Abstract
VO2max (maximal oxygen consumption), a validated measure of aerobic fitness, has been associated with better cerebral artery compliance and measures of brain morphology, such as higher cortical thickness (CT) in frontal, temporal and cingular cortices, and larger grey matter volume (GMV) of the middle temporal gyrus, hippocampus, orbitofrontal cortex and cingulate cortex. Single sessions of physical exercise can promptly enhance cognitive performance and brain activity during executive tasks. However, the immediate effects of exercise on macro-scale properties of the brain’s grey matter remain unclear. We investigated the impact of one session of moderate-intensity physical exercise, compared with rest, on grey matter volume, cortical thickness, working memory performance, and task-related brain activity in older adults. Cross-sectional associations between brain measures and VO2max were also tested. Exercise did not induce statistically significant changes in brain activity, grey matter volume, or cortical thickness. Cardiovascular fitness, measured by VO2max, was associated with lower grey matter blood flow in the left hippocampus and thicker cortex in the left superior temporal gyrus. Cortical thickness was reduced at post-test independent of exercise/rest. Our findings support that (1) fitter individuals may need lower grey matter blood flow to meet metabolic oxygen demand, and (2) have thicker cortex.
Subject terms: Neuroscience, Cognitive ageing, Neuro-vascular interactions
Introduction
Cardiorespiratory fitness has been associated with individual differences in cognitive performance and brain integrity in older adults1,2. Regular (i.e. chronic) physical exercise has been associated with greater total grey matter volume (GMV)3,4, greater volume of the hippocampus3,5–8, and larger volume in more than 80% of all cortical regions5,7,9. Most of the above investigations have used valid measures of cognitive performance and brain volumes, but settled for feasible though rather imprecise measures of cardiorespiratory fitness. More direct measures of cardiorespiratory fitness can be achieved with measurement of the maximum amount of oxygen (VO2max) that an individual can utilize during maximal exercise10. VO2max has also been related with cortical thickness (CT) in frontal, temporal and cingular cortices11, as well as in the insula and precuneus12. The positive association between cardiovascular fitness and brain morphology was deemed to be more specific to the older rather than younger age13. However, a recent study in a large middle-aged adult sample (n = 2,103) reported a positive association of VO2max with total brain volume and GMV of the middle temporal gyrus, hippocampus, orbitofrontal cortex and cingulate cortex in the general population14. VO2max-associated greater GMV in the dorsal striatum has been proposed to be responsible for the better cognitive flexibility observed with higher VO2max.
Several different mechanisms have been so far proposed to explain the virtuous relationship between cardiovascular fitness and brain health in aging. Post-exercise astrocyte swelling, blood flow increases, and increases in the level of Brain-Derived Neurotrophic Factor (BDNF), a neurotrophin strongly implicated in brain plasticity and capable of inducing rapid changes in CT and synaptic density, have all been suggested to mediate the beneficial effects of fitness on neuroplasticity15–17. Indeed, a single session of 35 min of physical activity can already raise the levels of BDNF18,19. Transient positive effects on cognitive performance have also been reported already after single bouts of physical activity20–22. Working memory and executive functions have been reported to be particularly improved by physical activity, with effects lasting up to two hours after the cessation of the exercise bout23–25. Whether rapid morphological changes in GMV and CT can be induced after single sessions of physical exercise is, however, an open issue, as most of the studies of brain structure have had intervention designs of at least 4 weeks of physical exercise26. Nonetheless, task-related changes in measures of grey matter (GM) are known to occur very rapidly and can be detected already over hours or even a few minutes27–29. These effects might be mediated by rapid changes in blood flow30. Indeed, arterial blood has larger T1 (relaxation time) than brain tissues, therefore substantial perfusion changes can induce apparent tissue changes in the T1-weighted images30 that are typically used to assess brain volume and cortical thickness.
Fluctuations in cerebral blood flow have also been proposed to explain the changes in brain activity after single sessions of physical exercise16,17. Previous research suggested that improvements in performance following a single session of physical exercise are coupled with increases in the magnitude of functional brain activity during executive tasks mainly in frontal areas20,24,31; however, there are also reports showing that task-related functional activity in several other areas, such as the cerebellum25,32, hippocampus31,32, parietal cortex32, and insula20 is increased following acute physical exercise. Moreover, the exercise-induced changes in functional brain activity and executive performance remain unknown. These lacunae, as well as the variability in study protocols and the small number of studies available, warrant further studies on the short-term effect of physical exercise of brain activity33.
Aerobic fitness is likely to exert a regulatory role on cerebral blood flow in aging34, by modulating myocardial function35, leading to lower arterial stiffness36 and ultimately improving cerebral blood flow36,37. Accordingly, VO2max has been positively related with higher middle cerebral arteries compliance38,39 and with higher regional cerebral blood flow in patients with coronary artery disease40. By sustaining aerobic capacity, aerobic fitness may thus counteract the detrimental effect of aortic stiffness on cognitive aging41. However, the findings have been so far conflicting. One study reported the existence of a positive relationship between fitness and cerebral blood flow in females but not in males42. Another study did not detect any association of cerebrovascular reactivity or blood flow with VO2max43, and another reported even a negative relationship with VO2max38. A potential explanation for these discrepancies is that the positive effect of aerobic fitness on age-related decline may go beyond vascular effects and more directly involve neurons’ preservation and metabolism2, as suggested by the positive correlation between NAA levels (N-acetyl-aspartate, a brain-specific metabolite enhancing mitochondrial energy production) and cardiovascular fitness and working memory2.
The present study investigates the effect of acute physical exercise on the brain and cognition in older adults, and follows our previous work focusing on cognitive performance and cerebral blood flow44. In our former study, we found that grey matter blood flow (GMBF) (assessed with arterial spin labelling) decreased after 30 min of cycling at moderate intensity compared with 30 min of rest. Working memory performance, on the other hand, was unaffected by the intervention. In the present ancillary work, we focus on the effects of acute physical exercise, compared with rest, on grey matter measures (GMV, CT) and task-related brain activity in older adults. We hypothesized that the exercise group would exhibit larger GMV and mean CT, as well as higher task-related activity post-intervention, compared with the relaxation group. We also hypothesized that changes in brain activity and GM measures would partly depend on the underlying changes in GMBF. Additionally, we hypothesized that VO2max would be positively related with working memory performance, mean GMBF and GM morphological measures. The study was preregistered on the Open Science Foundation platform (https://osf.io/bxjkq; https://osf.io/jg8tz; https://osf.io/4mxgt).
Methods
Subjects
The study was approved by the ethics committee of region Stockholm in Sweden (Regionala Etikprövningsnämnden i Stockholm, Dnr: 2018/1340–31/2) and complied with the principles of the Declaration of Helsinki. Participants were recruited via advertisements in local newspapers and flyers in the Stockholm area between September and December 2018. The ads sought older volunteers, aged between 65 and 75 years, for a study investigating the effects of physical exercise and rest on brain function Inclusion criteria were: availability to attend all sessions; adequate hearing; normal or corrected vision; fluent Swedish (to ensure the understanding of the instructions); adequate mobility to ensure a safe performance of the physical exercise; right-handedness (to avoid handedness-related variability in brain structure and motor-related activity during the task45–49); weight below 120 kg. Exclusion criteria were: previous or current neurological diseases; a score below 26 on the Mini-Mental State Examination (MMSE)50; previous or current cardiovascular diseases (blood pressure up to 200/100 mmHg was allowed); history of brain damage or stroke; uncontrolled metabolic diseases; type I or pharmacologically-treated type II diabetes; current cancer, unless more than one year had passed since the treatment end; psychiatric illness, except for a history of mild to moderate depression and/or anxiety; history of head trauma resulting in loss of consciousness; previous participation in studies involving cognitive tests; presence of metal in the body; claustrophobia; sound sensitivity; neuromotor or musculoskeletal dysfunctions; cardiovascular-active medications; ongoing infections; chest pain. An introduction meeting was held at the Aging Research Center (Karolinska Institute, Stockholm) for eligible participants. Of the 73 individuals who had initially expressed their interest in the study, 50 joined the experimental session. One was excluded due to MRI signs suggestive of past, undiagnosed stroke. Recruitment and screening procedures have been described in more detail elsewhere44 (https://osf.io/bxjkq). The exercise group included 24 individuals, while the relaxation group consisted of 25 individuals. Demographic variables, physical activity, MMSE score, blood pressure, and subjective memory evaluation of the two groups were comparable (table S6, Appendix D, Supplementary Material).
Behavioural and demographic measures
In the introduction meeting, participants received detailed information concerning the study and signed the informed consent. Participants were blind to the hypothesis of the study (i.e. whether we expected moderate intensity physical exercise to be superior to rest in improving cognition and brain imaging measurements) in order to minimize the influence of participants’ expectations on the cognitive performance measurements. Demographic information was collected. All participants were administered the Edinburgh Handedness Inventory to confirm right-handedness and the Mini Mental State Examination (MMSE) to assess their cognitive status. The International Physical Activity Questionnaire (IPAQ)51 was used to estimate their habitual physical activity. Blood pressure was measured. Participants were also provided with instructions on how to perform the n-back task to be done in the main experimental session, and could try it out for 5 min.
Fitness assessment and calculation of work rate to be used during exercise
Fitness characterization was performed at the Åstrand Laboratory at the Swedish School of Sport and Health Sciences, GIH. Participants performed a submaximal incremental test on a cycle ergometer and a maximal incremental running test on a treadmill44. The submaximal test on the bike was used to identify the individual work rate and heart rate during biking at which the VO2 reached around 60% of the VO2max recorded during the maximal running test. This work rate is expected to result in a perceived level of exertion from 13–15 on the Borg RPE scale and a heart rate of around 60–70% of maximal individual heart rate. This individual work rate and heart rate was then used during biking in the exercise intervention of the experimental session.
The submaximal test was performed on a cycle ergometer (model 828E, Monark, Varberg, Sweden). The protocol started with 4 min cycling at a standard work rate (resistance of 0.5 kp, 60 revolutions per minute), after which the resistance was increased in steps of 0.5–1 kp, until reaching 60–80% of individual maximal capacity and a rate of perceived exertion (RPE) of around 16 (and not above 16). The maximal incremental running test on a treadmill was used to measure VO2 max, the gold standard for the metabolic measurement in applied human research. Participants warmed up for 5–10 min before starting the test. The treadmill was initially set to an incline of 1 degree and a comfortable speed (around RPE 12–13). Incline degree and/or speed were increased every minute until volitional exhaustion. VO2 max was measured using a computerized metabolic system (Jaeger Oxycon Pro, Hoechberg, Germany). The highest 30 s of registered values of VO2 were referred to as VO2 max. All procedures have been described in more detail elsewhere44 (https://osf.io/bxjkq).
Experimental session and MRI acquisition
The experimental session was performed at the Siemens Prisma 3-Tesla facility at the Karolinska University Hospital in Huddinge. Subjects randomized to the exercise group cycled on a stationary bike for 30 min, starting at the intensity estimated from the fitness characterization. Subjects randomized to the relaxation group were asked to lay down and relax for 30 min, while listening to relaxing music with water-sounds in the background. MRI scanning was carried out at pre-test, and 7 min after the exercise or rest session. This time was necessary to allow for the safe placement of the participants back in the scanner. GMBF was measured with an arterial spin labeling (ASL) acquisition (TR 4000 ms; TE = 12 ms; inversion time (TI) = 2000 ms; FOV 128 × 128; 1.9 × 1.9x4.5 mm3; slice thickness 4.5 mm; 32 slices). FMRI was acquired with a T2*-weighted echoplanar imaging sequence (TR = 2000 ms; TE = 30 ms; flip angle = 90°; slice thickness = 3.5 mm; voxel-size 3.5 mm3; slice spacing = 3.5 mm; 38 slices). Structural images were acquired via a MPRAGE (T1-weighted magnetization prepared gradient-echo) sequence (Repetition time (TR) = 2300 ms; Echo time (TE) = 2.01 ms; flip angle = 9°; field of view (FOV) 240 × 256; voxel sixe: 1 mm3; slice thickness 1 mm; 208 slices).
fMRI protocol
A figural n-back task was used to assess working memory performance during the fMRI (Fig. 1). The n-back task is a widely used task to measure working memory52, which is part of the executive functions domain. Executive functions are particularly sensitive to the effects of physical exercise20,24,31,53,54. The n-back task is one of the most used paradigms to assess executive (specifically, working memory) performance, and it is largely employed in brain imaging research given its easy implementation in the scanner environment52. The task consisted in alternating blocks with different cognitive load (1-back, 2-back, 3-back), administered three times each. In each block, the subjects were presented with a sequence of stimuli, and they were instructed to indicate when the current stimulus matched the one from n steps earlier in the sequence. Between blocks a 4-s fixation screen was displayed, indicating the rule for the forthcoming level. Each block lasted one minute. The accuracy on the task was calculated as: (correct hits + correct rejections)/total stimuli. The accuracy on each load and average accuracy across loads were calculated. Prior to pre-test imaging, a short practice (10 min) of the n-back task was performed when the subject was in the scanner. Due to a malfunctioning of the keyboard, n-back data of nine participants could not be recorded. Two extreme outliers on n-back performance were identified and excluded from the analyses44.
Figure 1.
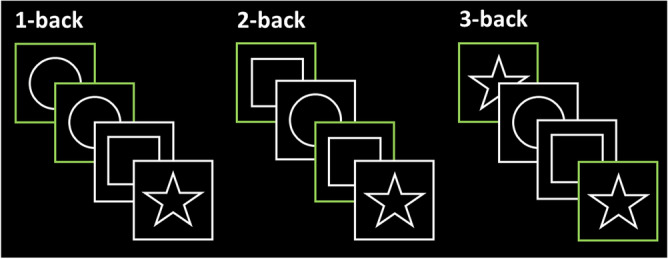
Figural n-back task. The task consisted in alternating blocks with different cognitive load (1-back, 2-back, 3-back), administered three times each. Each block lasted one minute.
Pre-processing of functional imaging data
The pre-processing of fMRI data is described in Appendix A, Supplementary Materials. Briefly, fMRI pre-processing steps for the BOLD signal analysis were carried out with Statistical Parametric Mapping 12 (SPM 12) and DPARSFA (http://rfmri.org/DPARSF) and included slice-timing, realignment, coregistration with the structural images, normalization, resampling to a 3 mm3 voxel-size and smoothing with an 8 mm full width at half maximum (FWHM) Gaussian kernel. As fMRI signal can be confounded by individual differences in the neurovascular coupling, especially in aging55, we additionally performed a network analysis with a graph theory approach, less sensitive to such confounds55. These analyses were included in the preregistration (https://osf.io/jg8tz), and are described in Appendix B, Supplementary Materials. Results are reported in figures S1, S2 and tables S1, S2 in Appendix B, Supplementary Materials.
Pre-processing of structural imaging data
Structural data were pre-processed with CAT12 (http://www.neuro.uni-jena.de/cat/). The cross-sectional pipeline was initially used to process data for the cross-sectional, correlational part of this project. The longitudinal pipeline was used for the longitudinal assessment. This consisted in: an initial inverse-consistent rigid registration; segmentation into GM, white matter and cerebrospinal fluid probability maps; non-linear spatial registration and averaging of the images; modulation; and smoothing with a 8 mm FWHM Gaussian kernel. Two data-points (post-test) presented with severe ring artefacts at visual inspection and were thus excluded from further analysis. Total GMV was calculated from the non-modulated image of the remaining subjects. CT and surface reconstruction were obtained from CAT12 as part of the longitudinal pipeline with a projection-based thickness (PBT) approach. Briefly, tissue segmentation is used to estimate the white matter distance, then local maxima (corresponding to the cortical thickness) are projected onto other GM voxels using a neighbouring algorithm depending on the white matter distance. Smoothing with a 12 mm FWHM Gaussian kernel was then applied to the surface images. More details on the pre-processing of structural imaging data can be found in Appendix A, Supplementary Material.
Pre-processing of ASL data
ASL data were checked for movement by calculating the root mean square signal change (DVARS) values, defined as the root mean square intensity difference of paired volumes (volume N to volume N + 1)46. Data-point with DVARS values exceeding the threshold of 75th percentile + 1.5 times the InterQuartile Range (IQR) were excluded due to excessive movement. One subject was excluded from the analysis as more than 30% of the acquisition was flagged for excessive movement (pre-test). ASL data were pre-processed with FSL (FMRIB Software Library). The oxford_asl command included in the BASIL (Bayesian Inference for Arterial Spin Labeling MRI) toolbox was used to process ASL data56. This automated pipeline includes motion correction, followed by the creation of subject-space tissue perfusion maps that are subsequently registered to the MNI standard space by applying the parameters generated during the registration of the subject’s structural image (see Appendix A, Supplementary Material). The calibrated perfusion map is then generated using the proton density weighted image, using the cerebrospinal fluid (CSF) as reference to calculate the equilibrium magnetisation of blood within a CSF mask. Calibrated perfusion images express GMBF in ml/100 g/min. The maps were smoothed with an 8 mm FWHM gaussian kernel. Prior to statistical analyses, all images were visually inspected to ensure the good quality of the pre-processing.
Results
Preregistered hypotheses (Hi) and follow-up research questions (RQi) are described below, along with the analyses used to address them (https://osf.io/bxjkq; https://osf.io/jg8tz; https://osf.io/4mxgt). As per pre-registration, the generalized-linear mixed model analyses for the longitudinal part of the project were performed with Statistical Package for Social Science (SPSS); cross-sectional correlational analyses were performed with R Studio. All hypotheses, follow-up research questions and corresponding analyses were pre-registered prior to the study (https://osf.io/bxjkq; https://osf.io/jg8tz; https://osf.io/4mxgt), regardless of their inclusion in the main text or in the Supplementary Materials. However, given the nature of the findings, some hypotheses became less meaningful, and were not expected to lead to the a priori hypothesized results. These hypotheses and corresponding analyses are reported in Appendix C, Supplementary Materials. Results are reported in tables S3, S4, S5 in Appendix C, Supplementary Materials.
Longitudinal analyses: effect of a single session of exercise vs rest on imaging measures
Effects of exercise on grey matter volume
H1
Total grey matter volume will increase after exercise compared with rest.
We used a generalized-linear mixed model with gamma distribution and robust estimation to test for effects of the group, session, and the group*session interaction (that directly tests our hypothesis) on total GMV. A random intercept model was built, where subjects represented the random factor, group was treated as between-subject factor, and session was treated as within-subject factor. The residual method was used for the estimation of denominator degrees of freedom. The threshold for significance was set at p < 0.05.
Group, session and the group*session interaction had no statistically significant effect on total GMV (table S7 and figure S3, Appendix D, Supplementary Materials).
RQ1
Is the effect of exercise on grey matter volume region-specific?
A flexible factorial model as implemented in SPM12 was used to test for main effects of the group, session, and group*session interaction on whole-brain, voxel-wise GMV. The flexible factorial model allows for mixed-model specification, where group was set as between-subject factor and session was set as within-subject factor. A preliminary uncorrected threshold of p < 0.001 was applied57. Voxels surviving such threshold were further corrected for family-wise error (FWE) rate at cluster level with a threshold of p < 0.0557.
No significant effects of the group, session, or group*session on voxel-wise GMV were found, indicating no effect of physical activity on GMV.
Effects of exercise on cortical thickness
H2
Mean cortical thickness will increase after exercise compared with rest.
Mean CT was not normally distributed according to the Shapiro-Wilks’s test for normality, nor was its log transformation. Thus, we applied a rank transformation to normal scores according to Rankit’s formula for proportional estimation: (r-1/2)/w ; where w is the number of observations and r is the rank, ranging from 1 to w. Rankit’s formula has been deemed to be more accurate than Blom, Tukey or Van der Warden58. A generalized-linear mixed model with robust estimation was used to test for effects of group, session, and group*session interaction (critical effect to test our hypothesis) on mean CT. Subjects represented the random factor (intercept model), group was entered as between-subject factor, and session was treated as within-subject factor. The residual method was used for the estimation of denominator degrees of freedom. The threshold for significance was set at p < 0.05.
No statistically significant effects of the group or session*group on mean CT were detected. Only the effect of session was statistically significant, indicating reduced mean CT at post-test independent of group (Cohen’s d = 0.114) (Table 1).
Table 1.
Statistical results for the group, session, and group by session effects on mean CT (n = 49).
| C.E | S.E | t | Adj. Sig | C.I. 95% (min, max) | |
|---|---|---|---|---|---|
| Group, F(1,90) = .001; p = .991 | |||||
| Exercise vs Rest | .003 | .276 | .012 | .991 | − .546, .552 |
| Session, F(1,90) = 5.935; p = .017 † | |||||
| Post-test vs Pre-test † | − .111 | .045 | − 2.436 | .017 | − .201, − .020 |
| Group*session F(1,90) = .379; p = .539 | |||||
| Exercise, post- vs pre-test | − .083 | .049 | − 1.687 | .095 | − .180, .015 |
| Rest, post- vs pre-test | − .139 | .077 | − 1.813 | .073 | − .291, .013 |
C.E., contrast estimate; S.E., standard error; df, degrees of freedom; C.I., confidence intervals.
†Statistically significant.
RQ2
Is the effect of exercise on cortical thickness region-specific, and is it associated to changes in GMBF?
CAT12 was used to test for a group*session [factors] effect on whole-brain, vertex-wise CT [dependent variable]. Group was treated as between-subject factor; session was entered as within-subject factor. A preliminary uncorrected threshold of p < 0.001 was applied57. Vertices surviving such threshold were further corrected for FWE rate at cluster level with a threshold of p < 0.0557.
Despite the overall reduction in mean CT, this vertex-wise exploratory analysis revealed a statistically significant increase in CT in the intracalcarine (visual) cortex (p FWE-corr = 0.004; 136 voxels; t = 5.05; Fig. 2). No effects of the intervention or intervention*session were found.
Figure 2.
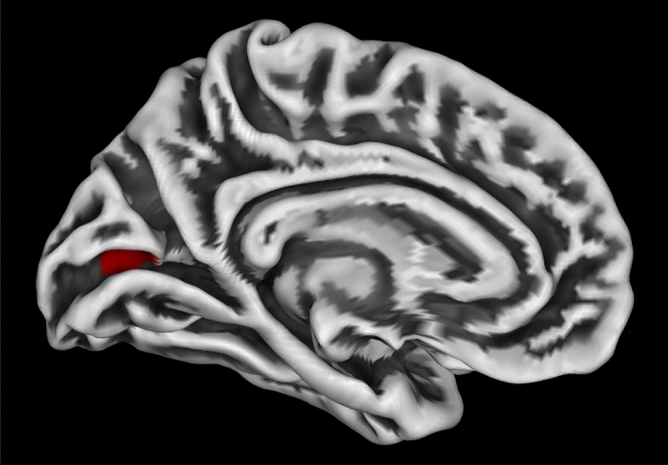
Cortical thickness increase in the visual cortex. The vertex-wise analysis revealed a cluster with pre- to post-test increased cortical thickness in the visual cortex (red) (p FWE-corr = .004). The figure was obtained with CAT12, overlaying the cluster on a surface rendering. MNI coordinates (x, y, z): −13, −77, 11. The Harvard–Oxford Cortical Structural Atlas was used to derive the location of the coordinates.
The same analysis was carried out by correcting for the regional GMBF of the visual cortex. The results were unchanged.
H3
Pre- to post-test changes in total grey matter volume and mean cortical thickness will correlate with changes in GMBF more strongly in the exercise group.
The difference in mean cortical thickness between sessions was computed (post-test vs pre-test). A positive difference reflected a post-test increase in mean cortical thickness. One subject was an extreme outlier on the mean cortical thickness and was excluded from the correlation analysis. Effects of the group, GMBF, and GMBF*group effects were tested with a generalized linear model with robust estimation. The group*GMBF changes was the critical effect that addresses our analysis. The threshold for significance was set at p < 0.05.
Changes in GMBF was not significantly associated with changes CT and group did affect the magnitude of the association in a statistically significant way.
Effects of exercise on brain activity
H4
Exercise will increase task-related brain activity compared with rest.
SPM 12 was used to analyse fMRI data. First-level analyses were carried out to generate the 2-back vs 1-back and 3-back versus 1-back contrasts, correcting for motion parameters. A preliminary one-sample t-test was carried out to ensure that the task was eliciting the expected effect on the BOLD signal. A full factorial model was then run to test for the effects of group, session, load, and all the two- and three-ways interactions on voxel-wise whole-brain activity during the figurative working memory task. The group*session and group*session*load interactions were the effects of interest. Group was set as between-subject factor; session and load were treated as within-subject factors. Two load levels were entered: the 2- vs 1- back contrast was set as first level; the 3- vs 1-back contrast was set as second level. The threshold for significance was set at p < 0.001, further corrected for multiple testing with a family-wise error (FWE) correction at cluster level with a threshold of p < 0.0557. Post-hoc tests were performed masked for the relevant significant effects.
The one-sample t-test showed widespread fronto-parietal task-related activity relative to both the 2-back versus 1-back and 3-back versus 1-back contrasts (Figure S4, S5 and table S8, Appendix D, Supplementary Materials). No statistically significant effects of the group, session, group*session or group*session*load interaction were found. Thus, brain activity did not show statistically significant changes from pre- to post-test, and was unaffected by the type of the intervention.
Cross-sectional analyses: associations between VO2max and pre-test cognitive and imaging measures
Association between VO2max and working memory
H5
VO2max will positively correlate with working memory performance.
ANOVA-repeated measures was used to test whether VO2max (independent variable) was associated with working memory performance (dependent variable). Prior to the analysis, n-back accuracy was tested for the presence of extreme outliers. One subject was an extreme outlier on the 1- and 2-back; another subject was an outlier on the 1-back task. No outliers were detected on the 3-back performance. Homogeneity of variance was inspected visually and formally tested with the Non-constant Variance (NCV) Score test (car package). Homogeneity of variance was confirmed on all loads (1-back, p = 0.744; 2-back, p = 0.253; 3-back, p = 0.969). Normality of the residuals was not met; however, given that ANOVA is quite robust to violations of the normality assumption59,60, repeated-measures ANOVA was applied. Nonetheless, we also analysed each load separately with generalized linear models with binomial distribution, selecting the number of correct answers as numerator and entering the total number of trials as denominator. All analyses were corrected by age and gender. A full factorial model (load, VO2max, load* VO2max) was carried out, where VO2max and load*VO2max were effects of interest. The threshold for significance was set at p < 0.05.
No statistically significant effect of VO2max or load*VO2max interaction on n-back accuracy was detected at the repeated-measure ANOVA, either at the uncorrected model nor when correcting for age and gender (table S5, Appendix C, Supplementary Material). When examining each load separately with generalized linear models, no significant associations between accuracy and VO2max were found at any load (p > 0.910).
Association between VO2max and grey matter blood flow
H6
VO2max will positively correlate with mean grey matter blood flow.
Prior to testing for an association between VO2max (independent variable) and mean GMBF (dependent variable), mean GMBF was tested for outliers. No extreme outliers were detected. Linearity of the relation and homogeneity of variance were visually verified. Additionally, the NCV test was applied to formally confirm homogeneity of variance (p = 0.266). The residuals approximated a normal distribution; however, gamma distribution was a better fit for mean GMBF (AIC = 308.082 vs AIC = 313.172). Therefore, a generalized linear model with gamma distribution (log-link) was used to test for associations between VO2max and mean GMBF. Age and gender were covariates in the analysis. The threshold for significance was set at p < 0.05.
VO2max displayed a statistically significant negative association with mean GMBF at the uncorrected model (p = 0.016; Confidence Intervals (CI) 95%: −0.04, −0.004) with a medium effect size (r = −0.35, corresponding to Cohen’s f = 0.37); a trend was still present after correcting for age and gender, though it only approached statistical significance (p = 0.08; r = −0.028, Cohen’s f = 0.29) (Table 2). This finding was in contrast with our prediction that VO2max would be positively associated with mean GMBF.
Table 2.
Associations between VO2max and morphological measures (n = 49).
| Estimate | Std. Error | T | P | C.I. 95% (min, max) | |
|---|---|---|---|---|---|
| GMBF | −.018 | .010 | −1.793 | .08 | − .038; .002 |
| GMV | −.0006 | .0005 | 1.192 | .240 | − .0016; .0004 |
| CT | .005 | .002 | 2.079 | .043 * | .001, .009 |
Corrected for age and gender.
RQ3
Is VO2max related with regional-specific GMBF?
Linear regression was used to test for a correlation between VO2max (independent variable) and whole-brain, voxel-wise GMBF (dependent variable). The analysis was corrected for age and gender. The threshold for significance was set at p < 0.001; a further FWE-correction at p < 0.05 was applied to voxels surviving the preliminary uncorrected threshold57. Post-hoc analyses were masked for the main effect of VO2max.
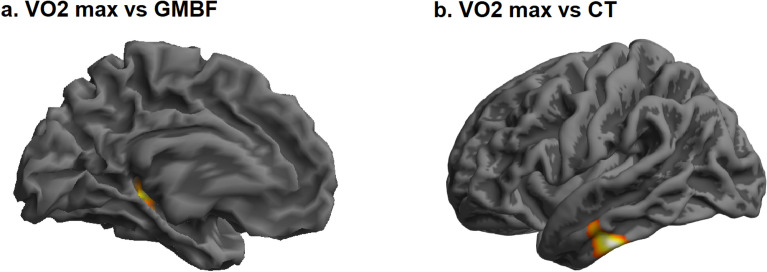
In this voxel-wise exploratory analysis, a statistically significant negative correlation between VO2max and GMBF (p FWE-corr = 0.0003) was detected in the left hippocampus (179 voxels, t = 5.64, MNI coordinates (x, y, z): −36, −34, 4) (Fig. 3a).
Figure 3.
Association between VO2max and brain measures. The figure shows the clusters were a statistically significant association was detected between VO2max and (a) GMBF and (b) CT, respectively. (a) VO2max was negatively associated with GMBF of the left hippocampus (p FEW-corr = .0003, t = 5.64, MNI coordinates (x, y, z): −36, −34, 4). (b) VO2max was, on the other hand, positively associated with the CT of the left superior temporal gyrus (STG) (p FWE-corr = .006, t = 5.18, MNI coordinates (x, y, z): −56, −21, −3). Clusters were superimposed on a surface rendering in SPM12. The Harvard–Oxford Cortical Structural Atlas was used to derive the location of the coordinates.
Association between VO2max and grey matter volume
H7
VO2max will positively correlate with total grey matter volume
Linear regression was used to test for associations between VO2max (independent variable) and total GMV (dependent variable), corrected for age, gender and total intracranial volume (TIV) (GM + white matter + cerebrospinal fluid). No extreme outliers on GMV were detected. Homogeneity of variance was met at the NCV test (p = 0.979) and the residuals were normally distributed. The threshold for significance was set at p < 0.05.
No statistically significant associations were detected between VO2max and total GMV at either the uncorrected model (p = 0.745; CI 95%: −0.0012, 0.0008; r = 0.14; Cohen’s f = 0.14), nor after correcting for age, gender and TIV (r = 0.11; Cohen’s f = 0.11) (Table 2).
RQ4. Is VO2max related with regional-specific grey matter volume?
Linear regression was used to test for a correlation between VO2max (independent variable) and whole-brain, voxel-wise GMV (dependent variable), correcting for age, gender and TIV. The threshold for significance was set at p < 0.001; a further FWE-correction at p < 0.05 was applied to voxels surviving the preliminary uncorrected threshold57. Post-hoc analyses were masked for the main effect of VO2max.
No statistically significant associations were detected between VO2max and voxel-wise GMV.
Association between VO2max and cortical thickness
H8
VO2max will positively correlate with mean cortical thickness
Linear regression was used to assess associations between VO2max (independent variable) and mean CT dependent variable, correcting for age and gender. No extreme outliers on mean cortical thickness were detected. The NCV test was used to verify the homogeneity of variance (p = 0.893). Residuals were normally distributed. The threshold for significance was set at p < 0.05.
VO2max had a statistically significant positive association with mean CT both at the uncorrected model (p = 0.039; CI 95%: 0.001, 0.009) and after correction for age and gender, with medium effect sizes (r = 0.30 and 0.33, corresponding to Cohen’s f = 0.31 and 0.34, at the uncorrected and corrected model, respectively) (Table 2).
RQ5
Is VO2max related with regional-specific CT?
CAT-12 was used to test for a correlation between VO2max (independent variable) and whole-brain, vertex-wise CT (dependent variable). The analysis was corrected for age and gender. The threshold for significance was set at p < 0.001; a further FWE-correction at p < 0.05 was applied to voxels surviving the preliminary uncorrected threshold57. Post-hoc analyses were masked for the main effect of VO2max.
In the vertex-wise analysis, VO2max had a statistically significant positive association with the CT in the left superior temporal gyrus (STG) (p FWE-corr = 0.006, 181 voxels, t = 5.18, MNI coordinates (x, y, z): −56, −21, −3) (Fig. 3b).
Discussion
We investigated the effects of moderate intensity physical exercise, relative to rest, on brain functional and structural measures, in older adults. A single session of moderate intensity physical exercise did not lead to appreciable changes in working memory performance, total GMV, CT or brain activity. However, mean CT was reduced at post-test independent of group, while regional CT was increased in the visual cortex. Cardiovascular fitness, measured as VO2max, was inversely associated with hippocampal blood flow and showed a trend for an inverse association with mean GMBF, in contrast with our hypotheses of a positive relation between VO2max and GMBF. On the other hand, VO2max and mean CT were positively associated in accordance with our hypothesis, and a positive association between VO2max and CT in the left STG was detected.
Brain morphological measures: effect of exercise and association with VO2max
We report positive associations of VO2max with mean CT as well as with CT of the left STG. The left STG has largely been implicated in speech processing and comprehension61,62 and in speech-related social cognition61,62; however, in recent years its role in social cognition and information processing has also been highlighted63–65. Although few studies focused on CT measures in relation to aerobic fitness, previous research has reported specific positive associations of VO2max with the GMV43,66 and CT of the left STG13,67. Moreover, moderate-intensity aerobic training may slow down cortical thinning in several brain areas in patients with Alzheimer’s Disease68, and mean CT is also positively related with physical activity habits in cognitively healthy individuals69. The beneficial effect of cardiorespiratory fitness on body composition, and specifically body fat reduction, has been proposed to mediate the positive relationship between fitness and mean CT, at least in developing children70. In fact, adiposity is related with thinner cortex during development70. In older adults, cardiovascular fitness is however more likely to counteract the detrimental effects of aging on brain health by promoting neurons’ preservation and metabolism2.
Interestingly, the association between VO2max and brain morphology was specific to CT, while total GMV did not show any statistically significant association with VO2max. CT and GMV measurements are related; however, GMV measurements also depend on cortical folding and surface area71–74, and are more closely related with the latter rather than with CT72. CT and surface area are in fact genetically and phenotypically independent72, making GMV a composite71,73,74 and thus less readily interpretable measure of brain morphology72. Interestingly, CT seems to be more sensitive to atrophy compared with GMV73,74, an aspect to be considered when focusing on older age. This may also explain why we could not detect any effect of exercise or session on GMV.
Mean CT was also reduced at post-test independent of group. CT reductions may be associated with pruning of superfluous or weak synapses and increased myelination, which may occur already over minutes during fast learning75, speeding up the learning process by eliminating synapses producing high-error spikes75. We did observe changes in working memory performance; thus, it is possible that synaptic pruning may be partially contributing to the general decrease in mean CT at post-test. However, it is worth noting that mean GMBF was reduced at post-test in our sample44, which may be a potential cause for apparent reductions in mean CT30. Arterial blood has in fact larger T1 (relaxation time) than brain tissues, and perfusion changes can induce signal changes in T1-weighted images30. Physical exercise initially induces transient increases in cerebral blood flow16,76, independent of the intensity of exertion. The post-exercise hypotension and consequential drop in cardiac output, coupled with sympathetically-mediated vasoconstriction reflexes aimed at protecting the brain from the damage of hyper-perfusion, then leads to a quick decrease in cerebral blood flow, detectable a few seconds after the cessation of the exercise bout16,76. We did not find a direct relationship between cortical thickness and GMBF, and reduced mean GMBF was only observed in the exercise group44 rather than independently of group as was the case for mean CT; however, pulse pressure was increased in our sample after the first scanning session44 regardless of the intervention, possibly reflecting stress in relation to the experimental procedure77. Thus, we cannot rule out that changes in mean GMBF may have confounded our results.
The finding of increased CT in the visual cortex is, on the other hand, in line with previous reports of early increases in visual CT occurring already over very short periods of visual stimulation27, such as that elicited in our sample by the working memory task performed prior to the structural acquisition. Previous studies have suggested that such rapid changes in brain morphology may be ascribed to dendritic spine plasticity in the visual cortex78, or to changes in blood flow27. However, correcting our analysis for GMBF in the visual cortex did not alter our results, and GMBF and CT of the visual cortex did not correlate, suggesting that GMBF may not be the sole contributor to the increased CT in the primary visual cortex. Further research is warranted to disentangle the nature of such signal changes. However, the notion that T1-weighted signal can alter so rapidly is crucial to MRI research. Global CT measurements likely display sufficient measurement stability to detect subtle changes that may occur over short time scales as a consequence of undetermined physiological processes. As such, strict protocol control is necessary to ensure reproducibility within longitudinal studies as well as across studies.
Summarizing, we expanded current research on aerobic fitness by using a more precise measure such as VO2max, and by investigating different measures of grey matter morphology. In particular, we report positive associations of VO2max with CT, a measure often overlooked in favour of GMV despite its indication as a more sensitive measure of grey matter modifications in older age compared with GMV. We also support recent reports on the occurrence of rapid changes in CT in response to visual stimuli, extending previous findings to an older population. The nature of such changes is still debated. In this context, we support recent views that variations in blood flow may not be the sole contributor to rapidly detectable alterations in CT measurements. Although we cannot assert with absolute certainty that true plasticity may be at play, we nonetheless suggest that CT measurements may be sensitive to subtle undetermined physiological changes occurring over short time scales.
Association between GMBF and VO2max
VO2max and cardiovascular fitness have been associated with higher middle cerebral artery compliance38,39 and higher cerebral blood flow in clinical40 and non-clinical populations34,36,79. Our study, however, supports more recent findings of negative associations between VO2max and resting GMBF38,43, specifically in the left hippocampus. Several mechanisms have been proposed to underlie discrepancies across studies. For example, differences in the VO2max range and cardiovascular health of the participants43, along with genetic variants potentially influencing brain perfusion, such as APoE4 variants associated with cerebral hyper-perfusion43, may all contribute to these somewhat conflicting literature findings. A recent hypothesis proposed by Furby et al.38 considers that fitter individuals may have more efficient gas exchange from the capillary bed due to increased vessel surface. This may lead to shorter diffusion distances and facilitate nutrient extraction, potentially reducing the amount of GMBF needed to meet metabolic oxygen demand38 and resulting in a negative association between GMBF and VO2max. Numerous preclinical studies have in fact demonstrated that regular physical exercise and aerobic fitness can increase brain vessel density80–82, also in the hippocampus80,83.
Brain activity during the working memory task: effects of exercise and association with VO2max
We did not find any effect of a single session of physical exercise on working memory-related brain activity, contrary to previous studies. However, most of these studies have focused on children32, adolescents31, or young adults20,25. The only study carried out on older participants reported increased brain activation in the IFG and IPL and decreased activation in the anterior cingulate cortex after acute exercise compared with rest, during a conflict monitoring task24. Whether the effects might depend on the type of task and the executive sub-domain targeted53,54, or whether confounding factors such as exercise intensity might be at play, remains to be elucidated.
We did not detect any association of VO2max with working memory accuracy on any load. While similar findings of no associations between cardiovascular fitness and working memory have been previously reported in middle-aged Swedish office workers84, several studies have pointed toward a positive association between VO2max and working memory performance, at least in healthy adolescents and younger adults85–88, and in older adults with mild to moderate cognitive impairment89. The absence of clinically evident cognitive impairment in our sample, the level and variability in their fitness levels, and the small sample size may all have contributed to this discrepancy. Indeed, n-back back accuracy data were missing on nine participants, further limiting our power to detect small effects in correlation analyses and preventing us from stratifying the participants according to their fitness level, an important confounder when investigating cognitive measures84.
Limitations and conclusions
This study is based on data from a project aiming at outlining the effect of acute physical exercise on the brain and cognition in older adults, and follows previous work focusing on cognitive performance and cerebral blood flow44. The sample size was somewhat small, limiting our power to detect small effects. Sample size was determined by statistical power analyses (G*Power), as reported in the preregistration (https://osf.io/m62rp/wiki/Power%20analysis/). However, n-back accuracy data were missing on nine participants, and seven subjects had to be excluded from our fMRI analysis due to movement, reducing our power in corresponding analyses. This might have contributed to the Moreover, we only assessed brain morphology twice, at pre- and post-test. Therefore, we cannot define the time-course and duration of the CT changes we observed, nor whether the effect of physical activity on brain morphology may be transient or sustained. While the mechanisms underlying those effects may be relatively transient, if repeated during habitual exercise, they may still induce sustained effects. Therefore, transient effects may be upstream causes of sustained effects. Future studies may be designed to acquire follow-up measurements of cerebral blood flow, brain morphology and cognition days or even weeks apart, to assess the time-course of brain changes induced by a single, acute bout of exercise. Such studies may also consider collecting device-based measures of physical activity and heart rate in the 24 h period prior to the acute exercise session.
Despite these limitations, we can conclude that a single session of moderate intensity exercise did not lead to appreciable changes in brain activity, working memory performance or total GMV in older individuals, compared to rest. VO2max, was associated with higher mean CT and higher CT in the left STG, but not with GMV. This supports the proposition that cardiovascular fitness is related with brain health in aging, and suggests that CT may be a more sensitive measure in older age compared with GMV. Furthermore, a negative association between VO2max and GMBF, particularly in the left hippocampus, was detected, reinforcing recent theories holding that fitter individuals may require lower GMBF levels to meet metabolic oxygen demand due to increased vessel surface and subsequent better capacity for gas exchange and nutrient extraction. Worth noting, associations between VO2max and imaging measures were limited to small, focal clusters. This may possibly indicate a lack of power to detect more widespread correlations, which might have been expected due to the global effect of aerobic activity and physical activity on brain circulation and morphology3–7,11,34,36,37.
We also support recent reports on the occurrence of rapid changes in CT in response to visual stimuli, extending previous findings to an older population. Regardless of the nature of such signal changes, the notion that T1-weighted signal can alter so rapidly is crucial to MRI research, hindering the reproducibility across studies using both structural and task-fMRI in the same session.
Supplementary Information
Acknowledgements
We thank Marie Helsing Västfjäll for her help with recruitment and organization, William Fredborg and Mattias Söderberg for their help with data collection, Eva Andersson for performing the health checks prior to cardiovascular testing and Henrik Petré for performing the cardiovascular testing. The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant Agreement No. 617280—REBOOT.
Author contributions
G.O. has run formal analyses on the data, wrote the original draft and handled the revision; J.N., B.G., A.L., M.E., M.L. were involved in the conceptualization and design of the study; A.L., O.T., M.E., M.L. were involved in data curation; G.O., J.N., B.G., A.L., A.W., O.T., M.E., M.L. contributed to the methodology of the study; M.E., M.L. obtained funding for the study; all authors revised the manuscript and contributed with critical intellectual content.
Funding
Open access funding provided by University of Gothenburg. The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007–2013)/ERC Grant Agreement No. 617280—REBOOT.
Data availability
Data are stored in compliance with the GDPR regulations. Data can be requested to the authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-96138-5.
References
- 1.Colcombe SJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc. Natl. Acad. Sci. USA. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotting K, Roder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci. Biobehav. Rev. 2013;37:2243–2257. doi: 10.1016/j.neubiorev.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Hamer M, Sharma N, Batty GD. Association of objectively measured physical activity with brain structure: UK Biobank study. J. Intern. Med. 2018;284:439–443. doi: 10.1111/joim.12772. [DOI] [PubMed] [Google Scholar]
- 4.Rovio S, et al. The effect of midlife physical activity on structural brain changes in the elderly. Neurobiol. Aging. 2010;31:1927–1936. doi: 10.1016/j.neurobiolaging.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Barnes JN, Corkery AT. Exercise improves vascular function, but does this translate to the brain? Brain Plast. 2018;4:65–79. doi: 10.3233/BPL-180075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kandola A, Ashdown-Franks G, Hendrikse J, Sabiston CM, Stubbs B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci. Biobehav. Rev. 2019 doi: 10.1016/j.neubiorev.2019.09.040. [DOI] [PubMed] [Google Scholar]
- 7.Liu-Ambrose T, Barha CK, Best JR. Physical activity for brain health in older adults. Appl. Physiol. Nutr. Metab. 2018;43:1105–1112. doi: 10.1139/apnm-2018-0260. [DOI] [PubMed] [Google Scholar]
- 8.Tyndall AV, et al. Protective effects of exercise on cognition and brain health in older adults. Exerc. Sport Sci. Rev. 2018;46:215–223. doi: 10.1249/JES.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 9.Batouli SAH, Saba V. At least eighty percent of brain grey matter is modifiable by physical activity: A review study. Behav. Brain Res. 2017;332:204–217. doi: 10.1016/j.bbr.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Hawkins MN, Raven PB, Snell PG, Stray-Gundersen J, Levine BD. Maximal oxygen uptake as a parametric measure of cardiorespiratory capacity. Med. Sci. Sports Exerc. 2007;39:103–107. doi: 10.1249/01.mss.0000241641.75101.64. [DOI] [PubMed] [Google Scholar]
- 11.Scheewe TW, et al. Exercise therapy, cardiorespiratory fitness and their effect on brain volumes: A randomised controlled trial in patients with schizophrenia and healthy controls. Eur. Neuropsychopharmacol. 2013;23:675–685. doi: 10.1016/j.euroneuro.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Reiter K, et al. Improved cardiorespiratory fitness is associated with increased cortical thickness in mild cognitive impairment. J. Int. Neuropsychol. Soc. 2015;21:757–767. doi: 10.1017/S135561771500079X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams VJ, et al. Cardiorespiratory fitness is differentially associated with cortical thickness in young and older adults. Neuroimage. 2017;146:1084–1092. doi: 10.1016/j.neuroimage.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wittfeld K, et al. Cardiorespiratory fitness and gray matter volume in the temporal, frontal, and cerebellar regions in the general population. Mayo Clin. Proc. 2020;95:44–56. doi: 10.1016/j.mayocp.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 15.Engeroff T, et al. Is objectively assessed sedentary behavior, physical activity and cardiorespiratory fitness linked to brain plasticity outcomes in old age? Neuroscience. 2018;388:384–392. doi: 10.1016/j.neuroscience.2018.07.050. [DOI] [PubMed] [Google Scholar]
- 16.Ogoh S, Ainslie PN. Cerebral blood flow during exercise: mechanisms of regulation. J. Appl. Physiol. 2009;1985(107):1370–1380. doi: 10.1152/japplphysiol.00573.2009. [DOI] [PubMed] [Google Scholar]
- 17.Smith JC, Paulson ES, Cook DB, Verber MD, Tian Q. Detecting changes in human cerebral blood flow after acute exercise using arterial spin labeling: Implications for fMRI. J. Neurosci. Methods. 2010;191:258–262. doi: 10.1016/j.jneumeth.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Hakansson K, et al. BDNF responses in healthy older persons to 35 minutes of physical exercise, cognitive training, and mindfulness: Associations with working memory function. J. Alzheimers Dis. 2017;55:645–657. doi: 10.3233/JAD-160593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilsson J, et al. Acute increases in brain-derived neurotrophic factor in plasma following physical exercise relates to subsequent learning in older adults. Sci. Rep. 2020;10:4395. doi: 10.1038/s41598-020-60124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehren A, et al. Intensity-dependent effects of acute exercise on executive function. Neural. Plast. 2019;2019:8608317. doi: 10.1155/2019/8608317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang YK, Labban JD, Gapin JI, Etnier JL. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012;1453:87–101. doi: 10.1016/j.brainres.2012.02.068. [DOI] [PubMed] [Google Scholar]
- 22.McMorris T, Hale BJ. Differential effects of differing intensities of acute exercise on speed and accuracy of cognition: A meta-analytical investigation. Brain Cogn. 2012;80:338–351. doi: 10.1016/j.bandc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Basso JC, Suzuki WA. The effects of acute exercise on mood, cognition, neurophysiology, and neurochemical pathways: A review. Brain Plast. 2017;2:127–152. doi: 10.3233/BPL-160040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Won J, Alfini AJ, Weiss LR, Callow DD, Smith JC. Brain activation during executive control after acute exercise in older adults. Int. J. Psychophysiol. 2019 doi: 10.1016/j.ijpsycho.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Li L, et al. Acute aerobic exercise increases cortical activity during working memory: a functional MRI study in female college students. PLoS ONE. 2014;9:e99222. doi: 10.1371/journal.pone.0099222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stimpson NJ, Davison G, Javadi AH. Joggin' the Noggin: towards a physiological understanding of exercise-induced cognitive benefits. Neurosci. Biobehav. Rev. 2018;88:177–186. doi: 10.1016/j.neubiorev.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Mansson KNT, et al. Viewing pictures triggers rapid morphological enlargement in the human visual cortex. Cereb. Cortex. 2020;30:851–857. doi: 10.1093/cercor/bhz131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nierhaus T, Vidaurre C, Sannelli C, Mueller KR, Villringer A. Immediate brain plasticity after one hour of brain-computer interface (BCI) J. Physiol. 2019 doi: 10.1113/JP278118. [DOI] [PubMed] [Google Scholar]
- 29.Kwok V, et al. Learning new color names produces rapid increase in gray matter in the intact adult human cortex. Proc. Natl. Acad. Sci. USA. 2011;108:6686–6688. doi: 10.1073/pnas.1103217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ge Q, et al. Short-term apparent brain tissue changes are contributed by cerebral blood flow alterations. PLoS ONE. 2017;12:e0182182. doi: 10.1371/journal.pone.0182182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metcalfe AW, et al. Effects of acute aerobic exercise on neural correlates of attention and inhibition in adolescents with bipolar disorder. Transl. Psychiatry. 2016;6:e814. doi: 10.1038/tp.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen AG, Zhu LN, Yan J, Yin HC. Neural basis of working memory enhancement after acute aerobic exercise: fMRI study of preadolescent children. Front. Psychol. 2016;7:1804. doi: 10.3389/fpsyg.2016.01804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herold F, Aye N, Lehmann N, Taubert M, Muller NG. The contribution of functional magnetic resonance imaging to the understanding of the effects of acute physical exercise on cognition. Brain Sci. 2020 doi: 10.3390/brainsci10030175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmerman B, et al. Cardiorespiratory fitness mediates the effects of aging on cerebral blood flow. Front. Aging Neurosci. 2014;6:59. doi: 10.3389/fnagi.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson NF, et al. Cardiorespiratory fitness modifies the relationship between myocardial function and cerebral blood flow in older adults. Neuroimage. 2016;131:126–132. doi: 10.1016/j.neuroimage.2015.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarumi T, et al. Central artery stiffness, neuropsychological function, and cerebral perfusion in sedentary and endurance-trained middle-aged adults. J. Hypertens. 2013;31:2400–2409. doi: 10.1097/HJH.0b013e328364decc. [DOI] [PubMed] [Google Scholar]
- 37.Brown AD, et al. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol. Aging. 2010;31:2047–2057. doi: 10.1016/j.neurobiolaging.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Furby HV, Warnert EA, Marley CJ, Bailey DM, Wise RG. Cardiorespiratory fitness is associated with increased middle cerebral arterial compliance and decreased cerebral blood flow in young healthy adults: A pulsed ASL MRI study. J. Cereb. Blood Flow Metab. 2019 doi: 10.1177/0271678X19865449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Witte E, et al. Exercise intensity and middle cerebral artery dynamics in humans. Respir. Physiol. Neurobiol. 2019;262:32–39. doi: 10.1016/j.resp.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacIntosh BJ, et al. Cardiopulmonary fitness correlates with regional cerebral grey matter perfusion and density in men with coronary artery disease. PLoS ONE. 2014;9:e91251. doi: 10.1371/journal.pone.0091251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kennedy G, et al. Physical fitness and aortic stiffness explain the reduced cognitive performance associated with increasing age in older people. J. Alzheimers Dis. 2018;63:1307–1316. doi: 10.3233/JAD-171107. [DOI] [PubMed] [Google Scholar]
- 42.Dougherty RJ, et al. Fitness, independent of physical activity is associated with cerebral blood flow in adults at risk for Alzheimer's disease. Brain Imaging Behav. 2019 doi: 10.1007/s11682-019-00068-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Intzandt B, et al. Higher cardiovascular fitness level is associated with lower cerebrovascular reactivity and perfusion in healthy older adults. J. Cereb. Blood Flow Metab. 2019 doi: 10.1177/0271678X19862873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olivo G, et al. Immediate effects of a single session of physical exercise on cognition and cerebral blood flow: A randomised controlled study of older adults. Neuroimage. 2021;225:117500. doi: 10.1016/j.neuroimage.2020.117500. [DOI] [PubMed] [Google Scholar]
- 45.Solodkin A, Hlustik P, Noll DC, Small SL. Lateralization of motor circuits and handedness during finger movements. Eur. J. Neurol. 2001;8:425–434. doi: 10.1046/j.1468-1331.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- 46.Dassonville P, Zhu XH, Uurbil K, Kim SG, Ashe J. Functional activation in motor cortex reflects the direction and the degree of handedness. Proc. Natl. Acad. Sci. USA. 1997;94:14015–14018. doi: 10.1073/pnas.94.25.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amunts K, et al. Asymmetry in the human motor cortex and handedness. Neuroimage. 1996;4:216–222. doi: 10.1006/nimg.1996.0073. [DOI] [PubMed] [Google Scholar]
- 48.Guadalupe T, et al. Differences in cerebral cortical anatomy of left- and right-handers. Front. Psychol. 2014;5:261. doi: 10.3389/fpsyg.2014.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hatta T. Handedness and the brain: A review of brain-imaging techniques. Magn. Reson. Med. Sci. 2007;6:99–112. doi: 10.2463/mrms.6.99. [DOI] [PubMed] [Google Scholar]
- 50.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 51.Craig CL, et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 52.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aguirre-Loaiza H, et al. Effect of acute physical exercise on executive functions and emotional recognition: Analysis of moderate to high intensity in young adults. Front. Psychol. 2019;10:2774. doi: 10.3389/fpsyg.2019.02774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alves CR, et al. Effects of acute physical exercise on executive functions: a comparison between aerobic and strength exercise. J. Sport Exerc. Psychol. 2012;34:539–549. doi: 10.1123/jsep.34.4.539. [DOI] [PubMed] [Google Scholar]
- 55.Schmithorst VJ, et al. Evidence that neurovascular coupling underlying the BOLD effect increases with age during childhood. Hum. Brain Mapp. 2015;36:1–15. doi: 10.1002/hbm.22608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chappell MA, Groves AR, Whitcher B, Woolrich MW. Variational Bayesian inference for a non-linear forward model. IEEE Trans. Signal Process. 2009;57:223–236. doi: 10.1109/TSP.2008.2005752. [DOI] [Google Scholar]
- 57.Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. Neuroimage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Solomon SR, Sawilowsky S. Impact of rank-based normalizing transformations on the accuracy of test scores. J. Mod. Appl. Stat. Methods. 2009;8:448–462. doi: 10.22237/jmasm/1257034080. [DOI] [Google Scholar]
- 59.Blanca MJ, Alarcon R, Arnau J, Bono R, Bendayan R. Non-normal data: Is ANOVA still a valid option? Psicothema. 2017;29:552–557. doi: 10.7334/psicothema2016.383. [DOI] [PubMed] [Google Scholar]
- 60.Ito, P. K. In Analysis of Variance Vol. 1 Handbook of Statistics (ed Krishnaiah, P. R.) Ch. 7, 199–236 (Elsevier B.V., 2020).
- 61.Wensing T, et al. Neural correlates of formal thought disorder: An activation likelihood estimation meta-analysis. Hum. Brain Mapp. 2017;38:4946–4965. doi: 10.1002/hbm.23706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yi HG, Leonard MK, Chang EF. The encoding of speech sounds in the superior temporal gyrus. Neuron. 2019;102:1096–1110. doi: 10.1016/j.neuron.2019.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Achiron A, et al. Superior temporal gyrus thickness correlates with cognitive performance in multiple sclerosis. Brain Struct. Funct. 2013;218:943–950. doi: 10.1007/s00429-012-0440-3. [DOI] [PubMed] [Google Scholar]
- 64.Alaerts K, et al. Age-related changes in intrinsic function of the superior temporal sulcus in autism spectrum disorders. Soc. Cogn. Affect Neurosci. 2015;10:1413–1423. doi: 10.1093/scan/nsv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maillet D, et al. Aging and the wandering brain: Age-related differences in the neural correlates of stimulus-independent thoughts. PLoS ONE. 2019;14:e0223981. doi: 10.1371/journal.pone.0223981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng G, et al. The effects of exercise on the structure of cognitive related brain regions: A meta-analysis of functional neuroimaging data. Int. J. Neurosci. 2019;129:406–415. doi: 10.1080/00207454.2018.1508135. [DOI] [PubMed] [Google Scholar]
- 67.d'Arbeloff T, et al. Is cardiovascular fitness associated with structural brain integrity in midlife? Evidence from a population-representative birth cohort study. Aging (Albany NY) 2020;12:20888–20914. doi: 10.18632/aging.104112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Um YH, et al. Effects of moderate intensity exercise on the cortical thickness and subcortical volumes of preclinical Alzheimer's disease patients: A pilot study. Psychiatry Investig. 2020;17:613–619. doi: 10.30773/pi.2020.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee JS, et al. Combined effects of physical exercise and education on age-related cortical thinning in cognitively normal individuals. Sci. Rep. 2016;6:24284. doi: 10.1038/srep24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Esteban-Cornejo I, et al. Fitness, cortical thickness and surface area in overweight/obese children: The mediating role of body composition and relationship with intelligence. Neuroimage. 2019;186:771–781. doi: 10.1016/j.neuroimage.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 71.Kong L, et al. Comparison of grey matter volume and thickness for analysing cortical changes in chronic schizophrenia: A matter of surface area, grey/white matter intensity contrast, and curvature. Psychiatry Res. 2015;231:176–183. doi: 10.1016/j.pscychresns.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 72.Winkler AM, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage. 2009;48:371–380. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lemaitre H, et al. Normal age-related brain morphometric changes: Nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol. Aging. 2012;33(617):e611–619. doi: 10.1016/j.neurobiolaging.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spiess R, George R, Cook M, Diehl PU. Structural plasticity denoises responses and improves learning speed. Front. Comput. Neurosci. 2016;10:93. doi: 10.3389/fncom.2016.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Querido JS, Sheel AW. Regulation of cerebral blood flow during exercise. Sports Med. 2007;37:765–782. doi: 10.2165/00007256-200737090-00002. [DOI] [PubMed] [Google Scholar]
- 77.Trapp M, et al. Impact of mental and physical stress on blood pressure and pulse pressure under normobaric versus hypoxic conditions. PLoS ONE. 2014;9:e89005. doi: 10.1371/journal.pone.0089005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schmidt S, et al. Experience-dependent structural plasticity in the adult brain: How the learning brain grows. Neuroimage. 2020;225:117502. doi: 10.1016/j.neuroimage.2020.117502. [DOI] [PubMed] [Google Scholar]
- 79.Thomas BP, et al. Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. J. Magn. Reson. Imaging. 2013;38:1177–1183. doi: 10.1002/jmri.24090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Al-Jarrah M, Jamous M, Al Zailaey K, Bweir SO. Endurance exercise training promotes angiogenesis in the brain of chronic/progressive mouse model of Parkinson's Disease. NeuroRehabilitation. 2010;26:369–373. doi: 10.3233/NRE-2010-0574. [DOI] [PubMed] [Google Scholar]
- 82.Swain RA, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- 83.Morland C, et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 2017;8:15557. doi: 10.1038/ncomms15557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pantzar A, Jonasson LS, Ekblom O, Boraxbekk CJ, Ekblom MM. Relationships between aerobic fitness levels and cognitive performance in Swedish office workers. Front. Psychol. 2018;9:2612. doi: 10.3389/fpsyg.2018.02612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Newson RS, Kemps EB. Cardiorespiratory fitness as a predictor of successful cognitive ageing. J. Clin. Exp. Neuropsychol. 2006;28:949–967. doi: 10.1080/13803390591004356. [DOI] [PubMed] [Google Scholar]
- 86.Erickson KI, et al. Beyond vascularization: aerobic fitness is associated with N-acetylaspartate and working memory. Brain Behav. 2012;2:32–41. doi: 10.1002/brb3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Bruijn AGM, Hartman E, Kostons D, Visscher C, Bosker RJ. Exploring the relations among physical fitness, executive functioning, and low academic achievement. J. Exp. Child Psychol. 2018;167:204–221. doi: 10.1016/j.jecp.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 88.Ross N, Yau PL, Convit A. Obesity, fitness, and brain integrity in adolescence. Appetite. 2015;93:44–50. doi: 10.1016/j.appet.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Volkers KM, Scherder EJ. Physical performance is associated with working memory in older people with mild to severe cognitive impairment. Biomed. Res. Int. 2014;2014:762986. doi: 10.1155/2014/762986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are stored in compliance with the GDPR regulations. Data can be requested to the authors.