Graphical abstract
Keyword: COVID-19, The traditional Chinese herbal medicine, Anti-viral, Anti-inflammatory, Reverse finding target
Abstract
Traditional Chinese herbal compound prescription in Xuanfei Baidu Tang (XBT) has obvious effects in the treatment of COVID-19. However, its effective compounds and targets for the treatment of COVID-19 remain unclear. Computer-Aided Drug Design is used to virtually screen out the anti-inflammatory or anti-viral compounds in XBT, and predict the potential targets by Discovery Studio 2020. Then, we searched for COVID-19 targets using Genecards databases and Protein Data Bank (PDB) databases and compared them to identify targets that were common to both. Finally, the target we screened out is: TP53 (Tumor Protein P53). This article also shows that XBT in the treatment of COVID-19 works in a multi-link and overall synergistic manner. Our results will help to design the new drugs for COVID-19.
1. Introduction
At the end of 2019, the novel coronavirus disease (COVID-19) appeared and caused global concern. It is a highly infectious disease characterized by respiratory symptoms [1]. COVID-19 pandemic has caused an unprecedented and uncontrollable health crisis. Since the outbreak of this disease, it has been characterized by strong infectivity, long treatment time after infection, and high mortality of patients with severe illness [2], [3], [4], [5], [6], [7], [8], [9], [10], [11]. The main physiological and pathological feature of severe COVID-19 is “cytokine storm”, also known as inflammatory storm [12]. It is an immune response produced by a positive feedback loop between cytokines and immune cells, and it is also the state which the body's immune system has evolved from “self-protection” to “over-protection” [13], [14]. Therefore, the outbreak of inflammation is the core pathological factor leading to aggravation and even death of patients in lung injury induced by COVID-19 [15], [16], [17]. Over expression and release of pro-inflammatory cytokines will lead to tissue damage [18], [19], [20], [21], [22], [23], [24]. In response to the inflammatory mechanism caused by the novel coronavirus (SARS-CoV-2) infection, a variety of methods have emerged to treat COVID-19, such as oxygen therapy, plasma therapy, drug (lopi-navir, favipiravir, ribavirin, PegIFN-α2a, traditional Chinese herbal compound prescription) therapy [25], [26]. Various traditional Chinese herbal compound prescriptions have been proved to be highly effective in the treatment of COVID-19 and have been widely used in the market and clinic, such as Sijunzi Decoction, Yupingfeng Powder, Buzhong Yiqi Decoction, Xuanfei Baidu Tang (XBT) and so on. In this article, we studies XBT in terms of anti-inflammatory or anti-viral effects. XBT is composed of 13 traditional Chinese herbal medicines, namely Ephedra, Bitter almond, Coix Seed, Atractylodes, Patchouli, Artemisia annua, Polygonum cuspidatum, Verbena, Reed root, Semen Lepidii, Exocarpium, licorice and Gypsum. XBT is a traditional Chinese herbal compound prescription for the treatment of anti-epidemic, which is designed for the pathological characteristics of wet toxin [27]. It has the effects of inhibiting viral infections, reducing inflammatory factors, and promoting the absorption of lung inflammation. Because of its outstanding efficacy, it is widely used as a recommended prescription in clinical practice [28].
Computer-Aided Drug Design (CADD) is to design and optimize lead compounds through calculating and estimating the relationship between ligand and receptor based on computer chemistry [29]. With the development of CADD, our understanding of disease pathogenesis, drug signal transduction pathways, target interactions, and other aspects is becoming more and more mature [30], [31], [32]. The modules of drug analysis, synthesis design, and drug molecular optimization in Discovery Studio 2020 (DS2020) make the later experimental operation more convenient. This not only reduces the cost of money and time, but also improves the safety of medicines. CADD builds a bridge between traditional Chinese medicine and modern pharmacology, and plays an important role in scientific development.
XBT is widely used in the treatment of clinical diseases [27], [33], [34], but its effective compounds and targets for the treatment of COVID-19 need to be further clarified. In this study, we aim to use DS2020 to screen out the anti-inflammatory or anti-viral compounds and targets in XBT, which will make contribution to treat COVID-19.
2. Experimental section
2.1. Screening out compounds in XBT
The Traditional Chinese Medicines Database is currently the world's largest non-commercial Chinese medicine database. This online database contains more than 20,000 purified compounds of 453 Chinese medicine ingredients [35], [36]. Using the database to obtain plant information, the components of traditional Chinese herbal medicines in XBT were screened separately. The “Plant Source” field in query mode was activated, entering the following informations (Ephedra, Bitter almond, Coix Seed, Atractylodes, Patchouli, Artemisia annua, Polygonum cuspidatum, Verbena, Reed root, Semen Lepidii, Exocarpium, licorice, and Gypsum) one by one. Then execute the query and output the ID of all compounds related to each traditional Chinese herbal medicines, as well as their name, source, structure, efficacy, etc. After exporting the data of all compounds, we only keep the compounds that have the functions of “anti-inflammatory” or “anti-virus”.
2.2. Optimization of small molecules and prediction of absorption, distribution, metabolism, excretion and toxicity (ADMET) properties
In view of the uncertain numbers of ligands and isomers, we used Prepare Ligands to modify the compound system, and then optimized the structure of small molecule ligands through the Minimize Ligands function. The optimized small molecules need to be evaluated by the prediction of ADMET properties. In the early stage of drug development, compounds can be predicted and selected based on the properties of the drug’s ADMET. The properties of ADMET we mentioned refer to the absorption, distribution, metabolism, excretion, and toxicity of drug molecules in the body [37], [38]. This operation can lower the expense of excessive structural modification and recombination in the later stage, and can improve the success rate of medical research and development to a certain extent. The ADMET properties that can be calculated in DS2020 include: aqueous solubility, blood brain barrier penetration (BBB), Cytochrome P450 2D6 inhibition [39], hepatotoxicity, Human intestinal absorption (HIA) and plasma protein binding [40], [41]. After the job is completed, open the ADMET plot window. Because the point within the 99% confidence interval means that the predictive properties of this molecule are reliable, it is necessary to delete the BBB model and the HIA model points outside the 99% confidence interval. For a more comprehensive analysis of drug toxicity, toxicological properties prediction process (TOPKAT) is required after the prediction of ADMET properties.
2.3. TOPKAT
TOPKAT is based on the 2D structure of the molecule to calculate and verify the toxicity and environmental effects of the compound[42]. Toxicity prediction has the characteristics of low cost and high efficiency in drug development and drug risk reduction. Enter the compounds retained after the ADMET screening, and the factors we need to consider in this screening are: mutagenicity, aerobic biodegradability, oral LD50 in rats, and rodent carcinogenicity [43], [44]. After the results are obtained, we need to verify whether all compounds are true in the ranges of “With Optimum Prediction Space (OPS)” and “Within OPS Limits”. Among them, “With OPS” evaluates whether the prediction results are in the best prediction space of the model, and “Within OPS Limits” evaluates whether the prediction result is within the best prediction space limit of the model. If “With OPS” and “Within OPS Limits” are both true for one compound, this compound is credible and will been kept.
2.4. Ligand small molecule filtration
According to the Lipinski Rule of Five and Veber Rule [45], [46], we evaluated the druggability of the compounds obtained after the preliminary screening. The Lipinski Rule of Five includes: the number of hydrogen bond donors does not exceed 5, the molecular weight does not exceed 500, the number of hydrogen bond acceptors does not exceed 10, the molecular weight does not exceed 500, and AlogP (The upper limit of the logarithm of the octanol–water partition coefficient) is not more than 5. The Veber Rule includes: The number of rotatable keys does not exceed 10, the polar surface area does not exceed 140 Å, and the sum of hydrogen bond donors and acceptors does not exceed 12. The range of these parameters correlates with the degree of oral availability of the drug.
After the evaluation, the small molecule compounds that do not meet the criteria are deleted, and the small molecules with good druggability are retained.
2.5. Performing DS2020 reverse finding target
After the prediction and screening of reverse finding target, the higher matching value of the pharmacophore and the compound, the more reliable it is. Then the targets corresponding to the pharmacophores are more likely to be the target of the compounds. The Fit Value of the entered compounds and pharmacophores can be got through the calculated results pages of PharmacophoreFits link and the “LigandProfiler” heat map.
2.6. Target point network construction
First, we use reverse finding target technology to get the targets that have anti-inflammatory or antiviral effects in XBT. Then we use Genecards databases (https://www.genecards.org) and Protein Data Bank (PDB) databases to search all targets related to COVID-19 with the key word of “SARS-CoV-2”. By comparing the results of these two parts, we can find anti-inflammatory or antiviral targets related to COVID-19 in XBT. Finally, we use Cytoscape 3.8.0 to establish the interaction network diagram of “XBT-Compounds-Targets-Efficacy”. After the construction is completed, it can clearly show the active components of each the traditional Chinese herbal medicine, as well as the common targets related to anti-inflammation or antivirus in these components.
3. Results and discussion
3.1. Screening the compounds of XBT in the traditional Chinese medicines Database
In the Traditional Chinese Medicines Database, if you query with “Ephedra” as the key word, the output results show that there are 53 compounds related to ephedra, including 2 compounds with anti-inflammatory effects and 0 compounds with antiviral effects. By analogy, it can be seen from table (Table 1, Table 2 ) that a total of 46 compounds from 13 traditional Chinese herbal medicines have been screened. According to the screening results, we have not found any compound with anti-inflammatory or anti-viral effects in 5 traditional Chinese herbal medicines (Gypsum, Coix Seed, Verbena, Reed root and Semen Lepidii). Then they will be excluded in the next calculation process. At this time there are only 8 traditional Chinese herbal medicines left.
Table 1.
The compounds screened out in Traditional Chinese Medicines Database.
| Name | ID | Activities | Screened Compounds |
|---|---|---|---|
| Ephedra | 6816 | anti-inflammatory | (4S,5R) Ephedroxane |
| 11,642 | anti-inflammatory | Isoquercitrin | |
| Bitter almond | 12,849 | antiviral | Linalool |
| 5699 | anti-inflammatory | Dihydroquercetin | |
| 7278 | anti-inflammatory | Eriodictyol | |
| 7821 | anti-inflammatory | Flavoxanthin | |
| Atractylodes | 1965 | anti-inflammatory | Atractylenolide I |
| 1971 | anti-inflammatory | Atractylone | |
| 7495 | anti-inflammatory | (+)-Eudesma-4(15),7(11)-dien-8-one | |
| 20,569 | anti-inflammatory | Syringin | |
| Patchouli | 16,498 | antiviral | Pachypodol |
| Artemisia annua | 2044 | antiviral | Axillarin |
| 4354 | antiviral | Cumaldehyde | |
| 12,849 | antiviral | Linalool | |
| 18,376 | antiviral | Quercetin-3-methyl ether | |
| 19,777 | antiviral | Sesamin | |
| 3743 | anti-inflammatory | Cirsiliol | |
| 7951 | anti-inflammatory | Friedelan-3-one | |
| 11,259 | anti-inflammatory | d-Isoborneol | |
| 11,260 | anti-inflammatory | l-Isoborneol | |
| 11,642 | anti-inflammatory | Isoquercitrin | |
| 13,137 | anti-inflammatory | Luteolin | |
| 17,377 | anti-inflammatory | beta-Pinene | |
| 19,540 | anti-inflammatory | Scoparone | |
| 19,545 | anti-inflammatory | Scopolin | |
| 19,983 | anti-inflammatory | beta-Sitosterol | |
| 19,087 | antiviral,anti-inflammatory | Rutin |
Table 2.
The compounds screened out in Traditional Chinese Medicines Database (continued).
| Name | ID | Activities | Screened Compounds |
|---|---|---|---|
| Polygonum cuspidatum | 3308 | antiviral | (+)-Catechin |
| 1367 | anti-inflammatory | Anthraquinone | |
| 8095 | anti-inflammatory | Gallic acid | |
| 10,887 | anti-inflammatory | Hyperin | |
| 11,642 | anti-inflammatory | Isoquercitrin | |
| 19,087 | antiviral, anti-inflammatory | Rutin | |
| 18,411 | antiviral, anti-inflammatory | Quercitrin | |
| Exocarpium | 15,286 | antiviral,anti-inflammatory | Naringin |
| licorice | 6402 | antiviral | 3,3′-Dimethylquercetin |
| 2455 | anti-inflammatory | 6,8-Bis(C-beta-glucosyl)-apigenin | |
| 8841 | anti-inflammatory | Glycyrrhetinic acid | |
| 11,505 | anti-inflammatory | Isoliquiritin | |
| 11,642 | anti-inflammatory | Isoquercitrin | |
| 12,766 | anti-inflammatory | Licochalcone A | |
| 12,907 | anti-inflammatory | Liquiritic acid | |
| 17,403 | anti-inflammatory | Pinocembrin | |
| 19,983 | anti-inflammatory | beta-Sitosterol | |
| 8846 | antiviral,anti-inflammatory | Glycyrrhizic acid | |
| 19,087 | antiviral,anti-inflammatory | Rutin |
3.2. Optimization of small molecules and prediction of ADMET properties
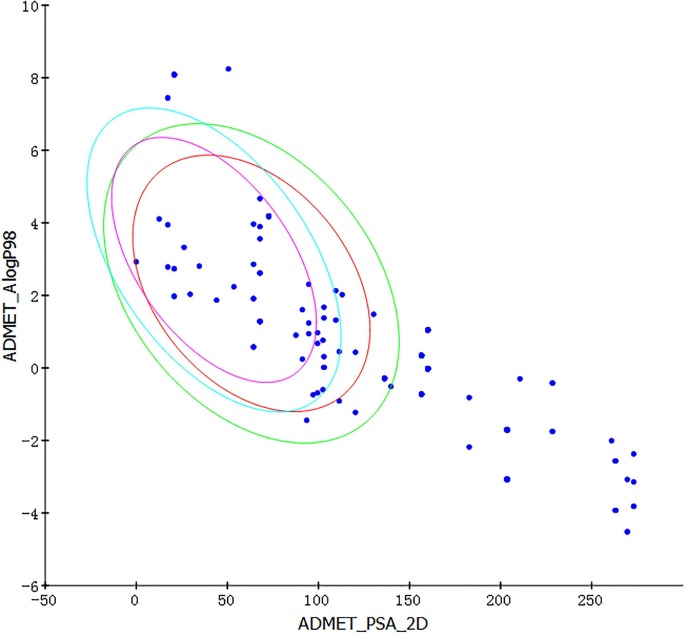
After the small molecule optimization process, the ADMET property prediction is carried out. Most drugs need to be discontinued and search for other candidate compounds during the development process. The main reason for this result is that the prediction results of ADMET properties are unreliable. From Fig. 1 , we can see that if a compound is reliable, the points shown in the prediction must be within the blue ellipse (99% confidence interval of the BBB model) and the green ellipse (99% confidence interval of the HIA model). Therefore, as shown in the Fig. 1, only 1 compound remains in Ephedra. In the same way, finally, 1 compound is retained in Bitter almond, 3 compounds in Atractylodes, 13 compounds in Artemisia annua, 3 compounds in Polygonum cuspidatum, and 30 compounds in Licorice. The compounds in Patchouli all meet the requirements of ADMET, while the compounds in Exocarpium do not meet the requirements of ADMET. Therefore, Exocarpium is excluded from the next calculation.
Fig. 1.
ADMET property prediction results of 8 kinds of traditional Chinese herbal medicines (Ephedra, Bitter almond, Atractylodes, Artemisia annua, Polygonum cuspidatum, Licorice, Patchouli, Exocarpium).
3.3. TOPKAT and ligand small molecule filtration
The calculated result is exported to PDF format. Open the calculated result (Table 3 , Table 4 ) to check whether all the properties and OPS components of each small molecule are within the expected range. Then, we remove compounds that do not meet the requirements. After screening, 1 compound is retained in Ephedra, 0 compound in Bitter almond, 1 compound in Atractylodes, 6 compounds in Patchouli, 8 compounds in Artemisia annua, 2 compounds in Polygonum cuspidatum, and 25 compounds in licorice. Therefore, Bitter almond is excluded from the next calculation. At this time there are only 6 traditional Chinese herbal medicines left. Then evaluate through the two configuration principles of Lipinski Rule of Five and Veber Rule, and delete small molecules that do not meet the evaluation criteria in the results. The output results showed that the types and quantities of compounds retained in each traditional Chinese herbal medicine remained unchanged.
Table 3.
Toxicity properties of the most promising compounds.
| Compounds | Rodent Carcinogenicity |
Ames | Rat Oral LD50 |
Aerobic Biodegradability |
|---|---|---|---|---|
| (4S,5R) Ephedroxane | Non-Carcinogen | 0.668916 | 0.991625 | 0.562176 |
| (+)-Eudesma-4(15), 7(11)-dien-8-one |
Non-Carcinogen | 0.122225 | 2.38961 | 0.724887 |
| Pachypodol1 | Non-Carcinogen | 0.669673 | 1.14754 | 0.481777 |
| Pachypodol2 | Non-Carcinogen | 0.512067 | 0.866841 | 0.621833 |
| Pachypodol3 | Non-Carcinogen | 0.599349 | 0.381132 | 0.490207 |
| Pachypodol4 | Non-Carcinogen | 0.704909 | 0.393169 | 0.471899 |
| Pachypodol5 | Non-Carcinogen | 0.577077 | 0.299591 | 0.616676 |
| Pachypodol6 | Non-Carcinogen | 0.661746 | 0.452941 | 0.536422 |
| Cirsiliol1 | Non-Carcinogen | 0.157938 | 0.24355 | 0.538137 |
| Cirsiliol2 | Non-Carcinogen | 0.603944 | 0.220941 | 0.572221 |
| Cirsiliol3 | Non-Carcinogen | 0.380653 | 0.133455 | 0.550077 |
| Scoparone | Non-Carcinogen | 0.636048 | 1.14097 | 0.7564 |
| Luteolin1 | Non-Carcinogen | 0.238779 | 0.194719 | 0.467705 |
| Luteolin2 | Non-Carcinogen | 0.630162 | 0.149287 | 0.513354 |
| Luteolin2Quercetin-3- methyl ether |
Non-Carcinogen | 0.689358 | 0.163154 | 0.475555 |
| Sesamin | Non-Carcinogen | 0.535598 | 0.489369 | 0.609635 |
| Gallic acid | Non-Carcinogen | 0.687047 | 0.736671 | 0.404672 |
| Anthraquinone | Non-Carcinogen | 0.776107 | 2.33633 | 0.171863 |
| Glycyrrhetinic acid1 | Non-Carcinogen | 8.87E-06 | 1.27097 | 0.730006 |
| Glycyrrhetinic acid2 | Non-Carcinogen | 8.87E-06 | 1.27097 | 0.730006 |
Table 4.
Toxicity properties of the most promising compounds (continued).
| Compounds | Rodent Carcinogenicity | Ames | Rat Oral LD50 |
Aerobic Biodegradability |
|---|---|---|---|---|
| Liquiritic acid1 | Non-Carcinogen | 8.87E-06 | 1.27097 | 0.730006 |
| Liquiritic acid2 | Non-Carcinogen | 8.87E-06 | 1.27097 | 0.730006 |
| Liquiritic acid3 | Non-Carcinogen | 8.87E-06 | 1.27097 | 0.730006 |
| Liquiritic acid4 | Non-Carcinogen | 8.87E-06 | 1.27097 | 0.730006 |
| Liquiritic acid5 | Non-Carcinogen | 8.87E-06 | 1.27097 | 0.730006 |
| Liquiritic acid6 | Non-Carcinogen | 8.87E-06 | 1.27097 | 0.730006 |
| Liquiritic acid7 | Non-Carcinogen | 8.87E-06 | 1.27097 | 0.730006 |
| Liquiritic acid8 | Non-Carcinogen | 8.87E-06 | 1.27097 | 0.730006 |
| Pinocembrin1 | Non-Carcinogen | 0.0779542 | 0.544404 | 0.535875 |
| Pinocembrin2 | Non-Carcinogen | 0.627138 | 0.419365 | 0.631557 |
| Pinocembrin3 | Non-Carcinogen | 0.594698 | 0.41769 | 0.654128 |
| Pinocembrin4 | Non-Carcinogen | 0.167847 | 0.301578 | 0.542622 |
| Pinocembrin5 | Non-Carcinogen | 0.621283 | 0.229593 | 0.644483 |
| Pinocembrin6 | Non-Carcinogen | 0.594698 | 0.41769 | 0.654128 |
| Pinocembrin7 | Non-Carcinogen | 0.627138 | 0.419365 | 0.631557 |
| Pinocembrin8 | Non-Carcinogen | 0.167847 | 0.301578 | 0.542622 |
| Pinocembrin9 | Non-Carcinogen | 0.0779542 | 0.544404 | 0.535875 |
| Pinocembrin10 | Non-Carcinogen | 0.621283 | 0.229593 | 0.644483 |
| 3,3′-Dimethylquercetin1 | Non-Carcinogen | 0.631479 | 0.528344 | 0.518521 |
| 3,3′-Dimethylquercetin2 | Non-Carcinogen | 0.550035 | 0.636029 | 0.622739 |
| 3,3′-Dimethylquercetin3 | Non-Carcinogen | 0.55875 | 0.218344 | 0.493588 |
| 3,3′-Dimethylquercetin4 | Non-Carcinogen | 0.688634 | 0.180996 | 0.502659 |
| 3,3′-Dimethylquercetin5 | Non-Carcinogen | 0.604492 | 0.21979 | 0.609962 |
3.4. Reverse finding target
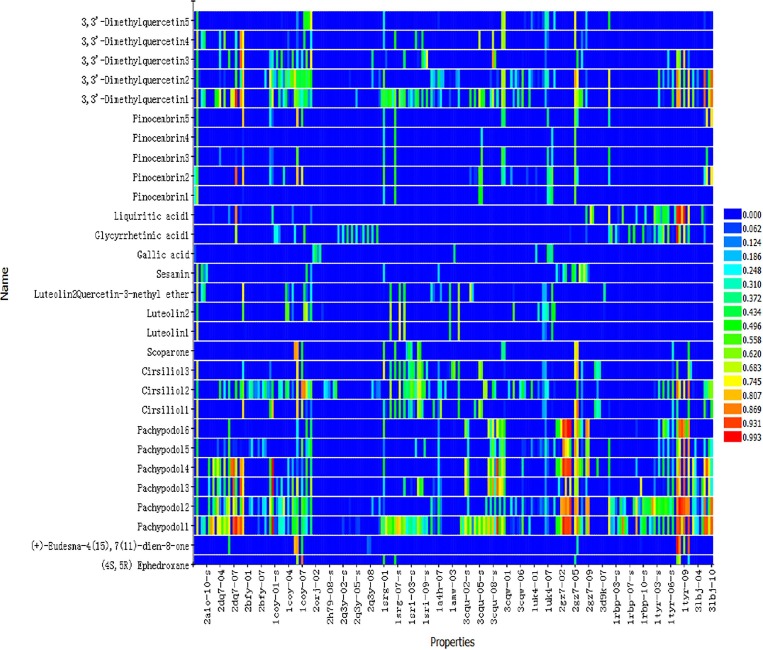
In order to show the matching degree of the pharmacophore with all the compounds participating in the test more concisely, we used the “Ligand Profiler” heat map (Fig. 2 ) to represent it. In Fig. 2, the horizontal axis represents the pharmacophores, and the vertical axis represents the compounds screened from traditional Chinese herbal medicines. The color changes from red to blue, indicating that the Fit Value is gradually decreasing. In order to explain the meaning of Fig. 2 more clearly, we take Ephedra as an example. After a series of screening, we extracted (4S, 5R) Ephedroxane from Ephedra. Because the pharmacophore with higher matching degree is red and yellow, the fitted value corresponding to 1coy is the highest. Therefore, the target corresponding to 1coy is very likely to be the target of (4S, 5R) Ephedroxane. It can be seen from Fig. 2, Fig. 3 that 29 compounds in XBT through reverse finding target were found.
Fig. 2.
Reverse finding target results of 6 kinds of traditional Chinese herbal medicines (Ephedra, Atractylodes, Patchouli, Artemisia annua, Polygonum cuspidatum, Licorice). The horizontal axis represents the pharmacophores, and the vertical axis represents the compounds obtained through reverse finding target. (4S,5R) Ephedroxane is from Ephedra; (+)-Eudesma-4(15),7(11)-dien-8-one is from Atractylodes and 3,3′-Dimethylquercetin1-5 are from licorice, et al (more information is shown in Table 6).
Fig. 3.
(1). The structures of 29 compounds. (2). The structures of 29 compounds. (3). The structures of 29 compounds. (4). The structures of 29 compounds.
3.5. Target point network construction
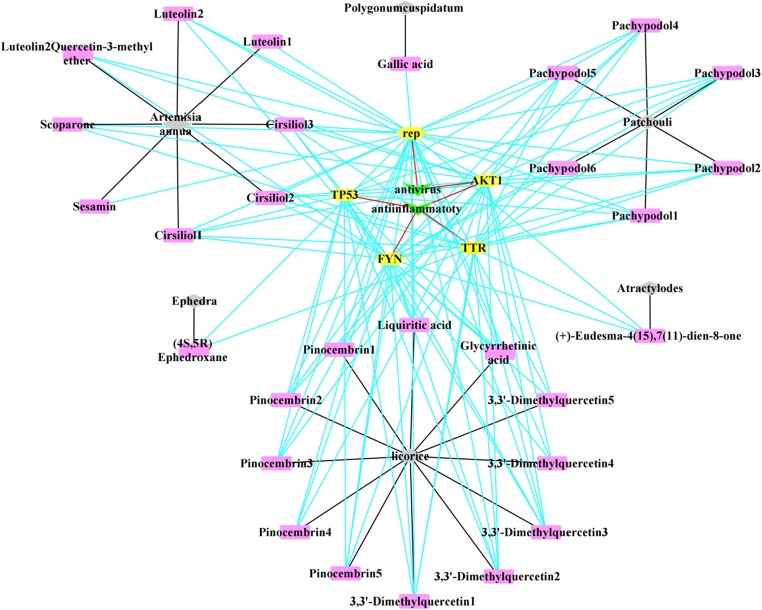
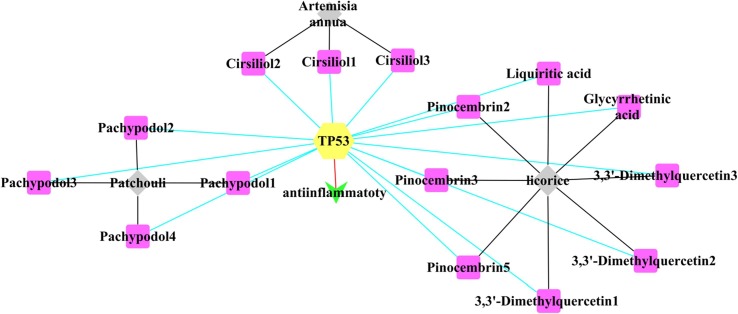
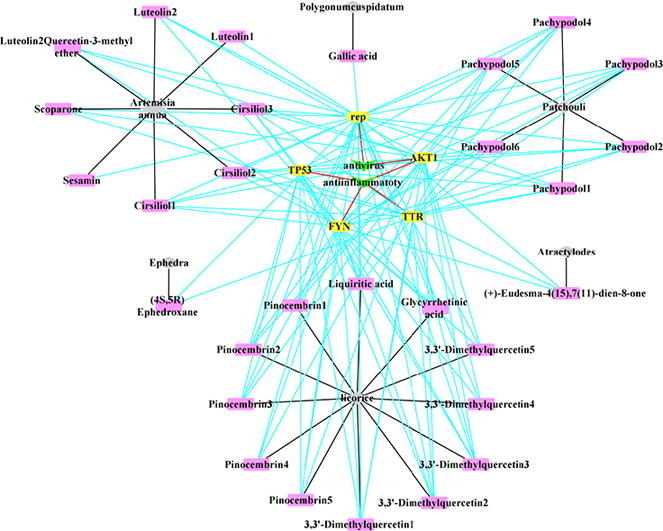
The reverse finding target technology was used to obtain pharmacophores whose corresponding targets were been predicted. The targets of XBT are rep (ATP- dependent DNA helicase Rep), TTR (Transthyretin), AKT1 (AKT Serine/Threonine Kinase 1), FYN (FYN Proto-Oncogene/Src Family Tyrosine Kinase) and TP53 (Tumor Protein P53). As the potential targets: TP53, FYN, FYN are against inflammation, rep is against virus, and AKTI is against inflammation and virus (Table 5 ). We compared these targets (rep, TTR, AKT1, FYN and TP53) with the targets searched by Genecards databases and PDB databases, and found the common target TP53 for COVID-19 (Fig. 4, Fig. 5 ).
Table 6.
Compounds in each traditional Chinese herbal medicine obtained by reverse finding target technology.
| Name | Screened compounds |
|---|---|
| Ephedra | (4S,5R) Ephedroxane |
| Atractylodes | (+)-Eudesma-4(15),7(11)-dien-8-one |
| licorice | Glycyrrhetinic acid |
| Liquiritic acid | |
| 3,3′-Dimethylquercetin1 | |
| 3,3′-Dimethylquercetin2 | |
| 3,3′-Dimethylquercetin3 | |
| 3,3′-Dimethylquercetin4 | |
| 3,3′-Dimethylquercetin5 | |
| Pinocembrin1 | |
| Pinocembrin2 | |
| Pinocembrin3 | |
| Pinocembrin4 | |
| Pinocembrin5 | |
| Patchouli | Pachypodol1 |
| Pachypodol2 | |
| Pachypodol3 | |
| Pachypodol4 | |
| Pachypodol5 | |
| Pachypodol6 | |
| Polygonum cuspidatum | Gallic acid |
| Artemisia annua | Cirsiliol1 |
| Cirsiliol2 | |
| Cirsiliol3 | |
| Scoparone | |
| Luteolin1 | |
| Luteolin2 | |
| Luteolin2Quercetin-3-methyl ether | |
| Sesamin |
Table 5.
All pharmacophores and their corresponding targets obtained by reverse finding target technology.
| Pharmacophore | Target(gene name) | Efficacy |
|---|---|---|
| 1tyr-01, 1tyr-01-s, 1tyr-02, 1tyr-02-s, 1tyr-03, 1tyr-03-s, 1tyr-04, 1tyr-05, 1tyr-05-s, 1tyr-06, 1tyr-06-s, 1tyr-07, 1tyr-08, 1tyr-08-s, 1tyr-09, 1tyr-09-s, 1tyr-10, 1tyr-10-s | 1tyr(TTR) | anti-inflammatory |
| 1uk4-01,1uk4-02,1uk4-03,1uk4-04,1uk4-05,1uk4-06,1uk4-07, 1uk4-08, 1uk4-09, 1uk4-10 | 1uk4(rep) | antiviral |
| 2gz7-01, 2gz7-01-s, 2gz7-02, 2gz7-03, 2gz7-03-s, 2gz7-04, 2gz7-04-s, 2gz7-05, 2gz7-05-s, 2gz7-06, 2gz7-07, 2gz7-07-s, 2gz7-08, 2gz7-08-s, 2gz7-09 | 2gz7(rep) | antiviral |
| 2dq7-01, 2dq7-02, 2dq7-02-s, 2dq7-03, 2dq7-04, 2dq7-05, 2dq7-05-s, 2dq7-06, 2dq7-06-s, 2dq7-07, 2dq7-07-s, 2dq7-08, 2dq7-09, 2dq7-10 | 2dq7(FYN) | anti-inflammatory |
| 2x0v-01 | 2x0v(TP53) | anti-inflammatory |
| 2x0u-01 | 2x0u(TP53) | anti-inflammatory |
| 3cqu-01, 3cqu-01-s, 3cqu-02, 3cqu-02-s, 3cqu-03, 3cqu-03-s, 3cqu-04, 3cqu-04-s, 3cqu-05, 3cqu-05-s, 3cqu-06, 3cqu-06-s, 3cqu-07, 3cqu-07-s, 3cqu-08, 3cqu-08-s, 3cqu-09, 3cqu-09-s, 3cqu-10, 3cqu-10-s | 3cqu(AKT1) | antiviral, anti-inflammatory |
| 3cqw-01, 3cqw-02, 3cqw-03, 3cqw-04, 3cqw-05, 3cqw-06, 3cqw-06-s, 3cqw-07, 3cqw-08, 3cqw-09, 3cqw-10, 3cqw-10-s | 3cqw(AKT1) | antiviral, anti-inflammatory |
Fig. 4.
“XBT-Compounds-Targets-Efficacy” Interaction Network Diagram. 6 traditional Chinese herbal medicines (Ephedra, Atractylodes, Patchouli, Artemisia annua, Polygonum cuspidatum, Licorice) in XBT are marked in gray; 29 compounds screened from 6 traditional Chinese herbal medicines with anti-inflammatory or antiviral effects are marked in pink; 5 targets are marked in yellow, and the properties of the targets are marked in green. The black line represents that a certain compound comes from a certain traditional Chinese herbal medicine; the blue line represents the interaction between the compound and the target, and the red line represents a certain target is against inflammation or virus. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
“3 traditional Chinese herbal medicines-Compounds-Target-anti-inflammatory” Interaction Network Diagram related to COVID-19. 3 traditional Chinese herbal medicines (licorice, Artemisia annua, Patchouli) in XBT are marked in gray; 15 compounds screened from 3 traditional Chinese herbal medicines with anti-inflammatory or antiviral effects are marked in pink; 1target (TP53) is marked in yellow, and the property of the target is marked in green. The black line represents that a certain compound comes from a certain traditional Chinese herbal medicine; the blue line represents the interaction between the compound and the target, and the red line represents TP53 is against inflammation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
As shown in Fig. 4, there are 29 compounds from 6 traditional Chinese herbal medicines (Ephedra, Atractylodes, Patchouli, Artemisia annua, Polygonum cuspidatum, Licorice) that have anti-inflammatory or antiviral effects in XBT. As shown in Fig. 5, Cirsiliol1-3 (extracted from Artemisia annua), Pachypodol1-4 (extracted from Patchouli), Liquiritic acid, Glycyrrhetinic acid, 3,3′-Dimethylquercetin1-3, Pinocembrin2, Pinocembrin3, Pinocembrin5 (extracted from Patchouli) could be used as candidate compounds for the treatment of COVID-19.
4. Conclusion
Based on the anti-inflammatory or anti-viral effects, we used DS2020 to optimize, screen out small molecule and predict ADMET, toxicological properties for various traditional Chinese herbal medicines in XBT. The pharmacophores are collected by reverse finding target, and then the targets are predicted. From Table 5, we found that the anti-inflammatory or antiviral targets corresponding to the compounds of XBT were mainly: rep, TTR, AKT1, FYN, TP53. Among them, TP53 is highly related to COVID-19. In addition, among these targets, each target is connected to two or more compounds, indicating that the same target can also be controlled by multiple compounds at the same time. The calculated results are expected to be used in the design of new drugs for the treatment of COVID-19.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by Natural Science Foundation of Shandong, China [Grant No. ZR2019MC004], the High-end Talent Team Construction Foundation [Grant No. 108-10000318] and the High-end Full-time Innovative Talent Introduction Foundation “two-hundred plans” of Yantai.
References
- 1.Sun F., Zhu J., Tao H., Ma Y., Jin W. A systematic review involving 11,187 participants evaluating the impact of COVID-19 on anxiety and depression in pregnant women. J. Am. Med. Assoc. 2021;42(2):91–99. doi: 10.1080/0167482X.2020.1857360. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed S.H., Sahi A., Al-Roomi R.A., Al-Karkhi I. Introduction to COVID-19, history, impact, symptoms and prevention, Pakistan. J, Med. Health Sci. 2020;14(2):1528–1534. [Google Scholar]
- 3.Zhao Y., Cui C., Zhang K., Liu J., Xu J., Nisenbaum E., Huang Y., Qin G., Chen B., Hoffer M., Blanton S.H., Telischi F., Hare J.M., Daunert S., Shukla B., Pahwa S.G., Jayaweera D.T., Farmer P.E., Rio C.D., Liu X., Shu Y. COVID19: A Systematic Approach to Early Identification and Healthcare Worker Protection. Front. Public Health. 2020;8(8):205–209. doi: 10.3389/fpubh.2020.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fogarty H., Townsend L., Cheallaigh C.N., Bergin C., Martin-Loeches I., Browne P., Bacon C.L., Gaule R., Gillett A., Byrne M., Ryan K., O’Connell N., O’Sullivan J.M., Conlon N., O'Donnell J.S. COVID19 coagulopathy in Caucasian patients. British J. Haematol. 2020;189(6):1044–1049. doi: 10.1111/bjh.16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ammar A., Brach M., Trabelsi K., Chtourou H., Boukhris O., Masmoudi L., Bouaziz B., Bentlage E., How D., Ahmed M., Müller P., Müller N., Aloui A., Hammouda O., Paineiras-Domingos L.L., Braakman-Jansen A., Wrede C., Bastoni S., Pernambuco C.S., Mataruna L., Taheri M., Irandoust K., Khacharem A., Bragazzi N.L., Chamari K., Glenn J.M., Bott N.T., Gargouri F., Chaari L., Batatia H., Ali G.M., Abdelkarim O., Jarraya M., Abed K.E., Souissi N., Gemert-Pijnen L.V., Riemann B.L., Riemann L., Moalla W., Gómez-Raja J., Epstein M., Sanderman R., Schulz S.V.W., Jerg A., Al-Horani R., Mansi T., Jmail M., Barbosa F., Ferreira-Santos F., Šimunič B., Pišot R., Gaggioli A., Bailey S.J., Steinacker J.M., Driss T., Hoekelmann A. Effects of COVID-19 Home Confinement on Eating Behaviour and Physical Activity: Results of the ECLB-COVID19 International Online Survey. Nutrients. 2020;12(6):1583–1590. doi: 10.3390/nu12061583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vlachakis D., Papakonstantinou E., Mitsis T., Pierouli K., Diakou I., Chrousos G., Bacopoulou F. Molecular mechanisms of the novel coronavirus SARS-CoV-2 and potential anti-COVID19 pharmacological targets since the outbreak of the pandemic. Food Chem. Toxicol. 2020;146:111805–111807. doi: 10.1016/j.fct.2020.111805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ammar A., Brach M., Trabelsi K., Chtourou H., Boukhris O., Masmoudi L., Bouaziz B., Bentlage E., How D., Ahmed M. Effects of COVID-19 Home Confinement on Eating Behaviour and Physical Activity: Results of the ECLB-COVID19 International Online Survey. Nutrients. 2020;12(6):1583–1594. doi: 10.3390/nu12061583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowan N.J., Laffey J.G. Challenges and solutions for addressing critical shortage of supply chain for personal and protective equipment (PPE) arising from Coronavirus disease (COVID19) pandemic - Case study from the Republic of Ireland. Sci. Total Environ. 2020;725:138532–198551. doi: 10.1016/j.scitotenv.2020.138532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Rohaimi A.H., Otaibi F.A. Novel SARS-CoV-2 outbreak and COVID19 disease; a systemic review on the global pandemic. Genes Dis. 2020;7(4):491–501. doi: 10.1016/j.gendis.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eubank S., Eckstrand I., Lewis B., Venkatramanan S., Barrett C.L. Commentary on Ferguson, et al., “Impact of Non-pharmaceutical Interventions (NPIs) to Reduce COVID-19 Mortality and Healthcare Demand”. Bull. Math. Biol. 2020;82(4):52–54. doi: 10.1007/s11538-020-00726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman D.T., Himanshu Sharma B.A., Finkelstein M., Timothy Carlon M., Marinelli B., Doshi A.H., Ms B.N.D., Lookstein R. The Role of Telemedicine in the Maintenance of IR Outpatient Evaluation and Management Volume During the COVID-19 Global Pandemic. J. Vasc. Interv. Radiol. 2021;32(3):479–481. doi: 10.1016/j.jvir.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouédraogo D., Tiendrébéogo W., Kaboré F., Ntsiba H. COVID-19, chronic inflammatory rheumatic disease and anti-rheumatic treatments. Clin. Rheumatol. 2020;39(10224):2069–2075. doi: 10.1007/s10067-020-05189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ronconi G., Teté G., Kritas S.K., Gallenga C.E., Conti P. SARS-CoV-2, which induces COVID-19, causes kawasaki-like disease in children: role of pro-inflammatory and anti-inflammatory cytokines. J. Biol. Regul. Homeost. Agents. 2020;34(3):767–773. doi: 10.23812/EDITORIAL-RONCONI-E-59. [DOI] [PubMed] [Google Scholar]
- 14.Bellanti J.A., Settipane R.A. The allergist/immunologist, the Janus gatekeeper of inflammation, COVID-19 and beyond. Allergy Asthma Proc. 2020;41(6):395–396. doi: 10.2500/aap.2020.41.200084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J. Relation between Cardiac Injury and Elevated Levels with Severe COVID-19 of Inflammatory Biomarkers in Patients. Cardiovascular Innovations Applications. 2020;5(3):165–172. [Google Scholar]
- 16.Liu B.M., Martins T.B., Peterson L.K., Hill H.R. Clinical significance of measuring serum cytokine levels as inflammatory biomarkers in adult and pediatric COVID-19 cases: A review. Cytokine. 2021;142(5):155478–155479. doi: 10.1016/j.cyto.2021.155478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni M., Tian F.B., Xiang D.D., Yu B. Characteristics of inflammatory factors and lymphocyte subsets in patients with severe COVID-19. J. Med. Virol. 2020;92(11):2600–2606. doi: 10.1002/jmv.26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandel M., Harari G., Gurevich M., Achiron A. Cytokine prediction of mortality in COVID19 patients. Cytokine. 2020;134:15190–15204. doi: 10.1016/j.cyto.2020.155190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frisullo G., Bellavia S., Scala I., Piano C., Morosetti R., Brunetti V., Calabresi P., Marca G.D. Stroke and COVID19: Not only a large-vessel disease. J. Stroke Cerebrovascular Dis. 2020;29(10):105074–105076. doi: 10.1016/j.jstrokecerebrovasdis.2020.105074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raza S.S., Khan M.A. Mesenchymal Stem Cells: A new front emerge in COVID19 treatment: Mesenchymal Stem Cells therapy for SARS-CoV2 viral infection. Cytotherapy. 2020;12:25–36. [Google Scholar]
- 21.Qian G., Zhang Y., Xu Y., Hu W., Hall I.P., Yue J., Lu H., Ruan L., Ye M., Mei J. Reduced Inflammatory Responses to SARS-CoV-2 Infection in Children Presenting to Hospital with COVID-19 in China. Social Sci. Electronic Publishing. 2021;34:100831. doi: 10.1016/j.eclinm.2021.100831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dd A., Ih A., Ak A., Ieis B., Fgc D., Ka A. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? - ScienceDirect. Microbial Pathogenesis. 2021;153:104799. doi: 10.1016/j.micpath.2021.104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conti P., Ronconi G., Caraffa A., Gallenga C.E., Kritas S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by COVID-19: anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34(2):11–15. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 24.Mozafari N., Azadi S., Mehdi-Alamdarlou S., Ashrafi H., Azadi A. Inflammation: A bridge between Diabetes and COVID-19, and possible management with sitagliptin. Medical Hypotheses. 2020;143:110111. doi: 10.1016/j.mehy.2020.110111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu Y., Huiqiang W., Yuhuan L. Research progress on therapeutic drugs for corona virus disease 2019. Acta Pharmaceutica Sinica. 2020;55(06):1081–1090. [Google Scholar]
- 26.Tongai L., Li W., Dujun Z., Chunfeng L., Jigang C., Chen Y. Research Progress of Convalescent Plasma Treatment in Coronavirus Disease 2019. Practical J. Cardiac Cerebral Pneumal Vascular Dis. 2021;29(07):9–12. [Google Scholar]
- 27.Chengcheng G., Huachen J., Yunlun L. Manifestation of Strengthening Body and Dispelling Pathogenic Factors Rule in TCM with“Three Medicines and Three Prescriptions”. J. Liaoning Univ. TCM. 2020;22(10):159–163. [Google Scholar]
- 28.Wang H., Song X., Wang D., Ma X., Zou X., Miao J., Wang Y., Yang W. The potential mechanism of the treatment of new type of coronavirus pneumonia was discussed based on the pharmacological and molecular interaction of the network. J. Hainan Med. College. 2020;26(18):1361–1372. [Google Scholar]
- 29.Huang H.J., Yu H.W., Chen C.Y., Hsu C.H., Chen Y.C. Current developments of computer-aided drug design. J. Taiwan Inst. Chem. Eng. 2010;41(6):623–635. [Google Scholar]
- 30.Gaudêncio S.P., Florbela P. A Computer-Aided Drug Design Approach to Predict Marine Drug-Like Leads for SARS-CoV-2 Main Protease Inhibition. Marine Drugs. 2020;18(12):13–17. doi: 10.3390/md18120633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Araújo R.S.A.D., Silva-Junior E.F.D., Aquino T.M.D., Scotti M.T., Ishiki H.M., Scotti L., Mendonça-Junior F.J.B. Computer-Aided Drug Design Applied to Secondary Metabolites as Anticancer Agents. Curr. Topics Med. Chem. 2020;20(19):1677–1703. doi: 10.2174/1568026620666200607191838. [DOI] [PubMed] [Google Scholar]
- 32.Daina A., Rhrig U.F., Zoete V. Computer-Aided Drug Design for Cancer Therapy. Syst. Med. 2021;2:386–401. [Google Scholar]
- 33.Li R., Hou Y., Huang J., Pan W., Ma Q., Shi Y., Li C., Zhao J., Jia Z., Jiang H., Zheng K., Huang S., Dai J., Li X., Hou X., Wang L., Zhong N., Yang Z. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacological Res. 2020;156:104761. doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi W., Xiang L., Jun-hua Z., Rui X., Jing-yang Q., Xiao-hui Z., Han Z., Qing-quan L., Xiao-hui F., Yi-yu C., Bo-li Z. Mechanism of Xuanfei Baidu Tang in treatment of COVID-19 based on network pharmacology. Chinese J. Traditional Chinese Med. 2020;45(10):2249–2256. doi: 10.19540/j.cnki.cjcmm.20200325.401. [DOI] [PubMed] [Google Scholar]
- 35.Sida J., Qiuji C., Bingwei N., Yingying C., Ying T., Weiping C., Zong C.Y. Databases for facilitating mechanistic investigations of traditional Chinese medicines against COVID-19. Pharmacol. Res. 2020;159(12):142–151. doi: 10.1016/j.phrs.2020.104989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tao L., Li A.X., You P.M., Ke Z.W., Ma Y. Screening of new non-nucleoside reverse transcriptase inhibitors of HIV-1 based on traditional Chinese medicines database. Chin. Chem. Lett. 2009;20(11):1386–1388. [Google Scholar]
- 37.Dong J., Wang N.-N., Yao Z.-J., Zhang L., Cheng Y., Ouyang D., Lu A.-P., Cao D.-S. ADMETlab: a platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J. Cheminformatics. 2018;10(1):29–36. doi: 10.1186/s13321-018-0283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng F., Li W., Zhou Y., Shen J., Wu Z., Liu G., Lee P.W., Tang Y. admetSAR: a comprehensive source and free tool for assessment of chemical ADMET properties. J. Chem. Information Modeling. 2012;52(11):3099–3105. doi: 10.1021/ci300367a. [DOI] [PubMed] [Google Scholar]
- 39.Roy P.P., Roy K. QSAR Studies of CYP2D6 Inhibitor Aryloxypropanolamines Using 2D and 3D Descriptors. Chem. Biol. Drug Des. 2009;73(4):442–455. doi: 10.1111/j.1747-0285.2009.00791.x. [DOI] [PubMed] [Google Scholar]
- 40.Feixiong C., Weihua L., Yadi Z., Jie S., Zengrui W., Guixia L., Lee P.W., Yun T. admetSAR: a comprehensive source and free tool for assessment of chemical ADMET properties. J. Chem. Information Modeling. 2012;52(11):3099–3105. doi: 10.1021/ci300367a. [DOI] [PubMed] [Google Scholar]
- 41.Gleeson M.P. Generation of a set of simple, interpretable ADMET rules of thumb. J. Med. Chem. 2008;51(4):817–834. doi: 10.1021/jm701122q. [DOI] [PubMed] [Google Scholar]
- 42.Ma X.H., Wang R., Xue Y., Li Z.R., Yang S.Y., Wei Y.Q., Chen Y.Z. Advances in Machine Learning Prediction of Toxicological Properties and Adverse Drug Reactions of Pharmaceutical Agents. Curr. Drug Saf. 2008;3(2):100–114. doi: 10.2174/157488608784529224. [DOI] [PubMed] [Google Scholar]
- 43.Zhu H., Tropsha A., Fourches D., Varnek A., Papa E., Gramatica P., Öberg T., Dao P., Cherkasov A., Tetko I.V. Combinatorial QSAR Modeling of Chemical Toxicants Tested against Tetrahymena pyriformis. J. Chem. Information Modeling. 2008;48(4):766–784. doi: 10.1021/ci700443v. [DOI] [PubMed] [Google Scholar]
- 44.Diaza R.G., Manganelli S., Esposito A., Roncaglioni A., Manganaro A., Benfenati E. Comparison of in silico tools for evaluating rat oral acute toxicity. SAR QSAR Environ. Res. 2015;26(1):1–27. doi: 10.1080/1062936X.2014.977819. [DOI] [PubMed] [Google Scholar]
- 45.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 1997;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 46.Tice C.M. Selecting the right compounds for screening: does Lipinski's Rule of 5 for pharmaceuticals apply to agrochemicals? Pest Manage. Sci. 2001;57(1):3–16. doi: 10.1002/1526-4998(200101)57:1<3::AID-PS269>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]