Abstract
Inflammatory responses arise as an outcome of tissues or organs exposure towards harmful stimuli like injury, toxic chemicals or pathogenic microorganism. It is a complex cascade of immune mechanism to overcome from tissue injury and to initiate the healing process by recruiting various immune cells, chemical mediators such as the vasoactive peptides and amines, pro-inflammatory cytokines, eicosanoids and acute-phase proteins to prevent tissue damage and ultimately complete restoration of the tissue function. The cytokines exhibits a central function in communication between the cells, inflammatory response initiation, amplification and their regulation. This review covers the importance of inflammatory responses; the significance of cytokines in inflammation and numerous inflammatory disorders/ailments due to the abrupt expression of cytokines and the hyper-inflammatory response or cytokine storm associated with poor prognosis in COVID-19 pandemic. Also highlighting the importance of naturally derived anti-inflammatory metabolites to overcome the side-effects of currently prevailing anti-inflammatory drugs.
Keywords: Cytokines, Inflammatory response, Interleukins, Interferon
Abbreviations: ARDS, Acute respiratory distress syndrome; AHR, Airway hyper-responsiveness; AD, Alzheimer's disease; ACE2, Angiotensin converting enzyme 2; APC, Antigen-presenting cells; CAMs, Cell adhesion molecules; CINCA, Chronic infantile neurologic cutaneous articular; CLL, Chronic lymphocytic leukemia; CNTF, Ciliary neurotrophic factor; COVID-19, Corona Virus Disease 2019; CRP, C-reactive protein; CAPS, Cryopyrin-associated periodic syndromes; CRS, Cytokine release syndrome; CTL, Cytotoxic T-cells; DMARD, Disease modifying antirheumatic drug; DIRA, Disorder associated with IL-1Ra; EPO, Erythropoietin; FCU, Familial cold urticaria; FCAS, Familial cold-associated syndrome; FMF, Familial Mediterranean fever; GI, Gastro-Intestinal; GM-CSF, Granulocyte/macrophage colony-stimulating factor; HBV, Hepatitis-B virus; HCV, Hepatitis-C virus; HIDS, Hyper immunoglobulin D and periodic fever syndrome; IFNγR, IFN-γ receptors; IL-1R, IL-1 receptor; IL-6R, IL-6 receptor; IBD, Inflammatory bowel diseases; IFNγ, Interferon-γ; IL-1β, Interleukin-1β; IL-6, Interleukin-6; ICAM, Intracellular adhesion molecule; JAK, Janus kinase; LIF, Leukemia inhibitory factor; MHC, Major histocompatibility complex; MKD, Mevalonate kinase deficiencies; MA, Mevalonic aciduria; MERS, Middle East Respiratory Syndrome; MAPK, Mitogen-activated protein kinase; MWS, Muckle Wells syndrome; MODS, Multiple organ dysfunction syndromes; NK, Natural Killer cells; NOMID, Neonatal onset multisystem inflammatory disease; NF-κB, Nuclear factor kappa-B; OSM, Oncostatin M; PD, Parkinson's disease; RA, Rheumatic arthritis; SARS, Severe Acute Respiratory Syndrome; SARS-CoV-2, Severe acute respiratory syndrome corona virus 2; STAT, Signal transducer and activator of transcription; TPO, Thrombopoietin; TNFR, TNF receptor; TLR, Toll like receptors; TGF, Transforming growth factor; TRAPS, Tumor necrosis factor receptor-associated periodic syndrome; TNF-α, Tumor necrosis factor-α; WBC, White blood cells; X-SCID, X-linked severe combined immunodeficiency
Graphical abstract
Introduction
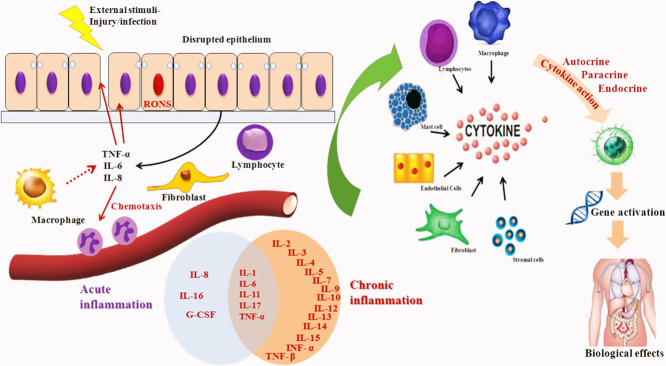
Inflammatory responses are mainly composed of complex cascade of interactions among pro and anti-inflammatory mediators. Their balance decides the successful outcome after an injury. The process of inflammation is a vital response required for the successful recovery from injury, trauma (surgically induced), sepsis and infections. The inflammatory responses were triggered to bring about the recruitment of blood leucocytes, tissue macrophage activation and production of series of mediators. In clinical aspects of trauma or sepsis, the unregulated synthesis and interaction of pro-inflammatory mediators can cause multiple organ dysfunctions. The inflammatory response to various harmful stimuli like pathogens, toxic chemicals or irritants (Medzhitov, 2010), damaged cells. Thereby acts to resolve by removing deleterious stimuli and establish the healing process (Ferrero-Miliani et al., 2007). Therefore, inflammation is body's natural protection mechanism that is essential to healthiness (Nathan et al., 2010). Nevertheless, it's a normal phenomenon which gradually progresses to acute (Ling et al., 2014) and later to chronic (Isailovic et al., 2015). In acute inflammatory process, usually cellular and molecular events and their controlled and combined interactions effectively minimize the prevailing injury/infection. This alleviation procedure contributes towards the restoration of the normal tissue homeostasis. However, uncontrolled acute inflammation gradually progress to chronic, causative for variety of chronic inflammatory diseases like neurodegenerative disorders, cancer, and cardiovascular diseases (Zhou et al., 2016).
Inflammation is defined clinically at the tissue level categorized by ‘redness, swelling, heat, pain, and loss of function’. The recognition of inflammation features back to antiquity. It was documented by Celsus in the 1st century AD that ancients known about tissue response to injury and sorted accordingly by rubor (redness due to increased hyperemia), tumor (swelling of the affected region by leakage of proteins to the interstitial space and thereby increased microvasculature permeability, calor (heat, related with increased metabolic rate of chemical mediators of inflammation and elevated blood flow rate to the injury site) and dolor (pain, due to changes associated with nerve endings and perivasculature). In 1850 by Rudolf Virchow introduced the 5th cardinal signal of inflammation, Functio laesa (dysfunction of the organs involved). The significant events that occur in microcirculation during the phase of inflammation includes the increased vascular permeability changes, leukocytes recruitment and their accumulation, release of mediators for initiating inflammatory responses (Ferrero-Miliani et al., 2007; Chertov et al., 2000). Inflammation is highly regulated by multitude of mediators and regulators which comprises growth factors, cytokines, complement, eicosanoids (prostaglandins, thromboxanes) and peptides. The discovery of the mediators has made a breakthrough to realize the underlying principle concerned with basic regulation of the inflammatory process as well as to unraveling its ambiguity associated in various disease conditions.
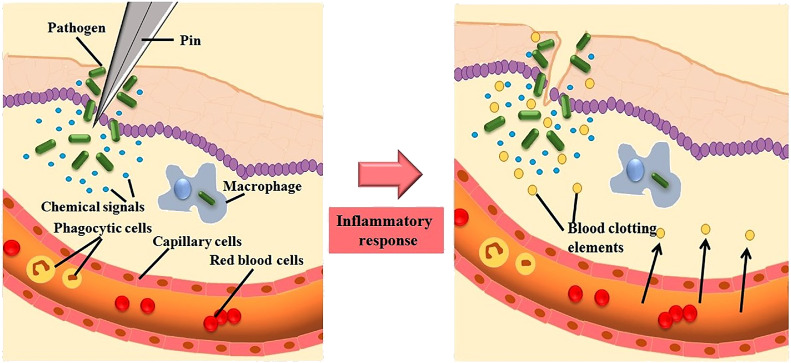
Over the past five decades, it has witnessed a quick expansion and advancement in inflammatory disease research. The understanding of activation of inflammatory process and also their complexity in intracellular signaling, ability to co-ordinate the various cells in the signaling pathways and to perform their dedicated roles in inflammatory site lead a greater advancement in inflammatory research. During tissue injury, the body triggers various chemical mediators and stimulate signaling cascade to heal the affected site of injury. This signal also activates leukocytes and attracts them towards the injured tissue via chemotaxis (Fig. 1 ). These stimulated leukocytes release cytokines that can persuade the inflammatory responses (Jabbour et al., 2009).
Fig. 1.
Inflammatory response mediated after an injury.
Cytokines are key inflammatory modulators, engaging in both acute and chronic inflammation through a multifaceted and often apparently opposing network of interactions. Enhanced perceptive of these regulated pathways help to promote more precise identification of the agents of inflammation and also in inflammatory disease management. Cytokines are produced by CD4 and CD8 cells, macrophages irrespective of responses generated by endogenous and exogenous antigens or towards bacterial products. Inflammation governed by pro and anti- inflammatory cytokine production possibly will aid to understand the disease progress within the body. For the development of therapeutic potential, deeper insight into the endogenous mechanisms that regulate the inflammatory response is beneficial.
Inflammatory response mechanism
The inflammatory responses is a controlled, co-ordinated stimulation of signaling pathways regulating the levels of mediators involved in inflammation at the tissue site and recruiting inflammatory cells from blood to maintain tissue homeostasis (Lawrence, 2009). Inflammation is normally a general phenomenon of pathogenesis of many chronic disease conditions together with neurodegenerative, bowel diseases and cardiovascular, arthritis, cancer and diabetes (Libby, 2007). These chains of dynamic responses include both cellular and vascular events by the stimulation of specific mediators. Thereby changes the physical location of leukocytes, plasma and fluids towards the site of inflammation. A collection of mediators and signaling molecules (eg- histamines, prostaglandins, free radicals generated from nitrogen and oxygen, serotonin and leucotrienes) are released principally by the immune cells for commencing the events of inflammation (Anwikar et al., 2010). The inflammatory responses is mediated mainly by two events i) acute and ii) chronic; each is triggered by different mechanism (Serhan et al., 2015). The occurrences of microvasculature during injury/infection is rapid and ultimately leads to vasodilatation and create the blood vessels more permeable and allowing mediators for inflammation to enter and produces edema in interstitial space (Porter, 2013).
The white blood cells (WBC) are essential for inflammatory response, gets infiltrated from the circulatory system (Goljan, 2018; Kumar et al., 2017) by a class of chemotactic constituents like endotoxins of microbial origin carrying, C5a complement fragments, amino terminal N-formyl methionyl groups, cytokines, platelet activating factors, histamines, leukotriene B recruits intense leukocyte infiltration within minutes to the site of injury (Bitencourt et al., 2013). The successive cellular events encompass attachment to the microvascular endothelium (Nourshargh et al., 2010). The Cell adhesion molecules (CAMs) are concerned in the mobilization pathway and include intracellular adhesion molecule (ICAM)−1, ICAM-2, integrins and selectins. P-selectin, E-selectin released by endothelial cells and L-selectin secreted by white blood cells (WBC's) are the three families of selectins (Springer et al., 2012). The elevated affinity of adhesion presented on WBC's in the endothelium is initiated by the co-ordination between integrins (CDII/CD18) and adhesion molecules (CAM1 and CAM2) expressed on endothelial as well as WBC respectively (Ogra et al., 2012). Extravasations and transendothelial migration are highly complex event that happens when WBC migrate towards subendothelial space after a short stationary period (Sies, 2020). The chronic event inflammation is distinguished from the infiltration of mononuclear cells (eg-lymphocytes and monocytes), fibroblast proliferation, connective tissue and collagen fiber formation, which eventually leads to granuloma formation (Gleeson et al., 2011). Proteases, nitrogen and reactive oxygen species released from the infiltrated inflammatory cells attribute the chronic inflammation during tissue degeneration (Murakami et al., 2012). The alteration in the genomic expression of p53 has been approved as a marker for various chronic inflammatory diseases including rheumatoid arthritis, cancer and inflammatory bowel disorders (Ogrunc et al., 2014; Kong, 2013; Bachanova et al., 2020).
A diverse array of chemical mediators is released from inflammatory cells, circulatory system and from the injured tissue site to modulate the control of inflammatory responses (Halliwell et al., 2015). The major class of chemical mediators includes the vasoactive amines (serotonin and histamine), bradykinin peptides and eicosanoids (prostaglandins, thromboxanes and leukotrienes). To maintain the acute phase inflammatory response, histamine is released from the mast cells (Gilfillan et al., 2011). Serotonin produced from the decarboxylation of tryptophan and has major 4 serotonin receptors to execute the biological functions. Similar to serotonin and histamine, bradykinin increases the prostaglandin production which is physiologically active lipids molecules released and creates pain locally (Hsieh, 2014). Arachidonic acid, the major component of all phospholipids on cell membrane, affords as a substrate for the synthesis of eicosanoids an important inflammatory mediator (Mak et al., 2013). It comprises the products of cyclooxygenases (thromboxanes and prostaglandins), 5-lipoxygenase (5-hydroxyeicosatetraenoic acid and leukotriene) and 12-lipoxygenase (12-hydroxyeicosatetraenoic acid) (Piomelli et al., 2013; Lieberman et al., 2013). Another class of cytosolic oligomer of multiprotein that can induce inflammation in response to infection is inflammasome.
Several chronic diseases are associated with common inflammatory mediators/signals and their targeted regulatory pathways in their pathogenesis. An inflammatory stimulus triggers the intracellular signaling pathways thereby allowing and activate the synthesis and release of inflammatory mediators. Microbial products and cytokines interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) acts as primary stimuli and initiates inflammation through interacting with the toll like receptors (TLR)s, IL-1 receptor (IL-1R), IL-6 receptor (IL-6R), and the TNF receptor (TNFR) reported by Kaminska in 2005 (Kaminska, 2005). The important intracellular signaling pathways triggered after receptor activations are nuclear factor kappa-B (NF-κB), mitogen-activated protein kinase (MAPK) and Janus kinase (JAK) - signal transducer and activator of transcription (STAT) pathways (Hendrayani et al., 2016; Kyriakis et al., 2001; Henríquez-Olguín et al., 2015).
Cytokines have the potential to regulate the inflammatory pathway to adjust the immune response towards infection or inflammation through complex network of interactions. Therefore in contrast, uncontrolled production of cytokines leads to damaged tissue, changes in hematological parameters, organ dysfunction/failure and ultimately death (Czaja, 2014; Liu et al., 2016). The deeper insight towards the regulation of cytokine pathways would allow precise recognition of their role in inflammation and thereby providing new treatment strategies in inflammatory diseases.
Cytokines as chemical mediators
Cytokines are low molecular weight glycoproteins with the size of 30 kDa that are secreted by WBC and other various cells in response to several external or internal stimuli. Some cytokines possess their own roles or functions and others are regulatory proteins which aids assist in maintaining the expansion of immune effector cells. Cytokines are important in cell signalling and are involved in cell to cell communication. In 2003, Watford group reported that Interleukins, chemokines, interferons, tumour necrosis factor, lymphokines, are the examples of cytokines depending on their respective role, secretion, or by target of action but hormones or growth factors are usually not included (Watford et al., 2003). Chemokines are the class of cytokines with chemoattractant property and are secreted proteins that influence the immune system.An extensive range of cells, including immune cells like macrophages, T & B lymphocytes, mast cells, as well as fibroblasts, endothelial cells and various stromal cells produce and secrete cytokines. More than one type of cells can produce given cytokines (Lackie, 2010; Ibelgaufts, 2013). The cytokines act through binding to their receptors and are crucial in preserving the equilibrium in the immune system. They can regulate the development, progression, maturation and sensitivity of a particular cell population as well as can adapt the balance between cell-based and humoral immune responses. According to the study reported by Ibelgaufts affirms that cytokines can improve or restrict the action of other cytokines in very complicated ways (Ibelgaufts, 2013).
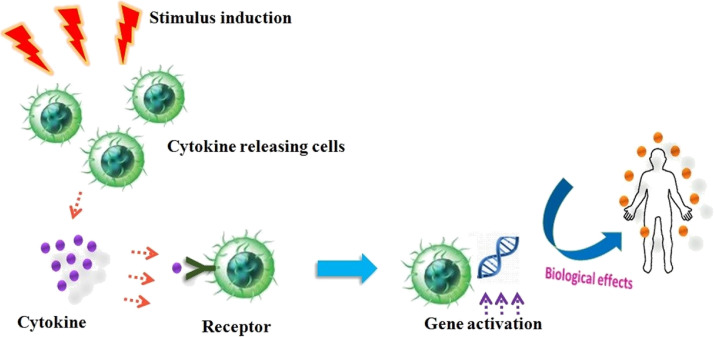
Cytokines are implicated and obligatory for regulation of hematopoiesis by controlling in cellular proliferation and separation and also involved in wound healing (Owen et al., 2013). Most of the cytokines contain its own corresponding receptors on cell-surface; receptor activation triggers cascade of signals intracellularly that directs to alteration in cell functions and their consequent action. The cascade of signal induction may comprise the activation or suppression of several genes and their corresponding transcription factors that altogether brings the generation of additional cytokines. These additional cytokines thereby amplify the surface receptors of additional molecules and can initiate feedback inhibition by suppression of its effect (Akira et al., 2004). Cytokines attach to the target cell membrane receptor and concurrently activate the pathways involved in signal-transduction that eventually modify the gene expression on the targeted cells which result in variation of specific biological responses (Khan, 2008) (Fig. 2 ).
Fig. 2.
Stimulus induction and biological effects of cytokines.
Cytokines play a very crucial task in coordinating and organizing typical immune homeostasis, maturation and in adapting defense against various contagious disease conditions (Fresno et al., 1997). It is stated by Dinarello that an elevated level of cytokines signifies activation of cytokine related pathways linked to inflammatory responses or disease development (Dinarello, 2000). The unexpected consequence of cytokine secretion are related to many disease conditions ranging from major depression and Alzheimer's disease to several malignacies whose intensity fluctuate from their normal physiological state. The various level cytokines in plasma can provide predictive insights on the inflammatory processes involved in autoimmune disorders (Miller et al., 2009). In a variety of immune and inflammatory disorders, they are regarded as effective therapeutic targets and agents for specific cytokine antagonists (Luer et al., 1996).
Properties of cytokines
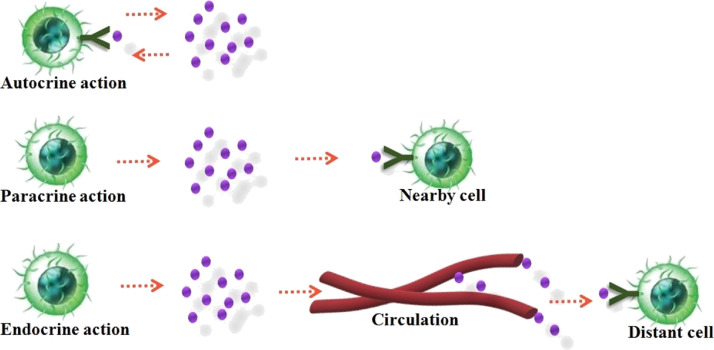
The cytokine productions are frequently transient and are strongly synchronized and coordinated. Cytokines have a high affinity for their receptors with extremely higher dissociation constants varying from 10– 10 - to 10–12 M. The homeostatic concentration of cytokines is very lower even to picomolar levels in body fluids owing to their high biological activity (Schenk et al., 2001). Their function in the bloodstream and extracellular fluids is only for a minimal period due to their shorter half-life (Owen et al., 2013). A specific cytokines bind to their corresponding receptors on the target cell membrane and mediates cell to cell communications by exhibiting autocrine, paracrine and endocrine actions (Wilson et al., 2002). The secreted cytokine binds with the receptors on the membranes within the very same cell that exude it in autocrine action. It put forth paracrine action by attaching to the target cell receptors on a near to the producer cell. In rare condition, cytokines connect to target cells in a distant part of the body exhibiting endocrine action (Fig. 3 ). A small quantity of cytokines is required to elicit a biological response. Another essential property of cytokines is to control the strength and extent of the immune responses by activating or inhibiting the commencement, proliferation, and discrimination of various cells. They also regulate immunological responses, hematopoietic growth and development, cell-to-cell communication and signaling as well as host response to contagious agents and inflammatory stimuli and thus acting as mediators and modulators within extremely localized environments (Lefkowitz et al., 2001).
Fig. 3.
Action of cytokines on the target cells.
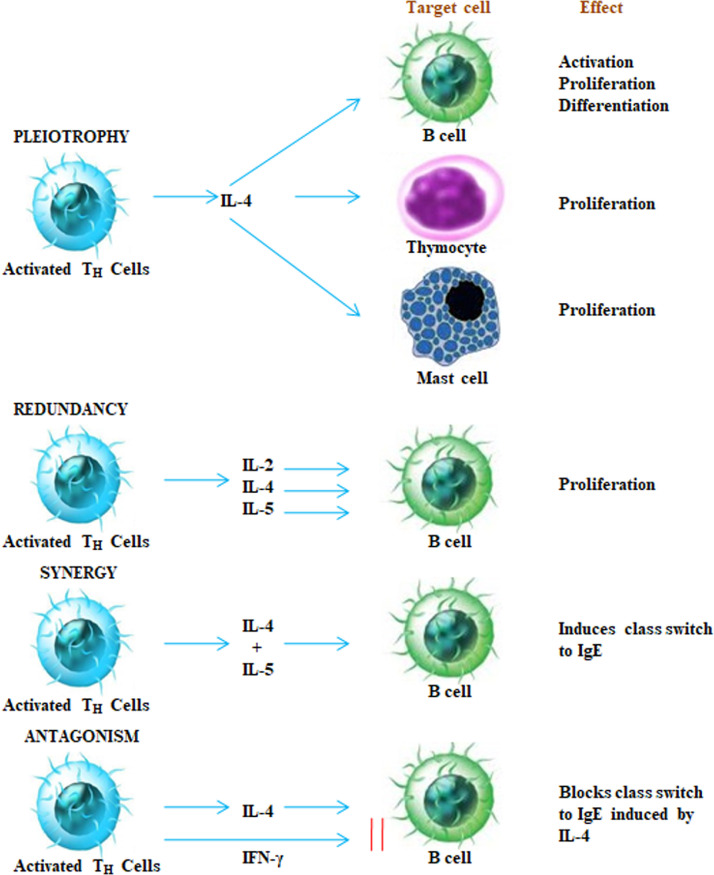
Cytokines has the capability to interconnect with each other in several multifaceted ways. They demonstrate the characteristic of “pleiotrophy, redundancy, synergy, antagonism and cascade induction” that allows to co-ordinate and control the cellular activities in an organized manner. Pleotropic activity is when a specific cytokine has diverse biological effects on distinct target cells. When two or more than two cytokines arbitrate related activity they are supposed to be exhibiting redundancy. The collective impact of two cytokines on cell function is more beneficial than the action of a single cytokine is referred as synergistic activity. In certain conditions, cytokines shows antagonism, which means that the effect of one cytokine inhibits or compensate the effect of a further cytokine as illustrated in Fig. 4 . In cascade induction, one cytokine acts on a specific target cell and leads to the release of one or more other cytokines which in turn persuades other target cells to produce and release further cytokines.
Fig. 4.
Target cells and effects of cytokines.
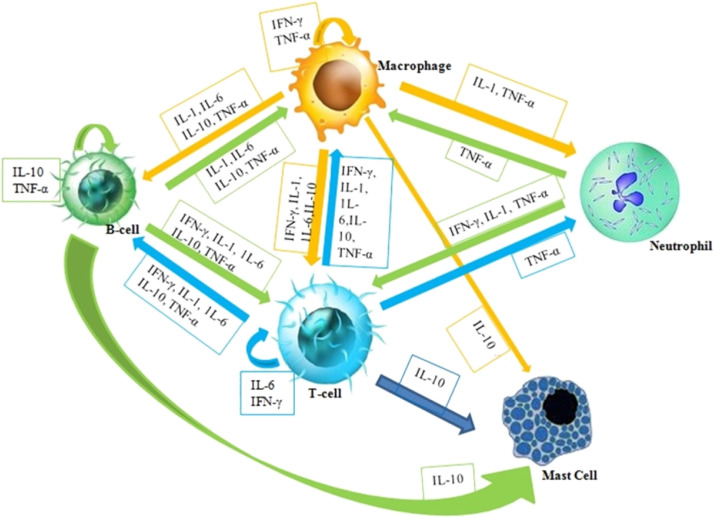
Cytokines contribute towards similar functions with hormones and growth factors but distinguishing between these 3 mediators are often indistinct. Hormones generally, circulate in nanomolar (10−9 M) concentrations, but cytokines constitutes in circulation only picomolar (10−12 M) level with shorter life span, but their concentration can elevate up to 1000 times during trauma and infections. In normal physiological condition, growth factors are often produced essentially, whereas specific stimuli are requisite for cytokines and hormones to get secreted. These released hormones by particular glands can tend to have specific effect on one or a few kind of intended cells. Apparently, cytokines are too frequently released and possibly attached to, a wide variety of cells (Owen et al., 2013). Their physiological effect regularly depends upon their respective concentration of several cytokines (Wakita et al., 2001). Thus cytokine levels illustrate the importance of recognizing the cytokine influence working as networks in an interconnected and coordinated manner (Fig. 5 ).
Fig. 5.
The illustration of cytokine networking between different cell types in the immune system and their molecular communication.
Classification of cytokines
Classification based on structure
The structural studies have made the classification of cytokines. They are categorized into four groups, such as the hematopoietin, interferon, chemokine and the tumour necrosis factor families. According to Cohen et al., cytokines could be categorized based on the origin of cell, their broad range of actions, the group of activity in which they sustain and regulate, the cells on which they are targeting, or the characteristics of ligand-receptor interaction (Cohen et al., 1996).
Based on the structural homology, there are four types of cytokines: The cytokines involved in the ‘four-α-helix bundle family’, hold a cluster of 4 alpha-helices with three-dimensional structure. It is the largest family of cytokines and comprise several cytokines other than involved in immunological responses such as erythropoietin (EPO) and thrombopoietin (TPO) and a few affiliates in this group share a typical gamma chain in their receptors as reported by Tonges and co-workers in their work (Tonges et al., 2007). The IL-2 sub-family, the interferon sub-family, and IL-10 sub-family are subsets of this family. The four alpha-helix bundle cytokines are grouped into a long and short chain cytokines (Rozwarski et al., 1994). IL-1 family mainly consists of IL-1 and IL-18. The IL-17 family has not yet thoroughly characterized. Some cytokines encompass a particular impact in advancement of T-cells proliferation, which may result in cytotoxicity (Arai et al., 1990). Another family, Cysteine–Knot cytokines includes transforming growth factor-beta superfamily- TGFβ1, TGFβ2 and TGFβ3.
Classification based on function
The classification of cytokines in which they are divided those to improve biological and cellular immune responses as type 1 including TNFα, IFN-γ etc. and type 2 with IL-4, 10, 13, TGF-β etc. that contributes to enhance antibody responses (Ouaguia et al., 2014). The effect of cytokines in one these subsets appear to get inhibited by cytokines in the other. The tendency of this associated dysregulation is of intensive research to identify the probable role played by these cytokines in the pathogenesis of autoimmune disorders (Jin et al., 2007). Majority of the inflammatory cytokines can induce oxidative stress (Vlahopoulos et al., 1999; David et al., 2007). The ability of some cytokines to trigger themselves for the secretion of other cytokines (Chokkalingam et al., 2013; Carpenter et al., 2002; Tian et al., 2005) leads to enhanced oxidative stress that finally results in chronic inflammation including fever and production of acute-phase protein in the liver such as IL-1,6,12 and IFN- alpha (Chokkalingam et al., 2013). Interferon-γ (IFNγ) is a prominent cytokine in the etiology and development of numerous autoimmune diseases and is also essential for defence against Mycobacterium tuberculosis and for other several intracellular microorganisms. In 2007 Dinarello reported that interleukin-2 is desirable for the production of cytotoxic T-cells (CTL) and outline the basis for several vaccines. But these cytokines are responsible to evoke the graft versus host reactions and restrict the accomplishment of bone marrow transplantation (Dinarello, 2007). Zhang (2007) et al., stated that cytokines are also involved in anti-inflammatory pathway and may be used to relieve pathological pain caused due to swelling or peripheral nerve injury. These pathways are regulated by both pro and anti-inflammatory cytokines (Zhang et al., 2007). Each individual cytokines perform specific role depending upon cell type and function.
Cytokines receptors
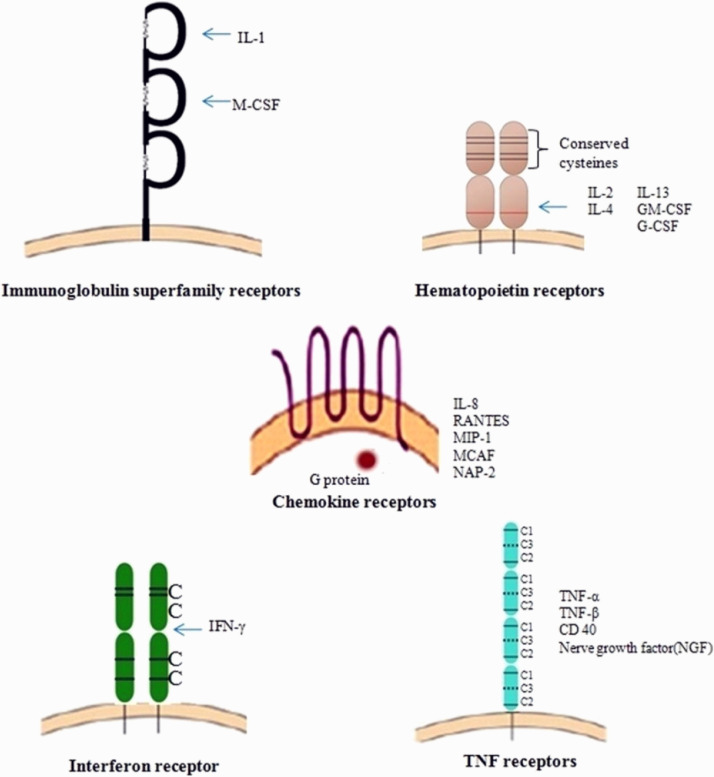
A wide range of cells expresses the cytokine receptors, which ultimately affect a varied range of cells. The biological and cellular effects of cytokines were exerted through various receptors found on the membranes of receptive target cells. These receptors comprises of an extracellular, a cytoplasmic and a single membrane-spanning domains. The extracellular domain (ECD) consists of conserved amino acid sequence motifs, and these motifs comprise four preserved cysteine residues and 2 polypeptide chains. One chain corresponds to α subunit specific cytokine, and the other is signal-transducing β subunit. The receptors for all the different cytokines belong to one of the five receptor protein family (Fig. 6 ).
-
I)
Immunoglobulin (Ig) superfamily receptors
-
II)
Class-I cytokine receptor family /hematopoietin receptor family
-
III)
Class-II cytokine receptor family /interferon receptor family
-
IV)
TNF receptor family and
-
V)
Chemokine receptor family
Fig. 6.
Representation of receptor families (Anwikar and Bhitre, 2010).
The class I cytokine receptor family includes the majority of the cytokine-binding receptors that functions both in the immunological and hematopoietic systems. In the extracellular domain, the members in this receptor family have conserved sequence of amino acid motifs consisting of 4 conserved positioned cysteine amino acid residues (CCCC) and a conserved sequence of tryptophan serine any amino acid-tryptophan serine (WSXWS, where X is the non-conserved amino acid). Apart from class I cytokine receptor family, otherwise known as hematopoietin receptor family, the receptors for all the cytokines identified as hematopoietins. The CCCC motifs are conserved in class II cytokine receptors, but the ‘WSXWS’ motif is lack in class I cytokine receptors (Owen et al., 2013). Multiple subunits are another common trait shared by both class I and II cytokine receptor families, with one subunit binding unique cytokine molecules and the other mediating signal transduction. These two functions however are not always restricted to one subunit or the other.
A number of sub-families of class I cytokine receptors have been recognized each with a signal-transducing subunit that are identical across all the receptors in a subfamily. The IL-5, 3, and GM-CSF receptors are the members of GM-CSF receptor subfamily. A considerable frequency of redundancy is exhibited by IL-3, 5, and GM-CSF. The progenitor and hematopoietic stem cells, and monocytes are activated by IL-3 and GM-CSF thereby, successive megakaryocyte differentiation. All the above three cytokines are involved in the induction of eosinophil proliferation and basophil degranulation accompanied with histamine release. The IL-6, 11, oncostatin M (OSM), leukemia inhibitory factor (LIF), and ciliary neurotrophic factor (CNTF) receptors are included in the IL-6 receptor sub-family. But this subfamily displayed overlapping biological activities after attachment of the cytokines to their respective receptors: OSM, LIF and IL-6 stimulate liver hepatocytes for the synthesis and production of acute-phase proteins and the segregation of myeloid leukemia cells into macrophages. The IL-6, LIF, and CNTF affect neuronal development, and IL-6, IL-11, and OSM stimulate megakaryocyte maturation and platelet production. The study reported by Khawar and Co-workers states that IL-1, 6, 8, and TNF-alpha are pro-inflammatory cytokines and chemokines that establish an environment that facilitates disease advancement (Khawar et al., 2015). These cytokines exhibit the property of chemoattractants and are not secreted by immune regulatory cells, but by stromal cells, tumor cells and also by tumour-associated macrophages.
The growth factors, such as VEGF and FGF, as well as basic matrix-degrading proteases are secreted by tumor associated cells (Rempel et al., 2000). The IL-2R subfamilies of receptors for IL-2, 4, 7, 9, and 15 have a general signal-transducing γ chain and each has a distinctive cytokine-specific α chain. IL-2 and 15 are trimers and commonly shares an IL-2R β chain. The monomeric IL-2R α exhibits lower affinity for IL-2, the IL-2R αβ (dimeric) demonstrated medium affinity, and trimeric IL-2R αβγ binds extremely with high affinity towards IL-2. The IL-2Rα chain (Tac) expressed by all activated T-cells excluding the resting T-cells. The IL-2R βγ is constitutively expressed in low levels by resting T cells and NK cells (Sprague et al., 2009). Henderson et al. reported that the defect in the IL-2R family γ chain results in X-linked severe combined immunodeficiency (X-SCID) which exhibits loss of activity from this family of cytokines (Henderson et al., 1998).
Cytokines that regulate innate immune responses
The innate immunity emphasizes to the non-specific defence mechanisms that appear to be in action instantly or due to sudden onset of an antigen's appearance within hours in the body. In this mechanism which involves the physical barriers such as skin, chemical mediators present in the blood, and immune cells of the immune system that attack foreign cells in the body. Cytokines imparts a crucial responsibility in innate immune responses. The detailed cytokines involved are mentioned below;
Tumor necrosis factor (TNF)
TNF (17 kDa) positioned at 6p21.33 signifies a multifunctional pro-inflammatory cytokine which activates signalling pathways for cell survival, cellular differentiation, inflammatory responses and apoptosis. It has been reported that, they are primarily secreted by macrophages, lymphoid cells, fibroblasts, mast cells and has the ability to cause cell death of certain cancer cell lines (Kawasaki et al., 2002). Beutler et al., reported that TNF is an effective endogenous pyrogen causing febrile response by direct interaction or stimulation attained from interleukin-1 secretion and is concerned in the initiation of cachexia. The stimulation of cell proliferation and differentiation can also occur at certain conditions after cachexia release (Beutler et al., 1985). In 1997 Marino et al., reported that upon immune system activation, TNF is produced which can put forth considerable cytotoxicity on various tumor cell lines and tumor necrosis in animal models (Marino et al., 1997). TNF can be divided into two molecular species; TNF-α and TNF-β activated by interferons.
The TNF ligand family members interact with their cognate membrane receptors to exert biological functions making up the TNF receptor (TNF-R) family (Locksley et al., 2001). During 1998, Naismith and co workers reported that TNF-R1 [TNF receptor type 1-CD120a; p55/60] and TNF-R2 [TNF receptor type 2-CD120b; p75/80] are two TNF–R family receptors that bind to membrane-integrated TNF (mem TNF), soluble TNF [sTNF] and also to the secreted homotrimeric molecule lymphotoxin-α (LTα) (Naismith et al., 1998). TNF-R1 is constitutively expressed in most tissues, while TNF-R2 expression is of exceptionally regulated and found primarily in cells of the immune system. The TNF-R1 appears to be the key mediator of TNF signalling in the vast majority of cells, TNF-R2 is a significant factor in the lymphoid system (Grell et al., 1995). TNFR1 is activated by TNF-α in most human tissues, while TNFR2 is mainly expressed in immune cells and activated by both TNF-α and β. TNF-α exhibits its pro-inflammatory signals after binding to the cell surface receptors, TNFR1 (p55) and TNFR2 (p75). It was reported by Englaro et al., that activation of TNFα triggers TNFR1 receptor to get trimerize thereby permitting death domains located on the cytoplasmic region of the protein TNFR1 to attach (Englaro et al., 1999).
After cell activation, TNF is expressed mainly on monocytes and T-cells, but by direct cell to cell interaction it mediates cell damage (Camussi et al., 1991). TNF are biologically active, enhances the production of prostaglandin E2 (PGE2) and collagenase that serves as an in vivo autocrine growth modulator for human chronic lymphocytic leukemia (CLL) cells moreover acts as growth factor for normal human fibroblast cells. It is further referred to as an autocrine growth modulator for neuroblastoma cells, with IL-4 inhibiting the autocrine growth-promoting operation (Wiemann and Starnes, 1994). TNF-α exhibits the ability to protect hematopoietic progenitors against cytotoxic agents and irradiation, recommending some probable therapeutic claim in aplasia induced by bone marrow transplantation or chemotherapy (Moreb et al., 1992).
Interleukin-1 (IL-1)
IL-1 (17 kDa) is one of the most important inflammatory cytokine, situated at 2q13 position. It is primarily secreted by monocytes, fibroblasts, dendritic cells, and tissue macrophages. Moreover in certain instances it is also expressed by NK cells, B lymphocytes and epithelial cells. IL-1 facilitates stimulation and enhanced expression levels of endothelial cells associated adhesion factors for the transmigration of immunocompetent cells, such as phagocytes and lymphocytes towards the site of infection. It has the direct effect on the thermoregulatory centre at hypothalamus, there in which it activates the febrile responses leading to rise in body temperature thereby considered as an endogenous pyrogen. Other than the capability to induce pyrexia, IL-1 can aggravate increased pain sensitivity (hyperalgesia), hypotension and vasodilatation (Simi et al., 2007). IL1-α and β are two functionally similar forms of IL-1 that are determined by the expression of two different genes where IL1-α in mice whereas IL1-β is major type in humans (Auron et al., 1985). Interleukin-1 receptors (IL-1R) are divided into type I and II receptors.
The IL-1 type I receptor is mainly concerned in transmitting the inflammatory effects of IL-1 while type II receptors counteracts by competing for IL-1 binding site to suppress the of IL-1 activity. Lo et al. studied and described the interaction of transmembrane IL-1 receptor accessory protein (IL1RAP) as IL-1R is required for triggering IL-1 signal transduction (Lo et al., 1996). One of the key biological attributes of IL-1 is the activation of T-helper cells that induce the secretion of IL-2 and produce large amount of IL-1 inhibitor by virus-infected macrophages thereby promoting opportunistic infections as well as to support the cell transformation in patients with T-cell maturation defects. It directly acts on B-cells, thereby promoting proliferation and immunoglobulin synthesis furthermore functions as one of the primary factors that make B-cells accessible to IL-5 (Koyama et al., 1988).
Interleukin-12 (IL-12)
IL-12 is a pro-inflammatory cytokine molecule with a molecular mass of 70 kDa comprising of two covalently-linked subunits of IL-12 p35 (35 kDa) expressed on 3q25.33 and IL-12 p40 (40 kDa) on 5q33.3 chromosome position. It is measured as a vital immunoregulatory cytokine and released mainly by antigen-presenting cells (APCs). IL-12 encompasses multiple biological activities and prominently involved in the early non-specific innate resistance and apparently the subsequent antigen-specific adaptive immunity (Presky et al., 1996). It is clearly reported in the study conducted by Trinchieri et al. that upon activation, IL-12 binds to a membrane receptor complex constituting two subunits: IL-12R β1 and IL-12R β2, which are class I cytokine receptor family members including IL-6, IL-11 and leukocyte inhibitory factor (LIF) related glycoprotein gp130 (Trinchieri et al., 2003). The macrophages, dendritic cells and less significantly human B cells produce IL-12 but not produced by murine B cells after subsequent CD40 ligation (Bost et al., 1999). The production of IL-12 is highly regulated by positive and negative feedback mechanisms with the involvement of Th1 cytokines (e.g., IFN-γ), Th2 cytokines (e.g., IL-10) and type 1 IFN (Bonecchi et al., 1998). These cytokines are also involved in T cell trafficking and migration by activating functional adhesion molecules such as P- and E-selectin ligand expressed on Th1 cells but not in Th2 cells(Flesch et al., 1995).
Interleukin-10 (IL-10)
IL-10 pleiotropic immunoregulatory cytokine (37 kDa), positioned at 1q32.1 chromosome that defend against infection-associated autoimmunity, immunopathology and allergic reactions. Initially IL-10 was termed as ‘cytokine-synthesis inhibitory factor’. It down regulates the production of IFN-γ, IL-2 and other pro-inflammatory cytokines thereby act as an anti- inflammatory or immunosuppressive cytokine (Fiorentino et al., 1989; Moore et al., 1993). The IL-10 receptor is a multifaceted efficient tetramer made of two identical ligand-binding subunits (IL10R1) that are expressed in activated hematopoietic cells and the other two identical accessory signaling subunits (IL10R2) expressed on the majority of cells and tissues (Moore et al., 2001). Del Prete and co-workers demonstrated in their study that IL-10 hinder the synthesis of Th1 but not affect the Th2 cytokine production antagonized by IL-4 and was shown to be a physiological competitor of IL-12 (Del Prete et al., 1993). According to Pecanha et al. IL-10 hinders the secretion of immunoglobulins by T-cell independent antigens that are stimulated specifically by IL-5 but not by IL-2 (Pecanha et al., 1992).
Cytokines that regulate adaptive immune responses
The adaptive immune response is also referred to be acquired immune response, is a sub-category in the immune system constituting systemic, specialized cells and processes that eradicates pathogens by preventing their growth. In the vertebrates, the acquired immune system is one of the major immunity strategies found and creates immunological memory cells after an initial response to a specific pathogen, and can enhance the immunological responses while subsequent encounters with that pathogen.
Interleukin-2 (IL-2)
IL-2 (15 kDa) positioned at 4q27 is a 133 amino acid residue single polypeptide chain. Subsequent binding of immune regulatory T helper cells to Antigen-presenting cells (APC) induces IL-2 synthesis and are released by CD4+ cells. The IL-2 interaction with its receptors on T cells stimulates the proliferation and differentiation that finally leads to the release of additional cytokines. The production of CD8+ cytolytic T cells is triggered by IL-2, which contributes an essential role in anti-viral responses thereby boosts the effector function of NK cells. It improves the ability of the immune system to target tumor cells and thereby interfere with inhibiting the blood flow towards the tumors. The proliferation of activated B-cells is thus enhanced in the presence of IL-4. IL-2 put its direct involvement on T and B-cells, correspondingly functions as a central regulator of immune responses in anti-inflammatory responses and tumor monitoring. It also exhibits additional effects on other cellular immune system components including B-cells, macrophages. Thereby triggers the release of other soluble mediators, including TNF-α, β and IFN-γ which contributes antitumor activity of IL-2 as well as dose-related toxicity. IL-2 is found to be effective in some patients with antibody deficiency, probably caused due to lack of T-cell assistance for B-cells (Waguespack et al., 1994).
Interleukin-4 (IL-4)
IL-4 (18 kDa) pleiotropic cytokine about 129 amino acids located at 5q31.1. They are usually produced by mast cells, TH2 cells and NK cells, specialized subsets of T cells, basophils and eosinophils. It controls the regulation of antigen-activated naive T cells differentiation and production of other TH2 type cytokines comprising IL-5, IL-10 and IL-13. An additional property of IL-4 is to suppress the TH1 cell production. It is requisite for further production of IgE and the principal cytokines that creates isotype switching of B cells from IgG expression to IgE. It arbitrate its effects on specific IL-4 receptors that are expressed on several tissues, including hematopoietic cells, endothelium, epithelial cells, hepatocytes, fibroblasts, neurons and muscles (Daniela et al., 2017). IL-4 receptor binds to α chain of IL-4 with high affinity. This cytokine plays a crucial role in the etiology and progression of chronic lymphocytic leukemia, which is discriminated by the abnormal accumulation of long-lived and slow-dividing monoclonal B-cells. They are possibly arrested at the intermediate stage of their differentiation by preventing malignant B-cells from proliferation and finally to death (Dancescu et al., 1992).
Interleukin-5 (IL-5)
TH2 lymphocytes predominantly secretes IL-5 (45–50 kDa) located at 5q31.1. They are normally found in mast cells and eosinophils and directly control the growth, differentiation, and longevity of eosinophils. It facilitates eosinophil migration, tissue localization and its own function, there by blocking programmed cell death (apoptosis). Eosinophils contribute a major role in the pathogenesis of allergic reaction and asthma and defend against helminths and arthropods. The IL-5 receptor is a heterodimer of α and β subunits. The α subunit is highly specific, while the β subunit is common shared to IL-3, IL-5, and granulocyte/macrophage colony-stimulating factor (GM-CSF) receptors and essential for signal transduction (Daniela et al., 2017). IL-5 stimulates the production of IgA and accelerates proliferation and differentiation of antigen-induced B lymphocytes. TH2 cytokines such as IL-4 and IL-5 plays a central role in the induction of airway eosinophilia and airway hyper-responsiveness (AHR). It is the foremost player in influencing and maintaining the eosinophilic airway inflammation (Daniela et al., 2017).
Interferon-γ (IFN-γ)
IFN-γ (50 kDa) located at 12q15 is produced by activated T lymphocytes (TH1 and CD8+ cells), NK cells, B cells, T cells and professional APCs modulates a number of components and mediators of the immune response. The IFN- γ activates mononuclear phagocytes and augments the expression of both MHC class I and II molecules. It results in the up regulation of MHC class I molecules which plays an essential role for host defence against intracellular pathogens. Thereby imparting improved susceptibility towards recognition and subsequent activation of cytolytic T cells activated cell-mediated immune response. IFN-γ inhibits cell growth, proliferation and induces co-stimulatory molecules on the macrophages, which boosts cell-mediated immunity. As a result it leads to the activation and enhancement of anti-tumor and antimicrobial activity possessed by granulocytes, mononuclear phagocytes, and NK cells.
The two ligand-binding IFNγR1 chains associated with two signal-transducing IFNγR2 chains and connected signaling machinery are the effectual IFN-γ receptors (IFNγR). IFNγR1 chain usually a supplementary whereas IFNγR2 chain which is constitutively expressed but its expression levels controlled according to the state of cellular differentiation or activation is the limiting factor in IFN-γ responsiveness. IFN-γ attributes anti-viral and anti-parasitic activities and as well hinders the proliferation of a number of normal and transformed cells. IFN-γ synergizes with TNF-α and β in attenuating the proliferation of various cell types. While comparing with other interferons, the more prominent growth inhibitory activity is exhibited by IFN-γ. It inhibits the synthesis of collagens by myofibroblasts and proliferation of endothelial cells. It acts as capillary growth inhibitor mediated by myofibroblasts and fibroblast growth factors.
Interleukin-13 (IL-13)
IL-13 (15 kDa) were initially recognized for its effects on monocytes and B cells, which incorporates isotype switching from IgG to IgE, thereby inhibiting inflammatory cytokines and enhance MHC class II expression. They belong to α-helix superfamily as IL-4, and genes located 12 kb apart on chromosome 5q31.1. Primarily, IL-13 appeared similar to IL-4 until its specific effector capabilities were recognized. However, IL-13 and IL-4 exhibits a number of similar effects (Daniela et al., 2017). For signal transduction, IL-13 requires signalling molecules associated with large intracellular domain the receptor subunit, IL-4Rα, a heterodimeric receptor complex IL-13 plays an indispensable role towards resistance to the majority of Gastro-Intestinal (GI) nematodes. It controls production of mucus, inflammatory responses, fibrosis and tissue remodelling. For a number of pathological conditions including idiopathic pulmonary fibrosis, asthma, ulcerative colitis and cancer IL-13 is considered as therapeutic targets. TH1 responses are supposed to inhibit by IL-13, which will hinder the ability of the host to eradicate the invading pathogens. The role played by IL-13 in the etiology/ pathogenesis of allergic disease and asthma has gathered widespread consideration (Daniela et al., 2017).
Transforming growth factor-β (TGF-β)
TGF-β (25 kDa), is a regulatory molecule with numerous effects on cell proliferation, differentiation, migration and survival that influence multiple biological processes, including development, fibrosis, wound healing, carcinogenesis, and also restrain immune response in the periphery to prevent autoimmune responses. It's a pleiotropic cytokine located at 19q13.2 and the family of TGF-β contains the three closely related isoforms, namely TGF-β1, β2 and β3, which are produced as large latent, non-functional complexes with proper folding. After interaction with critical partners like latent TGF-β binding proteins (LTBPs) or fibronectin the secretion/release from storage sites is controlled by disulfide bonds or by many different activation factors (Dennler et al., 2002). TGF-β family members consist of type I and type II heterotetrameric transmembrane receptors which initiate intracellular signalling. Types I and II have an N-glycosylated extracellular domain that is rich in cysteine residues, one transmembrane and an intracellular serine/ threonine kinase domain which was reported by Su et al., in 1991. TGF-β is the most potent known growth inhibitor for normal and transformed epithelial cells, endothelial cells, lymphoid cells, fibroblasts, neuronal cells, hepatocytes and keratinocytes. TGF-β has mainly suppressive effects on the immune system since it inhibits the IL-2 dependent proliferation of T cells and B-lymphocytes and inhibits B-cell maturation. It also holds back the interferon-induced cytotoxic activity of natural killer cells, the function of cytotoxic T-lymphocytes and the proliferation of the precursors of lymphokine-activated killer cells (Su et al., 1991).
Cytokines inhibitors
The biological activities of cytokine have been reported in several studies by a large number of proteins. Cytokine antagonists are molecules which bind to cytokines or their receptors thereby inhibiting their activity (Schreiber et al., 2010). The cytokine inhibitors which arise from the enzymatic cleavage of the extracellular domain of cytokine receptors are found in the bloodstream and extracellular fluids as soluble antagonists. IL-1 receptor antagonist (IL-1Ra) is highly specific for IL-1 creates hindrance in binding of IL-1α and IL-1β to their receptor. The production and secretion level of IL-1Ra plays a role in regulating the intensity of the inflammatory response. The continuous shedding of membrane receptor fragments are competitively involved for cytokine binding in throughout immune response. Some viruses also produce cytokine-binding proteins or cytokine mimicking molecules thereby influence cytokine actions. For example, the Vaccinia virus causative agent for smallpox and cowpox releases soluble molecule which binds to IFNγ.
In contrast, Epstein-Barr virus causing infectious mononucleosis encodes a molecule homologous to IL-10 that suppresses immune function in the host system (Phillips, 2002). The molecules secreted by viruses that mimic cytokines allow the viruses to manoeuvre the immune response in the ways that aids the continued survival of the pathogen. It is an interesting and powerful adaptation that some viruses have to undergone in their ongoing struggle to overcome the alarming barrier of host immune response.
Cytokines as drugs
Pro-inflammatory cytokines has the potential to mediate several biological processes and are highly controlled and regulated in the body. Chronic uncontrolled release of particular cytokines can initiate and derive much pathological conditions such as autoimmune disorders and neoplasia. Over the past decades the activity of inflammatory cytokines are regulated or controlled either supplementation by recombinant anti-inflammatory cytokines or neutralizing using blocking antibodies. The discovery of IL-1 by Auron and co-workers (Wojdasiewicz et al., 2014), is employed to treat cancer patients undergoing chemotherapy and additionally used for patients suffering from anaemia. Since IL-1 exhibits neutrophilic effects, it is assumed possibly to restore neutrophil counts in neutropenic patients to normal level (Iizumi et al., 1991; Crown et al., 1991; Walsh et al., 1992). But IL-1 treatment resulted in severe side effects such as fever, rigours, joint aches, fatigue, headache, and nausea as it is a potent pro-inflammatory cytokine. IL-1 and 2 showed possible natural immuno-stimulanting ability to combat the immune deficiency of AIDS. The hypothesis that immune-stimulant cytokines could help neutralize the immune-suppression of cancer and AIDS is supported by various clinical and experimental studies. Patients with anaemia and bone marrow failure are routinely administered with Erythropoietin (EPO). IFN-α another cytokine involved in response to viral infections and are preferred to patients suffering with hepatitis B, and C and for the treatment of multiple sclerosis IFN-β for is effective (Dinarello, 2007). The PEGylated form of IFNα is given to increase antiviral immunity by enhancing CD8+ cell response in chronic hepatitis-B virus (HBV) and hepatitis-C virus (HCV) (Zoulim et al., 2016); Dienstag et al., 2006) and besides providing an emergency treatment for acute HCV. The major host defense responses against viral infection are the antibody interaction and virus neutralization. In Fab fragmented mechanism, that generally involves the monovalent antibodies with single antigen binding site and has the ability to block endogenous immunoglobulins on cells. The study reported by Crowe et al., in 2001 demonstrated a potent virus neutralizing activity mediated by the virus antigen binding fragments (Fab) (Crowe et al., 2001). The PEGylated Fab fragment against TNF-α was found to be effective against Crohn disease (Bourne et al., al.,2008).
During the initial period for treating rheumatic arthritis (RA) etanercept was the only approved drug. Later, Infliximab anti-TNF antibodies were first approved for the treating severe Crohn's disease and also for rheumatic arthritis (RA). Adalimumab is an approved drug for rheumatoid and psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, moderate-to-severe chronic psoriasis, moderate-to-severe hidradenitis suppurativa, Crohn's disease, juvenile idiopathic arthritis, and non-infectious uveitis. Anakinra, a recombinant nonglycosylated form of IL-1Ra approved in 2001 for rhematoid arthritic patients for those not responding to any other anti rheumatoid drugs, like disease modifying antirheumatic drug (DMARD). Afelimomab treatment in patients of severe sepsis showed significant reduction in IL-6, TNF-α expression levels and severity of organ dysfunction (Panacek et al., 2004). For twelve weeks treatment with etanercept, a TNF-α antagonist in patients of refractory asthma showed significant improvement in asthma control and systemic inflammation (Morjaria et al., 2008). Blocking IL-6 signalling explained much beneficial effects in both experimental and human disease models. Further inhibition of IL-6 using tocilizumab could reverse or prevent symptoms typically associated with rheumatic diseases (Lipsky, 2006).
Recently Zhang et al., reported from their clinical data suggesting that there are mild or severe cytokine storms in severe patients with COVID-19 infection, which is a significant reason for mortality. Therefore, the timely treatment for cytokine storm has become an essential part of rescuing severe patients. The specific phenomenon namely the cytokine release syndrome (CRS) is mainly initiated and controlled by the cytokine Interleukin-6 (IL-6) which accelerates the disease severity. So blocking the IL-6 signal transduction pathway by Tocilizumab is expected to be a new treatment strategy method for severely affected patients (Zhang et al., 2020a). HER2-specific monoclonal antibody- Trastuzumab (Herceptin) used as effective treatment for adenocarcinoma of the stomach or the gastroesophageal junction as well as for aggressive form of breast cancer (Hudis et al., 2007). For the treatment of asthma, antibodies against cytokines like IL-4, 5 and 13 are used and TNF-α, IL-10 &11, anti- IL-12 and used for the preventing psoriasis. Anti-inflammatory cytokines, like IL-10 and IL-11, are used for curing Crohn's disease and ulcerative colitis (Zidek et al., 2009). The counterbalancing of endogenously produced IL-4 in bronchial asthma patients is attained after treatment with altrakincept, a recombinant human soluble IL-4 receptor (Condos et al., 1997). The pre-clinical study conducted by Alison et al. provides a beneficial treatment strategy with combination of cytokine inhibitor, interleukin 1 receptor antagonist and PEGylated tumor necrosis factor 1 against rheumatoid arthritis (Bendele et al., 2000).
Cytokines and associated inflammatory disorders
The abrupt and over expression of cytokine signaling contributes to pathogenesis and its related complexity in several disease conditions (Mehta et al., 1998). The up-regulated expression level of IL-1 and IL-6 is observed in lupus nephritis, rheumatoid arthritis, type 1 diabetes, psoriasis and, sclerosis considered as the chronic inflammatory and autoimmune disorders (Rosa et al., 2008; Park et al., 2007; Portugal-Cohen et al., 2012; Brugos et al., 2012; Kawaguchi et al., al.,1999). TNF-α illustrate a pivotal role in cascading inflammatory reactions in both systemic and localized level. It exhibit direct involvement in the pathogenesis of many systemic diseases. It is of greater significance that the appropriate level of TNF- α is beneficial for maintaining the key homeostatic functions of normal cells like cell proliferation, necrosis and apoptosis (MacEwan, 2002). TNF-α triggers the lipid mediators on vascular epithelial cell expression promoting oedema as well as eliciting immune cell infiltration by activating leukocyte adhesion molecules (Bradley, 2008). Several studies have pointed out the importance of anti-TNF-α therapy for inflammatory diseases. A highly effective therapeutics for synovial inflammation by TNF-α blockers was accomplished only because of the greater insight about the TNF-α role to attribute inflammation (Feldmann et al., 2010).
Certain precise chemokines and their receptors are observed in their elevated form in various inflammatory disease conditions. The high detection level of IL-8 in synovial fluid from patients with several types of rheumatic arthritis (Seitz et al., 1992) and also in mucosal secretion with active ulcerative colitis (Mahida et al., 1992) was remarkably observed. In psoriasis, pathogenesis is connected with inflammation mediated by chemokine and lymphocyte recruitment in the skin due to infiltration of T cells by CXCR3 ligand (Flier et al., 2001). For determining the role of distinct chemokine in particular disease, the identification of prominent chemokine receptor distribution of leukocytes contribute a better approach towards the treatment strategy. Relatively new sort of auto-inflammatory disorders consist of a group of inherited diseases affecting the innate immune system. The hereditary recurrent fever syndromes are mainly associated with a group of six disorders. The auto-inflammatory disorders share common similarity with auto immune ailment categorized by intense recurrence of fever, rash and joint swelling and carry the risk of amyloidosis that leads to accumulation of fatal proteins in organs. The most prevalent and severe autoinflammatory disorder is the Familial Mediterranean fever (FMF) and some other types are neonatal-onset multisystemic inflammatory disease (NOMID) syndrome, tumour necrosis factor receptor associated periodic syndrome (TRAPS), mevalonate kinase deficiencies (MKD), moderate hyper immunoglobulin D and periodic fever syndrome (HIDS), severe mevalonic aciduria (MA), familial cold urticaria (FCU) otherwise known as familial cold-associated syndrome (FCAS) (Savic et al., 2012), Muckle Wells syndrome (MWS) and disorder associated with IL-1Ra (DIRA) (Aksentijevich et al., 2009). These disorders exhibited recurrence of heightened local and systemic inflammation and abrupt innate immune system function.
The periodic attack of pyrexia and acute phase inflammation associated with serositis generalize Familial Mediterranean fever (FMF) (Farasat et al., 2008). During the disease condition, leukocytosis, increased level of erythrocyte sedimentation rate and acute phase reactants such as C-reactive protein, fibrinogen and serum amyloid A were observed (Sohar et al., 1967; Baykal et al., 2003; Gang et al., 1999). The symptoms of FMF is due to the mutation to the pyrin protein which leads to the over activation of the pro inflammatory pathway or suppression of the anti-inflammatory route which potentially affects the protein complex system. Tumor necrosis factor receptor-associated periodic syndrome (TRAPS) an exceptional autosomal dominant disease arising with recurrent periodic fever and common after (FMF) globally (Hull et al., 2002). The disease is characterized accordingly on the basis of frequent fever, migratory myalgia, rash, abdominal pain, and periabdominal oedema. Different mutations associated with the TRAPS results in distinct activities of NK-κB family subunit thereby generation of unique cytokine release profile (Nedjai et al., 2011). The cryopyrin-associated periodic syndromes (CAPS) comprise Neonatal onset multisystem inflammatory disease (NOMID) otherwise recognized as chronic infantile neurologic cutaneous articular (CINCA) syndrome. This usually affects the tissues and organ system including the eyes, skin, joints, and finally central nervous system. Almost children develop the initial symptoms within first six week after birth. The health issues follows as fever, joint damage, meningitis, loss of vision and hearing and mental retardation. This disease condition is rigorous and finally leads to death in affected children (Kitley et al., 2010). The lack of interleukin-1 receptor antagonist (DIRA) has been linked with auto inflammatory disorders caused by mutation in the IL1RN gene that encodes interleukin receptor antagonist and has a significant impact on the skin and bone (Aksentijevich et al., 2009). Swelling of the bone tissues, resulting in severe pain, discomfort and deformity along with inflammation of periosteum and pustules are all the fatal signs for children with this condition.
Inflammatory bowel diseases (IBD) condition of inflammation of gastrointestinal tract (Műzes et al., 2012) characterized by chronic, self destructive exhibited in Crohn's disease and ulcerative colitis. It is also evidenced that there is an elevated level of pro-inflammatory cytokines IL-1, IL-6, IL-8 and TNF-α in the mucosal immune system (Sartor, 1994; Elson et al., 1995). In allergic conditions especially in asthma inflammation plays a major role in disease progression in which chemokine CCL 11 (eotaxin) and their receptors recruit eosinophils to the lungs (Pope et al., 2005). Another common multi-centre inflammatory disorder is the Behcet's disease with warning sign comprising oral and genital canker ulcers or sores and ocular inflammation. In some patients symptoms varies from arthritis, skin problem, inflammation of digestive tract, spinal cord and brain (Paovic et al., 2013). Whilst the exact route cause of Bachet's is not recognized but major symptoms originated from the inflammation of the blood vessels. Elevated levels of IL-1, 2, 4, 6, 8, 10, 13, 18 and TNF- α (Kulaber et al., 2007; Musabak et al., 2006) cytokine release in the serum and patients with Bachet's disease had higher levels of IL-15 in cerebral spinal fluid. Rheumatoid arthritis recognized by the presence of rheumatoid factor (auto-antibodies) is an autoimmune disease and also leads to tissue damage (Feldmann et al., 2010) by chronic inflammation. Deregulated production of cytokines in the synovial tissue was identified as the initial possible factor in rheumatoid arthritis pathogenesis (Buchan et al., 1988). The onset of many neurodegenerative disorders is associated with inflammation.
The greater advancement in knowledge of cytokines in brain physiology has led to unravel its harmful effect due to over expression and also their association with neurodegenerative diseases like Parkinson's disease (PD) and Alzheimer's disease (AD). The progressive complication in the above mentioned neurodegenerative disorders are mainly due to the multifactorial changes assorted by associated inflammatory molecules involved in the disease progression. The most frequent age related neurodegenerative movement disorder Parkinson's disease (Schapira, 2009) with slowed motions, restless tremor, muscle rigidity and posture instability are the common symptoms (Martí et al., 2013; Berardelli et al., 2013). It is characterized with the progressive dopaminergic neuron degeneration. Both molecular and cellular mechanisms are accountable for the Alzheimer's disease that have been widely studied and are mainly due to the inflammation caused in the brain plays a crucial role in disease advancement. According to research, implies that peripheral infection can influence the inflammation of the central nervous system including chronic periodontitis which are directly linked with Alzheimer's disease (Stein et al., 2012). It must be of important consideration that attempts made to treat neuroinflammatory responses and neurological disease should avoid as it creates unwanted aftereffect to both the brain and the immune system.
COVID-19 associated hyper immune response and cytokine storm
The world has encountered the coronavirus outburst of infection that threatened the worldwide pandemic in 2002–2003 with Severe Acute Respiratory Syndrome (SARS) and in 2011 by Middle East Respiratory Syndrome (MERS). The causative viral agents in both situations (SARS-CoV and MERS-CoV) were newly recognized as zoonotic origin coronavirus in the genus Betacoronavirus. By the end of 2019, another coronavirus outbreak causing respiratory-associated illness was identified in Wuhan, Hubei, China, the disease now officially called "Corona Virus Disease 2019; COVID-19". Majority of COVID-19 patients were without any symptoms or some people experienced medium to severe respiratory infection and difficulty in breathing. Eventhough, in some cases lethal cases with multi-organ and systemic expression like septic shock, sepsis and multiple organ dysfunction syndromes (MODS) have also been observed (Yang et al., 2020). Cytokine storms (CS), one of the major characteristic events observed with elevated or uncontrollable release of pro-inflammatory cytokine level that can triggered various infectious or non-infectious diseases (Gu et al., 2019), and severely damaged multiple organs. The COVID-19 outbreak was declared by ‘World Health Organization’ as a pandemic, with a crude mortality rate of around 2.3% (Novel, 2020). In general, for several viruses like influenza A, HIV and cytomegalovirus adipose tissue serves as a reservoir and is speculated also for the COVID-19 virus (Kassir, 2020). So it serves as an important source of IL-6 and also its receptor, IL-6R. Therefore, adipose tissue can provide as a reservoir for IL-6 activation and cascading signals for viral multiplication and infection. Zhang et al. reported that cytokine storm can be triggered by several infectious, rheumatic diseases, and in tumor immunotherapy, and it usually leads to systemic inflammation, and multiple organ failure (Zhang et al., 2020a).
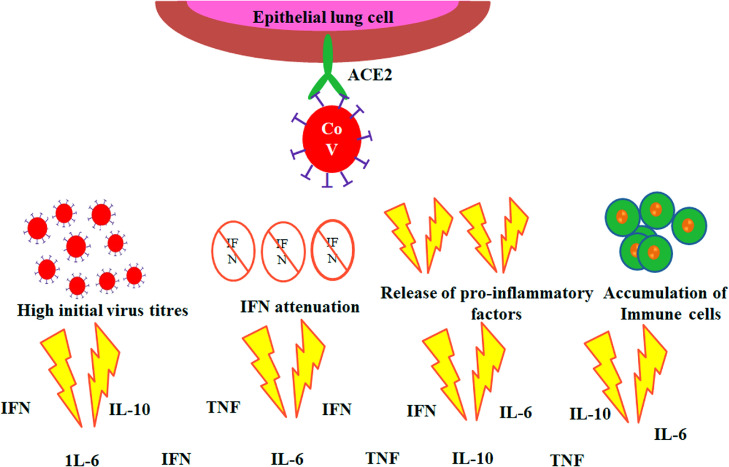
It is reported by several studies that the hyper-inflammatory response induced by severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) is a main causative reason for disease severity and death. Nevertheless, there are currently considerable prognostic biomarkers of pathogenic inflammation to target immunological pathways are critically lacking. A hyper-inflammatory syndrome or a “cytokine storm” thought to be the reason for poor COVID-19 diagnosis. In view towards the point of care to quantify cytokine levels at is necessary for the better understanding the disease progression. Many of the patients stayed behind to be asymptomatic but in others severe pneumonia developed and later followed by acute respiratory distress syndrome (ARDS). Currently, cytokine storm is considered as the useful prognosis biomarker and this urged the demanding overexpose of immunomodulatory drugs with the expectation of halting the disease progression and thus improving the symptoms (Zhao, 2020). The efficiency and counter effects of the treatment are yet to be investigated for predicting the time and dosage of the prevailing drugs to get success. In this present situation, there is an urgent need for the development of methods to check the cytokine release profile in COVID-19 patients. This will enable to detect the worsening condition of COVID-19 patient to diagnose and treat them before getting seriously ill. Measuring of cytokine levels also can make available with personalized drugs to control inflammation and to monitor their efficacy. SARS-CoV-2 infects epithelial lung cells using specific interactions with the angiotensin converting enzyme 2 (ACE2). Strong efforts are being made worldwide to better realize cytokine storm that can characterize the disease progression from severe pneumonia to ARDS (Fig. 7 ) which indicates as the major factors (Channappanavar et al., 2017; de la Rica et al., 2020; Coperchini et al., 2020).
Fig. 7.
Schematic representation of events leading to ‘cytokine storm’ in COVID-19.
In seriously ill COVID-19 patients, elevated level of pro-inflammatory cytokines such as IL-1, IL-6, 8, and tumor necrosis factor alpha (TNF-α) in the sera has been observed. (Qin et al., 2020; Huang et al., 2020; Wong et al., 2004; Kim et al., al.,2016; Wang et al.,2020; Chen et al.,2020; Yuan et al., al.,2020; Klein et al., 2020; G. Chen et al., 2020; Dong et al., 2020). So this indication can be considered for the detection of cytokines as good prognosis biomarkers as they can point out the disease progression from severe or critical in COVID-19 condition. The IL-10 most predominant anti-inflammatory cytokines has also been found in COVID-19 patient sera (Klein et al., 2020; G. Chen et al., 2020; Dong et al., 2020; W. Wang et al., 2020). It is also identified from other hyper-inflammatory syndromes that dysregulated inflammation is generally accompanied by immune suppression, (Patricio et al., 2019) making high risk for opportunistic infections from bacteria, fungi, or even from latent human cytomegalovirus (CMV) in these patients (Alba-Patiño et al., 2019; Mansfield et al., 2015; Monneret et al., 2011). The phenomenon of “Immunosenescence” (Bektas et al., 2019) contributes a critical role within the age-associated severity of COVID-19. Immunosenescence creates or prepare the innate immune response to be more active, triggers and evoke the amount of natural killer cells (NK) and ultimately releasing pro-inflammatory cytokines, such as Interleukin 6 (IL-6), Tumor Necrosis Alpha (TNF-α), and C-reactive protein (CRP). Eventually resulting in a chronic, low-grade inflammation developing a phenomenon termed as "inflammaging” (Franceschi et al., 2018). “Inflammaging” is considered to be a major risk factor for both mortality and morbidity in the elderly people. Fabbri et al. reported that older people due to severity of infection, there is a more rapid increase in IL-6 levels over time that significantly increases the risk of higher number of chronic diseases or multi-morbidity as compared to other people with high baseline levels with slower increase in IL-6 secretion over time period (Fabbri et al., 2015). The pro-inflammatory cytokine IL-6 is secreted by macrophages during the early acute phase of an inflammatory response. During this acute phase immune response, another downstream inflammatory marker, CRP is also released with respect to IL-6 (Heinrich et al., 1990).
COVID-19 has become a global problem that necessitates various expert technology to execute and co-ordinate in a variety of scenarios. Simultaneously, there is a general consensus view that the virus cannot be eliminated until a novel vaccine is developed and administered. In the meantime, new strategies are required to alleviate the COVID-19 burden. There are currently no specific antiviral therapies available, but efforts to develop anti-viral therapies and a vaccine are urgently desirable. It is therefore difficult to predict a medication system due to the variable symptoms and severity exhibited in different individual by the same COVID-19 virus.
Significance of naturally derived plant compounds to reduce inflammation
Inflammatory responses underlay the route of many chronic inflammatory disease conditions that is the major concern leading to increased global mortality and morbidity status. Even though highly efficient drugs are available to abate inflammation, their devastating side effects and high cost limits their treatment efficacy. As an alternative treatment strategy, natural products provide immense lead active molecules to combat for inflammation with diverse action and lesser side effects. Several diseases are associated with chronic inflammation such as cardiovascular and neurodegenerative disorders, cancer, trauma, sepsis, rheumatic arthritis, ulcerative colitis etc. Understanding the etiology of the disease condition is essential for the detection and development of medicinal natural derivatives for curing the ailments.
Several naturally derived secondary metabolites from different sources such as plants, microbes and marine algae acts as an excellent approach to eliminate the disease progression. One of the major plant metabolite is alkaloids, containing nitrogen as an integral part of the ring structure (Ziegler et al., 2008). Alkaloids are widely investigated for their pharmacological benefits in different study models. For example Cryptolepine, derived from Cryptolepis sanguinolenta reported to have the property to decrease the expression of IL-1β, TNF-α, IL-6, PGE2 in LPS induced microglia cells. mRNA and protein level expression of iNOS and COX2 were also found to be reduced and thereby inhibited the activation of NF-κB, p38 MAPK (Olajide et al., 2013).
Flavonoids are phenolic compounds having 15 carbon skeleton with 2 aromatic rings and a cyclohexane ring. They exhibit a very prominent role in reducing inflammation by suppressing the release of pro-inflammatory cytokine mediating antioxidant mechanisms as well as immunomodulatory activity on transcription factors such as NF-κB, activator protein 1 and nuclear factor erythroid 2-related factor 2 (Nrf2). Examples for flavonoids that exhibit potential anti-inflammatory activities include the quercetin, rutin, kaempferol, rhamnetin (Lutz et al., 2014, 2015). Another class of biologically active compound includes the terpenoids, containing two or more isoprene units. It is reported that approximately 60 % of known natural products are terpenoids and possess a range of pharmacological properties. Several studies suggest that terpenoids exhibit their pharmacological ability by modulating NF-κB signaling pathway (Souza et al., 2014). Parthenolide is a sesquiterpene lactones a potent NF-κB signaling inhibitor. Its therapeutic benefits in inflammatory conditions like rheumatoid arthritis (Liu et al., 2015), neuroinflammation/degeneration and colitis are well reported (Zhao et al., 2012). Plant derived natural products that can target endolysosomes and mTOR signaling might provide beneficial effects against COVID-19 (Khan et al., 2021).
Conclusion
Cytokines upholds a complex balance in the immune system as ubiquitous biomolecules by acting as important mediators connecting the majority of immune cells. Cytokines nearly influence every biological process including hematopoiesis, embryonic development, disease pathogenesis, non-specific responses towards infection etc. Thus regulation and maintenance of immune homeostasis is crucial for good health and many disease conditions; any disturbance to this equilibrium may results in many chronic pathological conditions. So to understand the involvement of each cytokines is of crucial as they play key role like central mediators for many inflammatory diseases. It is also important that an alternative system of anti-inflammatory therapeutics from natural products especially from plants can provide lead molecules economically with diverse action and lesser side effects. Thus it is imperative that cytokines may be useful biomarkers for health and disease condition and also can be used in various aspects of prognostics, diagnostic and as therapeutic agents.
Data availability
The authors declared that the research data referred to correctly cited in the manuscript's reference section.
CRediT authorship contribution statement
KB. Megha: Writing – review & editing. X. Joseph: Writing – review & editing. V. Akhil: Writing – review & editing. PV. Mohanan: Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no conflict of interests.
Acknowledgements
The authors wish to express their thanks to the Director and Head, Biomedical Technology Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology (Govt. of India), Trivandrum, Kerala, India for their support and for providing the infrastructure to carry out this work. PVM thank Department of Science and Technology, Govt. of India, New Delhi for financial support (DST/TDT/DDP-04/2018(G).
References
- Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Aksentijevich I., Masters S.L., Ferguson P.J., Dancey P., Frenkel J., van Royen-Kerkhoff A., Laxer R., Tedgård U., Cowen E.W., Pham T.H., Booty M. An autoinflammatory disease with deficiency of the interleukin-1–receptor antagonist. N. Engl. J. Med. 2009;360:2426–2437. doi: 10.1056/nejmoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba-Patiño A., Adrover-Jaume C., de la Rica R. Nanoparticle Reservoirs for Paper-Only Immunosensors. ACS sens. 2019;5:147–153. doi: 10.1021/acssensors.9b01937. [DOI] [PubMed] [Google Scholar]
- Anwikar S., Bhitre M. Study of the synergistic anti-inflammatory activity of Solanum xanthocarpum Schrad and Wendl and Cassia fistula Linn. Int. J. Ayurveda Res. 2010;1:167–171. doi: 10.4103/0974-7788.72489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K.I., Lee F., Miyajima A., Miyatake S., Arai N., Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu. Rev. Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Auron P.E., Rosenwasser L.J., Matsushima K., Copeland T., Dinarello C.A., Oppenheim J.J., Webb A.C. Human and murine interleukin 1 possess sequence and structural similarities. J. Mol. Cell. Immunol. 1985;2:169–177. [PubMed] [Google Scholar]
- Bachanova V., Perales M.A., Abramson J.S. Modern management of relapsed and refractory aggressive B-cell lymphoma: a perspective on the current treatment landscape and patient selection for CAR T-cell therapy. Blood. Rev. 2020;40 doi: 10.1016/j.blre.2019.100640. [DOI] [PubMed] [Google Scholar]
- Baykal Y., Saglam K., Yilmaz M.I., Taslipinar A., Akinci S.B., Inal A. Serum sIL-2r, IL-6, IL-10 and TNF-α level in familial Mediterranean fever patients. Clin. Rheumatol. 2003;22:99–101. doi: 10.1007/s10067-002-0682-1. [DOI] [PubMed] [Google Scholar]
- Bektas A., Schurman S.H., Gonzalez-Freire M., Dunn C.A., Singh A.K., Macian F., Cuervo A.M., Sen R., Ferrucci L. Age-associated changes in human CD4+ T cells point to mitochondrial dysfunction consequent to impaired autophagy. Aging (Albany NY) 2019;11:9234–9263. doi: 10.18632/aging.102438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendele A.M., Chlipala E.S., Scherrer J., Frazier J., Sennello G., Rich W.J., Edwards III C.K. Combination benefit of treatment with the cytokine inhibitors interleukin-1 receptor antagonist and PEGylated soluble tumor necrosis factor receptor type I in animal models of rheumatoid arthritis. Arthritis Rheum. 2000;43:2648–2659. doi: 10.1002/1529-0131(200012)43:12<2648::AID-ANR4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Berardelli A., Wenning G.K., Antonini A., Berg D., Bloem B.R., Bonifati V., Brooks D., Burn D.J., Colosimo C., Fanciulli A., Ferreira J. EFNS/MDS-ES recommendations for the diagnosis of Parkinson's disease. Eur. J. Neurol. 2013;20:16–34. doi: 10.1111/ene.12022. [DOI] [PubMed] [Google Scholar]
- Beutler B., Greenwald D., Hulmes J.D., Chang M., Pan Y.C., Mathison J., Ulevitch R., Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985;316:552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- Bitencourt C.S., Bessi V.L., Huynh D.N., Ménard L., Lefebvre J.S., Lévesque T., Hamdan L., Sohouhenou F., Faccioli L.H., Borgeat P., Marleau S. Cooperative role of endogenous leucotrienes and platelet-activating factor in ischaemia–reperfusion-mediated tissue injury. J. Cell. mol. med. 2013;17:1554–1565. doi: 10.1111/jcmm.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonecchi R., Bianchi G., Bordignon P.P., D'Ambrosio D., Lang R., Borsatti A., Sozzani S., Allavena P., Gray P.A., Mantovani A., Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost K.L., Ramp W.K., Nicholson N.C., Bento J.L., Marriott I., Hudson M.C. Staphylococcus aureus infection of mouse or human osteoblasts induces high levels of interleukin-6 and interleukin-12 production. J. Infect. Dis. 1999;180:1912–1920. doi: 10.1086/315138. [DOI] [PubMed] [Google Scholar]
- Bourne T., Fossati G., Nesbitt A. A PEGylated Fab' fragment against tumor necrosis factor for the treatment of Crohn disease: exploring a new mechanism of action. BioDrugs. 2008;22(5):331–337. doi: 10.2165/00063030-200822050-00005. [DOI] [PubMed] [Google Scholar]
- Bradley J.R. TNF-mediated inflammatory disease. J. Pathol. 2008;214:149–160. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- Brugos B., Vincze Z., Sipka S., Szegedi G., Zeher M. Serum and urinary cytokine levels of SLE patients. Pharmazie. 2012;67:411–413. doi: 10.1691/ph.2012.1694. [DOI] [PubMed] [Google Scholar]
- Buchan G., Barrett K., Turner M., Chantry D., Maini R.N., Feldmann M. Interleukin-1 and tumour necrosis factor mRNA expression in rheumatoid arthritis: prolonged production of IL-1 alpha. Clin. Exp. Immunol. 1988;73:449–455. [PMC free article] [PubMed] [Google Scholar]
- Camussi G., Albano E., Tetta C., Bussolino F. The molecular action of tumor necrosis factor-α. Eur. J. Biochem. 1991;202:3–14. doi: 10.1111/j.1432-1033.1991.tb16337.x. [DOI] [PubMed] [Google Scholar]
- Carpenter L.R., Moy J.N., Roebuck K.A. Respiratory syncytial virus and TNFalpha induction of chemokine gene expression involves differential activation of Rel A and NF-kappaB1. BMC Infect. Dis. 2002;2:5. doi: 10.1186/1471-2334-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Perlman S. Vol. 39. Springer; Berlin Heidelberg: 2017. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology; pp. 529–539. (Seminars in Immunopathology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., Men D., Huang Q., Liu Y., Yang B., Ding J. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin. Infect. Dis. 2020;71:1937–1942. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertov O., Yang D., Howard O.M., Oppenheim J.J. Leukocyte granule proteins mobilize innate host defenses and adaptive immune responses. Immunol. Rev. 2000;177:68–78. doi: 10.1034/j.1600-065x.2000.17702.x. [DOI] [PubMed] [Google Scholar]
- Chokkalingam V., Tel J., Wimmers F., Liu X., Semenov S., Thiele J., Figdor C.G., Huck W.T. Probing cellular heterogeneity in cytokine-secreting immune cells using droplet-based microfluidics. Lab. Chip. 2013;13:4740–4744. doi: 10.1039/c3lc50945a. [DOI] [PubMed] [Google Scholar]
- Cohen M.C., Cohen S. Cytokine function: a study in biologic diversity. Am. J. Clin. Pathol. 1996;105:589–598. doi: 10.1093/ajcp/105.5.589. [DOI] [PubMed] [Google Scholar]
- Condos R., Rom W.N., Schluger N.W. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-γ via aerosol. Lancet. 1997;349:1513–1515. doi: 10.1016/s0140-6736(96)12273-x. [DOI] [PubMed] [Google Scholar]
- Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine & Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe J.E., Jr, Suara R.O., Brock S., Kallewaard N., House F., Weitkamp J.H. Genetic and structural determinants of virus neutralizing antibodies. Immunol Res. 2001;23(2–3):135–145. doi: 10.1385/ir:23:2-3:135. [DOI] [PubMed] [Google Scholar]
- Crown J., Jakubowski A., Kemeny N., Gordon M., Gasparetto C., Wong G., Sheridan C., Toner G., Meisenberg B., Botet J. A phase I trial of recombinant human interleukin-1 beta alone and in combination with myelosuppressive doses of 5-fluorouracil in patients with gastrointestinal cancer. Blood. 1991;78:1420–1427. [PubMed] [Google Scholar]
- Czaja A.J. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World. J. Gastroenterol. 2014;20:2515–2532. doi: 10.3748/wjg.v20.i10.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancescu M., Rubio-Trujillo M., Biron G., Bron D., Delespesse G., Sarfati M. Interleukin 4 protects chronic lymphocytic leukemic B cells from death by apoptosis and upregulates Bcl-2 expression. J. Exp. Med. 1992;176:1319–1326. doi: 10.1084/jem.176.5.1319. 10.1084/jem.176.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniela P., Barbara M., Elisabetta S., Fabio R. Bovine lactoferrin a nutritional supplement for downregulation of inflammatory response in cutaneous disorder. Clin. Exp. Dermatol. CED-126. 2017 [Google Scholar]
- David F., Farley J., Huang H., Lavoie J.P., Laverty S. Cytokine and Chemokine Gene Expression of IL-1β Stimulated Equine Articular Chondrocytes. Vet. Surg. 2007;36:221–227. doi: 10.1111/j.1532-950x.2007.00253.x. [DOI] [PubMed] [Google Scholar]
- Del Prete G., De Carli M., Almerigogna F., Giudizi M.G., Biagiotti R., Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150(2):353–360. [PubMed] [Google Scholar]
- Dennler S., Goumans M.J., Ten Dijke P. Transforming growth factor β signal transduction. J. leukoc. Biol. 2002;71:731–740. doi: 10.1189/jlb.71.5.731. [DOI] [PubMed] [Google Scholar]
- de la Rica R., Borges M., Gonzalez-Freire M. COVID-19: in the eye of the cytokine storm. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.558898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienstag J.L., McHutchison J.G. American gastroenterological association technical review on the management of hepatitis C. Gastroenterology. 2006;130:231–264. doi: 10.1053/j.gastro.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Dinarello C.A. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- Dinarello C.A. Historical insights into cytokines. Eur. J. Immunol. 2007;37:34–45. doi: 10.1002/eji.200737772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong T., Santos S., Yang Z., Yang S., Kirkhus N.E. Sputum and salivary protein biomarkers and point-of-care biosensors for the management of COPD. Analyst. 2020;145(5):1583–1604. doi: 10.1039/c9an01704f. [DOI] [PubMed] [Google Scholar]
- Elson C.O., Sartor R.B., Tennyson G.S., Riddell R.H. Experimental models of inflammatory bowel disease. Gastroenterol. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- Englaro W., Bahadoran P., Bertolotto C., Buscà R., Dérijard B., Livolsi A., Peyron J.F., Ortonne J.P., Ballotti R. Tumor necrosis factor alpha-mediated inhibition of melanogenesis is dependent on nuclear factor kappa B activation. Oncogene. 1999;18:1553–1559. doi: 10.1038/sj.onc.1202446. [DOI] [PubMed] [Google Scholar]
- Fabbri E., An Y., Zoli M., Simonsick E.M., Guralnik J.M., Bandinelli S., Boyd C.M., Ferrucci L. Aging and the burden of multimorbidity: associations with inflammatory and anabolic hormonal biomarkers. J Gerontol A: Biol. Sci. Med. Sci. 2015;70:63–70. doi: 10.1093/gerona/glu127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farasat S., Aksentijevich I., Toro J.R. Autoinflammatory diseases: clinical and genetic advances. Arch. Dermatol. 2008;144:392–402. doi: 10.1001/archderm.144.3.392. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Maini R.N. Anti-TNF therapy, from rationale to standard of care: what lessons has it taught us? J. Immunol. 2010;185:791–794. doi: 10.4049/jimmunol.1090051. [DOI] [PubMed] [Google Scholar]
- Ferrero-Miliani L., Nielsen O.H., Andersen P.S., Girardin S.E. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin. Exp. Immunol. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D.F., Bond M.W., Mosmann T.R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I.E., Hess J.H., Huang S., Aguet M., Rothe J., Bluethmann H., Kaufmann S.H. Early interleukin 12 production by macrophages in response to mycobacterial infection depends on interferon gamma and tumor necrosis factor alpha. J. Exp. Med. 1995;181:1615–1621. doi: 10.1084/jem.181.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier J., Boorsma D.M., van Beek P.J., Nieboer C., Stoof T.J., Willemze R., Tensen C.P. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J. Pathol. 2001;194:398–405. doi: 10.1002/1096-9896(200108)194:4<397::aid-path899>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Franceschi C., Garagnani P., Parini P., Giuliani C., Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018;14:576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- Fresno M., Kopf M., Rivas L. Cytokines and infectious diseases. Immunol. Today. 1997;18:56–58. doi: 10.1016/s0167-5699(96)30069-8. [DOI] [PubMed] [Google Scholar]
- Gang N., Drenth J.P., Langevitz P., Zemer D., Brezniak N., Pras M., Van der Meer J.W., Livneh A. Activation of the cytokine network in familial Mediterranean fever. J. Rheumatol. 1999;26:890–897. [PubMed] [Google Scholar]
- Gilfillan, A.M. and Metcalfe, D. eds., 2011. Mast cell biology: contemporary and emerging topics (Vol. 716). Springer Science & Business Media.
- Gleeson M., Bishop N.C., Stensel D.J., Lindley M.R., Mastana S.S., Nimmo M.A. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- Goljan, E.F., 2018. Rapid Review Pathology E-Book.
- Grell M., Douni E., Wajant H., Löhden M., Clauss M., Maxeiner B., Georgopoulos S., Lesslauer W., Kollias G., Pfizenmaier K., Scheurich P. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- Gu Y., Hsu A.C.Y., Pang Z., Pan H., Zuo X., Wang G., Zheng J., Wang F. Role of the innate cytokine storm induced by the influenza A virus. Viral immunol. 2019;32:244–251. doi: 10.1089/vim.2019.0032. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J.M. Oxford University Press; USA: 2015. Free Radicals in Biology and Medicine. [Google Scholar]
- Heinrich P.C., Castell J.V., Andus T. IL-6 and the acute phase response. Biochem. J. 1990;265:621–636. doi: 10.1042/bj2650621. 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson A., Calame K. Transcriptional regulation during B cell development. Annu.Rev.immunol. 1998;16:163–200. doi: 10.1146/annurev.immunol.16.1.163. [DOI] [PubMed] [Google Scholar]
- Hendrayani S.F., Al-Harbi B., Al-Ansari M.M., Silva G., Aboussekhra A. The inflammatory/cancer-related IL-6/STAT3/NF-κB positive feedback loop includes AUF1 and maintains the active state of breast myofibroblasts. Oncotarget. 2016;7:41974–41985. doi: 10.18632/oncotarget.9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henríquez-Olguín C., Altamirano F., Valladares D., López J.R., Allen P.D., Jaimovich E. Altered ROS production, NF-κB activation and interleukin-6 gene expression induced by electrical stimulation in dystrophic mdx skeletal muscle cells. Biochim. Biophys. Acta. 2015;1852:1410–1419. doi: 10.1016/j.bbadis.2015.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh F.H. Primer to the immune response. Anna. Allerg. Asthma. Im. 2014;113:333. doi: 10.1016/j.anai.2014.06.005. [DOI] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudis C.A. Trastuzumab—Mechanism of action and use in clinical practice. N. Eng. J. Med. 2007;357:39–51. doi: 10.1056/nejmra043186. [DOI] [PubMed] [Google Scholar]
- Hull K.M., Drewe E., Aksentijevich I., Singh H.K., Wong K., Mcdermott E.M., Dean J., Powell R.J., Kastner D.L. The TNF receptor-associated periodic syndrome (TRAPS): emerging concepts of an autoinflammatory disorder. Medicine (Baltimore) 2002;81:349–368. doi: 10.1097/00005792-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Ibelgaufts, H., 2013. Cytokines. In: cytokines & Cells Online Pathfinder Encyclopedia.
- Iizumi T., Sato S., Iiyama T., Hata R., Amemiya H., Tomomasa H., Yazaki T., Umeda T. Recombinant human interleukin-1 beta analogue as a regulator of hematopoiesis in patients receiving chemotherapy for urogenital cancers. Cancer. 1991;68:1520–1523. doi: 10.1002/10970142(19911001)68:7<1520::AIDCNCR2820680710>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Isailovic N., Daigo K., Mantovani A., Selmi C. Interleukin-17 and innate immunity in infections and chronic inflammation. J.autoimmun. 2015;60:1–11. doi: 10.1016/j.jaut.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Jabbour H., Sales K., Catalano R., Norman J. Inflammatory pathways in female reproductive health and disease. Reproduction. 2009;138:903–919. doi: 10.1530/REP-09-0247. [DOI] [PubMed] [Google Scholar]
- Jin Y., Mailloux C.M., Gowan K., Riccardi S.L., LaBerge G., Bennett D.C., Fain P.R., Spritz R.A. NALP1 in vitiligo-associated multiple autoimmune disease. N. Engl. J. Med. 2007;356:1216–1225. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta. 2005;1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Kassir R. Risk of COVID-19 for patients with obesity. Obes. Rev. 2020;21(6):e13034. doi: 10.1111/obr.13034. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y., Hara M., Wright T.M. Endogenous IL-1α from systemic sclerosis fibroblasts induces IL-6 and PDGF-A. J. Clin. Investig. 1999;103:1253–1260. doi: 10.1172/JCI4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H., Taira K. A functional gene discovery in the Fas- mediated pathway to apoptosis by analysis of transiently expressed randomized hybrid-ribozyme libraries. Nucleic. Acids Res. 2002;15:3609–3614. doi: 10.1093/nar/gkf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, M.M., 2008. Role of cytokines. In: khan MM, Amsterdam: elsevier.
- Khan N., Chen X., Geiger J.D. Possible Therapeutic Use of Natural Compounds Against COVID-19. J Cell Signal. 2021;2(1):63–79. [PMC free article] [PubMed] [Google Scholar]
- Khawar B., Abbasi M.H., Sheikh N. A panoramic spectrum of complex interplay between the immune system and IL-32 during pathogenesis of various systemic infections and inflammation. Eur. J. Med. Res. 2015;20:7. doi: 10.1186/s40001-015-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.S., Choe P.G., Park W.B., Oh H.S., Kim E.J., Nam E.Y., Na S.H., Kim M., Song K.H., Bang J.H., Park S.W. Clinical progression and cytokine profiles of Middle East respiratory syndrome coronavirus infection. J. Korean Med. Sci. 2016;31:1717–1725. doi: 10.3346/jkms.2016.31.11.1717. 10.3346/jkms.2016.31.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitley J.L., Lachmann H.J., Pinto A., Ginsberg L. Neurologic manifestations of the cryopyrin-associated periodic syndrome. Neurology. 2010;74:1267–1270. doi: 10.1212/wnl.0b013e3181d9ed69. [DOI] [PubMed] [Google Scholar]
- Klein S., Cortese M., Winter S.L., Wachsmuth-Melm M., Neufeldt C.J., Cerikan B., Stanifer M.L., Boulant S., Bartenschlager R., Chlanda P. SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography. Nat. commun. 2020;11:5885. doi: 10.1038/s41467-020-19619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong A.N.T., editor. Inflammation, Oxidative stress, and cancer: Dietary Approaches For Cancer Prevention. CRC Press; 2013. [Google Scholar]
- Koyama N., Harada N., Takahashi T., Mita S., Okamura H., Tominaga A., Takatsu K. Role of recombinant interleukin-1 compared to recombinant T-cell replacing factor/interleukin-5 in B-cell differentiation. Immunology. 1988;63:277–283. [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Abbas A.K., Aster J.C. Elsevier Health Sciences; 2017. Robbins Basic Pathology E-Book. [Google Scholar]
- Kulaber A., Tugal-Tutkun I., Yentür S.P., Akman-Demir G., Kaneko F., Gül A., Saruhan-Direskeneli G. Pro-inflammatory cellular immune response in Behçet's disease. Rheumatol. Int. 2007;27:1113–1118. doi: 10.1007/s00296-007-0367-9. [DOI] [PubMed] [Google Scholar]
- Kyriakis J.M., Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Lackie J. Oxford University Press; 2010. A Dictionary of Biomedicine. [Google Scholar]
- Lawrence T. The nuclear factor B pathway in inflammation. Inflammation biology group. Cold Spring Harb. Perspect. Biol. 2009;1:1–10. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz D.L., Lefkowitz S.S. Macrophage–neutrophil interaction: a paradigm for chronic inflammation revisited. Immunol .Cell Biol. 2001;79:502–506. doi: 10.1046/j.1440-1711.2001.01020.x. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr. Rev. 2007;65:140–146. doi: 10.1111/j.1753-4887.2007.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Lieberman. M. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2013. Marks. AD. & Peet. A. Marks' Basic Medical biochemistry: a Clinical Approach; pp. 723–742. [Google Scholar]
- Ling S., Feng T., Ji K., Tian Y., Li Y. Inflammation to cancer: the molecular biology in the pancreas. Oncol Lett. 2014;7:1747–1754. doi: 10.3892/ol.2014.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P.E. Interleukin-6 and rheumatic diseases. Arthritis Res. Ther. 2006;8:4. doi: 10.1186/ar1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Wang Y., Wang Y., Ning Q., Zhang Y., Gong C., Zhao W., Jing G., Wang Q. Dexmedetomidine attenuates inflammatory reaction in the lung tissues of septic mice by activating cholinergic anti-inflammatory pathway. Int. Immunopharmacol. 2016;35:210–216. doi: 10.1016/j.intimp.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Liu Q., Zhao J., Tan R., Zhou H., Lin Z., Zheng M., Romas E., Xu J., Sims N.A. Parthenolide inhibits pro-inflammatory cytokine production and exhibits protective effects on progression of collagen-induced arthritis in a rat model. Scand J Rheumatol. 2015 May;44(3):182–191. doi: 10.3109/03009742.2014.938113. [DOI] [PubMed] [Google Scholar]
- Locksley R.M., Killeen N., Lenardo M.J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Lo Y.Y., Wong J.M., Cruz T.F. Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases. J. Biol. Chem. 1996;271:15703–15707. doi: 10.1074/jbc.271.26.15703. [DOI] [PubMed] [Google Scholar]
- Luer M.S., Rhoney D.H., Hughes M., Hatton J. New pharmacologic strategies for acute neuronal injury. Pharmacotherapy. 1996;16:830–848. doi: 10.1002/j.1875-9114.1996.tb03000.x. [DOI] [PubMed] [Google Scholar]
- Lutz J.A., Kulshrestha M., Rogers D.T., Littleton J.M. A nicotinic receptor-mediated anti-inflammatory effect of the flavonoid rhamnetin in BV2 microglia. Fitoterapia. 2014;98:11–21. doi: 10.1016/j.fitote.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz J.A., Carter M., Fields L., Barron S., Littleton J.M. The Dietary Flavonoid Rhamnetin Inhibits Both Inflammation and Excitotoxicity During Ethanol Withdrawal in Rat Organotypic Hippocampal Slice Cultures. Alcohol Clin Exp Res. 2015 Dec;39(12):2345–2353. doi: 10.1111/acer.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEwan D.J. TNF receptor subtype signalling: differences and cellular consequences. Cell. Signal. 2002;14:477–492. doi: 10.1016/s0898-6568(01)00262-5. [DOI] [PubMed] [Google Scholar]
- Mahida Y.R., Ceska M., Effenberger F., Kurlak L., Lindley I., Hawkey C.J. Enhanced synthesis of neutrophil-activating peptide-I/interleukin-8 in active ulcerative colitis. Clin. Sci. 1992;82:273–275. doi: 10.1042/cs0820273. [DOI] [PubMed] [Google Scholar]
- Mak T.W., Saunders M.E., Jett B.D. Newnes; 2013. Primer to the Immune Response. [Google Scholar]
- Mansfield S., Grießl M., Gutknecht M., Cook C.H. Sepsis and cytomegalovirus: foes or conspirators? Med. Microbiol. Immunol. 2015;204:431–437. doi: 10.1007/s00430-015-0407-0. 10.1007/s00430-015-0407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino M.W., Dunn A., Grail D., Inglese M., Noguchi Y., Richards E., Jungbluth A., Wada H., Moore M., Williamson B., Basu S. Characterization of tumor necrosis factor-deficient mice. PNAS USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí M.J., Tolosa E. New guidelines for diagnosis of Parkinson disease. Nat. Rev. Neurol. 2013;9:190–191. doi: 10.1038/nrneurol.2013.47. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Mehta K., Aggarwal B.B. Springer; Dordrecht: 1998. Recombinant Organisms As Source of Cancer biotherapeutics. Principles of Cancer Biotherapy; pp. 51–77. [Google Scholar]
- Miller A.H., Maletic V., Raison C.L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K.W., O'garra A., Malefyt R.D., Vieira P., Mosmann T.R. Interleukin-10. Annu. Rev. Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.00112. [DOI] [PubMed] [Google Scholar]
- Moore K.W., de Waal Malefyt R., Coffman R.L., O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu.Rev.Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Monneret G., Venet F., Kullberg B.J., Netea M.G. ICU-acquired immunosuppression and the risk for secondary fungal infections. Med. Mycol. 2011;49:17–23. doi: 10.3109/13693786.2010.509744. [DOI] [PubMed] [Google Scholar]
- Moreb J., Zucali J.R. The therapeutic potential of interleukin-1 and tumor necrosis factor on hematopoietic stem cells. Leuk. Lymphoma. 1992;8:267–275. doi: 10.3109/10428199209051006. [DOI] [PubMed] [Google Scholar]
- Morjaria J.B., Chauhan A.J., Babu K.S., Polosa R., Davies D.E., Holgate S.T. The role of a soluble TNFα receptor fusion protein (etanercept) in corticosteroid refractory asthma: a double blind, randomised, placebo controlled trial. Thorax. 2008;63:584–591. doi: 10.1136/thx.2007.086314. [DOI] [PubMed] [Google Scholar]
- Murakami M., Hirano T. The molecular mechanisms of chronic inflammation development. Front.Immunol. 2012;3:323. doi: 10.3389/fimmu.2012.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musabak U., Pay S., Erdem H., Simsek I., Pekel A., Dinc A., Sengul A. Serum interleukin-18 levels in patients with Behçet's disease. Is its expression associated with disease activity or clinical presentations? Rheumatol. Int. 2006;26:545–550. doi: 10.1007/s00296-005-0029-8. [DOI] [PubMed] [Google Scholar]
- Műzes G., Molnár B., Tulassay Z., Sipos F. Changes of the cytokine profile in inflammatory bowel diseases. World. J. Gastroenterol. 2012;18:5848. doi: 10.3748/wjg.v18.i41.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith J.H., Sprang S.R. Modularity in the TNF-receptor family. Trends. Biochem. Sci. 1998;23:74–79. doi: 10.1016/s0968-0004(97)01164-x. [DOI] [PubMed] [Google Scholar]
- Nathan C., Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Nedjai B., Hitman G.A., Church L.D., Minden K., Whiteford M.L., McKee S., Stjernberg S., Pettersson T., Ranki A., Hawkins P.N., Arkwright P.D. Differential cytokine secretion results from p65 and c-Rel NF-κB subunit signaling in peripheral blood mononuclear cells of TNF receptor-associated periodic syndrome patients. Cell. Immunol. 2011;268:55–59. doi: 10.1016/j.cellimm.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Nourshargh S., Hordijk P.L., Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat. Rev. Mol. Cell. Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- Novel C.P.E.R.E. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua liu xing bing xue za zhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- Ogra P.L., Mestecky J., Lamm M.E., Strober W., McGhee J.R., Bienenstock J. Academic Press; 2012. Handbook of Mucosal Immunology. [Google Scholar]
- Ogrunc M., Di Micco R., Liontos M., Bombardelli L., Mione M., Fumagalli M., Gorgoulis V.G., di Fagagna F.D.A. Oncogene-induced reactive oxygen species fuel hyperproliferation and DNA damage response activation. Cell. Death. Differ. 2014;21:998–1012. doi: 10.1038/cdd.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olajide O.A., Bhatia H.S., de Oliveira A.C., Wright C.W., Fiebich B.L. Inhibition of Neuroinflammation in LPS-Activated Microglia by Cryptolepine. Evid. Based. Complement. Alternat. Med. 2013;2013(2013) doi: 10.1155/2013/459723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouaguia L., Mrizak D., Renaud S., Moralès O., Delhem N. Control of the inflammatory response mechanisms mediated by natural and induced regulatory T-cells in HCV, HTLV-1-, and EBV-associated cancers. Mediators. inflamm. 2014;564296 doi: 10.1155/2014/564296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J.A., Punt J., Stranford S.A., Jones P.P. WH Freeman & Company; New York, USA: 2013. Kuby Immunology. [Google Scholar]
- Panacek E.A., Marshall J.C., Albertson T.E., Johnson D.H., Johnson S., MacArthur R.D., Miller M., Barchuk W.T., Fischkoff S., Kaul M., Teoh L. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F (ab′) 2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit. Care Med. 2004;32:2173–2182. doi: 10.1097/01.ccm.0000145229.59014.6c. [DOI] [PubMed] [Google Scholar]
- Paovic J., Paovic P., Sredovic V. Behcet's disease: systemic and ocular manifestations. BioMed Research Intl. 2013 doi: 10.1155/2013/247345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.Y., Pillinger M.H. Interleukin-6 in the pathogenesis of rheumatoid arthritis. Bull. NYU. Hosp. Jt. Dis. 2007;65:4–10. [PubMed] [Google Scholar]
- Patricio P., Paiva J.A., Borrego L.M. Immune response in bacterial and Candida sepsis. Eur. J. Microbiol. Immunol. 2019;9:105–113. doi: 10.1556/1886.2019.00011. 10.1556/1886.2019.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecanha L.M., Snapper C.M., Lees A., Mond J.J. Lymphokine control of type 2 antigen response. IL-10 inhibits IL-5-but not IL-2-induced Ig secretion by T cell-independent antigens. J. Immunol. 1992;148:3427–3432. [PubMed] [Google Scholar]
- Phillips R.E. Immunology taught by Darwin. Nat. immunol. 2002;3:987–989. doi: 10.1038/ni1102-987. [DOI] [PubMed] [Google Scholar]
- Piomelli D. Springer Science & Business Media; 2013. Arachidonic Acid in Cell Signaling. [Google Scholar]
- Pope S.M., Zimmermann N., Stringer K.F., Karow M.L., Rothenberg M.E. The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J. Immunol. 2005;175:5341–5350. doi: 10.4049/jimmunol.175.8.5341. [DOI] [PubMed] [Google Scholar]
- Porter S. Elsevier Health Sciences; 2013. Tidy's Physiotherapy E-Book. [Google Scholar]
- Portugal-Cohen M., Horev L., Ruffer C., Schlippe G., Voss W., Oron M., Soroka Y., Frušić-Zlotkin M., Milner Y., Kohen R. Non-invasive skin biomarkers quantification of psoriasis and atopic dermatitis: cytokines, antioxidants and psoriatic skin auto-fluorescence. Biomed. Pharmacother. 2012;66:293–299. doi: 10.1016/j.biopha.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Presky D.H., Yang H., Minetti L.J., Chua A.O., Nabavi N., Wu C.Y., Gately M.K., Gubler U. A functional interleukin 12 receptor complex is composed of two β-type cytokine receptor subunits. Proc. Natl. Acad. Sci. USA. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with COVID-19 in Wuhan. Clin. Infect. Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel S.A., Dudas S., Ge S., Gutiérrez J.A. Identification and localization of the cytokine SDF1 and its receptor, CXC chemokine receptor 4, to regions of necrosis and angiogenesis in human glioblastoma. Clin. Cancer. Res. 2000;6:102–111. [PubMed] [Google Scholar]
- Rosa J.S., Flores R.L., Oliver S.R., Pontello A.M., Zaldivar F.P., Galassetti P.R. Sustained IL-1α, IL-4, and IL-6 elevations following correction of hyperglycemia in children with type 1 diabetes mellitus. Pediatr. Diabetes. 2008;9:9–16. doi: 10.1111/j.1399-5448.2007.00243.x. [DOI] [PubMed] [Google Scholar]
- Rozwarski D.A., Gronenborn A.M., Clore G.M., Bazan J.F., Bohm A., Wlodawer A., Hatada M., Karplus P.A. Structural comparisons among the short-chain helical cytokines. Structure. 1994;2:159–173. doi: 10.1016/s0969-2126(00)00018-6. [DOI] [PubMed] [Google Scholar]
- Sartor R.B. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterol. 1994;106:533–539. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- Savic S., Dickie L.J., Battellino M., McDermott M.F. Familial Mediterranean fever and related periodic fever syndromes/autoinflammatory diseases. Curr. Opin. Rheumatol. 2012;24:103–112. doi: 10.1097/bor.0b013e32834dd2d5. [DOI] [PubMed] [Google Scholar]
- Schapira A.H. Molecular and clinical pathways to neuroprotection of dopaminergic drugs in Parkinson disease. Neurology. 2009;72:44–50. doi: 10.1212/wnl.0b013e3181990438. [DOI] [PubMed] [Google Scholar]
- Schenk T., Irth H., Marko-Varga G., Edholm L.E., Tjaden U.R., Van der Greef J. Potential of on-line micro-LC immunochemical detection in the bioanalysis of cytokines. J. Pharmaceut. Biomed. 2001;26:975–985. doi: 10.1016/s0731-7085(01)00464-2. [DOI] [PubMed] [Google Scholar]
- Schreiber G., Walter M.R. Cytokine–receptor interactions as drug targets. Curr opin Chem Biol. 2010;14:511–519. doi: 10.1016/j.cbpa.2010.06.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz M., Dewald B., Ceska M., Gerber N., Baggiolini M. Interleukin-8 in inflammatory rheumatic diseases: synovial fluid levels, relation to rheumatoid factors, production by mononuclear cells, and effects of gold sodium thiomalate and methotrexate. Rheumatol. Int. 1992;12:159–164. doi: 10.1007/bf00274936. [DOI] [PubMed] [Google Scholar]
- Serhan C.N., Dalli J., Colas R.A., Winkler J.W., Chiang N. Protectins and maresins: new pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta. 2015;1851:397–413. doi: 10.1016/j.bbalip.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H. Oxidative stress: concept and some practical aspects. Antioxidants. 2020;9:852. doi: 10.3390/antiox9090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simi A., Tsakiri N., Wang P., Rothwell N.J. Interleukin-1 and inflammatory neurodegeneration. Biochem. Soc. Trans. 2007;35:1122–1126. doi: 10.1042/bst0351122. [DOI] [PubMed] [Google Scholar]
- Sohar E., Gafni J., Pras M., Heller H. Familial Mediterranean fever: a survey of 470 cases and review of the literature. Am. J. Med. 1967;43:227–253. doi: 10.1016/0002-9343(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Souza M.T., Almeida J.R., Araujo A.A., Duarte M.C., Gelain D.P., Moreira J.C., dos Santos M.R., Quintans-Júnior L.J. Structure–activity relationship of terpenes with anti-inflammatory profile – a systematic review. Basic Clin Pharmacol Toxicol. 2014;115(3):244–256. doi: 10.1111/bcpt.12221. Sep. [DOI] [PubMed] [Google Scholar]
- Sprague A.H., Khalil R.A. Inflammatory cytokines in vascular dysfunction and vasculardisease. Biochem.Pharmacol. 2009;78 doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer, T.A., Anderson, D.C., Rosenthal, A.S. and Rothlein, R.eds., 2012. Leukocyte Adhesion Molecules: proceedings of the First International Conference on:" Structure, Function and Regulation of Molecules Involved in Leukocyte Adhesion", Held in Titisee, West Germany, September 28-October 2, 1988.287.
- Stein P.S., Steffen M.J., Smith C., Jicha G., Ebersole J.L., Abner E., Dawson III, D. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer's disease. Alzheimer's Dement. 2012;8:196–203. doi: 10.1016/j.jalz.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H.C., Leite-Morris K.A., Braun L., Biron C.A. A role for transforming growth factor-beta 1 in regulating natural killer cell and T lymphocyte proliferative responses during acute infection with lymphocytic choriomeningitis virus. J. Immunol. 1991;147:2717–2727. [PubMed] [Google Scholar]
- Tian B., Nowak D.E., Brasier A.R. A TNF-induced gene expression program under oscillatory NF-κB control. BMC Genomics. 2005;6:137. doi: 10.1186/1471-2164-6-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonges L., Schlachetzki M., Weishaupt J.C., J.H., Bahr M. Hematopoietic cytokines-on the verge of conquering neurology. Curr. Mol. Med. 2007;7:157–170. doi: 10.2174/156652407780059186. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Vlahopoulos S., Boldogh I., Casola A., Brasier A.R. Nuclear Factor-κB–Dependent Induction of Interleukin-8 Gene Expression by Tumor Necrosis Factor-α: evidence for an Antioxidant Sensitive Activating Pathway Distinct From Nuclear Translocation. Blood. 1999;94:1878–1889. doi: 10.1182/blood.V94.6.1878. [DOI] [PubMed] [Google Scholar]
- Waguespack P.J., Banks W.A., Kastin A.J. Interleukin-2 does not cross the blood-brain barrier by a saturable transport system. Brain Res. Bull. 1994;34:103–109. doi: 10.1016/0361-9230(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Wakita T., Shintani F., Yagi G., Asai M., Nozawa S. Combination of inflammatory cytokines increases nitrite and nitrate levels in the paraventricular nucleus of conscious rats. Brain. Res. 2001;905:12–20. doi: 10.1016/S0006-8993(01)02346-0. [DOI] [PubMed] [Google Scholar]
- Walsh C.E., Liu J.M., Anderson S.M., Rossio J.L., Nienhuis A.W., Young N.S. A trial of recombinant human interleukin-1 in patients with severe refractory aplastic anaemia. Br. J. Haem. 1992;80:106–110. doi: 10.1111/j.1365-2141.1992.tb06408.x. [DOI] [PubMed] [Google Scholar]
- Wang W., He J., Wu S. The definition and risks of cytokine release syndrome-like in 11 COVID-19-infected pneumonia critically ill patients: disease characteristics and retrospective analysis. J. Infect. Dis. 2020:jiaa387. doi: 10.1093/infdis/jiaa387. 10.1093/infdis/jiaa387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan. China. Jama. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watford W.T., Moriguchi M., Morinobu A., O'Shea J.J. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361–368. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- Wiemann B., Starnes C.O. Coley's toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol. Therapeut. 1994;64:529–564. doi: 10.1016/0163-7258(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Wilson C.J., Finch C.E., Cohen H.J. Cytokines and cognition—The case for a head-to-toe inflammatory paradigm. J. Am. Geriatr. Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- Wojdasiewicz P., Poniatowski Ł.A., Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators. Inflamm. 2014;2014 doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C.K., Lam C.W.K., Wu A.K.L., Ip W.K., Lee N.L.S., Chan I.H.S., Lit L.C.W., Hui D.S.C., Chan M.H.M., Chung S.S.C., Sung J.J.Y. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet. Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Zou R., Zeng L., Kou S., Lan J., Li X., Liang Y., Ding X., Tan G., Tang S., Liu L. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm. Res. 2020:1–8. doi: 10.1007/s00011-020-01342-0. 10.1007/s00011-020-01342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.M., An J. Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 2007;45:27–37. doi: 10.1097/aia.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int. J. Antimicrob Agents. 2020;105982 doi: 10.1016/j.ijantimicag.2020.105982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.J., Xiang J.Y., Liu L., Huang X.L., Gan H.T. Parthenolide, an inhibitor of the nuclear factor-κB pathway, ameliorates dextran sulfate sodium-induced colitis in mice. Int Immunopharmacol. 2012;12(1):169–174. doi: 10.1016/j.intimp.2011.11.007. JanEpub 2011 Dec 11. PMID: 22155740. https://doi.org/10.1016/j.intimp.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Hong Y., Huang H. Triptolide attenuates inflammatory response in membranous glomerulo-nephritis rat via downregulation of NF-κB signaling pathway. Kidney. Blood. Press. Res. 2016;41:901–910. doi: 10.1159/000452591. [DOI] [PubMed] [Google Scholar]
- Ziegler J., Facchini P.J. Alkaloid biosynthesis: metabolism and trafficking. Annu. Rev. Plant Biol. 2008;59:735–769. doi: 10.1146/annurev.arplant.59.032607.092730. [DOI] [PubMed] [Google Scholar]
- Zidek Z., Anzenbacher P., Kmoníčková E. Current status and challenges of cytokine pharmacology. Br. J. pharmacol. 2009;157:342–361. doi: 10.1111/j.1476-5381.2009.00206.x. 10.1111/j.1476-5381.2009.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoulim F., Lebossé F., Levrero M. Current treatments for chronic hepatitis B virus infections. Curr Opin Virol. 2016;18:109–116. doi: 10.1016/j.coviro.2016.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declared that the research data referred to correctly cited in the manuscript's reference section.