Abstract
Background
MR elastography can determine organ-related stiffness, which reflects the degree of fibrosis. Liver stiffness increases in cirrhosis, and stiffness increases further post-prandially due to increased portal blood in-flow. Non-selective beta-blockers (NSBB) reduce the portal venous inflow, but their effect on liver and spleen stiffness are disputed.
Aims
To assess whether MR elastography of the liver or spleen reflects the severity of cirrhosis, whether treatment with NSBB changes liver and spleen stiffness, and whether changes in stiffness can predict the effect of NSBB on portal pressure.
Methods
Fifty-two patients with cirrhosis underwent liver vein catheterization and two-dimensional (2D) MR elastography on separate days. Thirty-six of the patients had a hepatic venous pressure gradient (HVPG) of ≥12mmHg and were tested prior to, and after, intravenous infusion of NSBB using HVPG measurement and MR elastography.
Results
HVPG showed a strong, positive, linear relationship with liver stiffness (r2=0.92; p<0.001) and spleen stiffness (r2=0.94; p<0.001). The cut-off points for identifying patients with a HVPG ≥12mmHg was 7.7kPa for liver stiffness (sensitivity 0.78, specificity 0.64) and 10.5kPa for spleen stiffness (sensitivity 0.8, specificity 0.79).
Intravenous administration of NSBB significantly decreased spleen stiffness by 6.9% (CI: 3.5;10.4, p<0.001), but NSBB had no consistent effect on liver stiffness. However, changes in spleen stiffness was not related to the HVPG response (p=0.75).
Conclusions:
2D MR elastographic estimation of liver or spleen stiffness reflects the degree of portal hypertension in patients with liver cirrhosis, but changes in stiffness after NSBB do not predict the effect on HVPG.
Keywords: portal hypertension, liver cirrhosis, imaging, beta-blockers, magnetic resonance elastography
Lay summary
Magnetic resonance elastography (MR elastography) is a non-invasive method to assess liver fibrosis but the method has been sparsely investigated in patients with cirrhosis. The present cohort study shows that MR elastography of liver stiffness and spleen stiffness can estimate the degree of portal hypertension in patients with cirrhosis. Spleen stiffness is redued after treatment with non-selective betablockers (NSBB), but without capability to predict the treatment response.
The results provide additional evidence of MR-elastography as a clinically relevant technique for assessment of severity of portal hypertension and a potential tool for assessment of pharmacological response of new treatments of portal hypertension.
Introduction
Chronic liver disease often leads to fibrosis, cirrhosis and portal hypertension with further development of complications1. Elastography-based techniques are the most widely used noninvasive imaging methods for evaluating liver fibrosis2. They are performed with either an ultrasound or magnetic resonance (MR) technique. Both techniques assess fibrosis indirectly by measuring the mechanical properties of the tissue, and elastography is capable of discriminate cirrhosis from other, less severe liver diseases3,4. Nevertheless, evidence about MR elastography as a technique for estimating portal pressure in patients with cirrhosis has been sparsely investigated5,6
Patients with cirrhosis often receive treatment with non-selective beta-blockers (NSBB), which reduce portal venous inflow and hepatic vascular filling, thereby reducing the hepatic venous pressure gradient (HVPG) and likely reducing liver stiffness (LS) and spleen stiffness (SS)7. Unfortunately, only 40–50% of cirrhotic patients respond to NSBB treatment with a decrease in HVPG of ≥10% or to a HVPG ≤12 mmHg and non-responders suffer a three-fold increased risk of variceal bleeding8,9. Currently, response to NSBB is measured during a liver vein catheterization, which is an invasive and costly procedure that at present has no reliable, noninvasive alternatives10.
The few ultrasound-based elastography studies that have been carried out have shown a heterogeneous effect of NSBB on LS and SS11–15 and, to our knowledge, no study has previously evaluated the effects of NSBB on LS and SS using MR elastography. In this study, we therefore aimed to evaluate whether: (1) MR elastography of the spleen or liver can be used to classify the degree of portal hypertension, (2) intravenously administered NSBB can affect LS and SS in patients with severe portal hypertension, and (3) changes in MR elastography can predict the treatment effect of NSBB on HVPG.
Materials and Methods
Patients and study design
For this study, we prospectively included 52 patients with cirrhosis who were between 18 and 76 years old. Including period between April 2017 and December 2020. The diagnosis of cirrhosis was confirmed based on either liver histology or accepted clinical, biochemical, and ultrasonographic findings. All patients underwent a detailed medical history, physical examination, oesophagogastroduodenoscopy, liver vein catheterization, and MR elastography of the liver and spleen. SS was not available in eight patients because of technical failure (four), absence of spleen measurements (three), and a previous splenectomy in one patient. Six of those eight patients were in the NSBB test group. LS could not be measured in one patient due to iron overload. This patient was not in the NSBB test group. Oesophagogastroduodenoscopy was not performed on one patient with a HVPG of 8 mmHg. Thirty-six of the patients were included in the NSBB test group and 33 of those had a HVPG of ≥12mmHg and high-risk gastroesophageal varices according to the Baveno VI criteria, or they had previously suffered variceal bleeding with a clinical indication for treatment with NSBB, as recommended by the EASL guidelines (European Association of the Study of the Liver)16,17. Three of the 36 patients did not have previous variceal bleeding or high risk varices, but they had a HVPG ≥12mmHg and were included in the NSBB test-group.
Exclusion criteria were hepatic encephalopathy greater than 1 (West Haven Criteria), NYHA greater than 2, arrythmia, kidney disease, dysregulated insulin-dependent diabetes mellitus, malignancies (apart from cutaneous basal cell carcinoma or malignancies without relapse within the preceding five years), gastrointestinal bleeding within the past two weeks, patients with a transjugular intrahepatic portosystemic shunt, contraindication with magnetic resonance imaging, pregnancy, or any condition that, in an investigator’s opinion, could impede a participant’s compliance or completion of the study.
All patients provided written informed consent before participation. The study protocol was approved by the Ethics Committee for Medical Research in Copenhagen (H-16048475) and performed in accordance with the guidelines established in the Helsinki Declaration. The clinical.trials.gov registration ID is NCT03438916.
Study schedule
All patients were investigated on two separate days with at least a three-day interval (mean 18 days (18.7±10.8, range 3;52)) between the liver vein catheterization and the MR elastography. Eventual treatment with NSBB was withdrawn at least five days prior to the study days and diuretics were paused in the morning at the days of examinations. Patients fasted for four hours prior to MR elastography and liver vein catheterization to avoid falsely elevated values.
The 36 patients with a HVPG ≥12mmHg underwent assessment of HVPG and MR elastography prior to and after the intravenous NSBB test.
Liver vein catheterization and test of NSBB response
Liver vein catheterization was performed under local analgesia with a balloon catheter that was advanced to a hepatic vein introduced from either the femoral or jugular vein. HVPG was measured indirectly as the difference between the wedged hepatic venous pressure (WHVP) and the free hepatic venous pressures (FHVP)18,19.
Mean HVPG was calculated using three successful measurements of the WHVP and FHVP. Response to NSBB was assessed using the Baveno VI criteria and was defined as a reduction in HVPG ≥10%, or to a HVPG <12mmHg after intravenous injection of propranolol (0.15 mg/kg body weight, up to a maximum dose of 15 mg)9,17. After measuring baseline HVPG, the propranolol (Dociton®, MIBE GmbH Arzneimittel, Brehna) was administered as an intravenous infusion over a five-minute period, with surveillance of heart rate and blood pressure. The HVPG measurements were repeated 15 minutes after the propranolol infusion had been completed.
Two-dimensional MR elastography
For MR elastography we used a Siemens Healthcare Avanto 1.5T scanner with two-dimensional gradient-echo sequence and through-plane motion-encoding gradient.
Patients were scanned in the supine position with two torso coils placed over their abdomen. The two passive drivers were secured with an elastic band over the body anterior to the liver and anterior lateral to the spleen. The passive drivers were connected by a flexible plastic tube to an active acoustic driver outside the MRI room. Vibrations at 60 Hz are generated by the active driver and delivered by the tube to the passive driver, transmitting the vibrations into the body, thereby producing shear waves in the liver and spleen. A two-dimensional (2D) gradient-recalled echo MR elastography pulse sequence was performed while the vibrations were transmitted, and an axial slice (5mm thick) was acquired during a 17-second breath-hold through the widest transverse dimension of the liver and spleen. The sequence was repeated two or three times at different slice locations, with a recovery period between the sequences. The information of the waves at each slice location was post-processed with multi-model direct inversion (MMDI) to generate quantitative cross-sectional maps (called ‘stiffness maps’ or ‘elastograms’) (Figure 1). One elastogram was generated at each of the slice locations. These maps display stiffness with a color scale in units of kilopascals (kPa).
Figure 1: Elastogram with confidence interval.
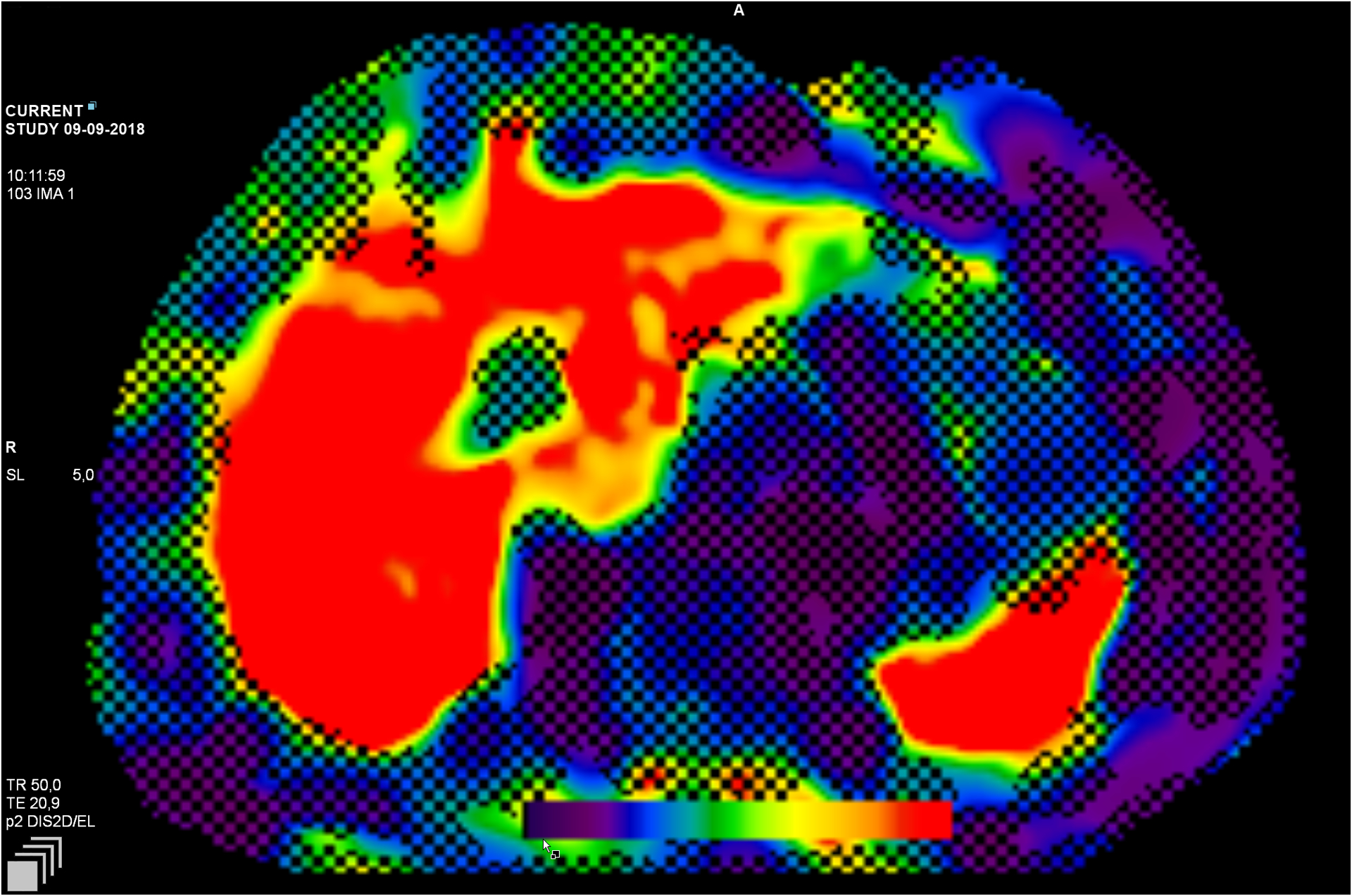
The pixilation (shading) illustrates the area of insufficient wave propagation needed to obtain a reliable estimate. The color scale illustrates the degree of liver fibrosis, from no fibrosis (purple) to cirrhosis (red)
One blinded expert with more than ten years of experience in liver and spleen MR elastography manually drew regions of interest (ROIs) using custom software, MRE Quant (Version 1.4). ROIs were drawn at each of the three slice locations in the right liver lobe and the spleen in which the corresponding wave images showed clearly observable wave propagation, avoiding organ edges, non-parenchyma tissue, and areas of wave interference or a confidence level of less than 0.95. The measurements were expressed as shear stiffness in kPa. It was the magnitude of calculated complex shear modulus, which represents both elasticity and viscosity of the tissue, here mentioned as liver or spleen stiffness. Mean liver and spleen stiffness was calculated by averaging the stiffness values across the ROIs for all of the slice locations for each organ.
Within the MR scanner the beta-blocker test was performed similarly to the procedure involving the liver vein catheterization with respect to the dosage of propranolol and timing.
Statistical analyses
Data from the whole population were analyzed for baseline results and the 36 patients receiving NSBB were analyzed separately. Quantitative variables are presented as mean values with standard deviation (SD) and categorical variables as n (%). Changes in stiffness were calculated as both an absolute change (the stiffness after NSBB minus the stiffness at baseline) and as a relative change (the stiffness after NSBB divided by the stiffness at baseline, multiplied by 100, minus 100), resulting in the decrease, expressed as a percentage.
Associations between HVPG and baseline LS value, absolute changes and relative changes were analyzed using linear regression models. Models were fitted without intercept and the baseline model was additionally adjusted for bilirubin and ALT, that is known confounders in MR elastography. Association between a HVPG ≥12mmHg and the LS baseline value, absolute change and relative change were analyzed using a logistic regression model, again with the baseline model adjusted for bilirubin and ALT (alanine aminotransferase). Similar models were fitted with SS instead of LS. Changes in LS and SS were analyzed by paired t-test. Estimates of models and tests are reported as means or odds ratios (OR) with 95% confidence intervals (CI) and p-values.
The normality assumption of residuals from the linear regression models were evaluated by Q-Q plots and the R-squared value was calculated to evaluate the variation. The fit of the logistic regression models was evaluated using the Hosmer-Lemeshow goodness of fit test.
Tests were based on two-sided probability, and a p smaller than 0.05 was considered to be statistically significant.
Receiver Operating Characteristic (ROC) and area under the curve (AUC) analyses were calculated and using the maximum sensitivity and specificity (Youden’s index) to reveal suggested cut-off values for MR elastography for identifying the risk of severe portal hypertension (HVPG ≥ 12mmHg), or to identify the response to NSBB. All analyses were performed using R 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline patient characteristics are shown in Table 1, including the whole cohort of 52 patients and the 36 patients who also underwent the NSBB test. Those undergoing the NSBB test were sub-divided into NSBB responders (n=19) and NSBB non-responders (n=17).
Table 1:
Clinical and biochemical characteristics of 52 patients with cirrhosis
| Total cohort | Patients with non-selective betablockers test (n=36) | ||||
|---|---|---|---|---|---|
| All patients (n = 52) |
All§
(n = 36) |
Responders (n = 19) |
Non-responders (n = 17) |
||
| Age (years) | 60.2 ± 9.5 | 59.9 ± 9.1 | 60.4 ±11.1 | 59.4 ± 6.5 | |
| Gender (n = male) | 28 (53.8) | 20 (55.6) | 9 (47.4) | 11 (64.7) | |
| Etiology (n (%)) | |||||
| Alcohol | 35 (67.3) | 28 (77.8) | 13 (68.4) | 15 (88.2) | |
| NASH | 6 (11.5) | 2 (5.6) | 1 (5.3) | 1 (5.9) | |
| Cryptogenic | 3 (5.8) | 2 (5.6) | 2 (10.5) | ||
| Hepatitis B or C | 2 (3.8) | 1 (2.8) | 1 (5.9) | ||
| Other or a combination | 6 (11.5) | 3 (8.3) | 3 (15.8) | ||
| Decompensated cirrhosis# (n (%)) | 33 (63.5) | 29 (80.6) | 16 (84.2) | 13 (76.5) | |
| Diuretic treatment | 27 (51.9) | 24 (66.7) | 13 (68.4) | 11 (64.7) | |
| Secondary prophylaxisΔ | 19 (36.5) | 19 (52.7) | 10 (52.6) | 9 (52.9) | |
| Child Pugh score | 6.6 ± 1.7 | 6.9 ± 1.7 | 6.8 ± 1.6 | 7.1 ± 1.8 | |
| A | 30 (57.7) | 16 (44.4) | 9 (47.4) | 7 (41.2) | |
| B | 17 (32.7) | 16 (44.4) | 8 (42.1) | 8 (47.1) | |
| C | 5 (9.6) | 4 (11.1) | 2 (10.5) | 2 (11.8) | |
| MELD-Na score | 12.1 ± 4.5 | 13.2 ± 4.3 | 12.6 ± 3.8 | 13.8 ±4.9 | |
| HVPG (mmHg) | 15.1 ± 4.7 | 17.0 ± 3.9 | 16.7 ± 3.8 | 17.3 ± 4.1 | |
| Liver stiffness (kPa) | 9.7 ± 3.3 | 10.4 ± 3.4 | 9.9 ± 3.5 | 10.9 ± 3.2 | |
| Spleen stiffness (kPa) | 12.0 ± 2.8 | 12.8± 2.6 | 13.3 ± 2.7 | 12.3 ± 2.4 | |
| Bilirubin (μmol/L) | 24.6 ± 24.6 | 27.0 ± 23.4 | 32.2 ± 29.0 | 21.2 ± 13.4 | |
| ALT¤ (U/L) | 43.9 ± 27.3 | 39.2 ± 17.3 | 41.6 ± 16.0 | 36.4 ± 18.8 | |
Results are presented as mean, SD or n (%)
Non-selective betablocker response defined as a reduction of hepatic venous pressure gradient (HVPG) ≥ 10%, or a HVPG < 12mmHg, after intravenous propranolol infusion during liver vein catheterization
Decompensated with previous or current jaundice, overt hepatic encephalopathy, variceal bleeding or ascites
Treatment with beta-blockers because of previously variceal bleeding (esophageal or rectal varices)
alanine aminotransferase
The mean age of the cohort was 60 years and 54% of the patients were males. Sixty-seven percent of the patients had alcohol-related cirrhosis. Fifteen patients (29%) had active periodically or continuously alcohol abuse and 26 patients (50%) were alcohol abstainers at the time of inclusion. Eleven patients were without previous or current alcohol abuse (21%). According to the Child-Pugh classification, 58% belonged to Child-Pugh (CP) class A, 33% to class B, and 10% to class C. The mean MELD-Na score was 12 (model for end-stage liver disease), and the mean HVPG was 15 mmHg. Thirty-three patients had decompensated cirrhosis with previous or current jaundice, overt hepatic encephalopathy, variceal bleeding or ascites (63.5%) and nineteen had compensated cirrhosis (36.5%). Six patients had non-clinical significant portal hypertension (CSPH) (HVPG < 10mmHg), 46 patients had CSPH (six with a HVPG = 10–12mmHg and 40 with a HVPG ≥ 12mmHg). Tweenty-seven patients had ascites and were treated with diuretics. One patient with hepatitis B was treated with antiviral drugs before inclusion and continuously during the two visits. Thirteen patients had type-2 diabetes mellitus that required treatment, but only one patient had insulin-dependent diabetes mellitus.
Relationship between MR elastography and the severity of liver disease and portal hypertension
Across the entire cohort, the mean stiffnesses of the liver was 9.7 kPa, while for the spleen it was 12.0 kPa. Figure 2 illustrates the linear regression analysis with a positive linear correlation between HVPG and LS (r2 = 0.92) and SS (r2 = 0.94); the model is adjusted for bilirubin and ALT. As such, the linear association between 1 kPa in LS was equal to 1.4 mmHg (CI: 1.1;1.7, p<0.001) in HVPG and 1 kPa in SS was equal to 1.1 mmHg (CI: 0.9;1.4, p<0.001) in HVPG. Similar correlations with LS and SS were found for both Child-Pugh and MELD-Na scores (Supporting Information, Table 1).
Figure 2: Linear regression analysis between A: liver stiffness (kPa) and HVPG (mmHg). B: spleen stiffness (kPa) and HVPG (mmHg).
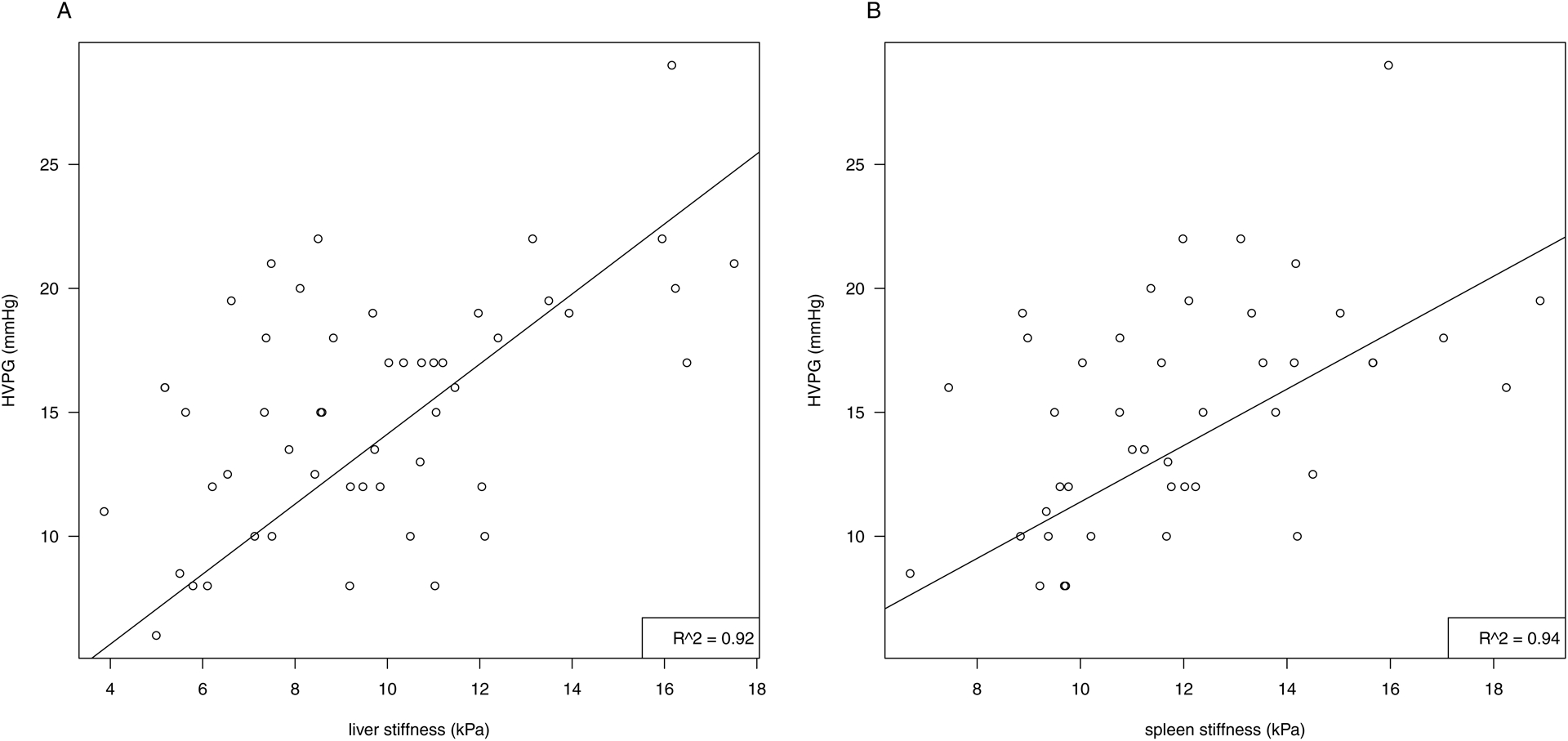
The linear regression model is adjusted for ALT and bilirubin
Abbreviations: HVPG: hepatic vein pressure gradient, kPa: kilopascal, ALT: alanine aminotransferase
There was a borderline-significant increase in the risk of having severe portal hypertension (HVPG ≥ 12mmHg) for increased values of SS (ORspleen=1.6, p=0.05); however, the risk for LS was not found to be significant (ORLiver=1.4, p=0.15).
The cut-off points for the prediction of a HVPG ≥12 mmHg was 7.7kPa for LS with a specificity and sensitivity of 0.64 and 0.78, respectively. The comparable cut-off point for SS was 10.5 kPa with a sensitivity and specificity of 0.80 and 0.79, respectively. ROC curves with AUC are shown in Figure 3.
Figure 3: Receiver Operating Characteristic (ROC) curve showing the prediction of severe portal hypertension with a HVPG ≥ 12mmHg for liver stiffness (A) and spleen stiffness (B).
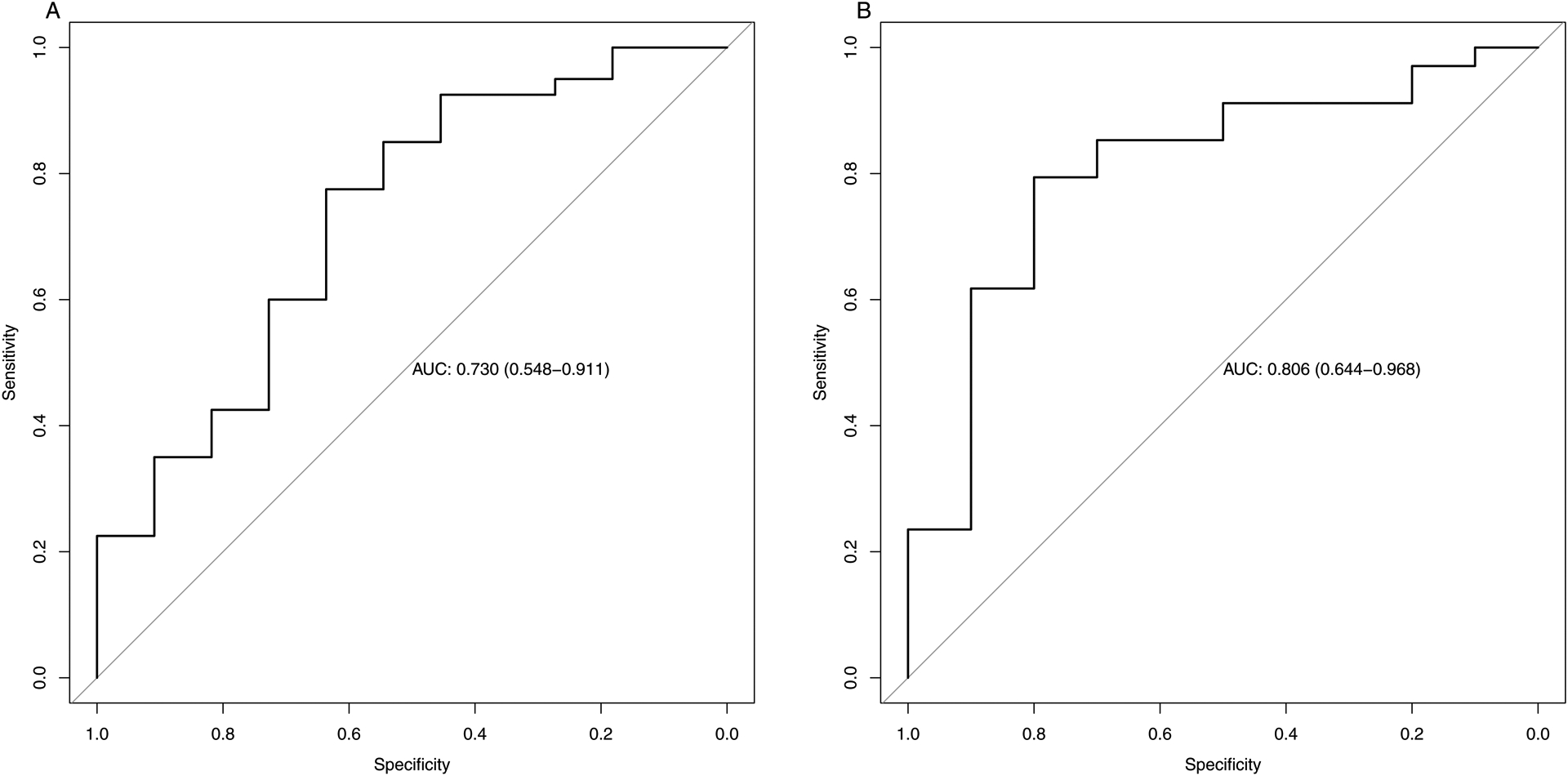
Optimal cut-off for liver stiffness was 7.7 kPa, which provided an area under the ROC curve of 0.73 (CI 0.55;0.91), a specificity of 0.64, and a sensitivity of 0.78 (A). The optimal cut-off for spleen stiffness was 10.5kPa and the area under the ROC curve was 0.81 (CI 0.64;0.97), with a specificity of 0.80 and a sensitivity of 0.79 (B)
The effect of NSBB on LS and SS
NSBB was without significant impact on LS, with a reduction in mean LS of 10.2 kPa to 9.9 kPa after NSBB. The mean decrease in LS was 0.3 kPa (CI: −0.1;0.6, p = 0.12) and the average percentual decrease was −1.0 % (CI: −1.9;5.5, p = 0.33). The SS significantly decreased from 12.9 kPa before NSBB to 12.0 kPa after NSBB, with a mean change of 1.0 kPa (0.5;1.5, p < 0.001). The average percentual decrease in SS was −6.9 % (CI: 3.5;10.4, p < 0.001) (Table 2).
Table 2:
Effects of non-selective beta-blockers on liver and spleen stiffness
| All patients (n = 36) |
p-value | Responder§
(n = 19) |
Non-responder§
(n = 17) |
p-value | ||
|---|---|---|---|---|---|---|
| Decrease in liver stiffness (kPa) | 0.3 (−0.1;0.6) | 0.12 | 0.4 (−0.1;0.8) | 0.1 (−0.4;0.6) | 0.48 | |
| Relative decrease in liver stiffness (%) | 1.0 (−2.7;4.7) | 0.59 | 2.4 (−1.2;6.1) | 0.7 (−8.1;6.7) | 0.41 | |
| Decrease in spleen stiffness (kPa) | 1.0 (0.5;1.5) | <0.001 | 1.1 (0.2;2.0) | 0.9 (0.5;1.3) | 0.76 | |
| Relative decrease in spleen stiffness (%) | 6.9 (3.5;10.4) | <0.001 | 6.8 (0.8;12.9) | 7.0 (3.7;10.3) | 0.96 |
Results are presented as absolute change (kPa) and relative change (%) with 95% confidence interval
Non-selective beta-blockers response defined as a reduction of hepatic venous pressure gradient (HVPG) ≥ 10%, or a HVPG < 12mmHg, after intravenous propranolol infusion during liver vein catheterization
Relationship between the effect of NSBB on HVPG and LS and SS
In the NSBB test cohort, 19 patients responded to the treatment (53%). There was no significant differences at baseline between responders to NSBB and non-responders in relation to age, HVPG, LS, SS, ALT, bilirubin, MELD, or CP.
Mean HVPG was reduced from 16.7mmHg to 13mmHg after NSBB in the patients with a NSBB response (mean delta 3.7mmHg) and from 17.3 mmHg to 16.8 mmHg in patients with non-response (mean delta 0.5mmHg).
We found no correlation between changes in HVPG and changes in LS (r2=0.06) after NSBB, while changes in SS did correlate with HVPG changes, but only weakly (r2=0.25) (Supporting Information, Figure 1). However, the association between changes in MR elastography for the spleen and the probability of a response to NSBB showed no significant OR (OR= 0.91, CI: 0.51;1.6, p = 0.75). All estimates for both LS and SS are summarized in Table 3.
Table 3:
Association between changes in liver or spleen stiffness and changes in HVPG or the probability of a response to non-selective beta-blockers
| Correlation | p-value | R2 | OR (CI) | p-value | ||
|---|---|---|---|---|---|---|
| Liver stiffness (kPa) | ||||||
| All | 0.76 (−0.33; 1.85) | 0.17 | 0.06 | 0.76 (0.36;1.6) | 0.47 | |
| Non-responders | 0.24 (−0.36; 0.83) | 0.41 | 0.05 | |||
| Responders | 1.16 (−0.82; 3.14) | 0.24 | 0.08 | |||
| Spleen stiffness (kPa) | ||||||
| All | 0.90 (0.3; 1.5) | <0.01 | 0.25 | 0.91 (0.51;1.62) | 0.75 | |
| Non-responders | 0.31 (−0.23; 0.85) | 0.24 | 0.11 | |||
| Responders | 1.06 (0.14; 1.97) | 0.03 | 0.29 |
Results are presented as correlation estimates with 95% confidence interval, correlation coefficient (r2) and p-value
OR with 95% confidence interval and p-value.
Non-selective beta-blockers (NSBB) response defined as a reduction of hepatic venous pressure gradient (HVPG) ≥ 10%, or a HVPG < 12mmHg, after intravenous propranolol infusion during liver vein catheterization
The cut-off for changes in SS after NSBB was 0.3 kPa or 2.6% to identify patients responding to NSBB, but the specificity and sensitivity of the cut-off point was 1 and 0.19, respectively. ROC curves and AUC for SS, and distribution plots for a response or no response, are shown in Figure 4.
Figure 4: Receiver Operating Characteristic (ROC) curve showing the effect of non-selective beta-blockers on liver stiffness (A) and spleen stiffness (B).
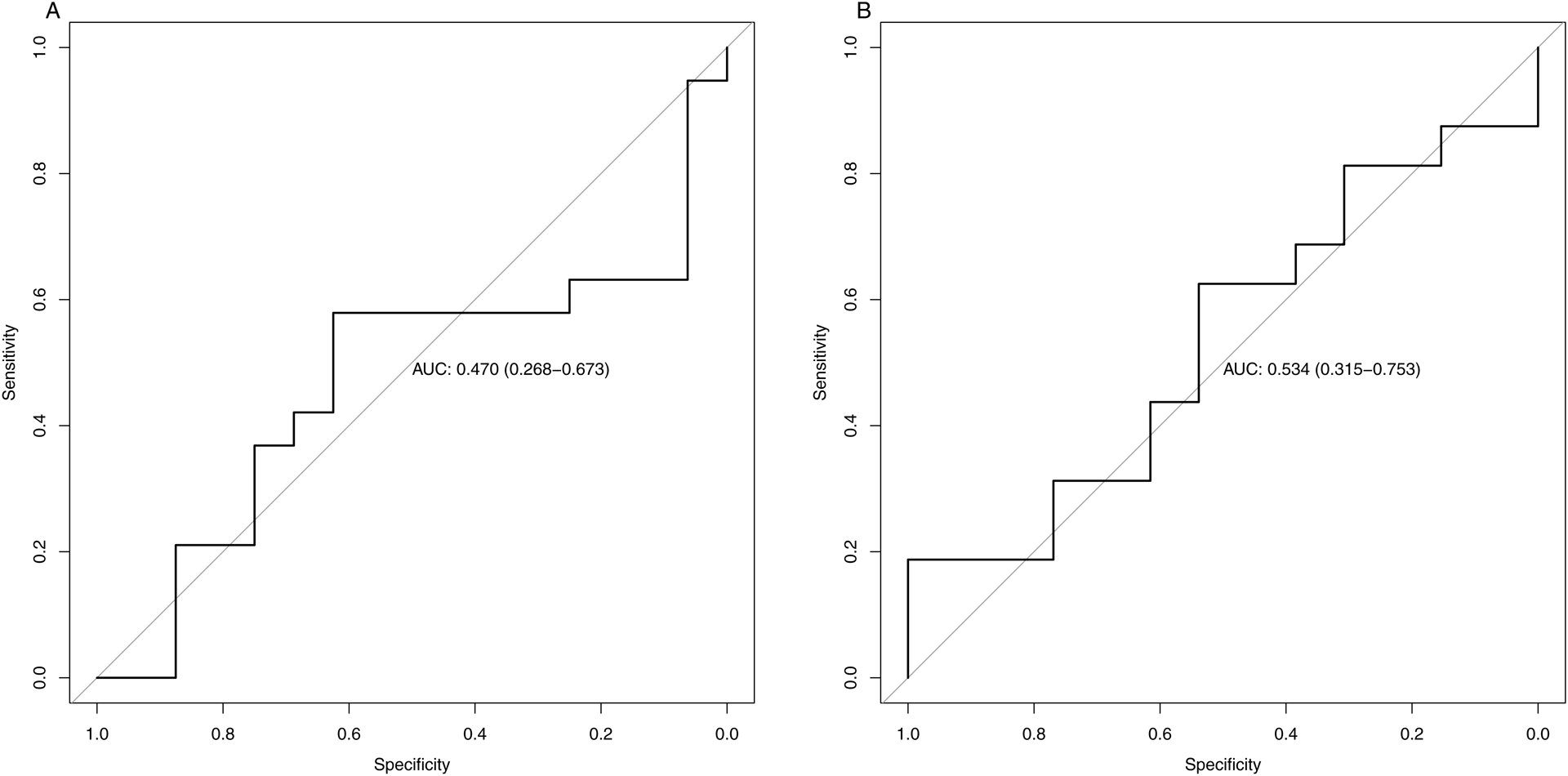
Non-selective beta-blockers (NSBB) response defined as a reduction of hepatic venous pressure gradient (HVPG) ≥ 10%, or a HVPG < 12mmHg, after intravascular propranolol infusion during liver vein catheterization.
Optimal cut-off for liver stiffness was a decrease of 0.3kPa after NSBB, which provided an area under the ROC curve of 0.47 (CI 0.27;0.67) (A) and a specificity of 0.62 and a sensitivity 0.58. Spleen stiffness optimal cut-off was an increase in spleen stiffness of 0.3 kPa after NSBB and the area under the ROC curve was 0.53 (CI 0.32;0.75) (B), with a specificity of 1.00 and a sensitivity 0.19
Discussion
The main findings of this prospective study are that (1) 2D MR elastography can be used to estimate portal pressure and predict severe portal hypertension; (2) NSBB has no significant impact on LS, but it reduces SS; (3) Changes in neither LS nor SS can predict whether patients with severe portal hypertension will respond to NSBB.
MR elastography has previously been proven to have excellent accuracy in differentiating between cirrhosis and less severe liver disease, and a meta-analysis comprising 26 studies showed that MR elastography had a high precision to stage liver fibrosis as compared to liver histology20. Nevertheless, the relationship between MR elastography and portal pressure has been sparsely studied, and all available studies are characterized by patient populations of fewer than 40 patients5,6,21,22.
Results of two MR elastography studies showed that SS, or the viscoelastic parameter of the spleen, was a better predictor of HVPG than LS5,6. In contrast, Wagner et al. found that LS correlated moderately well with HVPG, whereas SS did not21. The Wagner et al. cohort consisted primarily of patients without CSPH and evidence from studies of ultrasound-based elastography indicates that LS correlates better with a milder degree of portal hypertension and a HVPG less than 12mmHg11,23,24. One explanation for this could be that in severe portal hypertension other hemodynamic mechanisms, like the presence of a hyperdynamic circulation and pronounced peripheral vasodilation, contribute more to the increase in portal hypertension than the increase in hepatic vascular resistance, fibrogenesis, and consequently progression in LS.
In our study, we found significant correlations between HVPG and both LS and SS, but interestingly SS was a better predictor of severe portal hypertension than was LS. We recruited a broad spectrum of patients with respect to portal hypertension and, to our knowledge, our cohort represents the most extensive one assembled for investigating the relationship between MR elastography and portal pressure in patients with cirrhosis.
The effect of NSBB on LS and SS
Until now, no studies have investigated the effects of NSBB on LS and SS as measured with MR elastography. Our results indicate that NSBB has no effect on LS but reduces SS by an average of 6.9%.
Cirrhosis and portal hypertension are characterized by increased sinusoidal resistance to portal inflow. This is partly due to pronounced fibrogenesis with the development of regeneration nodules and intrahepatic sinusoidal vasoconstriction25,26. Cirrhosis and portal hypertension are also characterized by hyperdynamic circulation with increased cardiac output and splanchnic inflow. Thus, both static and dynamic components play a role in the development of portal hypertension, and the dynamic components in particular can be affected by beta-adrenergic blockade. NSBB blocks both beta-1 and beta-2 adrenoceptors and its effects on hepatic blood flow can be attributed to intrahepatic and extrahepatic vascular mechanisms. Thus, beta-1 adrenoceptors are located in the heart and NSBB reduces cardiac output by having a primarily negative chronotropic effect. Beta-2-receptors are primarily located in the vascular bed, leading to vascular relaxations, and NSBB thereby causes splanchnic vasoconstriction with a reduction of portal venous inflow and hepatic congestion27.
The absence of a response of NSBB on LS could be owing to the fact that NSBB only produces smaller changes in the intrahepatic resistance in patients with severe portal hypertension and the changes after NSBB are more pronounced in the extra-hepatic component, which cannot be measured precisely by MR elastography of the liver.
Our results are in line with other ultrasound-based studies which show that the effects of NSBB or combined alpha-betablockers on LS are heterogeneous – sometimes decreasing, other times increasing, LS11–15,28. The ultrasound-based study by Choi et al, found similar both increasing and decreasing LS after NSBB, but they showed a strong correlation between changes in LS and changes in HVPG. However, the mean follow-up time was 173.7 days and 9 of the 23 patients had aggravation with an increase in mean HVPG from 14.17 mmHg to 17.95 between baseline and follow-up despite betablocker treatment.
Despite having little effect on LS, NSBB was found to reduce SS. It is well known that portal hypertension leads to pooling of blood in the splanchnic area, and thereby to splenic congestion. This together with spleen tissue hyperplasia29, leads to increased SS, but because there is less fibrosis within the spleen compared to the liver, the dynamic changes in blood filling can be more pronounced and have a greater impact on SS than on LS. Since the primary effect of NSBB is a reduction in the portal inflow, it is to be expected that a reduction of the splenic blood volume will decrease SS. There may also be fewer confounding factors relating to the spleen than there are for the liver, such as liver congestion, biliary obstruction, and liver inflammation21. On the other hand, measurements of SS had a higher failure rate than those for LS30,31. This may be partly due to organ location and geometry32.
MR elastography and treatment response to NSBB
The NSBB-induced reduction in neither LS nor in SS could be used to predict the HVPG changes after NSBB. This is in contrast to the two aforementioned ultrasound-based studies showing that changes in SS were capable of predicting the effect of NSBB on HVPG12,13. In the cohorts of Kim et al. and Marasco et al., the beta-blockers were administered orally with carvedilol or propranolol, or just carvedilol, respectively. The mild alfa-1-adrenergic antagonist, in combination with the beta-blocker effect of carvedilol, is known to reduce HVPG beyond the effect of propranolol and may explain why these two studies showed significant associations between the response to beta-blockers and changes in SS27. The finging of a rather low correlation between changes in SS and HVPG could be due to the fact that the spleen enlargement is caused by a combination of congestion and spleen tissue hyperplasia, the last being a more static component.
Another possible explanation for the discrepant results is that in our study all patients had HVPG, LS, and SS assessed with acute NSBB response after intravenous propranolol, whereas the ultrasound-based studies have used the long-term response method where the HVPG measurements were repeated after 4 weeks of oral therapy with NSBB. The two different methods with intravenious vs. oral betablocker treatment may reveal different pathophysiological respons in LS changes and SS changes and thus influence the results. However, in a large study by Villanueva et al. the authors found a high correlation between the acute and the chronic changes in HVPG after NSBB and according to the Baveno VI recommendation both methods can be used to measure NSBB response9,17.
One of the strengths of our study is that the NSBB response was assessed with an average of 18 days between the MR-elastography and the liver vein catheterization. This design minimizes confounding factors, for with oral treatment and a longer time between the investigating days there is often a spontaneous improvement in the liver disease and a marked reduction in HVPG33. Furthermore, MR elastography is currently regarded as the most accurate noninvasive imaging technique for staging liver fibrosis and it has several advantages over ultrasound elastography, including operator-independency, better performance in obese patients, and the possibility of large-volume tissue sampling31,32,34–36.
Our study does have some limitations. First, our sample size was somewhat limited, yet it was deemed sufficient by our statistical power calculations. Second, the MR elastography method used to assess splenic stiffness has relevant limitations. While conventional 2D MR elastography has been established as a reliable technology for measuring liver stiffness, this version of the technique has known limitations for assessing the stiffness of other organs such as the spleen, where shear wavelength cannot be reliably measured in individual transverse 2D planes. A more advanced version of MR elastography known as 3D MR elastography is capable of assessing the shear wave field in three dimensional space37,38. 3D MR elastography technology is not yet widely available and was not an option on the MR-imaging system used in this study. However, the evidence of treatment effect on splenic stiffness observed in this work provides motivation for future studies to evaluate the potential of 3D MR elastography-based splenic stiffness measurements in predicting treatment response. Furthermore, we did not include ultrasound-based elastography together with MR-elastography due to the complexity of the design, although it would be extremely interesting to clarify if the two methods were able to find similar patterns in LS or SS changes after NSBB. Finally, the etiology of the cirrhosis in 67.3% of our patients was alcohol, while only 3.8% of patients’ cirrhosis was due to hepatitis. However, it is an important strength of our study that all patients were included prospectively, given intravenous NSBB infusions, underwent timely measurement of HVPG, and were scanned with the same MR-scanner. Moreover, this is the first MR elastography study to evaluate the effects of NSBB on LS and SS.
In conclusion, this study demonstrates that LS and SS, as measured with 2D MR elastography, correlate with the degree of portal hypertension and the severity of cirrhosis. Treatment with NSBB was associated with a significant decrease in SS, while having no clear impact on LS. The change in SS after NSBB did not reliably predict the effect on HVPG.
Supplementary Material
Acknowledgments
We sincerely thank the staff at the Centre for Functional and Diagnostic Imaging and Research and the Gastro Unit at Hvidovre University Hospital for their support. We would especially like to thank the excellent MR technicians, and the MR physicist Lasse R Søndergaard, for their impressive work with the MR elastography measurements. Furthermore, we wish to thank the Gastro Unit of Herlev Hospital for referring patients to the study coordinators.
This project has received financial support from Amager Hvidovre University Hospital Research Foundation and Ferring Pharmaceuticals A/S.
Conflicts of interest and financial support:
The study was supported by an independent research grant from Ferring Pharmaceuticals A/S and from Amager Hvidovre University Hospital Research Foundation.
The Mayo Clinic and authors RLE, MY, and JC have intellectual property and a financial interest related to this research. Furthermore, they are supported by the National Institutes of Health with NIH grants EB017197 (Yin) and EB001981 (Ehman).
Hartwig R. Siebner has received honoraria as a speaker from Sanofi Genzyme, Denmark and Novartis, Denmark, as consultant from Sanofi Genzyme, Denmark, Lophora, Denmark, and Lundbeck AS, Denmark, and as editor-in-chief (Neuroimage Clinical) and senior editor (NeuroImage) from Elsevier Publishers, Amsterdam, The Netherlands. He has received royalties as book editor from Springer Publishers, Stuttgart, Germany and from Gyldendal Publishers, Copenhagen, Denmark. Hartwig R. Siebner holds a five-year professorship in precision medicine at the Faculty of Health Sciences and Medicine, University of Copenhagen, which is sponsored by the Lundbeck Foundation (Grant Nr. R186-2015-2138).
Abbreviations
- MR
magnetic resonance
- NSBB
non-selective beta-blockers
- 2D
two dimensional
- HVPG
hepatic venous pressure gradient
- LS
liver stiffness
- SS
spleen stiffness
- WHVP
wedged hepatic venous pressure
- FHVP
free hepatic venous pressure
- kPa
kilopascal
- ROI
regions of interest
- ALT
alanine aminotransferase
- ROC
receiver operating characteristic
- AUC
area under the curve
- CP
child-pugh
- MELD
model for end-stage liver disease
- CSPH
clinical significant portal hypertension
Footnotes
The remaining authors have no conflicts of interest to declare
Trial registration number
H-16048475, Ethics Committee for Medical Research in Copenhagen
Clinical.trials.gov registration ID: NCT03438916
References
- 1.Møller S & Bendtsen F The pathophysiology of arterial vasodilatation and hyperdynamic circulation in cirrhosis. Liver International 38, 570–580 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Mathew RP & Venkatesh SK Imaging of Hepatic Fibrosis. Current Gastroenterology Reports 20, (2018). [DOI] [PubMed] [Google Scholar]
- 3.Manatsathit W et al. Accuracy of liver stiffness, spleen stiffness, and LS-spleen diameter to platelet ratio score in detection of esophageal varices: Systemic review and meta-analysis. Journal of Gastroenterology and Hepatology (Australia) 33, 1696–1706 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Babu AS et al. Elastography in chronic liver disease: Modalities, techniques, limitations, and future directions. Radiographics 36, 1987–2006 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronot M et al. Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. Eur. Radiol 24, (2014). [DOI] [PubMed] [Google Scholar]
- 6.Guo J et al. In vivo abdominal magnetic resonance elastography for the assessment of portal hypertension before and after transjugular intrahepatic portosystemic shunt implantation. Invest. Radiol 50, 347–351 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Bendtsen F, Henriksen JH & Sørensen TI Propranolol and haemodynamic response in cirrhosis. J. Hepatol 13, 144–8 (1991). [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Tsao G, Sanyal AJ, Grace ND & Carey W Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 46, 922–938 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Villanueva C et al. Acute Hemodynamic Response to β-Blockers and Prediction of Long-term Outcome in Primary Prophylaxis of Variceal Bleeding. Gastroenterology 137, 119–128 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Tsao G, Bosch J & Groszmann RJ Portal hypertension and variceal bleeding-Unresolved issues. Summary of an American Association for the study of liver diseases and European Association for the study of the liver single-topic conference. Hepatology 47, 1764–1772 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Reiberger T et al. Non-selective β-blockers improve the correlation of liver stiffness and portal pressure in advanced cirrhosis. J. Gastroenterol 47, 561–568 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Kim HY et al. Non-invasive response prediction in prophylactic carvedilol therapy for cirrhotic patients with esophageal varices. J. Hepatol 70, 412–422 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Marasco G et al. Spleen stiffness measurement for assessing the response to β-blockers therapy for high-risk esophageal varices patients. Hepatol. Int 14, 850–857 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Piecha F et al. Pharmacological decrease of liver stiffness is pressure-related and predicts long-term clinical outcome. Am. J. Physiol. - Gastrointest. Liver Physiol 315, G484–G494 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Zaghloul SG et al. Impact of non-selective beta blockers on portal hypertension and hepatic elasticity in hepatitis C virus-related liver cirrhosis. Drug Discov. Ther 13, 108–113 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Angeli P et al. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol 69, 406–460 (2018). [DOI] [PubMed] [Google Scholar]
- 17.de Franchis R & Faculty Baveno VI. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol 63, 743–52 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Hobolth L et al. Carvedilol or propranolol in portal hypertension? A randomized comparison. Scand. J. Gastroenterol 47, 467–474 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Abraldes JG, Sarlieve P & Tandon P Measurement of portal pressure. Clinics in Liver Disease 18, 779–792 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Kim YS, Jang YN & Song JS Comparison of gradient-recalled echo and spin-echo echo-planar imaging MR elastography in staging liver fibrosis: a meta-analysis. Eur. Radiol 28, 1709–1718 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Wagner M et al. Noninvasive prediction of portal pressure with MR elastography and DCE-MRI of the liver and spleen: Preliminary results. J. Magn. Reson. Imaging 48, 1091–1103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gharib AM et al. Magnetic resonance elastography shear wave velocity correlates with liver fibrosis and hepatic venous pressure gradient in adults with advanced liver disease. Biomed Res. Int 2017, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vizzutti F et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology 45, 1290–1297 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Hirooka M et al. Splenic elasticity measured with real-time tissue elastography is a marker of portal hypertension. Radiology 261, 960–968 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Yin M et al. Dynamic postprandial hepatic stiffness augmentation assessed with MR elastography in patients with chronic liver disease. Am. J. Roentgenol 197, 64–70 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moller S, Hobolth L, Winkler C, Bendtsen F & Christensen E Determinants of the hyperdynamic circulation and central hypovolaemia in cirrhosis. Gut 60, 1254–1259 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Zacharias AP et al. Carvedilol versus traditional, non-selective beta-blockers for adults with cirrhosis and gastroesophageal varices. Cochrane Database of Systematic Reviews 2018, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi SY et al. Shear-Wave elastography: A noninvasive tool for monitoring changing hepatic venous pressure gradients in patients with cirrhosis. Radiology 273, 917–926 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Bolognesi M, Merkel C, Sacerdoti D, Nava V & Gatta A Role of spleen enlargement in cirrhosis with portal hypertension. Digestive and Liver Disease 34, 144–150 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Friedrich-Rust M, Poynard T & Castera L Critical comparison of elastography methods to assess chronic liver disease. Nature Reviews Gastroenterology and Hepatology 13, 402–411 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Kim DW, Kim SY, Yoon HM, Kim KW & Byun JH Comparison of technical failure of MR elastography for measuring liver stiffness between gradient-recalled echo and spin-echo echo-planar imaging: A systematic review and meta-analysis. J. Magn. Reson. Imaging 51, 1086–1102 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Idilman IS, Li J, Yin M & Venkatesh SK MR elastography of liver: current status and future perspectives. Abdom. Radiol 45, 3444–3462 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bendtsen F, Henriksen JH & Sørensen TIA Long-term effects of oral propranolol on splanchnic and systemic haemodynamics in patients with cirrhosis and oesophageal varices. Scand. J. Gastroenterol 26, 933–939 (1991). [DOI] [PubMed] [Google Scholar]
- 34.Lefebvre T et al. Prospective comparison of transient, point shear wave, and magnetic resonance elastography for staging liver fibrosis. Eur. Radiol 29, 6477–6488 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Huwart L et al. Magnetic Resonance Elastography for the Noninvasive Staging of Liver Fibrosis. Gastroenterology 135, 32–40 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Kennedy P et al. Quantitative elastography methods in liver disease: Current evidence and future directions. Radiology 286, 738–763 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J et al. Quantitative assessment of portal hypertension with bi-parametric dual-frequency hepatic MR elastography in mouse models. Eur. Radiol (2020). doi: 10.1007/s00330-020-07341-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen AM et al. The Role of Three-Dimensional Magnetic Resonance Elastography in the Diagnosis of Nonalcoholic Steatohepatitis in Obese Patients Undergoing Bariatric Surgery. Hepatology 71, 510–521 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


