Summary
This review is intended to advance understanding of the anticoagulant function of an important plasma protein, Protein S. Despite 40+ years of research, we have not had a complete description of PS biology as it pertains to control of blood coagulation. However, the picture of PS function has become sharper with the recent discovery of FIXa inhibition by PS. Hemostasis mediated by PS now includes regulation of FIXa activity alongside the cofactor activities of PS in the TFPI/APC pathways. In addition, the direct inhibition of FIXa by PS suggests that PS, particularly a small derivative of PS, could be used to treat individuals with PS deficiencies or abnormalities that cause thrombotic complications.
Keywords: Protein S, Factor IXa, COVID-19, Hypercoagulability, Hypoxemia
Structured Abstract
Purpose of review
Protein S (PS) is an essential natural anticoagulant. PS deficiency is a major contributor to acquired hypercoagulability. Acquired hypercoagulability causes myocardial infarction, stroke, and deep vein thrombosis in millions of individuals. Yet, despite its importance in hemostasis, PS is the least understood anticoagulant. Even after 40 years since PS was first described, we are still uncovering information about how PS functions. The purpose of this review is to highlight recent findings that advance our understanding of the functions of PS and explain hypercoagulability caused by severe PS deficiency.
Recent findings
Protein S has long been described as a cofactor for Activated Protein C (APC) and Tissue Factor Pathway Inhibitor (TFPI). However, a recent report describes direct inhibition of Factor IXa (FIXa) by Protein S, an activity of PS that had been completely overlooked. Thrombophilia is becoming a more frequently reported disorder. Hereditary PS deficiency is an anticoagulant deficiency that results eventually in thrombophilia. In addition, PS deficiency is a predisposing factor for venous thromboembolism, but an effect of PS deficiency in arterial thrombosis, such as arterial ischemic stroke, is uncertain. Plasma PS concentration decreases in pregnant women. Inherited thrombophilias are important etiologies for recurrent pregnancy loss, and anticoagulation therapy is of benefit to women with recurrent pregnancy loss who had documented only PS deficiency.
Hypoxia is a risk factor for venous thromboembolism (VTE), and hypoxia downregulates plasma PS level. Importantly, COVID-19 can lead to hypoxemia because of lung damage from IL6-driven inflammatory responses to the viral infection. Because hypoxia decreases the abundance of the key anticoagulant PS, we surmise that the IL6-induced cytokine explosion combined with hypoxemia causes a drop in PS level that exacerbates the thrombotic risk in COVID-19 patients.
Introduction
Protein S (PS) is a γ-carboxyglutamate-containing plasma glycoprotein synthesized and secreted primarily by hepatocytes (1), endothelial cells (2), and Leydig cells (3). About 2.5% of circulating PS exists within platelet α-granules (4, 5); platelet PS is derived exclusively from expression within megakaryocytes (6).
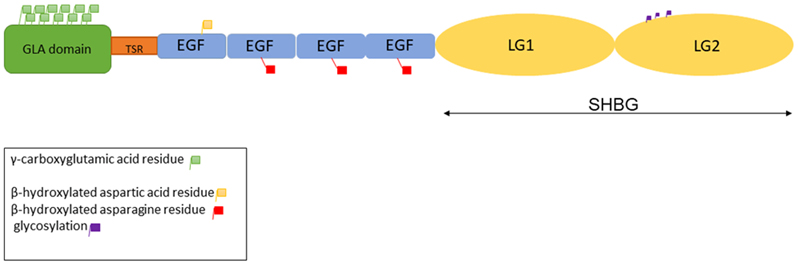
Protein S is encoded by a single PROS1 gene in humans (7). Human PROS1 is located on chromosome 3; the gene contains 11 introns (8). Protein S was the first blood coagulation protein mapped to a human chromosome (9). Protein S is synthesized as a 676 amino acid precursor protein that is processed to a mature multimodular protein of 635 amino acids. The mature protein is composed of a phospholipid binding γ-carboxyglutamic acid domain (GLA), a thrombin sensitive region, four Epidermal Growth Factor (EGF)-like domains, and a sex hormone binding globule (10-12) that contains two laminin G (LG)-type domains (13). Three different types of post-translationally modified amino acid residues are found in PS, 11 γ-carboxyglutamic acid residues in the GLA domain, a β-hydroxylated aspartic acid in the first EGF-like domain, and a β-hydroxylated asparagine in each of the other three EGF-like domains (Figure 1)(14). Gamma-carboxylation, the most-studied posttranslational modification, is essential for PS function.
Figure 1-.
The cartoon showing different domains of Protein S and sites for post translational modifications in different domains.
Protein S is a multifunctional protein at the intersection of blood coagulation, inflammation, and other cellular processes. Plasma PS concentration is 350 nM, 60% of which is bound to complement component 4 binding protein (C4BP) (15); the other 40% is termed as ‘free’ (16). Both forms of PS function as an anticoagulant; in addition, the C4BP-bound form of PS minimizes the effect of inflammation (13). Lastly, PS promotes efferocytosis (clearance of apoptotic cells by phagocytosis) through the TAM (Tyro-3, Axl and Mer) family of protein kinase receptors (17).
Protein S is a vital anticoagulant because homozygous PS deficiency causes severe thrombosis in newborns (18) and defects in vascular system development that may be fatal (19). However, despite 40 years of research, there was a lack of clarity about how PS exerts its anticoagulant activity. In hindsight, clarity was impaired because several proposed activities of PS as an anticoagulant were confounded by artifactual activity of PS multimers that form at low phospholipid concentration. More recent studies performed with physiological amounts of phospholipids have eliminated the effects of multimers and suggest three disparate functions of PS: 1) PS is a cofactor for Activated Protein C (APC) in inactivation of FVa and FVIIIa (20, 21), 2) PS is a cofactor for Tissue Factor Pathway Inhibitor (TFPI) in accelerating the inhibition of FXa (22, 23), and 3) PS binds and inhibits the function of FIXa in producing FXa from FX (24, 25).
Protein S Functions:
Protein S is a key negative regulator of blood coagulation, and it has a significant function in complement activation pathways (20). Protein S deficiency and dysfunction cause life-threatening thrombotic conditions, including neonatal purpura fulminans (18). Currently, the consensus is that PS inhibits coagulation either independently or by enhancing the anticoagulant function of Activated Protein C (APC).
Cofactor for APC
Protein S acts as a cofactor for APC by enhancing the inactivation of activated coagulation factors Va and VIIIa (20, 21, 26). Protein S amplifies APC’s inactivating function by enhancing cleavage at Arg 306 of FVa (27, 28). The EGF1 domain residue Asp95 and the GLA domain residue E36 of PS are crucial for the APC cofactor function of PS (29).
There are two APC-independent functions of PS that stood out once studies were performed with appropriate phospholipid concentrations and the results were confirmed in vivo.
Cofactor of TFPI
Protein S acts as a cofactor of Tissue Factor Pathway Inhibitor by stimulating the inhibition of FXa by TFPI by 4~10-fold (23, 30, 31). The Kunitz domain 3 of TFPI binds the LG1 domain of PS (32-34), and a direct TFPIα-protein S interaction is required for the enhanced inhibition of FXa.
Inhibition of Factor IXa Activity
In 2012, another anticoagulant function of PS was established by the finding that PS directly binds and inhibits the procoagulant FIXa in the presence and absence of FVIIIa (24, 25). This direct inhibitory activity suggested a novel regulatory function of PS in blocking thrombin generation maximally in the presence of FIXa and FVIIIa (24, 25, 35, 36).
Plautz et.al., showed that PS binds and inhibits FIXa in vivo by associating with the K132, K126, and R170 residues in the FIXa heparin-binding exosite; this interaction with PS regulates thrombin generation by inhibiting the intrinsic Xase complex (35). FIXa and PS also coimmunoprecipitated from plasma, corroborating their interaction in a physiological setting (25, 35). Importantly, Hemophilia B mice infused with a FIXa double mutant, K132A/R170A, that had full activity but could not bind to PS demonstrated an accelerated rate of fibrin clot formation compared with infusion of wild type FIXa (35). Crucially, disruption of the interaction between PS and FIXa caused an increased rate of thrombus formation in Hemophilia B mice (35). These data established that interaction of PS with the FIXa is an important APC-independent regulatory mechanism for the propagation phase of coagulation (35).
Regulation of Protein S:
Protein S deficiency is a genetic disorder caused by mutation in the PROS1 gene. Protein S deficiency has been difficult to study because of the peculiar PS biology. PS has an anticoagulant function but no enzymatic activity, and PS interacts with plasma components that operate in both hemostasis and inflammation. Heterozygous PS deficiency is an autosomal dominant condition because half the normal expression of PS increases the risk of thrombosis, as judged from familial and population studies. Heterozygous individuals have an increased risk of developing blood clots in the legs (deep venous thrombosis); the clots can break loose and travel to the lungs, an event that is termed pulmonary embolism. Rarely, two abnormal PS genes are inherited (congenital) that can cause widespread small clots and a life-threatening disease, purpura fulminans (18). Furthermore, murine PS knockouts are embryonic lethal (22) (23). However, a heterozygous genotype is not directly associated with lethality, unlike a homozygous genotype. Heterozygous PS deficiency is associated with increased risk of venous thrombosis (37-39) and pulmonary embolism (40).
Occasionally, PS deficiency may be acquired because of conditions such as kidney disease, malignancy, pregnancy, or the use of oral (estrogen-based) contraceptives (41, 42). Patients with chronic kidney disease who undergo hemodialysis experience hypercoagulability. There are reports that PS deficiency is a cause of hypercoagulability in these patients (43). Protein S is also an important signaling molecule that binds and activates a family of tyrosine kinase receptors known as TAM (Tyro3, Axl and Mer) receptors. TAM receptors are often upregulated in cancers. We recently reported that PS suppresses the growth of pancreatic cancer cells in vitro and that PROS1 (PS) gene expression is associated with better survival in pancreatic cancer (44). Acquired PS deficiency occurs in women with high levels of estrogen, either because of pregnancy or from the use of estrogen containing oral contraceptive pills (45-47). There was a report that estrogen represses PS production because the estrogen receptor (ERα) downregulates the activity of the PS gene promoter (46) .However, the details of this mechanism are not known. Sickle cell anemia is another seldom recognized cause of acquired PS deficiency (48). Patients with sickle cell disease bear a high risk of developing thromboembolic disorders, and PS deficiency was one of the major reasons for thromboembolism (49). It has been hypothesized that acquired PS deficiency in sickle cell disease is due to direct binding of PS to sickling red cells that express increased surface phosphatidylserine (50). In addition, PS expression is depressed in hypoxic conditions (51). Stabilization of HIF1α in the liver, a normal response to hypoxia, is associated with reduced PS expression and plasma level, which, in turn, increases the likelihood of thrombosis. HIF1α is a well-documented, general transcriptional activator, and Pilli et al. (51) showed that HIF1α directly downregulates PS expression (51). There is a report of a rare case of PS deficiency associated with assisted reproductive treatment with ovarian hyperstimulation syndrome (OHSS), following right neck venous thromboembolism (1). Thromboembolism is a life-threatening complication among women with OHSS; therefore, thrombophilia workup should be considered for women with thrombotic events.
Mutations of Protein S
Almost 300 different mutations have been identified in PROS1, spread over all exons, the promoter region, and within several introns (54). Most alterations are missense mutations, but several other types of mutations have been reported.
Here, we describe further three PROS1 mutations. We selected these mutations because they are unique and have been observed only recently. The molecular genetic analysis of a 23-year-old patient who experienced venous thrombosis showed a homozygous missense mutation, G664>A, in exon7 of PROS1 (52). This mutation results in replacement of glycine by arginine at codon 222, and the mutation causes a reduction in PS level (52). This alteration was the first reported mutation of PS codon 222 (52). Hereditary PS deficiency caused by a mutation/deletion in PROS1 is autosomal dominant, which means that an individual who inherits only one mutant PROS1 gene nevertheless has an increased chance of developing symptoms of PS deficiency. A recent report describes a heterozygous deletion of eight base pairs (Leu313Ser) in PROS1 exon 9 and a missense variant (Ser538Phe) in two different patients. These mutations resulted in PS deficiency and severe thrombophilic diathesis (53). Further, a rare C39>T mutation in the PROS1 5'UTR was reported to create a new upstream translation initiation codon that caused PS deficiency and venous thromboembolism (54).
Clinical Considerations:
Protein S – Clinical Perspectives
PS deficiency manifests clinically as thrombophilia, an imbalance of coagulation factors that leads to abnormal blood clotting. This pathophysiological disturbance puts patients at risk for disseminated intravascular coagulation, potentially causing disabilities such as cerebrovascular injury or chronic venous thromboembolism (VTE). We also consider unique case studies to discuss other recent developments of PS clinical research concerning pregnancy and management of PS deficiency. Lastly, Malas et al. reported that approximately 31% of COVID-19 patients admitted to the ICU experienced at least one thromboembolic event (55). Thus, it is important to discuss the possible correlation between COVID 19 morbidity and PS deficiency. Although there are certainly other factors that contribute to thrombophilia in severe COVID patients, when we consider the intersection of hypoxia, inflammation, and severity of symptoms associated with decreased PS, we suggest that Protein S likely contributes to thrombophilia in COVID-19 patients.
Estrogen Induced Acquired Protein S Deficiency
In general, estrogen-containing medications, such as oral contraceptives or hormone replacement therapy, induce a prothrombotic state that results from acquired PS deficiency. Pregnant women are at high risk for thromboembolic events because of estrogen-associated hypercoagulability [51]. Estrogen level steadily increases during pregnancy, which elevates the risk for clotting events [52]. Hereditary PS deficiency has long been a contraindication for contraceptive use because of the known effect of estrogen on downregulation of PS (46). The molecular mechanism of estrogen-induced PS deficiency has not yet been clearly defined.
Management of PS deficiency
Interestingly, a diminished PS level appears to correlate with lifestyle modifications to combat obesity and insulin resistance, despite overall reduction in thrombophilia with a decrease in prothrombotic factors. Recall that hypoxia can induce PS deficiency (51); thus, hypoxia secondary to intense exercise may be a factor in the negative correlation between lifestyle change and PS level. As for pharmaceutical management of PS deficiency, direct oral anticoagulants (DOAC) have been found to be efficient in treatment and prevention of VTE in patients with mutations in the PROS1 gene (56, 57). Traditionally, heparin or vitamin K antagonists, such as warfarin, have been used to prevent VTE; however, the DOACs described in these studies, rivaroxaban and apixaban, may offer more predictable outcomes in the case of PS deficiency. Another benefit of DOACs is that they offer fixed dosing, limited drug-drug interactions, and the potential to reverse anticoagulant activity with the administration of Andexanet alfa (58).
Protein S deficiency is associated with venous thromboembolism; however, it is unclear whether PS deficiency promotes arterial thrombosis, such as arterial ischemic stroke. Fearon et al. described a 79-year-old woman with PS deficiency due to a Trp149Cys mutation; this individual had an arterial thromboembolic event (59). Similarly, Naghavi et al. reported an effect of PS deficiency on arterial ischemic stroke (60). This group reported further that rivaroxaban was an effective option for prevention of recurrent arterial ischemic stroke caused by PS deficiency (60). Lastly, Ohashi et al. described a 59-year-old patient with ischemic stroke caused by a hereditary PS deficiency; the patient was treated with apixaban, another direct oral anticoagulant (57).
Further, Lou et al. described two patients with hereditary PS deficiency who had deep vein thrombosis even after warfarin therapy for two years (56). However, after the patients were treated with 20 mg/day rivaroxaban, they did not have any thromboembolic events even after one year follow up (56). A consensus should be reached for moving towards new oral anticoagulants such as rivaroxaban and enoxaparin-VKA, which can preserve PS level during clotting events.
COVID-19
Infection by SARS-CoV-2 not only results in hyperactivity of the immune system by a cytokine storm, but also the virus causes severe hypoxemia. We know hypoxia downregulates PS expression (51). Therefore, one may speculate that downregulation of PS during COVID-19 contributes to a hypercoagulable state (61). Several studies support the correlation between VTE in COVID-19 patients and decreased PS level (62) (63). Wood et.al. showed that free, but not total, PS level was depressed in severe COVID-19 patients (64). Unpublished data from our group supported the findings of Wood et al. (64).
Interestingly, Ruzicka et al. (65) hypothesized that the papain-like protease (PLpro) of SARS-CoV-2, the causative agent of COVID-19, may contribute to the coagulation and thrombotic dysregulation by dissolute cleavage of Protein S . A review of the three N-terminal cleavage sites recognized by the PL proteases of SARS viruses revealed a highly conserved LXGG motif. A BLAST search using the 10 amino acids that span the nsp3/4 cleavage site of CoV2 (IALKGG/KIVN) returned an eight amino acid “hit” within PROS1, including the core LXGG sequence (65). The “X” in the CoV2 polyprotein, a lysine, is replaced by a highly conserved arginine in Protein S. Therefore, it is rational to suggest that downregulation of PS during severe COVID 19 occurs by PLpro cleavage of PS.
Whether or not PS deficiency has a significant effect in the development of VTE, this research topic has potential for elucidating the mechanism of hypercoagulable states in SARS-infected patients. Investigators have identified reduced vitamin K levels as a potential modifiable risk (66) factor in patients with COVID-19, which may be pertinent because PS is a vitamin K-dependent protein. Measurement of free PS level in the circulation is not currently part of the standard blood work-up for COVID-19 patients, but such measurements are warranted, and they will be informative for treatment.
Conclusion
The hypercoagulable state associated with congenital deficiency of PS demonstrates the importance of this protein in regulation of hemostasis. Protein S acts as an anticoagulant by performing three different functions: 1) Cofactor for APC, 2) Cofactor for TFPI, and 3) Inhibitor of FIXa. Downregulation of FIXa function by PS has not been previously considered. The allure of this inhibitory property of PS is related to the fact that mutant forms of FIXa that lack the binding site for PS would then exhibit enhanced procoagulant activity, which has translational potential regarding therapies for FIX upregulation or PS deficiency. Pregnancy, estrogen containing contraceptives, hypoxia, and most probably COVID-19 infection are factors that cause acquired PS deficiency, which, in turn, causes VTE. Measurement of PS in plasma must be considered as a routine for COVID-19 and during pregnancy, so that initial symptoms due to PS deficiency can be treated in a timely manner with DOACs such rivaroxaban.
Key Points:
Protein S enhances the activities of Activated Protein C and Tissue Factor Pathway Inhibitor and inhibits the activity of activated Factor IX. Thus, Protein S occupies a central position in regulation of hemostasis.
Genetic deficiencies of Protein S, and acquired deficiencies of Protein S induced by, e.g., estrogen and hypoxia, are linked to a variety of venous and arterial thrombotic complications, including deep vein thrombosis and stroke.
SARS COV2 virus-induced Protein S deficiency may be responsible for the often fatal hypercoagulated state of COVID-19 patients.
Acknowledgements
The work was supported by the National Institute of Health HL118557 and HL151613 to RM.
References:
- 1.Biron-Andreani C, Raulet E, Pichard-Garcia L, Maurel P. Use of human hepatocytes to investigate blood coagulation factor. Methods Mol Biol. 2010;640:431–45. [DOI] [PubMed] [Google Scholar]
- 2.Fair DS, Marlar RA, Levin EG. Human endothelial cells synthesize protein S. Blood. 1986;67(4):1168–71. [PubMed] [Google Scholar]
- 3.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8(5):327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogura M, Tanabe N, Nishioka J, Suzuki K, Saito H. Biosynthesis and secretion of functional protein S by a human megakaryoblastic cell line (MEG-01). Blood. 1987;70(1):301–6. [PubMed] [Google Scholar]
- 5.Schwarz HP, Heeb MJ, Wencel-Drake JD, Griffin JH. Identification and quantitation of protein S in human platelets. Blood. 1985;66(6):1452–5. [PubMed] [Google Scholar]
- 6.Calzavarini S, Prince-Eladnani R, Saller F, Bologna L, Burnier L, Brisset AC, et al. Platelet protein S limits venous but not arterial thrombosis propensity by controlling coagulation in the thrombus. Blood. 2020;135(22):1969–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundwall A, Dackowski W, Cohen E, Shaffer M, Mahr A, Dahlback B, et al. Isolation and sequence of the cDNA for human protein S, a regulator of blood coagulation. Proc Natl Acad Sci U S A. 1986;83(18):6716–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long GL, Marshall A, Gardner JC, Naylor SL. Genes for human vitamin K-dependent plasma proteins C and S are located on chromosomes 2 and 3, respectively. Somat Cell Mol Genet. 1988;14(1):93–8. [DOI] [PubMed] [Google Scholar]
- 9.Watkins PC, Eddy R, Fukushima Y, Byers MG, Cohen EH, Dackowski WR, et al. The gene for protein S maps near the centromere of human chromosome 3. Blood. 1988;71(1):238–41. [PubMed] [Google Scholar]
- 10.Stenflo J Contributions of Gla and EGF-like domains to the function of vitamin K-dependent coagulation factors. Crit Rev Eukaryot Gene Expr. 1999;9(1):59–88. [PubMed] [Google Scholar]
- 11.Dahlback B, Hildebrand B, Malm J. Characterization of functionally important domains in human vitamin K-dependent protein S using monoclonal antibodies. J Biol Chem. 1990;265(14):8127–35. [PubMed] [Google Scholar]
- 12.Dahlback B. Purification of human vitamin K-dependent protein S and its limited proteolysis by thrombin. Biochem J. 1983;209(3):837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rigby A, Grant M. Protein S: a conduit between anticoagulation and inflammation. Crit Care Med 2004;32(5 Suppl):S336–41. [DOI] [PubMed] [Google Scholar]
- 14.Dahlback B Protein S and C4b-binding protein: components involved in the regulation of the protein C anticoagulant system. Thromb Haemost. 1991;66(1):49–61. [PubMed] [Google Scholar]
- 15.Hackeng TM, Dawson PE, Kent SB, Griffin JH. Chemical synthesis of human protein S thrombin-sensitive module and first epidermal growth factor module. Biopolymers. 1998;46(2):53–63. [DOI] [PubMed] [Google Scholar]
- 16.Dahlback B, Stenflo J. High molecular weight complex in human plasma between vitamin K-dependent protein S and complement component C4b-binding protein. Proc Natl Acad Sci U S A. 1981;78(4):2512–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80(4):661–70. [DOI] [PubMed] [Google Scholar]
- 18.Marlar RA, Neumann A. Neonatal purpura fulminans due to homozygous protein C or protein S deficiencies. Seminars in thrombosis and hemostasis. 1990;16(4):299–309. [DOI] [PubMed] [Google Scholar]
- 19.Burstyn-Cohen T, Heeb MJ, Lemke G. Lack of protein S in mice causes embryonic lethal coagulopathy and vascular dysgenesis. The Journal of clinical investigation. 2009;119(10):2942–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker FJ. Protein S and the regulation of activated protein C. Semin Thromb Hemost. 1984;10(2):131–8. [DOI] [PubMed] [Google Scholar]
- 21.Walker FJ. Regulation of activated protein C by protein S. The role of phospholipid in factor Va inactivation. J Biol Chem. 1981;256(21):11128–31. [PubMed] [Google Scholar]
- 22.Reglinska-Matveyev N, Andersson HM, Rezende SM, Dahlback B, Crawley JT, Lane DA, et al. TFPI cofactor function of protein S: essential role of the protein S SHBG-like domain. Blood. 2014;123(25):3979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NDONWI M, BROZE G JR. Protein S enhances the tissue factor pathway inhibitor inhibition of factor Xa but not its inhibition of factor VIIa–tissue factor. J Thromb Haemost 2008;6:1044–6. [DOI] [PubMed] [Google Scholar]
- 24.Chattopadhyay R, Sengupta T, Majumder R. Inhibition of intrinsic Xase by protein S: a novel regulatory role of protein S independent of activated protein C. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(10):2387–93. [DOI] [PubMed] [Google Scholar]
- 25.Plautz WE, Chattopadhyay R, Goldfeld EI, Samelson-Jones BJ, Pilli VS, Campello E, et al. Padua FIXa resistance to Protein S and a potential therapy for hyperactive FIXa. Thrombosis research. 2018;170:133–41. [DOI] [PubMed] [Google Scholar]
- 26.Dahlback B, Villoutreix BO. The anticoagulant protein C pathway. FEBS Lett. 2005;579(15):3310–6. [DOI] [PubMed] [Google Scholar]
- 27.Rosing J, Hoekema L, Nicolaes GA, Thomassen MC, Hemker HC, Varadi K, et al. Effects of protein S and factor Xa on peptide bond cleavages during inactivation of factor Va and factor VaR506Q by activated protein C. J Biol Chem. 1995;270(46):27852–8. [DOI] [PubMed] [Google Scholar]
- 28.Norstrom EA, Steen M, Tran S, Dahlback B. Importance of protein S and phospholipid for activated protein C-mediated cleavages in factor Va. J Biol Chem. 2003;278(27):24904–11. [DOI] [PubMed] [Google Scholar]
- 29.Ahnstrom J, Andersson HM, Canis K, Norstrom E, Yu Y, Dahlback B, et al. Activated protein C cofactor function of protein S: a novel role for a gamma-carboxyglutamic acid residue. Blood. 2011;117(24):6685–93. [DOI] [PubMed] [Google Scholar]
- 30.Hackeng TM, Rosing J. Protein S as cofactor for TFPI. Arteriosclerosis, thrombosis, and vascular biology. 2009;29(12):2015–20. [DOI] [PubMed] [Google Scholar]
- 31.Hackeng TM, Sere KM, Tans G, Rosing J. Protein S stimulates inhibition of the tissue factor pathway by tissue factor pathway inhibitor. Proc Natl Acad Sci U S A. 2006;103(9):3106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ndonwi M, Burlingame OO, Miller AS, Tollefsen DM, Broze GJ Jr., Goldberg DE. Inhibition of antithrombin by Plasmodium falciparum histidine-rich protein II. Blood. 2011;117(23):6347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ndonwi M, Tuley EA, Broze GJ Jr. The Kunitz-3 domain of TFPI-alpha is required for protein S-dependent enhancement of factor Xa inhibition. Blood. 2010;116(8):1344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Somajo S, Ahnstrom J, Fernandez-Recio J, Gierula M, Villoutreix BO, Dahlback B. Amino acid residues in the laminin G domains of protein S involved in tissue factor pathway inhibitor interaction. Thromb Haemost. 2015;113(5):976–87. [DOI] [PubMed] [Google Scholar]
- 35.Plautz WE, Sekhar Pilli VS, Cooley BC, Chattopadhyay R, Westmark PR, Getz T, et al. Anticoagulant Protein S Targets the Factor IXa Heparin-Binding Exosite to Prevent Thrombosis. Arteriosclerosis, thrombosis, and vascular biology. 2018;38(4):816–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pilli VS, Plautz W, Majumder R. The Journey of Protein S from an Anticoagulant to a Signaling Molecule. JSM Biochem Mol Biol. 2016;3(1). [PMC free article] [PubMed] [Google Scholar]
- 37.ten Kate MK, van der Meer J. Protein S deficiency: a clinical perspective. Haemophilia : the official journal of the World Federation of Hemophilia. 2008;14(6):1222–8. [DOI] [PubMed] [Google Scholar]
- 38.Mannucci PM, Tripodi A, Bertina RM. Protein S deficiency associated with "juvenile" arterial and venous thromboses. Thromb Haemost. 1986;55(3):440. [PubMed] [Google Scholar]
- 39.Bucciarelli P, Rosendaal FR, Tripodi A, Mannucci PM, De Stefano V, Palareti G, et al. Risk of venous thromboembolism and clinical manifestations in carriers of antithrombin, protein C, protein S deficiency, or activated protein C resistance: a multicenter collaborative family study. Arteriosclerosis, thrombosis, and vascular biology. 1999;19(4):1026–33. [DOI] [PubMed] [Google Scholar]
- 40.Kam RM, Tan AT, Chee TS, Wong J. Massive acute pulmonary embolism in protein S deficiency--a case report. Ann Acad Med Singap. 1994;23(3):396–9. [PubMed] [Google Scholar]
- 41.Kemkes-Matthes B. Acquired protein S deficiency. Clin Investig. 1992;70(6):529–34. [DOI] [PubMed] [Google Scholar]
- 42.Humphries JE. Thrombophilia and complex acquired deficiencies of antithrombin, protein C, and protein S. Semin Hematol. 1995;32(4 Suppl 2):8–16; discussion 7-8. [PubMed] [Google Scholar]
- 43.Ichinose M, Sasagawa N, Chiba T, Toyama K, Kayamori Y, Kang D. Protein C and protein S deficiencies may be related to survival among hemodialysis patients. BMC Nephrol. 2019;20(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pilli VS, Datta A, Dorsey A, Liu B, Majumder R. Modulation of protein S and growth arrest specific 6 protein signaling inhibits pancreatic cancer cell survival and proliferation. Oncol Rep. 2020;44(4):1322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Ommen CH, Fijnvandraat K, Vulsma T, Delemarre-Van De Waal HA, Peters M. Acquired protein S deficiency caused by estrogen treatment of tall stature. J Pediatr. 1999;135(4):477–81. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki A, Sanda N, Miyawaki Y, Fujimori Y, Yamada T, Takagi A, et al. Down-regulation of PROS1 gene expression by 17beta-estradiol via estrogen receptor alpha (ERalpha)-Sp1 interaction recruiting receptor-interacting protein 140 and the corepressor-HDAC3 complex. J Biol Chem. 2010;285(18):13444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carr ME Jr., Steingold KA, Zekert SL. Protein S levels during the normal menstrual cycle and during estrogen therapy for premature ovarian failure. Am J Med Sci. 1993;306(4):212–7. [DOI] [PubMed] [Google Scholar]
- 48.Francis RB Jr., Protein S deficiency in sickle cell anemia. J Lab Clin Med. 1988;111(5):571–6. [PubMed] [Google Scholar]
- 49.el-Hazmi MA, Warsy AS, Bahakim H. Blood proteins C and S in sickle cell disease. Acta Haematol. 1993;90(3):114–9. [DOI] [PubMed] [Google Scholar]
- 50.Balakrishnan Ridin, Hu Shaomin, Gil MR. Increased Incidence of Acquired Protein S Deficiency in Sickle Cell Disease. American Journal of Clinical Pathology. 2018; 150, (suppl_1,):S161. [Google Scholar]
- 51.Pilli VS, Datta A, Afreen S, Catalano D, Szabo G, Majumder R. Hypoxia downregulates protein S expression. Blood. 2018;132(4):452–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu J, Peng G, Ouyang Y. A novel mutation Gly222Arg in PROS1 causing protein S deficiency in a patient with pulmonary embolism. J Clin Lab Anal. 2020;34(4):e23111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Juhl D, Kuta P, Shneyder M, Wunsche F, Nowak-Gottl U. Two Novel Variants in the Protein S Gene PROS1 Are Associated with Protein S Deficiency and Thrombophilia. Acta Haematol. 2021;144(2):222–6. [DOI] [PubMed] [Google Scholar]
- 54.Labrouche-Colomer S, Soukarieh O, Proust C, Mouton C, Huguenin Y, Roux M, et al. A novel rare c.−39C>T mutation in the PROS1 5'UTR causing PS deficiency by creating a new upstream translation initiation codon. Clin Sci (Lond). 2020;134(10):1181–90. [DOI] [PubMed] [Google Scholar]
- 55.Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. EClinicalMedicine. 2020;29:100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lou J, Yin L, Ke X, Zhang L, Xu F, Liu Z. A case-report of two patients with hereditary protein S deficiency treated by rivaroxaban. Blood Coagul Fibrinolysis. 2020;31(6):405–9. [DOI] [PubMed] [Google Scholar]
- 57.Ohashi I, Wada S, Yoshino F, Kuwashiro T, Matsumoto S, Hotta T, et al. Ischemic Stroke with Protein S Deficiency Treated by Apixaban. J Stroke Cerebrovasc Dis. 2020;29(4):104608. [DOI] [PubMed] [Google Scholar]
- 58.Wigle P, Hein B, Bernheisel CR. Anticoagulation: Updated Guidelines for Outpatient Management. Am Fam Physician. 2019;100(7):426–34. [PubMed] [Google Scholar]
- 59.Fearon A, Pearcy P, Venkataraman S, Shah P. Protein S Deficiency and Arterial Thromboembolism: A Case Report and Review of the Literature. J Hematol. 2019;8(1):37–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naghavi S, Pourmohammadi A, Adibi I. Rivaroxaban in Recurrent Ischemic Stroke Due to Protein S Deficiency: A Case Report. Neurol Ther. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chatterjee S, Sengupta T, Majumder S, Majumder R. COVID-19: a probable role of the anticoagulant Protein S in managing COVID-19-associated coagulopathy. Aging (Albany NY). 2020;12(16):15954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stoichitoiu LE, Pinte L, Balea MI, Nedelcu V, Badea C, Baicus C. Anticoagulant protein S in COVID-19: low activity, and associated with outcome. Rom J Intern Med. 2020. This manuscript showed that Protein S is down regulated in COVID-19.
- 63.Zhang Y, Cao W, Jiang W, Xiao M, Li Y, Tang N, et al. Profile of natural anticoagulant, coagulant factor and anti-phospholipid antibody in critically ill COVID-19 patients. J Thromb Thrombolysis. 2020;50(3):580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.MB Martha MS Sim, Hollifield Melissa, Alfar Hammodah, Li Xian, Thornton Alice, Porterfield James Z, et al. Inflammation Drives Coagulopathies in Sars-Cov-2 Patients. Blood. 2020;136(S1):34–5. [Google Scholar]
- 65. Ruzicka JA. Identification of the antithrombotic protein S as a potential target of the SARS-CoV-2 papain-like protease. Thrombosis research. 2020;196:257–9. This study is the first that indicated Protein S as a potential target of the SARS-CoV2 papain like protease. The work suggested that the anticoagulant function of PS is defective in COVID-19
- 66. Dofferhoff ASM, Piscaer I, Schurgers LJ, Visser MPJ, van den Ouweland JMW, de Jong PA, et al. Reduced vitamin K status as a potentially modifiable risk factor of severe COVID-19. Clin Infect Dis. 2020. This study revealed that pneumonia-induced extrahepatic vitamin K depletion leads to accelerated elastic fiber damage and thrombosis in severe COVID-19 because of impaired activation of matrix Gla protein and endothelial Protein S, respectively.