’s disease. J Neurol 249Suppl 3, III/40–48. [DOI] [PubMed] [Google Scholar]
- Berg D, Godau J, Seppi K, Behnke S, Liepelt-Scarfone I, Lerche S, Stockner H, Gaenslen A, Mahlknecht P, Huber H, Srulijes K, Klenk J, Fassbender K, Maetzler W, Poewe W, group P.s., 2013. The PRIPS study: screening battery for subjects at risk for Parkinson’s disease. Eur J Neurol 20, 102–108. [DOI] [PubMed] [Google Scholar]
- Boesveldt S, Verbaan D, Knol DL, Visser M, van Rooden SM, van Hilten JJ, Berendse HW, 2008. A comparative study of odor identification and odor discrimination deficits in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society 23, 1984–1990. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Gedela S, Herath P, Constantine GM, Moore RY, 2008. Selective hyposmia in Parkinson disease: association with hippocampal dopamine activity. Neurosci Lett 447, 12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Muller ML, Kotagal V, Koeppe RA, Kilbourn MA, Albin RL, Frey KA, 2010. Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson’s disease. Brain 133, 1747–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K, 2006. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112, 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E, Yilmazer D, de Vos RA, Jansen EN, Bohl J, Jellinger K, 1994. Amygdala pathology in Parkinson’s disease. Acta neuropathologica 88, 493–500. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E, 2003. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of aging 24, 197–211. [DOI] [PubMed] [Google Scholar]
- Branigan B, Tadi P, 2020. Physiology, Olfactory, StatPearls, Treasure Island (FL). [PubMed]
- Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong B, Chance R, Macaulay IC, Chou HJ, Fletcher RB, Das D, Street K, de Bezieux HR, Choi YG, Risso D, Dudoit S, Purdom E, Mill J, Hachem RA, Matsunami H, Logan DW, Goldstein BJ, Grubb MS, Ngai J, Datta SR, 2020. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Science advances 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum MS, Kesslak JP, Lynch G, Chui H, Wu J, Sicotte N, Hazlett E, Teng E, Cotman CW, 1991. Temporal and hippocampal metabolic rate during an olfactory memory task assessed by positron emission tomography in patients with dementia of the Alzheimer type and controls. Preliminary studies. Archives of general psychiatry 48, 840–847. [DOI] [PubMed] [Google Scholar]
- Calhoun-Haney R, Murphy C, 2005. Apolipoprotein epsilon4 is associated with more rapid decline in odor identification than in odor threshold or Dementia Rating Scale scores. Brain Cogn 58, 178–182. [DOI] [PubMed] [Google Scholar]
- Canugovi C, Misiak M, Scheibye-Knudsen M, Croteau DL, Mattson MP, Bohr VA, 2015. Loss of NEIL1 causes defects in olfactory function in mice. Neurobiol Aging 36, 1007–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardesin A, Alobid I, Benitez P, Sierra E, de Haro J, Bernal-Sprekelsen M, Picado C, Mullol J, 2006. Barcelona Smell Test - 24 (BAST-24): validation and smell characteristics in the healthy Spanish population. Rhinology 44, 83–89. [PubMed] [Google Scholar]
- Cazzolla AP, Lovero R, Lo Muzio L, Testa NF, Schirinzi A, Palmieri G, Pozzessere P, Procacci V, Di Comite M, Ciavarella D, Pepe M, De Ruvo C, Crincoli V, Di Serio F, Santacroce L, 2020. Taste and Smell Disorders in COVID-19 Patients: Role of Interleukin-6. ACS chemical neuroscience 11, 2774–2781. [DOI] [PubMed] [Google Scholar]
- Chang SH, Chang YY, Wu LY, 2019. Gender differences in lifestyle and risk factors of metabolic syndrome: Do women have better health habits than men? J Clin Nurs 28, 2225–2234. [DOI] [PubMed] [Google Scholar]
- Chen W, Tan YY, Hu YY, Zhan WW, Wu L, Lou Y, Wang X, Zhou Y, Huang P, Gao Y, Xiao Q, Chen SD, 2012. Combination of olfactory test and substantia nigra transcranial sonopraphy in the differential diagnosis of Parkinson’s disease: a pilot study from China. Translational neurodegeneration 1, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho W, Heberlein U, Wolf FW, 2004. Habituation of an odorant-induced startle response in Drosophila. Genes, Brain and Behavior 3, 127–137. [DOI] [PubMed] [Google Scholar]
- Chou KL, Bohnen NI, 2009. Performance on an Alzheimer-selective odor identification test in patients with Parkinson’s disease and its relationship with cerebral dopamine transporter activity. Parkinsonism & related disorders 15, 640–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung TW, Sridhar S, Zhang AJ, Chan KH, Li HL, Wong FK, Ng MY, Tsang RK, Lee AC, Fan Z, Ho RS, Luk SY, Kan WK, Lam SH, Wu AK, Leung SM, Chan WM, Ng PY, To KK, Cheng VC, Lung KC, Hung IF, Yuen KY, 2020. Olfactory Dysfunction in Coronavirus Disease 2019 Patients: Observational Cohort Study and Systematic Review. Open forum infectious diseases 7, ofaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Yurgelun-Todd DA, 2013. Age-related changes in insula cortical thickness and impulsivity: significance for emotional development and decision-making. Developmental cognitive neuroscience 6, 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert HA, Bargmann CI, 1995. Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron 14, 803–812. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA, 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923. [DOI] [PubMed] [Google Scholar]
- Criaud M, Christopher L, Boulinguez P, Ballanger B, Lang AE, Cho SS, Houle S, Strafella AP, 2016. Contribution of insula in Parkinson’s disease: A quantitative meta-analysis study. Human brain mapping 37, 1375–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy I, Nordin S, Hummel T, 2014. Olfactory disorders and quality of life--an updated review. Chem Senses 39, 185–194. [DOI] [PubMed] [Google Scholar]
- Damm M, Pikart LK, Reimann H, Burkert S, Goktas O, Haxel B, Frey S, Charalampakis I, Beule A, Renner B, Hummel T, Huttenbrink KB, 2014. Olfactory training is helpful in postinfectious olfactory loss: a randomized, controlled, multicenter study. The Laryngoscope 124, 826–831. [DOI] [PubMed] [Google Scholar]
- Daramola OO, Becker SS, 2015. An algorithmic approach to the evaluation and treatment of olfactory disorders. Curr Opin Otolaryngol Head Neck Surg 23, 8–14. [DOI] [PubMed] [Google Scholar]
- DeTure MA, Dickson DW, 2019. The neuropathological diagnosis of Alzheimer’s disease. Molecular neurodegeneration 14, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanand DP, Liu X, Tabert MH, Pradhaban G, Cuasay K, Bell K, de Leon MJ, Doty RL, Stern Y, Pelton GH, 2008. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biological psychiatry 64, 871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, 1995. Handbook of Olfaction and Gustation (Vol 32, Pg 1432, 1995). Neurology 45, 1952–1952. [Google Scholar]
- Doty RL, 2007. Office Procedures for Quantitative Assessment of Olfactory Function. American Journal of Rhinology 21, 460–473. [DOI] [PubMed] [Google Scholar]
- Doty RL, 2012. Olfactory dysfunction in Parkinson disease. Nature reviews. Neurology 8, 329–339. [DOI] [PubMed] [Google Scholar]
- Doty RL, 2017. Olfactory dysfunction in neurodegenerative diseases: is there a common pathological substrate? Lancet Neurol 16, 478–488. [DOI] [PubMed] [Google Scholar]
- Doty RL, Frye RE, Agrawal U, 1989. Internal consistency reliability of the fractionated and whole University of Pennsylvania Smell Identification Test. Perception & Psychophysics 45, 381–384. [DOI] [PubMed] [Google Scholar]
- Doty RL, Kamath V, 2014. The influences of age on olfaction: a review. Front Psychol 5, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Tourbier I, Ng V, Neff J, Armstrong D, Battistini M, Sammel MD, Gettes D, Evans DL, Mirza N, Moberg PJ, Connolly T, Sondheimer SJ, 2015. Influences of hormone replacement therapy on olfactory and cognitive function in postmenopausal women. Neurobiol Aging 36, 2053–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Double KL, Rowe DB, Hayes M, Chan DK, Blackie J, Corbett A, Joffe R, Fung VS, Morris J, Halliday GM, 2003. Identifying the pattern of olfactory deficits in Parkinson disease using the brief smell identification test. Archives of neurology 60, 545–549. [DOI] [PubMed] [Google Scholar]
- Duff K, McCaffrey RJ, Solomon GS, 2002. The Pocket Smell Test: successfully discriminating probable Alzheimer’s dementia from vascular dementia and major depression. The Journal of neuropsychiatry and clinical neurosciences 14, 197–201. [DOI] [PubMed] [Google Scholar]
- E Ray Dorsey AE, Nichols Emma, Abd-Allah Foad, Abdelalim Ahmed, Adsuar Jose C, Ansha Mustafa Geleto, Brayne Carol, Choi Jee-Young J, Collado-Mateo Daniel, Dahodwala Nabila, Do Huyen Phuc, Edessa Dumessa, Endres Matthias, Fereshtehnejad Seyed-Mohammad, Foreman Kyle J, Gankpe Fortune Gbetoho, Gupta Rahul, Hankey Graeme J, Hay Simon I, Hegazy Mohamed I, Hibstu Desalegn T, Kasaeian Amir, Khader Yousef, Khalil Ibrahim, Khang Young-Ho, Kim Yun Jin, Kokubo Yoshihiro, Logroscino Giancarlo, Massano João, Ibrahim Norlinah Mohamed, Mohammed Mohammed A, Mohammadi Alireza, Moradi-Lakeh Maziar, Naghavi Mohsen, Nguyen Binh Thanh, Nirayo Yirga Legesse, Ogbo Felix Akpojene, Owolabi Mayowa Ojo, Pereira David M, Postma Maarten J, Qorbani Mostafa, Rahman Muhammad Aziz, Roba Kedir T, Safari Hosein, Safiri Saeid, Satpathy Maheswar, Sawhney Monika, Shafieesabet Azadeh, Shiferaw Mekonnen Sisay, Smith Mari, Szoeke Cassandra E I, Tabarés-Seisdedos Rafael, Truong Nu Thi, Ukwaja Kingsley Nnanna, Venketasubramanian Narayanaswamy, Villafaina Santos, Weldegwergs Kidu Gidey, Westerman Ronny, Wijeratne Tissa, Winkler Andrea S, Xuan Bach Tran, Yonemoto Naohiro, Feigin Valery L, Vos Theo, Murray Christopher J L, 2018. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. Neurology 17, 939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eibenstein A, Fioretti AB, Lena C, Rosati N, Amabile G, Fusetti M, 2005. Modern psychophysical tests to assess olfactory function. Neurological Sciences 26, 147–155. [DOI] [PubMed] [Google Scholar]
- Faucher C, Forstreuter M, Hilker M, Bruyne M.d., 2006. Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex and olfactory context. Journal of Experimental Biology 209, 2739–2748. [DOI] [PubMed] [Google Scholar]
- Gaugler J, James B, Johnson T, Marin A, Weuve J, Assoc A.s., 2019. 2019 Alzheimer’s disease facts and figures. Alzheimers & Dementia 15, 321–387. [Google Scholar]
- Genin E, Hannequin D, Wallon D, Sleegers K, Hiltunen M, Combarros O, Bullido MJ, Engelborghs S, De Deyn P, Berr C, Pasquier F, Dubois B, Tognoni G, Fievet N, Brouwers N, Bettens K, Arosio B, Coto E, Del Zompo M, Mateo I, Epelbaum J, Frank-Garcia A, Helisalmi S, Porcellini E, Pilotto A, Forti P, Ferri R, Scarpini E, Siciliano G, Solfrizzi V, Sorbi S, Spalletta G, Valdivieso F, Vepsalainen S, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossu P, Hanon O, Piccardi P, Annoni G, Seripa D, Galimberti D, Licastro F, Soininen H, Dartigues JF, Kamboh MI, Van Broeckhoven C, Lambert JC, Amouyel P, Campion D, 2011. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol Psychiatry 16, 903–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerde KV, Muller B, Skeie GO, Assmus J, Alves G, Tysnes OB, 2018. Hyposmia in a simple smell test is associated with accelerated cognitive decline in early Parkinson’s disease. Acta neurologica Scandinavica 138, 508–514. [DOI] [PubMed] [Google Scholar]
- Gocel J, Larson J, 2013. Evidence for loss of synaptic AMPA receptors in anterior piriform cortex of aged mice. Frontiers in aging neuroscience 5, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Diaz C, Martin F, Garcia-Fernandez JM, Alcorta E, 2018. The Two Main Olfactory Receptor Families in Drosophila, ORs and IRs: A Comparative Approach. Frontiers in cellular neuroscience 12, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Wang RD, Lian TH, Ding DY, Zhang YN, Zhang WJ, Li DN, Li LX, Li JH, Guan HY, Yu SY, Liu L, Hu Y, Zuo LJ, Yu QJ, Wang XM, Zhang W, 2020. Olfactory Dysfunction and Its Association With Neuropathologic Proteins in Cerebrospinal Fluid From Patients With Parkinson Disease. Frontiers in aging neuroscience 12, 594324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haehner A, Hummel T, Reichmann H, 2011. Olfactory Loss in Parkinson’s Disease. Parkinson’s Disease 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hånell A, Marklund N, 2014. Structured evaluation of rodent behavioral tests used in drug discovery research. Frontiers in Behavioral Neuroscience 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart A, 2006. Behavior. WormBook. [Google Scholar]
- Hart AC, Chao MY, 2010. From Odors to Behaviors in Caenorhabditis elegans, in: Menini A (Ed.), The Neurobiology of Olfaction. CRC Press/Taylor & Francis, Boca Raton (FL). [PubMed] [Google Scholar]
- Hasegawa-Ishii S, Shimada A, Imamura F, 2019. Neuroplastic changes in the olfactory bulb associated with nasal inflammation in mice. The Journal of allergy and clinical immunology 143, 978–989 e973. [DOI] [PubMed] [Google Scholar]
- He R, Zhao Y, He Y, Zhou Y, Yang J, Zhou X, Zhu L, Zhou X, Liu Z, Xu Q, Sun Q, Tan J, Yan X, Tang B, Guo J, 2020. Olfactory Dysfunction Predicts Disease Progression in Parkinson’s Disease: A Longitudinal Study. Frontiers in neuroscience 14, 569777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedner M, Larsson M, Arnold N, Zucco GM, Hummel T, 2010. Cognitive factors in odor detection, odor discrimination, and odor identification tasks. Journal of Clinical and Experimental Neuropsychology 32, 1062–1067. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Carpenter K, Cartwright H, Halliday GM, 2000. Loss of thalamic intralaminar nuclei in progressive supranuclear palsy and Parkinson’s disease: clinical and therapeutic implications. Brain : a journal of neurology 123 (Pt 7), 1410–1421. [DOI] [PubMed] [Google Scholar]
- Henkin RI, Schmidt L, Velicu I, 2013. Interleukin 6 in hyposmia. JAMA otolaryngology-- head & neck surgery 139, 728–734. [DOI] [PubMed] [Google Scholar]
- Herz RS, 2009. Aromatherapy Facts and Fictions: A Scientific Analysis of Olfactory Effects on Mood, Physiology and Behavior. International Journal of Neuroscience 119, 263–290. [DOI] [PubMed] [Google Scholar]
- Hilliard MA, Bargmann CI, Bazzicalupo P, 2002. C. elegans Responds to Chemical Repellents by Integrating Sensory Inputs from the Head and the Tail. Current Biology 12, 730–734. [DOI] [PubMed] [Google Scholar]
- Hirotsu T, Sonoda H, Uozumi T, Shinden Y, Mimori K, Maehara Y, Ueda N, Hamakawa M, 2015. A highly accurate inclusive cancer screening test using Caenorhabditis elegans scent detection. PloS one 10, e0118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JW, Keller A, Wong M, Jiang RS, Vosshall LB, 2017. SMELL-S and SMELL-R: Olfactory tests not influenced by odor-specific insensitivity or prior olfactory experience. Proc Natl Acad Sci U S A 114, 11275–11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T, Kobal G, Gudziol H, Mackay-Sim A, 2007. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies 264, 237–243. [DOI] [PubMed] [Google Scholar]
- Hummel T, Pfetzing U, Lotsch J, 2010. A short olfactory test based on the identification of three odors. J Neurol 257, 1316–1321. [DOI] [PubMed] [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G, 1997. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22, 39–52. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Arriagada PV, Van Hoesen GW, 1991. Pathologic changes in the olfactory system in aging and Alzheimer’s disease. Ann N Y Acad Sci 640, 14–19. [DOI] [PubMed] [Google Scholar]
- Igarashi KM, Ieki N, An M, Yamaguchi Y, Nagayama S, Kobayakawa K, Kobayakawa R, Tanifuji M, Sakano H, Chen WR, Mori K, 2012. Parallel Mitral and Tufted Cell Pathways Route Distinct Odor Information to Different Targets in the Olfactory Cortex. Journal of Neuroscience 32, 7970–7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TJ, Fraser C, Wang L, Walker V, O’Connor S, 2003. Psychophysical evaluation of responses to pleasant and mal-odour stimulation in human subjects; adaptation, dose response and gender differences. Int J Psychophysiol 48, 67–80. [DOI] [PubMed] [Google Scholar]
- Jennings D, Siderowf A, Stern M, Seibyl J, Eberly S, Oakes D, Marek K, Investigators P, 2017. Conversion to Parkinson Disease in the PARS Hyposmic and Dopamine Transporter-Deficit Prodromal Cohort. JAMA neurology 74, 933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, Wang Z, Yang T, Li Y, Gao S, Wu G, Jiang T, Liang P, 2019. Entorhinal Cortex Atrophy in Early, Drug-naive Parkinson’s Disease with Mild Cognitive Impairment. Aging and disease 10, 1221–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ME, Bergkvist L, Mercado G, Stetzik L, Meyerdirk L, Wolfrum E, Madaj Z, Brundin P, Wesson DW, 2020. Deficits in olfactory sensitivity in a mouse model of Parkinson’s disease revealed by plethysmography of odor-evoked sniffing. Sci Rep 10, 9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HJ, Shin I-S, Lee J-E, 2019a. Olfactory function in mild cognitive impairment and Alzheimer’s disease: A meta-analysis: Olfactory Function Deficit in MCI and AD. The Laryngoscope 129, 362–369. [DOI] [PubMed] [Google Scholar]
- Jung HJ, Shin IS, Lee JE, 2019b. Olfactory function in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Laryngoscope 129, 362–369. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Wilson DA, 2006. Olfactory Cortical Adaptation Facilitates Detection of Odors Against Background. Journal of neurophysiology 95, 1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass MD, Czarnecki LA, Moberly AH, McGann JP, 2017. Differences in peripheral sensory input to the olfactory bulb between male and female mice. Sci Rep 7, 45851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavoi BM, Jameela H, 2011. Comparative Morphometry of the Olfactory Bulb, Tract and Stria in the Human, Dog and Goat. Int J Morphol 29, 939–946. [Google Scholar]
- Kjelvik G, Evensmoen HR, Brezova V, Haberg AK, 2012. The human brain representation of odor identification. J Neurophysiol 108, 645–657. [DOI] [PubMed] [Google Scholar]
- Kobayakawa M, Tsuruya N, Kawamura M, 2017. Decision-making performance in Parkinson’s disease correlates with lateral orbitofrontal volume. Journal of the neurological sciences 372, 232–238. [DOI] [PubMed] [Google Scholar]
- Kondo K, Kikuta S, Ueha R, Suzukawa K, Yamasoba T, 2020. Age-Related Olfactory Dysfunction: Epidemiology, Pathophysiology, and Clinical Management. Frontiers in aging neuroscience 12, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács, Cairns, Lantos, 1999. β-Amyloid deposition and neurofibrillary tangle formation in the olfactory bulb in ageing and Alzheimer’s disease. Neuropathology and Applied Neurobiology 25, 481–491. [DOI] [PubMed] [Google Scholar]
- Krantz EM, Schubert CR, Dalton DS, Zhong W, Huang GH, Klein BE, Klein R, Nieto FJ, Cruickshanks KJ, 2009. Test-retest reliability of the San Diego Odor Identification Test and comparison with the brief smell identification test. Chem Senses 34, 435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Etoile ND, Bargmann CI, 2000. Olfaction and odor discrimination are mediated by the C. elegans guanylyl cyclase ODR-1. Neuron 25, 575–586. [DOI] [PubMed] [Google Scholar]
- Laska M, Salazar LTH., 2015. Olfaction in Nonhuman Primates, in: Doty RL (Ed.), Handbook of Olfaction and Gustation, 3 ed. Wiley Online Library, pp. 605–622. [Google Scholar]
- Lazarini F, Gabellec MM, Torquet N, Lledo PM, 2012. Early activation of microglia triggers long-lasting impairment of adult neurogenesis in the olfactory bulb. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 3652–3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Tian H, Grosmaitre X, Ma M, 2009. Expression Patterns of Odorant Receptors and Response Properties of Olfactory Sensory Neurons in Aged Mice. Chemical Senses 34, 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Oh JS, Ham JH, Lee JJ, Lee I, Lee PH, Kim JS, Sohn YH, 2015. Is normosmic Parkinson disease a unique clinical phenotype? Neurology 85, 1270–1275. [DOI] [PubMed] [Google Scholar]
- Leonhardt B, Tahmasebi R, Jagsch R, Pirker W, Lehrner J, 2019. Awareness of olfactory dysfunction in Parkinson’s disease. Neuropsychology 33, 633–641. [DOI] [PubMed] [Google Scholar]
- Li S, Li W, Wu X, Li J, Yang J, Tu C, Ye X, Ling S, 2019. Olfactory deficit is associated with mitral cell dysfunction in the olfactory bulb of P301S tau transgenic mice. Brain Res Bull 148, 34–45. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu J, Liu Y, Zhu J, Liu N, Zeng W, Huang N, Rasch MJ, Jiang H, Gu X, Li X, Luo M, Li C, Teng J, Chen J, Zeng S, Lin L, Zhang X, 2017. A distinct entorhinal cortex to hippocampal CA1 direct circuit for olfactory associative learning. Nat Neurosci 20, 559–570. [DOI] [PubMed] [Google Scholar]
- Liang C, Yang Z, Zou Q, Zhou M, Liu H, Fan J, 2019. Construction of an irreversible allergic rhinitis-induced olfactory loss mouse model. Biochem Biophys Res Commun 513, 635–641. [DOI] [PubMed] [Google Scholar]
- Liu AKL, Chau TW, Lim EJ, Ahmed I, Chang RC, Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RKB, 2019. Hippocampal CA2 Lewy pathology is associated with cholinergic degeneration in Parkinson’s disease with cognitive decline. Acta neuropathologica communications 7, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Umbach DM, Peddada SD, Xu Z, Troster AI, Huang X, Chen H, 2015. Potential sex differences in nonmotor symptoms in early drug-naive Parkinson disease. Neurology 84, 2107–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C, Arora S, Ben-Shlomo Y, Barber TR, Lawton M, Klein JC, Kanavou S, Janzen A, Sittig E, Oertel WH, Grosset DG, Hu MT, 2021. Olfactory Testing in Parkinson Disease and REM Behavior Disorder: A Machine Learning Approach. Neurology 96, e2016–e2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Z, Zheng S, Zhang X, Mai Y, Pan J, Hummel T, Hahner A, Zou L, 2021. Olfactory impairment as an early marker of Parkinson’s disease in REM sleep behaviour disorder: a systematic review and meta-analysis. Journal of neurology, neurosurgery, and psychiatry 92, 271–281. [DOI] [PubMed] [Google Scholar]
- Ma Y, Smith D, Hof PR, Foerster B, Hamilton S, Blackband SJ, Yu M, Benveniste H, 2008. In Vivo 3D Digital Atlas Database of the Adult C57BL/6J Mouse Brain by Magnetic Resonance Microscopy. Front Neuroanat 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado C, Reis-Silva T, Lyra C, Felicio L, Malnic B, 2018. Buried Food-seeking Test for the Assessment of Olfactory Detection in Mice. BIO-PROTOCOL 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlknecht P, Iranzo A, Hogl B, Frauscher B, Muller C, Santamaria J, Tolosa E, Serradell M, Mitterling T, Gschliesser V, Goebel G, Brugger F, Scherfler C, Poewe W, Seppi K, Sleep Innsbruck Barcelona G, 2015. Olfactory dysfunction predicts early transition to a Lewy body disease in idiopathic RBD. Neurology 84, 654–658. [DOI] [PubMed] [Google Scholar]
- Majid A, Burenhult N, Stensmyr M, de Valk J, Hansson BS, 2018. Olfactory language and abstraction across cultures. Philosophical Transactions of the Royal Society B: Biological Sciences 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese-Ragona R, Restivo DA, De Corso E, Vianello A, Nicolai P, Ottaviano G, 2020. Loss of smell in COVID-19 patients: a critical review with emphasis on the use of olfactory tests. Acta otorhinolaryngologica Italica : organo ufficiale della Societa italiana di otorinolaringologia e chirurgia cervico-facciale 40, 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek M, Linnepe S, Klein C, Hummel T, Paus S, 2018. High prevalence of olfactory dysfunction in cervical dystonia. Parkinsonism Relat Disord 53, 33–36. [DOI] [PubMed] [Google Scholar]
- Maresh A, Rodriguez Gil D, Whitman MC, Greer CA, 2008. Principles of Glomerular Organization in the Human Olfactory Bulb – Implications for Odor Processing. PLoS ONE 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin C, Vilas D, Langdon C, Alobid I, Lopez-Chacon M, Haehner A, Hummel T, Mullol J, 2018. Olfactory Dysfunction in Neurodegenerative Diseases. Current allergy and asthma reports 18, 42. [DOI] [PubMed] [Google Scholar]
- Marino-Sanchez F, Valls-Mateus M, Fragola C, de Los Santos G, Aguirre A, Alonso J, Valero J, Santamaria A, Rojas Lechuga MJ, Cobeta I, Alobid I, Mullol J, 2020. Pediatric Barcelona Olfactory Test 6 (pBOT-6): Validation of a Combined Odor Identification and Threshold Screening Test in Healthy Spanish Children and Adolescents. J Investig Allergol Clin Immunol 30, 439–447. [DOI] [PubMed] [Google Scholar]
- Martin C, Beshel J, Kay LM, 2007. An olfacto-hippocampal network is dynamically involved in odor-discrimination learning. Journal of neurophysiology 98, 2196–2205. [DOI] [PubMed] [Google Scholar]
- Masurkar AV, Devanand DP, 2014. Olfactory Dysfunction in the Elderly: Basic Circuitry and Alterations with Normal Aging and Alzheimer’s Disease. Current geriatrics reports 3, 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, Laue M, Schneider J, Brunink S, Greuel S, Lehmann M, Hassan O, Aschman T, Schumann E, Chua RL, Conrad C, Eils R, Stenzel W, Windgassen M, Rossler L, Goebel HH, Gelderblom HR, Martin H, Nitsche A, Schulz-Schaeffer WJ, Hakroush S, Winkler MS, Tampe B, Scheibe F, Kortvelyessy P, Reinhold D, Siegmund B, Kuhl AA, Elezkurtaj S, Horst D, Oesterhelweg L, Tsokos M, Ingold-Heppner B, Stadelmann C, Drosten C, Corman VM, Radbruch H, Heppner FL, 2021. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nature neuroscience 24, 168–175. [DOI] [PubMed] [Google Scholar]
- Menon C, Westervelt HJ, Jahn DR, Dressel JA, O’Bryant SE, 2013. Normative performance on the Brief Smell Identification Test (BSIT) in a multi-ethnic bilingual cohort: a Project FRONTIER study. Clin Neuropsychol 27, 946–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado-Gomez OF, Cordova-Davalos L, Garcia-Betanzo D, Rocha L, Alonso-Vanegas MA, Cienfuegos J, Guevara-Guzman R, 2018. Overexpression of inflammatory-related and nitric oxide synthase genes in olfactory bulbs from frontal lobe epilepsy patients. Epilepsy Res 148, 37–43. [DOI] [PubMed] [Google Scholar]
- Merrick C, Godwin CA, Geisler MW, Morsella E, 2014. The olfactory system as the gateway to the neural correlates of consciousness. Frontiers in psychology 4, 1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam RI, Moberg PJ, Mahr RN, Doty RL, 1998. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases. Archives of Neurology 55, 84–90. [DOI] [PubMed] [Google Scholar]
- Miller AC, Thiele TR, Faumont S, Moravec ML, Lockery SR, 2005. Step-Response Analysis of Chemotaxis in Caenorhabditis elegans. Journal of Neuroscience 25, 3369–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiak M, Vergara Greeno R, Baptiste BA, Sykora P, Liu D, Cordonnier S, Fang EF, Croteau DL, Mattson MP, Bohr VA, 2017a. DNA polymerase beta decrement triggers death of olfactory bulb cells and impairs olfaction in a mouse model of Alzheimer’s disease. Aging cell 16, 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiak MM, Hipolito MS, Ressom HW, Obisesan TO, Manaye KF, Nwulia EA, 2017b. Apo E4 Alleles and Impaired Olfaction as Predictors of Alzheimer’s Disease. Clin Exp Psychol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley AS, Rodriguez-Gil DJ, Imamura F, Greer CA, 2014. Aging in the olfactory system. Trends Neurosci 37, 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, 2009. Olfactory Bulb Mapping, Encyclopedia of Neuroscience. Elsevier, pp. 71–75. [Google Scholar]
- Morley JF, Cohen A, Silveira-Moriyama L, Lees AJ, Williams DR, Katzenschlager R, Hawkes C, Shtraks JP, Weintraub D, Doty RL, Duda JE, 2018. Optimizing olfactory testing for the diagnosis of Parkinson’s disease: item analysis of the university of Pennsylvania smell identification test. NPJ Parkinson’s disease 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RJ, Xu S, Zhuo J, Roys S, Pereira EFR, Albuquerque EX, Gullapalli RP, 2020. Postnatal Guinea Pig Brain Development, as Revealed by Magnetic Resonance and Diffusion Kurtosis Imaging. Brain Sci 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C, 2019. Olfactory and other sensory impairments in Alzheimer disease. Nature reviews. Neurology 15, 11–24. [DOI] [PubMed] [Google Scholar]
- Murphy C, Anderson JA, Markison S, 1994. Psychophysical Assessment of Chemosensory Disorders in Clinical Populations, in: Kurihara K, Suzuki N, Ogawa H (Eds.). Springer; Japan, pp. 609–613. [Google Scholar]
- Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM, 2002. Prevalence of olfactory impairment in older adults. JAMA 288, 2307–2312. [DOI] [PubMed] [Google Scholar]
- Nagayama S, Homma R, Imamura F, 2014. Neuronal organization of olfactory bulb circuits. Frontiers in Neural Circuits 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm R, Jones EA, Huang Y, 2019. Apolipoprotein E4, inhibitory network dysfunction, and Alzheimer’s disease. Mol Neurodegener 14, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro JF, Croteau DL, Jurek A, Andrusivova Z, Yang B, Wang Y, Ogedegbe B, Riaz T, Stoen M, Desler C, Rasmussen LJ, Tonjum T, Galas MC, Lundeberg J, Bohr VA, 2020. Spatial Transcriptomics Reveals Genes Associated with Dysregulated Mitochondrial Functions and Stress Signaling in Alzheimer Disease. iScience 23, 101556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimura Y, 2009. Evolutionary dynamics of olfactory receptor genes in chordates: interaction between environments and genomic contents. Hum Genomics 4, 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimura Y, Matsui A, Touhara K, 2014. Extreme expansion of the olfactory receptor gene repertoire in African elephants and evolutionary dynamics of orthologous gene groups in 13 placental mammals. Genome Res 24, 1485–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H, Shen L, Li T, Ren C, Ding S, Wang L, Zhang Z, Liu X, Zhang Q, Geng D, Wu X, Li H, 2018. Alpha-synuclein overexpression in the olfactory bulb initiates prodromal symptoms and pathology of Parkinson’s disease. Translational neurodegeneration 7, 25. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Niu H, Wang Q, Zhao W, Liu J, Wang D, Muhammad B, Liu X, Quan N, Zhang H, Zhang F, Wang Y, Li H, Yang R, 2020. IL-1beta/IL-1R1 signaling induced by intranasal lipopolysaccharide infusion regulates alpha-Synuclein pathology in the olfactory bulb, substantia nigra and striatum. Brain Pathol 30, 1102–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa M, Nei M, 2007. Evolutionary dynamics of olfactory receptor genes in Drosophila species. Proc Natl Acad Sci U S A 104, 7122–7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oboti L, Peretto P, 2014. How neurogenesis finds its place in a hardwired sensory system. Front Neurosci 8, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Pinto AV, Santos RM, Coutinho RA, Oliveira LM, Santos GB, Alho AT, Leite RE, Farfel JM, Suemoto CK, Grinberg LT, Pasqualucci CA, Jacob-Filho W, Lent R, 2014. Sexual dimorphism in the human olfactory bulb: females have more neurons and glial cells than males. PloS one 9, e111733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmquist E, Larsson M, Olofsson JK, Seubert J, Backman L, Laukka EJ, 2020. A Prospective Study on Risk Factors for Olfactory Dysfunction in Aging. J Gerontol A Biol Sci Med Sci 75, 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Kim H, Kim S, Cha H, 2021. The Association between Olfactory Function and Cognitive Impairment in Older Persons with Cognitive Impairments: A Cross-Sectional Study. Healthcare 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskin TR, Iqbal TR, Byrd-Jacobs CA, 2011. Olfactory Bulb Recovery Following Reversible Deafferentation with Repeated Detergent Application in the Adult Zebrafish. Neuroscience 196, 276–284. [DOI] [PubMed] [Google Scholar]
- Pellegrino R, Sinding C, de Wijk RA, Hummel T, 2017. Habituation and adaptation to odors in humans. Physiology & Behavior 177, 13–19. [DOI] [PubMed] [Google Scholar]
- Poellinger A, Thomas R, Lio P, Lee A, Makris N, Rosen BR, Kwong KK, 2001. Activation and habituation in olfaction--an fMRI study. NeuroImage 13, 547–560. [DOI] [PubMed] [Google Scholar]
- Prasanna Gandhi RB, Girish Arabale, Sunu Engineer, Sanjay Phadke, 2020. Olfactory Device for Large Scale Pre-screening for COVID-19. Trans Indian Natl Acad Eng 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP, 2013. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 9, 63–75 e62. [DOI] [PubMed] [Google Scholar]
- Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia A-S, McNamara JO, Williams SM, 2001. Trigeminal Chemoreception. Neuroscience. 2nd edition. [Google Scholar]
- Rahayel S, Frasnelli J, Joubert S, 2012. The effect of Alzheimer’s disease and Parkinson’s disease on olfaction: a meta-analysis. Behavioural brain research 231, 60–74. [DOI] [PubMed] [Google Scholar]
- Rawal S, Hoffman HJ, Chapo AK, Duffy VB, 2014. Sensitivity and Specificity of Self-Reported Olfactory Function in a Home-Based Study of Independent-Living, Healthy Older Women. Chemosensory perception 7, 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagh ZM, Noche JA, Tustison NJ, Delisle D, Murray EA, Yassa MA, 2018. Functional Imbalance of Anterolateral Entorhinal Cortex and Hippocampal Dentate/CA3 Underlies Age-Related Object Pattern Separation Deficits. Neuron 97, 1187–1198 e1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regensburger M, Prots I, Winner B, 2014. Adult hippocampal neurogenesis in Parkinson’s disease: impact on neuronal survival and plasticity. Neural plasticity 2014, 454696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Lamar M, Driscoll I, 2007. Vulnerability of the orbitofrontal cortex to age-associated structural and functional brain changes. Annals of the New York Academy of Sciences 1121, 562–575. [DOI] [PubMed] [Google Scholar]
- Rey NL, Wesson DW, Brundin P, 2018. The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol Dis 109, 226–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard MB, Taylor SR, Greer CA, 2010. Age-induced disruption of selective olfactory bulb synaptic circuits. Proceedings of the National Academy of Sciences of the United States of America 107, 15613–15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Moberg MJ, Turetsky BI, Brennan L, Kabadi S, Wolk DA, Moberg PJ, 2017. A quantitative meta-analysis of olfactory dysfunction in mild cognitive impairment. Journal of neurology, neurosurgery, and psychiatry 88, 226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RO, Christianson TJ, Kremers WK, Mielke MM, Machulda MM, Vassilaki M, Alhurani RE, Geda YE, Knopman DS, Petersen RC, 2016. Association Between Olfactory Dysfunction and Amnestic Mild Cognitive Impairment and Alzheimer Disease Dementia. JAMA Neurol 73, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson HM, Thomas JH, 2006. The putative chemoreceptor families of C. elegans. WormBook, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross GW, Petrovitch H, Abbott RD, Tanner CM, Popper J, Masaki K, Launer L, White LR, 2008. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol 63, 167–173. [DOI] [PubMed] [Google Scholar]
- Rumeau C, Nguyen DT, Jankowski R, 2016. How to assess olfactory performance with the Sniffin’ Sticks test((R)). European annals of otorhinolaryngology, head and neck diseases 133, 203–206. [DOI] [PubMed] [Google Scholar]
- Saiz-Sanchez D, De la Rosa-Prieto C, Ubeda-Banon I, Martinez-Marcos A, 2015. Interneurons, tau and amyloid-beta in the piriform cortex in Alzheimer’s disease. Brain structure & function 220, 2011–2025. [DOI] [PubMed] [Google Scholar]
- Sancandi M, Schul EV, Economides G, Constanti A, Mercer A, 2018. Structural Changes Observed in the Piriform Cortex in a Rat Model of Pre-motor Parkinson’s Disease. Frontiers in cellular neuroscience 12, 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Horie Y, 2020. Association Between Olfactory Impairment and Disease Severity and Duration in Parkinson’s Disease. Movement disorders clinical practice 7, 820–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scangas GA, Bleier BS, 2017. Anosmia: Differential diagnosis, evaluation, and management. Am J Rhinol Allergy 31, 3–7. [DOI] [PubMed] [Google Scholar]
- Schlosser RJ, Desiato VM, Storck KA, Nguyen SA, Hill JB, Washington BJ, Noonan TE, Lamira J, Mulligan JK, Rowan NR, Yoo F, Matthews LJ, Dubno JR, Soler ZM, 2020. A Community-Based Study on the Prevalence of Olfactory Dysfunction. Am J Rhinol Allergy, 1945892420922771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert CR, Cruickshanks KJ, Fischer ME, Huang GH, Klein BE, Klein R, Pankow JS, Nondahl DM, 2012. Olfactory impairment in an adult population: the Beaver Dam Offspring Study. Chemical senses 37, 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedghizadeh MJ, Hojjati H, Ezzatdoost K, Aghajan H, Vahabi Z, Tarighatnia H, 2020. Olfactory response as a marker for Alzheimer’s disease: Evidence from perceptual and frontal lobe oscillation coherence deficit. PloS one 15, e0243535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura B, Baggio HC, Solana E, Palacios EM, Vendrell P, Bargallo N, Junque C, 2013. Neuroanatomical correlates of olfactory loss in normal aged subjects. Behav Brain Res 246, 148–153. [DOI] [PubMed] [Google Scholar]
- Seo Y, Kim HS, Kang KS, 2018. Microglial involvement in the development of olfactory dysfunction. J Vet Sci 19, 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert J, Laukka EJ, Rizzuto D, Hummel T, Fratiglioni L, Backman L, Larsson M, 2017. Prevalence and Correlates of Olfactory Dysfunction in Old Age: A Population-Based Study. J Gerontol A Biol Sci Med Sci 72, 1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharer JD, Leon-Sarmiento FE, Morley JF, Weintraub D, Doty RL, 2015. Olfactory dysfunction in Parkinson’s disease: Positive effect of cigarette smoking. Movement disorders : official journal of the Movement Disorder Society 30, 859–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JK, Wroblewski KE, McClintock MK, Pinto JM, 2019. Olfactory dysfunction persists after smoking cessation and signals increased cardiovascular risk. Int Forum Allergy Rhinol 9, 977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MME, Mercer PBS, Witt MCZ, Pessoa RR, 2018. Olfactory dysfunction in Alzheimer’s disease Systematic review and meta-analysis. Dementia & neuropsychologia 12, 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokowski P, Karwowski M, Misiak M, Marczak MK, Dziekan M, Hummel T, Sorokowska A, 2019. Sex Differences in Human Olfaction: A Meta-Analysis. Front Psychol 10, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Stevens CF, 2019. Scaling Principles of Distributed Circuits. Curr Biol 29, 2533–2540 e2537. [DOI] [PubMed] [Google Scholar]
- St Jacques P, Dolcos F, Cabeza R, 2010. Effects of aging on functional connectivity of the amygdala during negative evaluation: a network analysis of fMRI data. Neurobiology of aging 31, 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowbridge BW, 2010. Linking Local Circuit Inhibition to Olfactory Behavior: A Critical Role for Granule Cells in Olfactory Discrimination. Neuron 65, 295–297. [DOI] [PubMed] [Google Scholar]
- Sultan-Styne K, Toledo R, Walker C, Kallkopf A, Ribak CE, Guthrie KM, 2009. Long-term survival of olfactory sensory neurons after target depletion. J Comp Neurol 515, 696–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg H, Døving K, Novikov S, Ursin H, 1982. A method for studying responses and habituation to odors in rats. Behavioral and Neural Biology 34, 113–119. [DOI] [PubMed] [Google Scholar]
- Sungnak W, Huang N, Becavin C, Berg M, Network HCALB, 2020. SARS-CoV-2 Entry Genes Are Most Highly Expressed in Nasal Goblet and Ciliated Cells within Human Airways. ArXiv. [Google Scholar]
- Suzukawa K, Kondo K, Kanaya K, Sakamoto T, Watanabe K, Ushio M, Kaga K, Yamasoba T, 2011. Age-related changes of the regeneration mode in the mouse peripheral olfactory system following olfactotoxic drug methimazole-induced damage. The Journal of comparative neurology 519, 2154–2174. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Teranishi M, Katayama N, Nakashima T, Sugiura S, Sone M, 2021. Relationship between cognitive impairment and olfactory function among older adults with olfactory impairment. Auris, nasus, larynx 48, 420–427. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Iino M, Takagi SF, 1975. Discrimination of odors in olfactory bulb, pyriform-amygdaloid areas, and orbitofrontal cortex of the monkey. Journal of neurophysiology 38, 1284–1296. [DOI] [PubMed] [Google Scholar]
- Terada T, Miyata J, Obi T, Kubota M, Yoshizumi M, Murai T, 2018. Reduced gray matter volume is correlated with frontal cognitive and behavioral impairments in Parkinson’s disease. Journal of the neurological sciences 390, 231–238. [DOI] [PubMed] [Google Scholar]
- Terroba Chambi C, Rossi M, Bril A, Vernetti PM, Cerquetti D, Cammarota A, Merello M, 2017. Diagnostic Value of Combined Acute Levodopa Challenge and Olfactory Testing to Predict Parkinson’s Disease. Mov Disord Clin Pract 4, 824–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer C, Mainland JD, 2017. The Olfactory System, Conn’s Translational Neuroscience. Elsevier, pp. 363–377. [Google Scholar]
- Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI, 1995. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83, 207–218. [DOI] [PubMed] [Google Scholar]
- Twick I, Lee JA, Ramaswami M, 2014. Olfactory Habituation in Drosophila—Odor Encoding and its Plasticity in the Antennal Lobe, Progress in Brain Research. Elsevier, pp. 3–38. [DOI] [PubMed] [Google Scholar]
- Ullmann JFP, Calamante F, Collin SP, Reutens DC, Kurniawan ND, 2015. Enhanced characterization of the zebrafish brain as revealed by super-resolution track-density imaging. Brain Struct Funct 220, 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasavada MM, Martinez B, Wang J, Eslinger PJ, Gill DJ, Sun X, Karunanayaka P, Yang QX, 2017. Central Olfactory Dysfunction in Alzheimer’s Disease and Mild Cognitive Impairment: A Functional MRI Study. Journal of Alzheimer’s disease : JAD 59, 359–368. [DOI] [PubMed] [Google Scholar]
- Vassilaki M, Christianson TJ, Mielke MM, Geda YE, Kremers WK, Machulda MM, Knopman DS, Petersen RC, Lowe VJ, Jack CR Jr., Roberts RO, 2017. Neuroimaging biomarkers and impaired olfaction in cognitively normal individuals. Ann Neurol 81, 871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayudhan L, Gasper A, Pritchard M, Baillon S, Messer C, Proitsi P, 2015. Pattern of Smell Identification Impairment in Alzheimer’s Disease. Journal of Alzheimer’s disease : JAD 46, 381–387. [DOI] [PubMed] [Google Scholar]
- Verschut TA, Carlsson MA, Hambäck PA, 2019. Scaling the interactive effects of attractive and repellent odours for insect search behaviour. Scientific Reports 9, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall LB, 2000. Olfaction in Drosophila. Current opinion in neurobiology 10, 498–503. [DOI] [PubMed] [Google Scholar]
- Wabnegger A, Schlintl C, Hofler C, Gremsl A, Schienle A, 2019. Altered grey matter volume in ‘super smellers’. Brain imaging and behavior 13, 1726–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, 2010. Active Sensing in Olfaction, in: Menini A (Ed.), The Neurobiology of Olfaction, Boca Raton (FL). [PubMed] [Google Scholar]
- Wang XY, Han YY, Li G, Zhang B, 2019. Association between autonomic dysfunction and olfactory dysfunction in Parkinson’s disease in southern Chinese. BMC Neurol 19, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S, 1973. Chemotaxis by the nematode Caenorhabditis elegans: identification of attractants and analysis of the response by use of mutants. Proceedings of the National Academy of Sciences of the United States of America 70, 817–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welniak-Kaminska M, Fiedorowicz M, Orzel J, Bogorodzki P, Modlinska K, Stryjek R, Chrzanowska A, Pisula W, Grieb P, 2019. Volumes of brain structures in captive wild-type and laboratory rats: 7T magnetic resonance in vivo automatic atlas-based study. PLoS One 14, e0215348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcroft KL, Cuevas M, Haehner A, Hummel T, 2017. Patterns of olfactory impairment reflect underlying disease etiology. Laryngoscope 127, 291–295. [DOI] [PubMed] [Google Scholar]
- Wilson DA, 2009. Olfactory Cortex Physiology, Encyclopedia of Neuroscience. Elsevier, pp. 95–100. [Google Scholar]
- Wilson RI, 2013. Early Olfactory Processing in Drosophila: Mechanisms and Principles. Annual review of neuroscience 36, 217–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Yu L, Schneider JA, Arnold SE, Buchman AS, Bennett DA, 2011. Lewy Bodies and Olfactory Dysfunction in Old Age. Chemical Senses 36, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward MR, Hafeez MU, Qi Q, Riaz A, Benedict RHB, Yan L, Szigeti K, Texas Alzheimer’s Research CC, 2018. Odorant Item Specific Olfactory Identification Deficit May Differentiate Alzheimer Disease From Aging. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 26, 835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Geng Z, Zhou S, Bai T, Wei L, Ji GJ, Zhu W, Yu Y, Tian Y, Wang K, 2019. Brain Structural Correlates of Odor Identification in Mild Cognitive Impairment and Alzheimer’s Disease Revealed by Magnetic Resonance Imaging and a Chinese Olfactory Identification Test. Frontiers in neuroscience 13, 842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Tully T, 2007. Segregation of Odor Identity and Intensity during Odor Discrimination in Drosophila Mushroom Body. PLOS Biology 5, e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Liu J, Wroblewski KE, McClintock MK, Pinto JM, 2020. Odor Sensitivity Versus Odor Identification in Older US Adults: Associations With Cognition, Age, Gender, and Race. Chem Senses 45, 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Kuan WL, Spillantini MG, 2016. Progressive tauopathy in P301S tau transgenic mice is associated with a functional deficit of the olfactory system. Eur J Neurosci 44, 2396–2403. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zhao X, Wang P, Mei F, Zhou J, Jin Y, McNutt MA, Yin Y, 2019. PTENalpha regulates endocytosis and modulates olfactory function. FASEB J 33, 11148–11162. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ji D, Yin J, Wang Z, Zhou Y, Ni H, Liu Y, 2019. Olfactory fMRI Activation Pattern Across Different Concentrations Changes in Alzheimer’s Disease. Front Neurosci 13, 786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, He Y, He R, Zhou Y, Pan H, Zhou X, Zhu L, Zhou X, Liu Z, Xu Q, Sun Q, Tan J, Yan X, Tang B, Guo J, 2020. The Discriminative Power of Different Olfactory Domains in Parkinson’s Disease. Frontiers in neurology 11, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziontz J, Bilgel M, Shafer AT, Moghekar A, Elkins W, Helphrey J, Gomez G, June D, McDonald MA, Dannals RF, Azad BB, Ferrucci L, Wong DF, Resnick SM, 2019. Tau pathology in cognitively normal older adults. Alzheimers Dement (Amst) 11, 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Wang W, Pan Y-W, Lu S, Xia Z, 2015. Methods to measure olfactory behavior in mice. Current protocols in toxicology / editorial board, Mahin D. Maines (editor-in-chief) … [et al.] 63, 11.18.11–11.18.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou YM, Lu D, Liu LP, Zhang HH, Zhou YY, 2016. Olfactory dysfunction in Alzheimer’s disease. Neuropsychiatr Dis Treat 12, 869–875. [DOI] [PMC free article] [PubMed] [Google Scholar]


