Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly transmissible virus with significant global impact, morbidity, and mortality. The SARS-CoV-2 virus may result in widespread organ manifestations including acute respiratory distress syndrome, acute renal failure, thromboembolism, and myocarditis. Virus-induced endothelial injury may cause endothelial activation, increased permeability, inflammation, and immune response and cytokine storm. Endothelial dysfunction is a systemic disorder that is a precursor of atherosclerotic vascular disease that is associated with cardiovascular risk factors and is highly prevalent in patients with atherosclerotic cardiovascular and peripheral disease. Several studies have associated various viral infections including SARS-CoV-2 infection with inflammation, endothelial dysfunction, and subsequent innate immune response and cytokine storm. Noninvasive monitoring of endothelial function and identification of high-risk patients who may require specific therapies may have the potential to improve morbidity and mortality associated with subsequent inflammation, cytokine storm, and multiorgan involvement.
Abbreviations and Acronyms: ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; IL, interleukin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF, tumor necrosis factor
Article Highlights.
-
•
Severe acute respiratory syndrome coronavirus 2 is a highly transmissible virus that has led to a global pandemic.
-
•
Severe acute respiratory syndrome coronavirus 2 may cause viral infection and injury of endothelial cells leading to endothelial dysfunction.
-
•
Viral-induced endothelial dysfunction may cause inflammation, a heightened immune response, and cytokine storm.
-
•
Endothelial injury and dysfunction may lead to widespread multiorgan manifestations of this disease.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a virulent and highly transmissible virus that has caused the coronavirus disease 2019 (COVID-19) pandemic with millions of infected patients.1, 2, 3 Mechanistically, excessive inflammation, oxidation, and an exaggerated immune response have been thought to be key players in the underlying pathophysiology of COVID-19. Subsequent cytokine storm and acute lung injury leading to acute respiratory distress carries significant morbidity and mortality. The SARS-CoV-2 virus infection can result in respiratory, cardiovascular, gastrointestinal, and central nervous system manifestations that have been well described.4 We postulate the role of virus-induced endothelial injury and subsequent widespread endothelial dysfunction in the pathogenesis of SARS-CoV-2 infection.5, 6, 7, 8 SARS-CoV-2, like other viruses, may directly injure endothelial cells, causing a heightened innate immune response, endothelial activation, loss of barrier function, and microvascular leakage leading to dysfunction in multiple organ beds.7, 8, 9, 10, 11
Endothelial dysfunction is a systemic disorder that is an early phase of atherosclerotic vascular disease and is thought to be due to endothelial cell injury.12 , 13 Endothelial dysfunction has been found to be associated with inflammation, coronary artery disease, and peripheral artery disease, as well as atherosclerotic risk factors including diabetes, hypertension, and hyperlipidemia.12, 13, 14 Physiologically, abnormal endothelial function results in abnormal microvascular function impairing microvessel tone and perfusion regulation and may result in vascular wall ischemia and neovascularization and subsequent recruitment of macrophages, inflammation, and plaque formation. Endothelial dysfunction has been associated with significant adverse events, including both major adverse cardiovascular events and nonvascular disease such as cancer.12 , 15 , 16 Several studies have associated inflammation, including both viral and nonviral inflammatory disorders, with endothelial dysfunction and clinical sequelae spanning multiple organ systems.13 , 17 , 18
Viral Endothelial Dysfunction
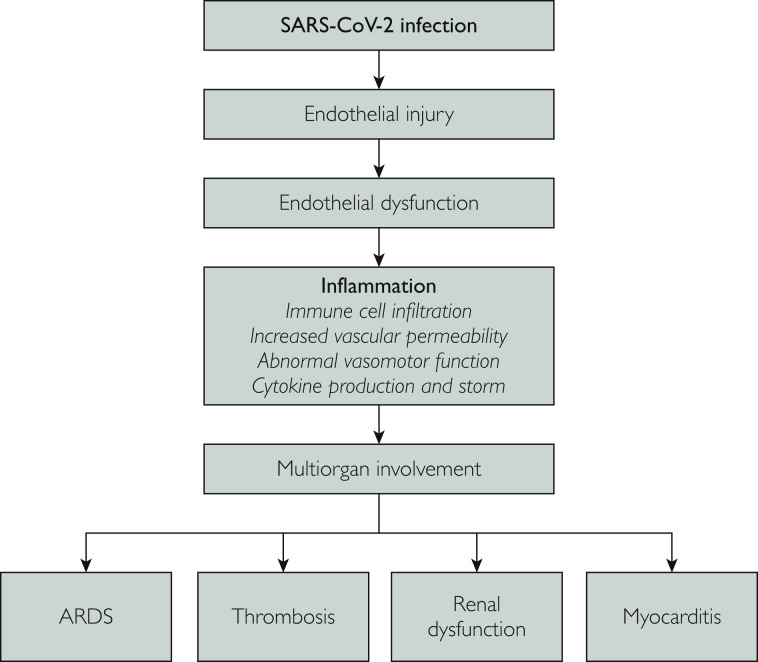
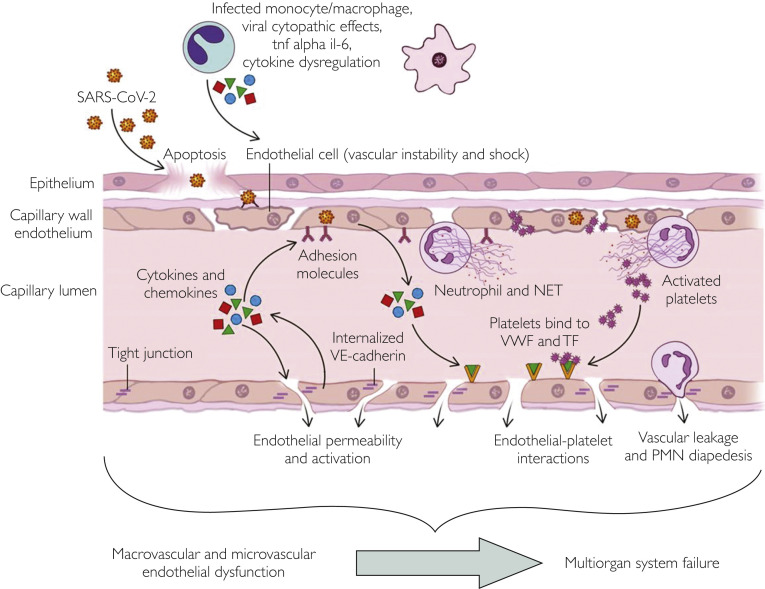
Although endothelial dysfunction is often a chronic process, in acute infection, viral endothelial cell injury can lead to diffuse and systemic endothelial dysfunction and activation of multiple immune-mediated, thrombogenic and inflammatory pathways that can lead to severe multiorgan involvement and subsequent morbidity and mortality (Figure 1 ).19, 20, 21, 22 Figure 2 depicts viral endothelial dysfunction and its potential effect on disrupting the vascular barrier responsible for homeostasis, well described in other viral infections. A similar pathophysiologic mechanism beginning at the endothelium is likely responsible for the widespread systemic effects of SARS-CoV-2 infection. Further understanding of the role of viral endothelial dysfunction may play a role in optimizing our understanding of the pathophysiology of SARS-CoV-2 infection while improving our ability to risk-stratify and predict increased morbidity and mortality in individuals with chronic impaired endothelial function. The endothelium plays a key role in maintaining a vascular barrier and thereby controlling platelet and immune cell interactions, mediating arteriolar capillary tone, and endothelial cell adherence. Viral infection of the endothelium may cause impaired microvascular perfusion, capillary injury, thrombocytopenia, and vascular leakage.23 Endothelial cells regulate vascular permeability and normally permit fluid transfer through a series of checks and balances to prevent lethal capillary leakage.8 Additionally, endothelial cell adherence plays a critical part in maintaining proper endothelial function. Figure 2 depicts the effect of viruses on the endothelium if these pathways are bypassed, with resulting vascular leakage, immune cell activation, and cytokine storm.6 , 7 , 19 , 24
Figure 1.
We postulate viral-induced endothelial dysfunction as the underlying pathophysiologic mechanism leading to the multisystem involvement seen with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The initial viral infection results in endothelial injury and abnormal function of the endothelium that activates inflammatory pathways with cytokine production and cytokine storm, immune cell infiltration, increased vascular permeability, abnormal vasomotor function, and microvascular ischemia, which in turn lead to multiorgan clinical manifestations including development of acute respiratory distress syndrome (ARDS), thrombosis, renal dysfunction, and myocarditis.
Figure 2.
This central illustration schematically illustrates our proposed mechanism of viral endothelial injury. The virus may infect the endothelial cell and monocytes, leading to cytopathic effects, increasing levels of cytotoxins including interleukin 6 (il-6) and tumor necrosis factor α (tnf alpha), and cytokine dysregulation, and cause platelet activation and alterations in platelet binding, vascular permeability, endothelial platelet interactions that may in turn cause widespread endothelial dysfunction, cytokine storm, heightened immune response, and multiorgan system involvement and potentially failure. NET, neutrophil extracellular trap; PMN, polymorphonuclear; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TF, tissue factor; VE, vascular endothelial; VWF, von Willebrand factor.
Several viruses previously have been found to cause endothelial injury, vascular dysfunction, and widespread manifestations across multiple organ systems by disrupting normal endothelial homeostasis as described in Figure 2. These viruses include dengue fever virus, hantavirus, HIV, and influenza virus, which have all been associated with endothelial injury leading to an inflammatory response, cytokine release, and vascular leakage with varying multiorgan involvement.20 , 22 , 25 , 26
The dengue virus is thought to be associated with endothelial injury, which in turn leads to widespread vascular leakage evident at day 3 to 6 after the onset of illness.27 , 28 This timing is similar to the onset of severe illness after SARS-CoV-2 infection. The dengue virus targets dendritic cells and monocytes/macrophages, which after becoming infected induce release of chemokines and cytokines that activate endothelial cells, increase vascular permeability, and impair vascular function and perfusion (Figure 2). In vitro studies have documented direct infection of endothelial cells by the dengue virus leading to production of inflammatory mediators and widespread endothelial dysfunction. Additionally, excessive endothelial activation is also responsible for activation of nuclear factor κB, another important mediator of vascular permeability. This in turn has been reported to lead to reduced multiorgan failure and morbidity and mortality.27, 28, 29, 30
Chronic HIV infection also is widely known to be associated with endothelial dysfunction, but there is evidence that even in the acute setting HIV can result in endothelial injury and dysfunction.22 , 26 , 31 In a study by Bush et al,32 early HIV infection was associated with early endothelial dysfunction that was reversed with the initiation of antiretroviral therapy. Moreover, persistent endothelial dysfunction was associated with delays in initiation of antiretroviral therapy, further supporting a role of endothelial dysfunction in early HIV infection. Mechanistically, HIV type 1 proteins have been found to dysregulate reactive oxygen species–generating enzymatic systems in the vasculature, inducing microvascular impairment. HIV infection has also been associated with increased expression of adhesion molecules and inflammatory cytokines including tumor necrosis factor (TNF) α and interleukin (IL) 6.26 , 33 Increased oxidative stress in the vascular wall may lead to an imbalance in the production of reactive oxygen species and vascular inflammation (Figure 2).22 , 25 , 32 , 34
A less commonly known tick-borne viral disease, severe fever with thrombocytopenia syndrome has also been associated with endothelial injury and dysfunction as evidenced by enhanced levels of several biomarkers of endothelial activation.5 , 35 Moreover, markers of endothelial dysfunction have been significantly associated with severity of disease and mortality.35
Hantavirus, like COVID-19, is also characterized by sudden flulike symptoms, fever, and often a hypotensive phase in which endothelial injury, impaired vasomotor function, and vascular permeability may result in severe elevation in inflammatory markers, cytokine storm, and subsequent low albumin levels, high serum creatinine concentrations, and cardiopulmonary/renal manifestations and shock.20 Zika virus has also previously been associated with microvascular dysfunction and may cause downstream neurologic clinical sequelae by infection of human brain microvascular endothelial cells leading to enhanced release of inflammatory cytokines and increased vascular permeability.36
Clinical manifestations of SARS-CoV-2 infection suggest that it too may act directly on the endothelium, eliciting a diffuse immune and inflammatory response, cytokine storm, impaired vascular function, and multiple organ involvement including, but not limited to, acute respiratory failure, acute renal failure, thrombosis, and myocarditis as described in other viral infections. Further investigation should be directed at noninvasive assessment of endothelial function in patients with COVID-19 infection and the role of endothelial biomarkers in risk stratification and prognostication. Such diagnostic interventions, if performed early, may have the ability to predict impending cytokine storm and clinical decompensation and may elucidate new therapeutic targets to reduce adverse events associated with SARS-CoV-2 infection.
Recent studies in this area have provided further support for this hypothesis that endothelial injury by the SARS-CoV-2 virus may be important in explaining the widespread clinical manifestations. Dupont et al37 have reported that excess soluble fms-like tyrosine kinase 1, which is a soluble inhibitor of the vascular endothelial growth factor pathway and a promoter of endothelial dysfunction, is associated with organ failure in patients with SARS-CoV-2 infection. Green38 has suggested increased endothelial dysfunction and nitric oxide deficiency as a key factor in COVID-19. Complement-mediated extracellular vesicle release has also been suggested as a measure of endothelial dysfunction and may help establish a prognosis for patients with COVID-19.39
Inflammation/Cytokine Storm
Cytokine storm has been found to be a clinical predictor of morbidity and mortality in several viral infections including influenza.40 Cytokine storm is also associated with significant morbidity and mortality in patients with COVID-19 infection, and this result may be driven by initial endothelial injury. Endothelial cells play a key role in orchestrating both immune cell infiltration and cytokine production leading to a cytokine storm.41 Viral infection of monocytes may lead to release of cytokines, which may in turn activate the endothelium. Additionally, viral infection may activate T cells or cause lysis of immune cells, inducing release of interferon γ and TNF-α, which in turn activate macrophages, dendritic cells, and other immune cells/endothelial cells to release inflammatory cytokines. Endothelial cells and macrophages can produce high levels of IL-6, which is a key therapeutic target in the SARS-CoV-2 virus. This in turn activates T cells and other immune cells, leading to a positive feedback system and cytokine storm, which is associated with significant morbidity and mortality (Figure 1).5 , 40 , 42 , 43
When endothelial cells undergo inflammatory activation secondary to viral infection or another cause, there is also increased expression of vascular cell adhesion molecule 1 and intercellular adhesion molecule 1, which promote monocyte adherence. Several proinflammatory cytokines such as IL-1β and TNF-α, acute phase protein, and C-reactive protein produced in response to IL-6 all play a key role in adhesion molecule expression, which also are thought to further contribute to development of a cytokine storm.44
Many patients with COVID-19 may have mild symptoms or even be asymptomatic; however, a significant proportion of patients with COVID-19 may experience severe respiratory illness requiring intensive care admission for management of multiorgan failure, driven by severe inflammation. Risk factors for more severe infection include underlying cardiovascular risk factors that may be associated with underlying preexisting endothelial dysfunction, potentially further increasing risk of severe presentation.45 Clinically, inflammatory markers have been used to herald impending clinical decompensation and have been closely affiliated with impending multiorgan failure. The inflammatory cytokine storm characteristic of COVID-19 infection is a key component of the disease and is being studied as a therapeutic target.45 , 46 Several medical therapies targeting cytokine release are being used, with some data suggesting clinical improvement as inflammatory markers decline. Further studies are necessary to identify the role of routine monitoring of endothelial function and endothelial markers in stable patients to predict impending cytokine storm. At this time, inflammatory markers are used as a harbinger of impending cytokine storm and clinical decompensation. These biomarker elevations often play a role in diagnosing underlying cytokine storm, but by then, it may be too late to reverse the underlying inflammatory pathway. Early identification of patients with impending cytokine storm and clinical decompensation using markers of endothelial injury and function may help guide novel therapies. Moreover, therapies targeted upstream may potentially have increased therapeutic benefit when compared with downstream therapies targeting inflammation.47
Acute Respiratory Distress Syndrome
Clinically, acute respiratory distress syndrome (ARDS) is a key component of SARS-CoV-2 infection and is characterized by shortness of breath, hypoxemia, and diffuse pulmonary edema, with most patients requiring mechanical ventilation.7 , 46 , 48 Acute respiratory distress syndrome has been reported to be caused by pulmonary endothelial injury.49, 50, 51 The pulmonary endothelium is a dynamic layer of cells that is a semipermeable barrier between pulmonary blood flow and the lung interstitium that regulate lung homeostasis, gas exchange, leukocyte adhesion, vasomotor tone, intravascular coagulation, and fluid trafficking and permeability.21 , 52 Pulmonary endothelial injury and dysfunction of this layer of key cells can result in ARDS, characterized by inflammation of the gas exchange surface of the lung and abnormal microvascular function and reactivity.53 This can cause increased endothelial permeability due to the formation of intercellular gaps between endothelial cells causing vascular leakage and pulmonary edema.53
Moreover, the pulmonary endothelium is a key regulator in the development of the cytokine storm in these patients and may similarly play an important role in patients with COVID-19 infection. Although endothelium in the normal lung plays a key role in preventing inflammation, injury can convert the endothelium to an activated proinflammatory phenotype that induces parenchymal inflammation.53 , 54 Infection with a virus can activate cytokines and induce a proinflammatory milieu and loss of normal endothelial homeostasis. Clinically, this results in a diffuse process affecting the entire lung parenchyma as a whole, as seen with SARS-CoV-2 infection and subsequent ARDS, as opposed to a localized insult from a bacterial pneumonia.18 , 21 , 49 , 54
Thrombosis
The endothelium plays an important role in preventing thrombosis by discouraging attachment of clotting proteins, as it expresses antiplatelet and anticoagulant agents that prevent platelet aggregation and fibrin formation. A wealth of data has previously shown that endothelial dysfunction is associated with increased risk of thrombosis.55 , 56 Thrombosis has been an important complication noted in patients admitted with COVID-19 pneumonia, and this too may be secondary to endothelial injury and subsequent endothelial dysfunction.55 , 57 Vascular injury to the endothelium in the setting of infection and significant subsequent inflammation may activate platelets, further damaging the endothelium and resulting in fibrin deposition and thrombus formation, often called thromboinflammation.17 , 55 , 58 Injury to the endothelium results in loss of protective molecules and expression of procoagulant activities, adhesive molecules, and mitogenic factors that can result in smooth muscle cell migration, proliferation, and thrombosis.56
Infection-induced thrombosis has been well described in the literature and is associated with macrovascular and microvascular complications including stroke, myocardial infarction, disseminated intravascular coagulation, and other forms of both arterial and venous thrombosis.58 The chance of development of thrombosis is thought to be directly correlated with the degree of inflammation, and thus cytokine storm and systemic inflammatory response syndrome are associated with increased risk of development of disseminated intravascular coagulation, in which a procoagulant milieu contributes to microthrombus formation in multiple microvascular beds in multiple organs. Infection has also been linked to posthospital thrombotic events including deep venous thrombosis and pulmonary embolism.9 Multiple cytokines including IL-1, IL-6, and IL-8 are all thought to play a key role in COVID-19 infection as well, as they recruit interferons and TNF, induce platelet activation, and create a proinflammatory and prothrombotic milieu.
Viral infection specifically has been robustly studied and found to induce inflammation and a procoagulant milieu that may result in stroke, myocardial infarction, and other forms of arterial and/or venous thrombosis and may explain the thrombotic complications associated with SARS-CoV-2 infection.55 , 58 , 59 The Ebola virus has been found to activate tissue factor in mononuclear phagocytes, leading to an up-regulation of the coagulation system with evidence of fibrin deposits in multiple organs and proximity of the infected cells and viral proteins to activated macrophages.60 In the mouse model of ARDS, the influenza virus was also found to be associated with increased platelet aggregation, abnormal vasomotor function, microvascular pulmonary thrombosis, endothelial damage, cytokine storm, and microvessel occlusion with clinical thrombosis noted.60 , 61 Trypanosoma cruzi–infected mice and dogs have been found to have occlusive thrombi and fibrin deposits in small epicardial and intramyocardial vessels as well as intramural thrombi.
SARS-CoV-2 infection has been associated with increased thrombosis, which has led to significant morbidity and mortality.62 , 63 Infection-induced thrombosis may be associated with preexisting risk factors including smoking, hyperlipidemia, and hypertension, but whether there is direct causation is unknown.55 In certain individuals, however, it appears that infection is especially associated with thrombosis, and further identification of these risk factors may help in risk stratification and prognostication to help answer important clinical questions involving the use of anticoagulation in patients infected with COVID-19. Moreover, the degree to which thrombosis is associated with endothelial injury and inflammation is important to further elucidate the role of clinical monitoring of endothelial injury in predicting future thrombosis. This advance may in turn help identify therapeutic targets and enhance understanding of indications for anticoagulation and/or other agents to reduce the risk of thrombosis.57
Renal Dysfunction
Acute renal failure is highly prevalent in patients with COVID-19 infection and is another important major cause of morbidity and mortality.50 Especially in patients with critical illness secondary to COVID-19 infection, it has been reported that many patients require renal replacement therapy.50 , 64 , 65
The kidney has a rich and diversified population of endothelial cells because the renal endothelium is an important part of transportation across the nephron, and the endothelium must survive in extremes of oxygenation and osmolality.65 The renal vascular endothelium may be targeted in several inflammatory processes including nephritis vasculitis and ischemic acute renal failure. Viral-induced endothelial dysfunction in the kidney may result in vasoconstriction, endothelial leakage, and increased vascular permeability, tissue edema, leukocyte adhesion to the endothelial cells, and microthrombosis, leading to increased renal vascular resistance that in turn alters global and regional blood flow.56 , 66 , 67 Thus, during sepsis with cytokine storm, an increase in renal vascular resistance may alter global and regional blood flow, contributing to changes in renal function.40 , 66 , 68 Therefore, endothelial dysfunction secondary to viral infection with COVID-19 may also explain the high prevalence of acute renal failure and the need for renal replacement therapy noted in many patients with COVID-19 infection, especially in the setting of cytokine storm.9 Such a phenomenon has been previously described in patients presenting with hantavirus infection, which leads to disrupted endothelial cells and loss of barrier function and vascular leakage causing hemorrhagic fever with renal syndrome characterized by acute renal failure with massive proteinuria.20
Myocarditis and Microvascular Injury
Myocarditis is yet another well-described manifestation of COVID-19 infection and is also likely driven by cardiomyocyte viral infection, endothelial injury, and inflammation.69 Myocardial thrombosis presenting as myocardial infarction has also been described. Myocarditis and myocardial thrombosis may be 2 separate entities or may be pathophysiologically intertwined.42
With viral infection, endothelial cells become injured and express adhesion molecules, antigen-presenting major histocompatibility complex molecules, and mount a cellular immune response leading to cardiac microvascular damage and dysfunction, diffuse myocardial inflammation, thrombus formation, and sometimes myocardial infarction.42 In autopsy reports, several viruses including dengue virus, hantavirus, herpes simplex virus, parvovirus B19, cytomegalovirus, coxsackievirus, and T cruzi have been found to infect the microvascular endothelial cells of the heart. Recently, it has also been seen with SARS-CoV-2 infection in several patients in whom histology revealed viral involvement of the endothelium.70 Thus, viral infection may lead to endothelial activation, damage, injury, and increased permeability, causing downstream macrovascular and microvascular manifestations.42 The increase in proinflammatory cytokines leads to an increase in endothelial adhesion molecules as well, which through a positive feedback mechanism may further exacerbate the infection. Ultimately, the severity of the autoimmune response to these cellular mechanisms may determine long-term prognosis. The inflammatory response secondary to SARS-CoV-2 viral infection may also cause increased vascular permeability due to enlarged intercellular gap junctions and extensive recruitment and extravasation of immune cells into the myocardium leading to myocardial edema.
In the acute phase of viral infection, viral replication occurs followed by a subacute phase during which an immune response results in a significant increase in cytokines and immune cell infiltration.42 As the chronic phase occurs, the virus is often cleared, myocardial inflammation resolves, and the myocardium remodels. Thus, myocardial damage secondary to viral myocarditis may be secondary to the virus itself or due to the activated immune system.42
Potential Clinical Implications
Our understanding of SARS-CoV-2 infection continues to evolve since the beginning of the pandemic. However, morbidity and mortality remain high with high rates of infection, and we continue to strive to understand risk factors that may predict increased risk. Additional risk factors for endothelial dysfunction including a history of cardiovascular disease, race, family history, and genetics may play a role in endothelial dysfunction. Like other viruses shown to involve the endothelium, there is evidence that SARS-CoV-2 may infect the endothelium and lead to impaired endothelial function, disruption of normal homeostasis of the endothelial barrier, and widespread endothelial dysfunction and systemic disease.11 , 37 , 39 Although currently diagnosis, risk stratification, and treatment focus on the downstream systemic clinical manifestations, assessment of endothelial function and identification of individuals with endothelial dysfunction through noninvasive endothelial function testing and additional biomarker evaluation may help identify high-risk individuals who may benefit from different prevention and treatment strategies.
Conclusion
The SARS-CoV-2 virus results in a severe infection with multiple organ system manifestations, leading to severe morbidity and mortality. Acute viral-induced endothelial dysfunction may be an underlying, unifying mechanism responsible for the widespread systemic manifestations seen with SARS-CoV-2 infection. Further investigation is necessary to better understand the roles of endothelial dysfunction, noninvasive assessment of endothelial function, and biomarkers of endothelial injury that may contribute to risk stratification and identifying patients prone to clinical decompensation. Although our current clinical approach is to target downstream therapeutic targets and temporize with renal replacement therapy, ventilator-assisted respiratory support, and medications, it is important to identify upstream pathways as therapeutic targets to be able to predict and manage harbingers of morbidity and mortality. Although few robust therapies for endothelial dysfunction exist, as a field we must work to devise treatments that can not only treat and prevent severe complications of viral infections but also manage the multitude of other macrovascular and microvascular diseases that are associated with an initial endothelial injury.
Footnotes
Potential Competing Interests: Dr Lilach Lerman has been a consultant for AstraZeneca and Weijian Technologies, Inc, and has received grants/has grants pending from Novo Nordisk. Dr Amir Lerman has been a consultant for Itamar Medical Ltd. The other authors report no competing interests.
References
- 1.Guan W., Ni Z., Hu Y., et al. China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020;55(5):105955. doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X. Epidemiology of Covid-19. N Engl J Med. 2020;382(19):1869. doi: 10.1056/NEJMc2005157. [DOI] [PubMed] [Google Scholar]
- 4.Patterson C., Sapontis J., Nicholson W.J., et al. OPEN CTO Study Group Impact of body mass index on outcome and health status after chronic total occlusion percutaneous coronary intervention: insights from the OPEN-CTO study. Catheter Cardiovasc Interv. 2021;97(6):1186–1193. doi: 10.1002/ccd.28928. [DOI] [PubMed] [Google Scholar]
- 5.Mackow E.R., Gorbunova E.E., Gavrilovskaya I.N. Endothelial cell dysfunction in viral hemorrhage and edema. Front Microbiol. 2015;5:733. doi: 10.3389/fmicb.2014.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhargavan B., Kanmogne G.D. Differential mechanisms of inflammation and endothelial dysfunction by HIV-1 subtype-B and recombinant CRF02_AG Tat proteins on human brain microvascular endothelial cells: implications for viral neuropathogenesis. Mol Neurobiol. 2018;55(2):1352–1363. doi: 10.1007/s12035-017-0382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aburawi E., Liuba P., Pesonen E., Ylä-Herttuala S., Sjöblad S. Acute respiratory viral infections aggravate arterial endothelial dysfunction in children with type 1 diabetes. Diabetes Care. 2004;27(11):2733–2735. doi: 10.2337/diacare.27.11.2733. [DOI] [PubMed] [Google Scholar]
- 8.De Lorenzo A., Escobar S., Tibiriçá E. Systemic endothelial dysfunction: a common pathway for COVID-19, cardiovascular and metabolic diseases. Nutr Metab Cardiovasc Dis. 2020;30(8):1401–1402. doi: 10.1016/j.numecd.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escher R., Breakey N., Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montone R.A., Iannaccone G., Meucci M.C., Gurgoglione F., Niccoli G. Myocardial and microvascular injury due to coronavirus disease 2019. Eur Cardiol. 2020;15:e52. doi: 10.15420/ecr.2020.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavriilaki E., Anyfanti P., Gavriilaki M., Lazaridis A., Douma S., Gkaliagkousi E. Endothelial dysfunction in COVID-19: lessons learned from coronaviruses. Curr Hypertens Rep. 2020;22(9):63. doi: 10.1007/s11906-020-01078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Widmer R.J., Lerman A. Endothelial dysfunction and cardiovascular disease. Glob Cardiol Sci Pract. 2014;2014(3):291–308. doi: 10.5339/gcsp.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi B.-J., Matsuo Y., Aoki T., et al. Coronary endothelial dysfunction is associated with inflammation and vasa vasorum proliferation in patients with early atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34(11):2473–2477. doi: 10.1161/ATVBAHA.114.304445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taher R., Sara J.D., Prasad M., et al. Elevated serum uric acid is associated with peripheral endothelial dysfunction in women. Atherosclerosis. 2019;290:37–43. doi: 10.1016/j.atherosclerosis.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Gasiorowska A., Talar-Wojnarowska R., Kaczka A., Borkowska A., Czupryniak L., Małecka-Panas E. Subclinical inflammation and endothelial dysfunction in patients with chronic pancreatitis and newly diagnosed pancreatic cancer. Dig Dis Sci. 2016;61(4):1121–1129. doi: 10.1007/s10620-015-3972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nastri C.O., Martins W.P., Reis F.J.C., Ferriani R.A. Breast cancer and endothelial dysfunction [in Portuguese] Rev Assoc Med Bras (1992) 2008;54(5):467–470. doi: 10.1590/s0104-42302008000500023. [DOI] [PubMed] [Google Scholar]
- 17.Apetrei E., Ciobanu-Jurcut R., Rugină M., Gavrilă A., Uscătescu V. C-reactive protein, prothrombotic imbalance and endothelial dysfunction in acute coronary syndromes without ST elevation. Rom J Intern Med. 2004;42(1):95–102. [PubMed] [Google Scholar]
- 18.Huertas A., Montani D., Savale L., et al. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19) [editorial]? Eur Respir J. 2020;56(1):2001634. doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong S.M., Darwish I., Lee W.L. Endothelial activation and dysfunction in the pathogenesis of influenza A virus infection. Virulence. 2013;4(6):537–542. doi: 10.4161/viru.25779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krautkrämer E., Zeier M., Plyusnin A. Hantavirus infection: an emerging infectious disease causing acute renal failure. Kidney Int. 2013;83(1):23–27. doi: 10.1038/ki.2012.360. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman G.A., Albertine K.H., Carveth H.J., et al. Endothelial activation in ARDS. Chest. 1999;116(1, suppl):18S–24S. doi: 10.1378/chest.116.suppl_1.18s. [DOI] [PubMed] [Google Scholar]
- 22.Mazzuca P., Caruso A., Caccuri F. HIV-1 infection, microenvironment and endothelial cell dysfunction. New Microbiol. 2016;39(3):163–173. [PubMed] [Google Scholar]
- 23.Amraei R., Rahimi N. COVID-19, renin-angiotensin system and endothelial dysfunction. Cells. 2020;9(7):1652. doi: 10.3390/cells9071652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodford J., Yeo T.W., Piera K.A., et al. Early endothelial activation precedes glycocalyx degradation and microvascular dysfunction in experimentally induced Plasmodium falciparum and Plasmodium vivax infection. Infect Immun. 2020;88(5) doi: 10.1128/IAI.00895-19. e00895-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricardo-Dukelow M., Kadiu I., Rozek W., et al. HIV-1 infected monocyte-derived macrophages affect the human brain microvascular endothelial cell proteome: new insights into blood-brain barrier dysfunction for HIV-1-associated dementia. J Neuroimmunol. 2007;185(1-2):37–46. doi: 10.1016/j.jneuroim.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cotter B.R. Endothelial dysfunction in HIV infection. Curr HIV/AIDS Rep. 2006;3(3):126–131. doi: 10.1007/BF02696656. [DOI] [PubMed] [Google Scholar]
- 27.Vitoria W.O., Thomé L.S., Kanashiro-Galo L., et al. Upregulation of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in renal tissue in severe dengue in humans: effects on endothelial activation/dysfunction. Rev Soc Bras Med Trop. 2019;52:e20180353. doi: 10.1590/0037-8682-0353-2018. [DOI] [PubMed] [Google Scholar]
- 28.Vervaeke P., Vermeire K., Liekens S. Endothelial dysfunction in dengue virus pathology. Rev Med Virol. 2015;25(1):50–67. doi: 10.1002/rmv.1818. [DOI] [PubMed] [Google Scholar]
- 29.Beatty P.R., Puerta-Guardo H., Killingbeck S.S., Glasner D.R., Hopkins K., Harris E. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med. 2015;7(304):304ra141. doi: 10.1126/scitranslmed.aaa3787. [DOI] [PubMed] [Google Scholar]
- 30.Malavige G.N., Ogg G.S. Pathogenesis of vascular leak in dengue virus infection. Immunology. 2017;151(3):261–269. doi: 10.1111/imm.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosepele M., Mohammed T., Mupfumi L., et al. HIV disease is associated with increased biomarkers of endothelial dysfunction despite viral suppression on long-term antiretroviral therapy in Botswana. Cardiovasc J Afr. 2018;29(3):155–161. doi: 10.5830/CVJA-2018-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bush K.N.V., Teel J.L., Watts J.A., et al. Association of endothelial dysfunction and antiretroviral therapy in early HIV infection. JAMA Netw Open. 2019;2(10):e1913615. doi: 10.1001/jamanetworkopen.2019.13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X., Chai H., Yao Q., Chen C. Molecular mechanisms of HIV protease inhibitor-induced endothelial dysfunction. J Acquir Immune Defic Syndr. 2007;44(5):493–499. doi: 10.1097/QAI.0b013e3180322542. [DOI] [PubMed] [Google Scholar]
- 34.Paladugu R., Fu W., Conklin B.S., et al. HIV Tat protein causes endothelial dysfunction in porcine coronary arteries. J Vasc Surg. 2003;38(3):549–555. doi: 10.1016/s0741-5214(03)00770-5. [DOI] [PubMed] [Google Scholar]
- 35.Li X.-K., Yang Z.-D., Du J., et al. Endothelial activation and dysfunction in severe fever with thrombocytopenia syndrome. PLoS Negl Trop Dis. 2017;11(8):e0005746. doi: 10.1371/journal.pntd.0005746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ismail A.A., Mahboob T., Samudi Raju C., Sekaran S.D. Zika virus modulates blood-brain barrier of brain microvascular endothelial cells. Trop Biomed. 2019;36(4):888–897. [PubMed] [Google Scholar]
- 37.Dupont V., Kanagaratnam L., Goury A., et al. Excess soluble fms-like tyrosine kinase 1 correlates with endothelial dysfunction and organ failure in critically ill coronavirus disease 2019 patients. Clin Infect Dis. 2021;72(10):1834–1837. doi: 10.1093/cid/ciaa1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green S.J. Covid-19 accelerates endothelial dysfunction and nitric oxide deficiency [letter] Microbes Infect. 2020;22(4-5):149–150. doi: 10.1016/j.micinf.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inal J. Complement-mediated extracellular vesicle release as a measure of endothelial dysfunction and prognostic marker for COVID-19 in peripheral blood [letter] Clin Hemorheol Microcirc. 2020;75(4):383–386. doi: 10.3233/CH-200958. [DOI] [PubMed] [Google Scholar]
- 40.Oldstone M.B.A., Rosen H. Cytokine storm plays a direct role in the morbidity and mortality from influenza virus infection and is chemically treatable with a single sphingosine-1-phosphate agonist molecule. Curr Top Microbiol Immunol. 2014;378:129–147. doi: 10.1007/978-3-319-05879-5_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teijaro J.R., Walsh K.B., Cahalan S., et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146(6):980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woudstra L., Juffermans L.J.M., van Rossum A.C., Niessen H.W.M., Krijnen P.A.J. Infectious myocarditis: the role of the cardiac vasculature. Heart Fail Rev. 2018;23(4):583–595. doi: 10.1007/s10741-018-9688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prasad M., Matteson E.L., Herrmann J., et al. Uric acid is associated with inflammation, coronary microvascular dysfunction, and adverse outcomes in postmenopausal women. Hypertension. 2017;69(2):236–242. doi: 10.1161/HYPERTENSIONAHA.116.08436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol. 2008;103(5):398–406. doi: 10.1007/s00395-008-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death [editorial] BMJ. 2020;368:m1198. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 46.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020;395(10223):496] Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell B., Moss C., Rigg A., Van Hemelrijck M. COVID-19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting? Ecancermedicalscience. 2020;14:1023. doi: 10.3332/ecancer.2020.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng Y., Liu W., Liu K., et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J (Engl) 2020;133(11):1261–1267. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Millar F.R., Summers C., Griffiths M.J., Toshner M.R., Proudfoot A.G. The pulmonary endothelium in acute respiratory distress syndrome: insights and therapeutic opportunities. Thorax. 2016;71(5):462–473. doi: 10.1136/thoraxjnl-2015-207461. [DOI] [PubMed] [Google Scholar]
- 50.Xu X., Yu C., Qu J., et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47(5):1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poor H.D., Ventetuolo C.E., Tolbert T., et al. COVID-19 critical illness pathophysiology driven by diffuse pulmonary thrombi and pulmonary endothelial dysfunction responsive to thrombolysis. Clin Transl Med. 2020;10(2):e44. doi: 10.1002/ctm2.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murphy L.S., Wickersham N., McNeil J.B., et al. Endothelial glycocalyx degradation is more severe in patients with non-pulmonary sepsis compared to pulmonary sepsis and associates with risk of ARDS and other organ dysfunction. Ann Intensive Care. 2017;7(1):102. doi: 10.1186/s13613-017-0325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sukriti S., Tauseef M., Yazbeck P., Mehta D. Mechanisms regulating endothelial permeability. Pulm Circ. 2014;4(4):535–551. doi: 10.1086/677356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gando S., Kameue T., Matsuda N., Sawamura A., Hayakawa M., Kato H. Systemic inflammation and disseminated intravascular coagulation in early stage of ALI and ARDS: role of neutrophil and endothelial activation. Inflammation. 2004;28(4):237–244. doi: 10.1023/b:ifla.0000049049.81688.fe. [DOI] [PubMed] [Google Scholar]
- 55.Loscalzo J. Oxidative stress in endothelial cell dysfunction and thrombosis. Pathophysiol Haemost Thromb. 2002;32(5-6):359–360. doi: 10.1159/000073600. [DOI] [PubMed] [Google Scholar]
- 56.Kakar P., Lip G.Y.H. Hypertension: endothelial dysfunction, the prothrombotic state and antithrombotic therapy. Expert Rev Cardiovasc Ther. 2007;5(3):441–450. doi: 10.1586/14779072.5.3.441. [DOI] [PubMed] [Google Scholar]
- 57.Bikdeli B., Madhavan M.V., Jimenez D., et al. Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up; JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keller T.T., Mairuhu A.T.A., de Kruif M.D., et al. Infections and endothelial cells. Cardiovasc Res. 2003;60(1):40–48. doi: 10.1016/s0008-6363(03)00354-7. [DOI] [PubMed] [Google Scholar]
- 59.Wang X., Sahu K.K., Cerny J. Coagulopathy, endothelial dysfunction, thrombotic microangiopathy and complement activation: potential role of complement system inhibition in COVID-19. J Thromb Thrombolysis. 2021;51(3):657–662. doi: 10.1007/s11239-020-02297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ashar H.K., Mueller N.C., Rudd J.M., et al. The role of extracellular histones in influenza virus pathogenesis. Am J Pathol. 2018;188(1):135–148. doi: 10.1016/j.ajpath.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beristain-Covarrubias N., Perez-Toledo M., Thomas M.R., Henderson I.R., Watson S.P., Cunningham A.F. Understanding infection-induced thrombosis: lessons learned from animal models. Front Immunol. 2019;10:2569. doi: 10.3389/fimmu.2019.02569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fournier M., Faille D., Dossier A., et al. Arterial thrombotic events in adult inpatients with COVID-19. Mayo Clin Proc. 2021;96(2):295–303. doi: 10.1016/j.mayocp.2020.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lippi G., Sanchis-Gomar F., Favaloro E.J., Lavie C.J., Henry B.M. Coronavirus disease 2019–associated coagulopathy. Mayo Clin Proc. 2021;96(1):203–217. doi: 10.1016/j.mayocp.2020.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watnick S., McNamara E. On the frontline of the COVID-19 outbreak: keeping patients on long-term dialysis safe. Clin J Am Soc Nephrol. 2020;15(5):710–713. doi: 10.2215/CJN.03540320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malyszko J. Mechanism of endothelial dysfunction in chronic kidney disease. Clin Chim Acta. 2010;411(19-20):1412–1420. doi: 10.1016/j.cca.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 66.Prasad N., Patel M.R. Infection-induced kidney diseases. Front Med (Lausanne) 2018;5:327. doi: 10.3389/fmed.2018.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kilickap M., Goksuluk H., Candemir B., et al. Evaluation of acute infection-induced endothelial dysfunction and its potential mediators. Acta Cardiol. 2011;66(5):581–587. doi: 10.1080/ac.66.5.2131082. [DOI] [PubMed] [Google Scholar]
- 68.Patschan S.A., Patschan D., Temme J., et al. Endothelial progenitor cells (EPC) in sepsis with acute renal dysfunction (ARD) Crit Care. 2011;15(2):R94. doi: 10.1186/cc10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fried J.A., Ramasubbu K., Bhatt R., et al. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141(23):1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]