Abstract
In Belgium, high-risk contacts of an infected person were offered PCR-testing irrespective of their vaccination status. We estimated vaccine effectiveness (VE) against infection and onwards transmission, controlling for previous infections, household-exposure and temporal trends. We included 301,741 tests from 25 January to 24 June 2021. Full-schedule vaccination was associated with significant protection against infection. In addition, mRNA-vaccines reduced onward transmission: VE-estimates increased to >90% when index and contact were fully vaccinated. The small number of viral-vector vaccines included limited interpretability.
1. Introduction
COVID-19 vaccines have shown efficacy against symptomatic disease in clinical trials [1], [2], [3] and real-life vaccine effectiveness (VE) has subsequently been confirmed in several large observational studies [4], [5], [6]. The VE on onwards transmission of COVID-19 is less well documented. A contact tracing system to limit the spread of COVID-19 in Belgium is in place since May 2020. A positive laboratory test will trigger a process in which the person with the positive test, referred to as index case, is asked to provide details on whereabouts and contacts. High-risk contacts (HRC) will subsequently be contacted and tested. National data from testing, contact tracing and vaccination are centralized in one data-warehouse. The data are pseudonomized and can be linked through a recoded national identification number of social security (NISS). From this data, we estimated VE against infection after high-risk contact and onwards transmission.
2. Data & methods
We included data collected through contact tracing on events from 25/01/2021 to 24/06/2021. During this period, ‘contact tracing’-procedures remained unchanged and independent of a person’s vaccination status. Events were defined as high-risk contacts (contacts for > 15′ at < 1,5m without face masks, or direct physical contact [7]), between an infected person and a susceptible person. Any person without a positive test (PCR or Antigen) in the past 90 days was considered susceptible. Testing of HRC via PCR was carried out as soon as possible. An additional PCR-test was collected seven days after exposure if the first test was negative, sooner if symptoms appeared.
Vaccination status was defined by the number of doses and type of vaccine received until 14 days before the date of last high-risk contact. We excluded events if a vaccine was received by either index case or HRC during this 14-day period. When this date was missing, the date of contact between HRC and the ‘contact tracing’-center was used. For descriptive purposes, we use the terms ‘partially’ (persons having received a single dose of a two-dose vaccine schedule) and ‘fully’ vaccinated.
There were 393,469 events during the study period. Events were excluded if missing NISS (N = 48,860), missing test result (N = 34,147) or vaccination in the 14-day period preceding the event (N = 10,089). We included 301,741 events associated with 131,283 index cases in the analysis. For 274,942 events (91%), both the index and HRC were unvaccinated. The other events included: 2.7% partially and 0.8% fully vaccinated index cases and 4% partially and 2.6% fully vaccinated HRC (Table 1 ). Based on positive PCR/antigen results > 90 days before the current event, 290 index cases and 697 HRC were previously infected. The average age of the study population was 33 years (standard deviation 19.4) and women represented 51.5%.
Table 1.
Vaccination status of the study population (HRC = high-risk contact).
| Total | ChadOx1 (AstraZenaca) |
BNT162b2 (Pfizer) |
mRNA-1273 (Moderna) |
Ad26.CoV2.S (J&J) |
|
|---|---|---|---|---|---|
| Index Cases | |||||
| Not vaccinated | 126,780 | – | – | – | – |
| Partially vaccinated | 3513 | 2,121 | 1264 | 106 | – |
| Fully vaccinated | 990 | 12 | 908 | 69 | 22 |
| HRC | |||||
| Not vaccinated | 281,592 | – | – | – | – |
| Partially vaccinated | 12,162 | 7,137 | 4444 | 507 | – |
| Fully Vaccinated | 7987 | 55 | 7275 | 652 | 74 |
We fitted the final test result associated with an event using a Bayesian logistic regression including the vaccination status of index and HRC and previous COVID-19 infections. We further included household-exposure and a factor representing the week on which the sample was taken.
followed a Bernoulli distribution. Prior distributions given to were normal with mean zero and standard deviation 100. Analysis was performed in R (4.0.5, using the R-Nimble package [8]).
VE was estimated from the probability of infection given vaccination status (, ), compared to a baseline scenario ().
VE was jointly estimated with the secondary attack-rates, allowing for a straightforward estimation of the 95% credibility intervals (95% CI).
3. Vaccine effectiveness
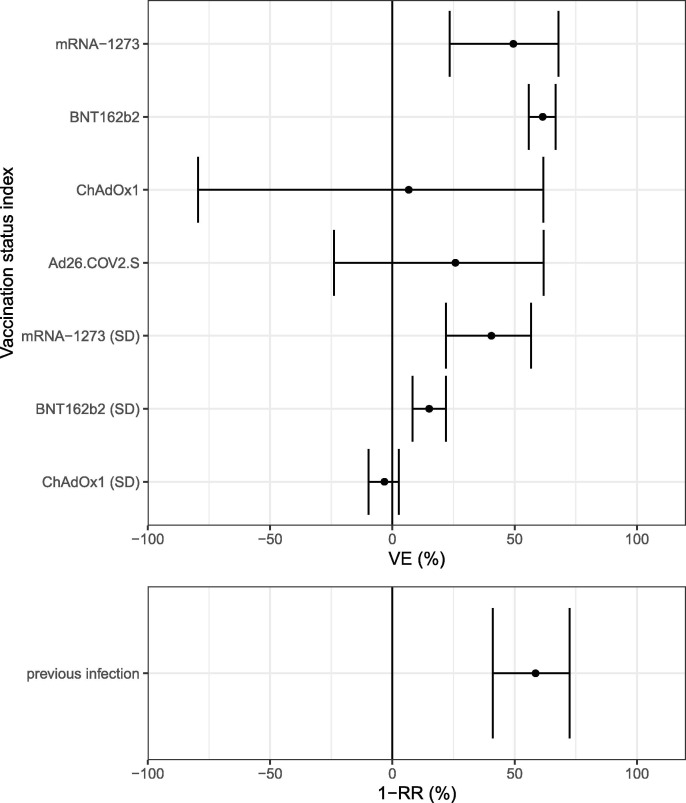
VE against infection for a fully vaccinated HRC and an unvaccinated index was estimated at 74% (95% CI 72–76) and 85% (95% CI 80–90) for the mRNA-vaccines BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) and 53% (95% CI 12–84) and 61% (95% CI 29–84) for ‘viral-vector’-vaccines ChAdOx1 (AstraZeneca) and Ad26.COV2.S (Janssen). There was no significant difference between protection by full-dose vaccination and previous infection. VE increased significantly between doses for BNT162b2 and mRNA-1273 (Fig. 1 ).
Fig. 1.
Vaccine Effectiveness-estimates (VE) and 95% credibility intervals against infection by vaccination status (upper) or by previous infection (lower) of the contact after high-risk contact (HRC) with an unvaccinated index (SD = single dose, RR = relative risk).
The VE against onwards transmission was estimated at 62% (95% CI 57–67) for BNT162b2 and 52% (95% CI 33–69) for mRNA-1273 for full vaccination. No significant effect against onward transmission was found for the ‘viral-vector’-vaccines, but credibility intervals were large. Vaccination with mRNA-vaccines had a similar effect as previous infection, but two doses were required to achieve this effect (Fig. 2 ).
Fig. 2.
Vaccine Effectiveness-estimates (VE) and 95% credibility intervals against onward transmission by vaccination status (upper) or by previous infection (lower) of the index after high-risk contact with an unvaccinated contact (SD = single dose, RR= relative risk).
We estimated VE for the combinations of the vaccination status of the index and HRC. The combined effects against infection and onward transmission led to high protection (Fig. 3 ).
Fig. 3.
Vaccine Effectiveness-estimates (VE) and 95% credibility intervals by vaccination status of index and high-risk contact (HRC). The background color represents the VE-estimate. Combinations in which both the index case and high-risk contact were partially vaccinated are in the grey box. Combinations in which both the index case and high-risk contact were fully vaccinated are in the black box (SD = single dose).
4. Discussion
Our study investigated vaccine-induced protection against infection and onward transmission after high-risk contact under extensive and systematic testing of symptomatic and asymptomatic contacts. The study period coincided with the early roll-out of Belgium’s vaccination campaign and all EMA-authorized vaccines were included. Belgium’s vaccination strategy prioritized nursing home residents and healthcare workers after which an age- and risk-based approach was taken to vaccinate the general population. There was a three- to five-week interval between doses for the mRNA-vaccines and a 12-week interval for ChAdOx1. For further details on the strategy we refer to the Scientific Institute of Public Health FAQ [9]. At the end of the study period, 33% of the adult Belgian population was fully vaccinated, 60% had received at least one dose. Strains sequenced during the study period were increasingly identified as B.1.1.7; from 33% during the first weeks to 80% by the end of the study period. The presence of other variants of concern was limited [10].
The period during which data was collected as well as the testing strategy under which it was collected, have to be kept in mind when comparing VE-estimates. Our data was collected under extensive testing which likely led to a high proportion of asymptomatic infections. HRC were asked about symptoms when contacted for contact tracing, but we could not distinguish between symptomatic and asymptomatic infections as symptoms might have appeared after the call. Studies that made the distinction, have reported lower VE against asymptomatic infection [11]. For example; in Israel for BNT162b2, VE was estimated at 97% (95% CI 96.7–97.2) for symptomatic infection and 91.5% (95% CI 90.7–92.2) for asymptomatic infection [5]. Our study population was mainly of working age, partly because schools and nursing homes had separate contact tracing. Björk et al. [12] reported effectiveness for BNT162b2 against infection in the working population and included high-risk, household contacts. Their estimates were in line with our study and significantly increased between doses from 42% (95% CI 14–63) to 86% (95% CI 72–94).
Observational studies on onward transmission were scarce at the time of writing. Shah et al. [13], estimated the effect on household transmission for healthcare workers and reported a hazard ratio of 0.7 (95% CI 0.63–0.78) for ChAdOx1 and BNT162b2. Harris et al. [14] investigated onwards transmission within households after vaccination of the index case. Their unadjusted OR for risk of infection in unvaccinated household members were: 0.55 (95% CI 0.46–0.67) for ChAdOx1 and 0.57 (95% CI 0.49–0.65) for BNT162b2. Since 93% of their vaccinated study population were partially vaccinated, their estimates are best compared to our OR-estimates for single doses: 1 (95% CI 0.95–1.10, ChAdOx1) and 0.93 (95% CI 0.83–1.02, BNT162b2) (see supplementary material).
Despite a considerable number of events included, only a limited number of vaccinated persons were included. This has several reasons. (1) Our study collected data when coverage was low. (2) Despite contact tracing being automatically triggered by a positive test, not all Belgian cases were included. E.g. collectivities such as nursing homes were prioritized in the vaccination strategy, but performed contact tracing through a distinct system. (3) We excluded events where the index or HRC were vaccinated within the ‘14-day’-period before the event. (4) Index and HRC often had similar ages (results not shown) which in combination with an age-based vaccination strategy and (5) vaccine-induced protection all led to the inclusion of mainly unvaccinated persons.
For the viral-vector vaccines the numbers were especially small which is reflected in the large 95% CIs from which no real conclusions can be drawn. Comparison between vaccine types is further limited by risk- and age-specific allocation, e.g. the first group to be vaccinated with ChAdOx1 were healthcare workers, which is not considered in this analysis.
We used a 14-day period before the last contact to define vaccination status. This is a common approach to evaluate protection given the 14-day period necessary for the protection to become effective [2]. We excluded events with a vaccination in this 14-day period to have the vaccination status correspond to the protection offered by the vaccine. Infection might however have occurred before the last contact in which case we underestimated VE. VE-estimates might further be biased because of missed previous infections. During the first wave, March-June 2020, testing was limited. In addition, breakthrough infections might be detected less than infections in unvaccinated persons [15], [16]. This would bias the analysis as it means we can no longer correct for previous infections. Persons included in this analysis have however not been vaccinated long which limits the likelihood of undiagnosed breakthrough infections. Finally, while the index case is a likely source of infection for its high-risk contact, other sources are not excluded. We could not exclude that an undiagnosed infection in a high-risk contact was the source of infection for the index case. For this analysis we assumed that the source of infection was not a confounder.
In conclusion, we found significant protection against infection after a high-risk contact by a full schedule of any of the vaccines currently administered in Belgium. Protection was comparable to protection obtained after infection. Significant effects on onward transmission in case of breakthrough infections were shown for mRNA-vaccines, leading to high protection when both the index and HRC were fully vaccinated. More data needs to be collected on viral-vector vaccines to estimate their effect on onward transmission.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We wish to dedicate this study to all persons who played a key role by providing and processing the data technically: especially physicians, clinical microbiology laboratories, and the colleagues of heealthdata.be. Without their dedication and efforts this study could not have been conducted.
Funding
This study was supported by the Belgian Federal Authorities through funding for the LINK-VACC project on vaccine surveillance.
Privacy and ethical committee
Data linkage and collection within the data-warehouse has been approved by the information security committee.
Contributions
Study design: TB, LC, HVO, CWT and DVC; Statistical analysis and figures: TB and KP; Data interpretation: LC, FH, KR, SQ, AD, RM, FL, AD, NH; TB, LC, CWT and DVC wrote the first draft.
All authors reviewed their data and critically revised the paper, as well as approved the final version of the paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.08.060.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine [Internet]. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet [Internet]. 2020 Dec 8 [cited 2020 Dec 14];0(0). Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32661-1/abstract [DOI] [PMC free article] [PubMed]
- 4.Moustsen-Helms IR, Emborg H-D, Nielsen J, Nielsen KF, Krause TG, Molbak K, et al. Vaccine effectiveness after 1st and 2nd dose of the BNT162b2 mRNA Covid-19 Vaccine in long-term care facility residents and healthcare workers – a Danish cohort study. medRxiv. 2021 Mar 9;2021.03.08.21252200.
- 5.Haas E.J., Angulo F.J., McLaughlin J.M., Anis E., Singer S.R., Khan F., et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. The Lancet [Internet]. 2021;397(10287):1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernal JL, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 variant. medRxiv. 2021 May 24;2021.05.22.21257658.
- 7.Sciensano. COVID-19 procedures [Internet]. [cited 2021 Jul 13]. Available from: https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_FAQ_ENG_final.pdf
- 8.de Valpine P., Turek D., Paciorek C.J., Anderson-Bergman C., Lang D.T., Bodik R. Programming With Models: Writing Statistical Algorithms for General Model Structures With NIMBLE. Journal of Computational and Graphical Statistics. 2017;26(2):403–413. [Google Scholar]
- 9.Sciensano. COVID-19 FAQ [Internet]. [cited 2021 Jul 13]. Available from: https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_FAQ_ENG_final.pdf
- 10.Weekly epidemiological report, COVID-19 [Internet]. [cited 2021 Jul 21]. Available from: http://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_Weekly%20report_20210625%20-%20NL_0.pdf
- 11.Ledford H. Six months of COVID vaccines: what 1.7 billion doses have taught scientists. Nature. 2021 Jun 4;594(7862):164–7. [DOI] [PubMed]
- 12.Björk J, Inghammar M, Moghaddassi M, Rasmussen M, Malmqvist U, Kahn F. Effectiveness of the BNT162b2 vaccine in preventing COVID-19 in the working age population – first results from a cohort study in Southern Sweden. medRxiv. 2021 Apr 21;2021.04.20.21254636.
- 13.Shah ASV, Gribben C, Bishop J, Hanlon P, Caldwell D, Wood R, et al. Effect of vaccination on transmission of COVID-19: an observational study in healthcare workers and their households. medRxiv. 2021 Mar 21;2021.03.11.21253275.
- 14.Harris R.J., Hall J.A., Zaidi A., Andrews N.J., Dunbar J.K., Dabrera G. Effect of Vaccination on Household Transmission of SARS-CoV-2 in England. N Engl J Med. 2021 doi: 10.1056/NEJMc2107717. Jun 23;0(0):null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chia PY, Ong SWX, Chiew CJ, Ang LW, Chavatte J-M, Mak T-M, et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine-breakthrough infections: a multi-center cohort study [Internet]. 2021 Jul [cited 2021 Aug 12] p. 2021.07.28.21261295. Available from: https://www.medrxiv.org/content/10.1101/2021.07.28.21261295v1 [DOI] [PMC free article] [PubMed]
- 16.Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. New England Journal of Medicine. 2021 Jul 28;0(0):null. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.