Abstract
Purpose:
To assess whether metformin is associated with dry age-related macular degeneration (dAMD) development.
Methods:
In this retrospective cohort study, patients enrolled in a nationwide U.S. medical insurance claims database from 2002–2016 were included if they had diabetes mellitus, were ≥55 years old, and were enrolled for ≥2 years without a prior AMD diagnosis. The primary exposure was metformin use analyzed as either active or prior use or cumulative metformin dosage over the study period. A time updating Cox proportional hazard regression was used to estimate the hazard ratio of dAMD incidence with metformin exposure.
Results:
Among 1,007,226 diabetic enrollees, 53.3% were female and 66.4% were white with a mean hemoglobin A1c of 6.8%. Of eligible enrollees, 166,115 (16.5%) were taking metformin at the index date. Over the study period, 29,818 (3.0%) participants developed dAMD. In the active versus prior use of metformin model, active use conferred an increased hazard of developing dAMD (HR, 1.08; 95% CI, 1.04–1.12) while prior use had a decreased hazard (HR, 0.95; 95% CI 0.92–0.98). The cumulative metformin dosage model showed a significant trend towards increased hazard of dAMD incidence with increasing cumulative dosage (p<0.001), with the lowest dosage quartile having decreased hazard of dAMD incidence (HR, 0.95; 95% CI, 0.91–0.99) and the highest having increased hazard (HR, 1.07; 95% CI, 1.01–1.13).
Conclusions:
Small, conflicting associations between metformin exposure and development of dAMD were observed depending on cumulative dosage and whether drug use was active, suggesting metformin did not substantially affect the development of dAMD.
Keywords: age-related macular degeneration, dry age-related macular degeneration, metformin, diabetes mellitus, retrospective cohort study
Introduction
Age-related macular degeneration (AMD) affects 6.5% of individuals in the U.S. over the age of 40 and is the leading cause of irreversible vision loss in the older adults [1–3]. AMD comes in two forms, with the initial form, dry AMD (dAMD), characterized by loss of the retinal pigment epithelium (RPE) and photoreceptors. To date, therapeutics for dAMD are lacking. Oxidative damage, inflammation, and outer retinal metabolic dysfunction are all stressors felt to contribute to RPE and photoreceptor loss in AMD [4–7]. Given this, modulation of photoreceptor and RPE metabolism as well as oxidative stress may represent a novel therapeutic paradigm to slow retinal degeneration and preserve vision in those afflicted with dAMD.
Metformin is a Food and Drug Administration approved oral hypoglycemic used in treatment of type 2 diabetes mellitus. The mechanisms of action of metformin are complex and not fully elucidated, but include activation of the adenosine monophosphate-activated protein kinase (AMPK) signaling pathway [8]. AMPK is an energy sensor and when activated functions to restore energy balance by down-regulating ATP consumption and up-regulating ATP production [9]. Metformin’s effects on metabolism have led to numerous studies looking at its protective properties in degenerative neurologic and cardiac disease, as well as cancer [10–14]. In ocular disease, evidence exists that metformin exposure reduces development of primary open angle glaucoma in diabetic patients [15]. Additionally, in animal models of retinal degeneration and early AMD, metformin was shown to be protective against RPE and photoreceptor degeneration and that these effects were mediated by AMPK signaling [16]. These results have generated interest in metformin as a therapeutic agent in retinal degenerative diseases, including dAMD [17].
Early retrospective studies on the effect of metformin exposure on development of AMD have shown mixed results [17–20]. Three studies, one case-control, one retrospective cohort, and one retrospective cross sectional, report that metformin exposure is associated with decreased AMD incidence [17–19]. In contrast, a third case-control study found no association with metformin exposure and AMD development [20]. Possibly contributing to the conflicting results is the fact that previous studies combined incident dAMD and neovascular AMD as the primary outcome of interest, rather than looking at these disease subsets with different pathogeneses separately. Additionally, control for diabetic severity varied widely in the different study designs. Currently, a Phase II randomized control trial investigating metformin intake to slow the progression of geographic atrophy in dAMD patients without diabetes is underway (NCT02684578) [21].
There remains an unmet need for therapeutics that target dAMD. Given the potential for favorable modulation of RPE and photoreceptor metabolism by metformin, and lack of consensus on metformin’s association with AMD in existing studies, we conducted an analysis of metformin exposure and dAMD in a U.S. insurance claims database.
Methods
Data Source
We accessed the Clinformatics™ Data Mart Database (OptumInsight, Eden Prairie, MN) which captures deidentified medical insurance claims data from enrollees in a nationwide U.S. healthcare network. This dataset included outpatient medical claims with associated International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10) diagnosis codes and Current Procedural Terminology (CPT) procedure codes, prescription medication fills, laboratory results, and demographic data for enrollees in the insurance plan. Data available for use in this study included patients in the database from January 1, 2002 to December 31, 2016 (15 years). The University of Pennsylvania Institutional Review Board declared this study exempt because it involves anonymized data with removal of protected health information. All research was conducted in adherence to the tenets of the Declaration of Helsinki.
Cohort Inclusion and Exclusion Criteria
Enrollees 55 years of age or older with at least 2 years of enrollment in the insurance plan (i.e. two-year lookback period) and a diagnosis of diabetes mellitus were included for analysis. An index date was set as the earliest date at which an enrollee met all three criteria. Exclusion criteria for the cohort were a history of any of the following diagnoses or procedures prior to the index date: AMD or choroidal neovascularization (CNVM), any other retinal disease that could be confused with AMD (sickle cell retinopathy, proliferative diabetic retinopathy, retinal artery or vein occlusion, vitreous hemorrhage, retinal edema, cystoid macular edema, serous retinal detachment, or other retinopathy), prior surgical or laser treatment for diabetic retinopathy, use of anti-VEGF intravitreal injection, and/or no prior eye care provider visit (see Supplemental Table 1 for all ICD-9, ICD-10, and CPT codes used in this study).
Outcomes and Covariates
The primary outcome of interest was an ICD-9 or 10 incident diagnosis code for dAMD any time after the index date (Supplemental Table 1). Covariates assessed at the time of the index date were sex, race, geographic location, yearly income, and education level. Time varying covariates were created for age, non-proliferative diabetic retinopathy status, hypertension, hypercholesterolemia, chronic kidney disease, anemia, stroke, chronic liver disease, chronic pulmonary disease, peripheral vascular disease, any malignancy and the diabetes complications severity index (DCSI). Time varying lab values were also used to assess hemoglobin A1c level and anemia. Time varying prescription coverage was also assessed for oral statin medications and insulin. The DCSI is a validated metric that is calculated using diagnosis codes from seven categories of diabetic complications [22]. We used a modified DCSI in this study, excluding the diabetic retinopathy category as this was separately controlled for in the analysis. All the aforementioned covariates were included in the final multivariable model.
Data analysis
Descriptive statistics were used to analyze the data, with continuous variables reported using mean and standard deviation and categorical variables using frequency and percentage. A time-dependent multivariable Cox proportional hazard regression was performed to determine the association between hazard of developing dAMD and current metformin exposure. Metformin exposure was modeled in a time varying fashion with a per day evaluation of prescription coverage accounting for whether a patient was receiving the medication or not. Historical metformin use was based on any history of prescription coverage prior to the index date. For the dose-dependent analysis of metformin, cumulative metformin exposure was calculated as daily metformin dose in milligrams multiplied by days supply and was analyzed categorically. In reporting the results the actual cumulative dose will be stated, but for ease of use, this information will also be translated into a “months usage” based on a medium dose of 1000mg/day in a 30-day month (30,000mg/month). This may or may not reflect the actual time in months as the dosing for metformin can vary dramatically. Patients were censored if any of the above exclusion criteria occurred, the patient exited from insurance plan or the end of observation was reached. All data analyses were performed using SAS software (version 9.4; SAS Institute Inc, Cary NC).
Results
1,007,226 eligible patients were included for final analysis after processing exclusion criteria (Figure 1). Approximately half (53.3%, 536,120) were female and 66.4% (668,580) of included patients were white. The mean baseline hemoglobin A1c was 6.8% (SD 1.4) and 7.9% (80,003) of participants had non-proliferative diabetic retinopathy (NPDR) at baseline. At the index date, 166,115 (16.5%) of included patients had an active metformin prescription. Over the study period, there were 29,818 (3.0%) instances of new dAMD among eligible enrollees. Table 1 describes baseline cohort characteristics.
Fig. 1.
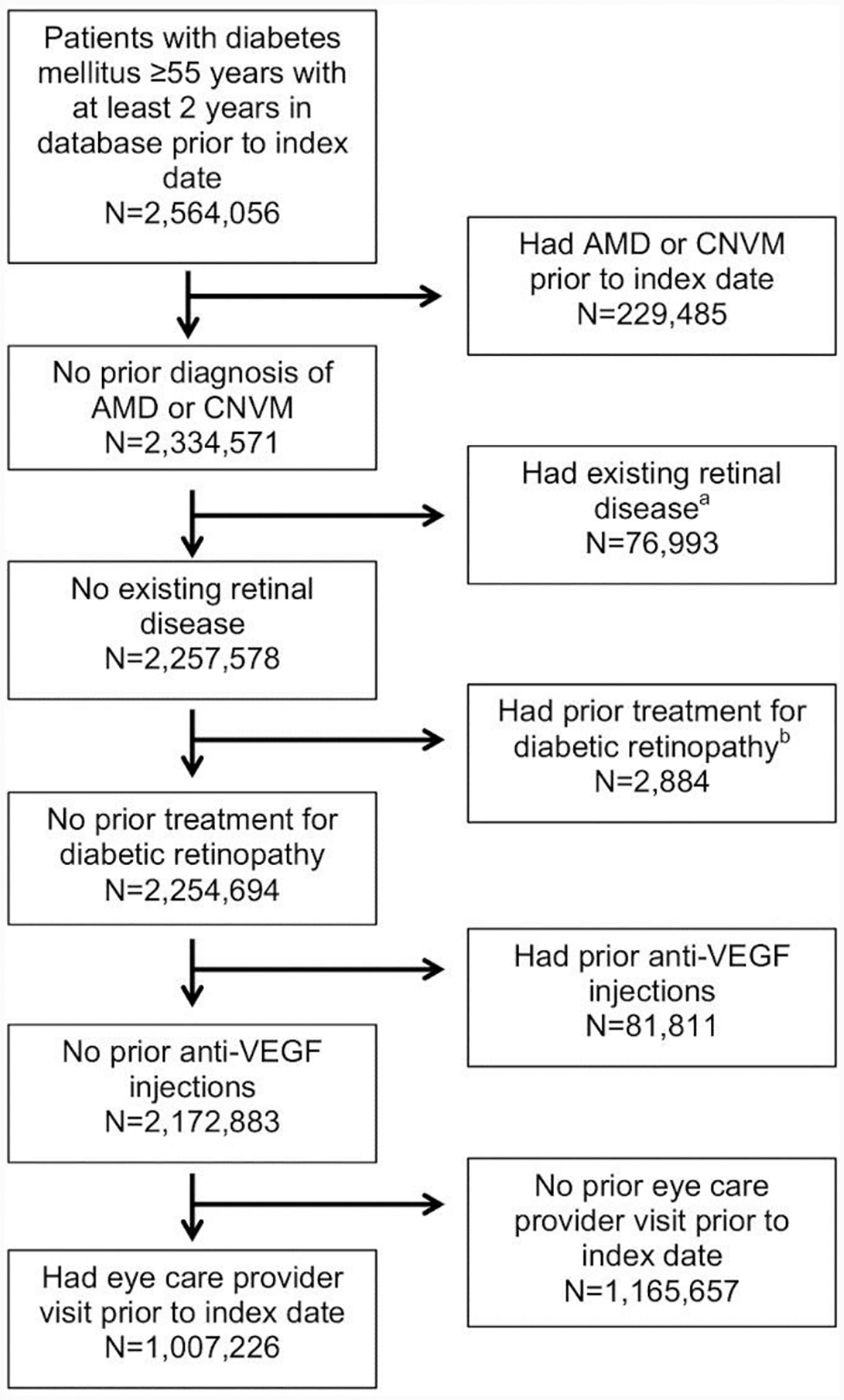
Flowchart demonstrating number of patients included and excluded from analysis
aDiagnoses include proliferative diabetic retinopathy, diabetic macular edema, vitreous hemorrhage, tractional retinal detachment, sickle cell retinopathy, “other retinopathy”, retinal artery or vein occlusion, cystoid macular edema, retinal edema, or separation of retinal layers.
bTreatments include vitrectomy or laser therapy.
Table 1:
Baseline Cohort Characteristics at Index Date.
| Demographics | No. (%) or Mean (SD) |
|---|---|
| Age | 67.5 (8.9) |
| Female sex | 536,120 (53.3%) |
| Race | |
| White | 668,580 (66.4%) |
| Asian | 33,151 (3.3%) |
| Black | 127,669 (12.7%) |
| Hispanic | 89,388 (8.9%) |
| Other/Unknown | 87,694 (8.7%) |
| Income | |
| <$40K | 249,422 (24.8%) |
| $40K-$49K | 74,388 (7.4%) |
| $50K-$59K | 68,116 (6.8%) |
| $60K-$74K | 85,251 (8.5%) |
| $75K-$99K | 106,550 (10.6%) |
| $100K+ | 185,462 (18.4%) |
| Unknown | 237,293 (23.6%) |
| Education | |
| <12th Grade | 7,235 (0.7%) |
| High School Diploma | 336,001 (33.4%) |
| <Bachelor Degree | 491,984 (48.9%) |
| ≥Bachelor Degree | 112,676 (11.2%) |
| Unknown | 58,586 (5.8%) |
| Geographic Division | |
| Mountain | 63,414 (6.3%) |
| Northeast | 163,179 (16.2%) |
| Pacific | 70,447 (7.0%) |
| South Atlantic | 289,546 (28.8%) |
| Southern Midwest | 157,707 (15.7%) |
| Upper Midwest | 259,685 (25.8%) |
| Unknown | 2,504 (0.2%) |
| NPDR | 80,003 (7.9%) |
| Hypertension | 869,713 (86.4%) |
| Hypercholesterolemia | 867,060 (86.1%) |
| Kidney Disease | |
| CKD | 201,993 (20.1%) |
| ESRD | 15,434 (1.5%) |
| Anemia a | |
| No | 262,246 (26.1%) |
| Yes | 79,697 (7.9%) |
| Unknown | 664,539 (66.0%) |
| DCSI b | 1.8 (1.9) |
| HbA1c | 6.8 (1.4) |
| Statin | 350,431 (34.8%) |
| Insulin use | 54,518 (5.4%) |
| Metformin | |
| Current use | 166,115 (16.5%) |
| Cumulative dose (mg) | 162,859.3 (383,963.8) |
| No. of outcome events | 29,818 (3.0%) |
Anemia: defined at hemoglobin <13.0 for males, <12.0 for females
DCSI: Diabetes complications severity index. Categories include nephropathy, neuropathy, cerebrovascular, cardiovascular, peripheral vascular, and metabolic complications, each scored 0,1,2 for severity with score totaled over all categories.
CKD: chronic kidney disease, ESRD: end-stage renal disease, HbA1c: hemoglobin A1C, NPDR: non-proliferative diabetic retinopathy
Multivariable, time updating, Cox proportional hazard regression modeling evaluated the hazard of developing dAMD after the index date (Table 2). Each increasing year of age was associated with an increased hazard of developing dAMD (HR, 1.09; 95% CI, 1.09–1.09; p<0.001). Males had a decreased hazard of dAMD development (HR, 0.87; 95% CI, 0.85–0.89; p<0.001). Black and Hispanic enrollees were less likely to receive a dAMD diagnosis during the study period compared to white enrollees (HR, 0.61; 95% CI, 0.58–0.63 and HR, 0.85; 95% CI, 0.81–0.89, respectively; p<0.001). Both the presence of NPDR and increased severity of diabetic complications based on the DSCI were associated with an increased hazard of new dAMD diagnosis (NPDR HR, 1.20; 95% CI, 1.15–1.24; p<0.001 and DCSI score HR, 1.04; 95% CI, 1.03–1.04; p<0.001).
Table 2:
Full model results showing HR associated with development of dAMD using a time-dependent multivariate Cox regression model.
| Variable* | Category | Hazard Ratio (95%CI) | P-value |
|---|---|---|---|
| Age | 1.09 (1.09, 1.09) | <0.001 | |
| Sex | Male | 0.87 (0.85, 0.89) | <0.001 |
| Unknown | 0.88 (0.37, 2.12) | ||
| Race | Hispanics | 0.85 (0.81, 0.89) | <0.001 |
| Black | 0.61 (0.58, 0.63) | ||
| Asian | 1.31 (1.23, 1.39) | ||
| Unknown | 1.06 (1.00, 1.13) | ||
| Income | $100K+ | 0.95 (0.91, 0.99) | 0.11 |
| $75K-$99K | 0.98 (0.94, 1.03) | ||
| $60K-$74K | 0.97 (0.93, 1.02) | ||
| $50K-$59K | 0.95 (0.90, 0.99) | ||
| $40K-$49K | 0.99 (0.95, 1.03) | ||
| Unknown | 1.01 (0.97, 1.04) | ||
| Education | High School Diploma | 1.26 (1.06, 1.49) | <0.001 |
| <Bachelor Degree | 1.22 (1.03, 1.45) | ||
| ≥Bachelor Degree | 1.11 (0.94, 1.32) | ||
| Geographic Division | Southern Midwest | 0.96 (0.92, 1.00) | <0.001 |
| South Atlantic | 1.09 (1.05, 1.13) | ||
| Pacific | 1.06 (1.01, 1.11) | ||
| Northeast | 1.16 (1.12, 1.21) | ||
| Mountain | 1.03 (0.98, 1.08) | ||
| Unknown | 0.69 (0.54, 0.90) | ||
| NPDR | 1.20 (1.15, 1.24) | <0.001 | |
| Insulin use | 0.99 (0.94, 1.05) | 0.75 | |
| Hypertension | 1.06 (1.01, 1.12) | 0.01 | |
| Hypercholesterolemia | 0.95 (0.92, 0.99) | 0.02 | |
| DSCI | 1.04 (1.03, 1.04) | <0.001 | |
| HbA1c | 0.97 (0.95, 0.99) | 0.003 | |
| Kidney disease | ESRD | 0.84 (0.76, 0.92) | <0.001 |
| CKD | 0.94 (0.92, 0.97) | ||
| Anemia | Unknown | 0.97 (0.94, 1.01) | 0.36 |
| Yes | 0.99 (0.94, 1.03) | ||
| Statin use | 1.13 (1.10, 1.16) | <0.001 | |
| Previous ischemic stroke or TIA | 0.98 (0.95, 1.01) | 0.22 | |
| Peripheral vascular disease | 1.03 (1.00, 1.06) | 0.0499 | |
| Previous intracranial hemorrhage | 0.96 (0.87, 1.07) | 0.46 | |
| Any malignancy | 1.05 (1.02, 1.08) | <0.001 | |
| Chronic liver disease | 1.20 (1.07, 1.35) | 0.002 | |
| Chronic pulmonary disease | 1.08 (1.06, 1.11) | <0.001 | |
| Metformin Cumulative Dose (mg) | > 1,430,000 | 1.07 (1.01, 1.13) | <0.001 |
| >720,000 and ≤1,430,000 | 1.07 (1.02, 1.12) | ||
| >290,000 and ≤720,000 | 0.99 (0.95, 1.04) | ||
| >0 and ≤290,000 | 0.95 (0.91, 0.99) | ||
| Metformin Exposure * | Current use | 1.08 (1.04–1.12) | <0.001 |
| Prior use | 0.95 (0.92–0.98) | 0.002 |
CKD: chronic kidney disease, DSCI: diabetes complications severity index, ESRD: end-stage renal disease, HbA1c: hemoglobin A1C, NPDR: non-proliferative diabetic retinopathy, TIA: transient ischemic attack
All results listed are from the metformin cumulative dose model except for the metformin exposure results which are from a model that includes exposure instead of the cumulative dose.
With regard to the exposure of interest, current metformin use during the study period had a small but significant increased hazard of developing dAMD (HR, 1.08; 95% CI, 1.04–1.12; p<0.001) however, when considering historical (and not current) metformin use a conflicting protective association was seen (HR, 0.95; 95% CI, 0.92–0.98; p=0.002). Within the metformin cumulative dosage model, dosages from >0 to ≤290,000 mg (or 9.7 months of use) conferred a decreased hazard (HR, 0.95; 95% CI, 0.91–0.99), while doses of 720,000 to ≤1,430,000mg (24.0 to 47.7 months; HR, 1.07; 95% CI, 1.02–1.12) and > 1,430,000mg (>47.7 months; HR, 1.07; 95% CI, 1.01–1.13) were associated with increased hazards of developing dAMD compared with non-users. Across all dosage groups, there was a trend of increasing hazard ratio with increasing total metformin exposure (p<0.001), though effect sizes were small (Table 2).
Discussion
AMD is of significant public health interest given its substantial burden of disease in an increasingly aged population. Dry AMD in particular continues to pose an unmet treatment need. Metformin is a first line drug for type 2 diabetes and is associated with few adverse effects. Based on its ability to metabolically reprogram cells and protect the outer retina in preclinical models of retinal degeneration and AMD [16], clinical studies have begun to examine the effect of metformin on AMD. However, the current literature on the association between metformin and AMD remains mixed [17–20]. In this study that used data from a large U.S. medical claims database, there were small conflicting associations between metformin exposure and development of dAMD depending on cumulative dosage and whether drug use was active or historical.
One interpretation of the dose-dependent results from this study could conclude metformin use increases the risk of dAMD development. While this possibility cannot be ruled out, it is important to note that the effect size is small (HR=1.08 or less) and was stable across the two highest levels of metformin dosing. If metformin were truly the instigating factor, the hazard would be expected to rise with increasing cumulative doses, which was not seen. One possible explanation for these findings is that of an observation bias. Clearly those that accumulate larger doses of metformin are more likely to be in the dataset longer, allowing more time to be diagnosed with dAMD. The same can be said of patients analyzed as current metformin users. In contrast, historic metformin use was protective against AMD, though again effect size was very small. These conflicting data do not make a compelling case for metformin having an impact, positive or negative, on dAMD incidence.
Metformin’s uniqueness as an initial treatment for type 2 diabetics can make it a difficult drug to study. Its ubiquitous use in this manner makes finding appropriate study control groups difficult and confounding that much more likely. To address this issue, we chose to only include diabetic patients, distinguishing our study from some of the previous ones [17,20]. Yet, even within diabetics there is likely something fundamentally different among metformin users versus non-users. One of these is severity of illness, with worse diabetic disease likely to have progressed past the stage where metformin is the best choice of treatment. This study prioritized having robust control for diabetic severity, using the DCSI score in addition to hemoglobin A1c and insulin use. The DCSI is a validated metric formulated from billing codes across seven different diabetic complication categories. This analysis used in this study modified the score to include six categories, excluding the category for retinopathy, which was controlled for separately. The DCSI has been shown to better predict clinically significant adverse events when compared to measures such as number of diabetic complications or duration of diabetic disease [22]. This draws a sharp contrast with previous studies that either did not address diabetic severity [17], or controlled for a combination of factors such as insulin use, chronic kidney disease (CKD), or presence or absence of any diabetic complications including diabetic retinopathy as surrogates for diabetes severity [18–20], which may have been inadequate markers of diabetic disease. This issue has particular importance as numerous studies have established an association of diabetes with AMD incidence [23–27]. Based on hemoglobin A1c and DCSI values, this study cohort represents relatively well-controlled diabetics, likely due in part to exclusion of those with more severe diabetic eye disease (proliferative diabetic retinopathy and macular edema).
As alluded to above, to date, few previous studies have examined the impact of metformin on AMD. In contrast to our study, the majority reported incidence of dAMD and nAMD combined as their primary outcome. The study herein examined the effect of metformin specifically on the development of dAMD, as has been experimentally modeled in a preclinical study that investigated the impact of metformin on RPE and photoreceptor health [16]. The pathogenesis of dAMD and nAMD are distinct and one might expect metformin to modulate these two disease subsets differently. The only other study to look at outcomes for dAMD independently had significant limitations, including a cross-sectional design with small sample size, and treatment of metformin exposure as a dichotomous variable without consideration for potential dose-response [19].
Our results are most consistent another study that assessed a metformin dose-response relationship, which similarly, was unable to find a consistent effect of metformin on AMD [20]. One of the strengths of our study was the use of time varying Cox regression analysis allowing for updates to patient covariate characteristics over the course of the study period and in particular the day-to-day use of metformin, which more closely modeled the patient characteristics at any given time. Dose-response relationships along with the ability to find associations in both forward looking cohort models as well as backward looking case-control studies are important factors in considering the ancillary effects of medications. Unfortunately, with regards to AMD, metformin has yet to clear this necessary bar.
Other results found in our study offer external validation of our findings. Development of dAMD was associated with increasing age and men had a lower hazard of dAMD development compared to women, findings previously demonstrated in the Blue Mountains Eye, Beaver Dam Eye, and Rotterdam studies [28–30]. With regards to race, there was a decreased hazard of dAMD development in black and Hispanic persons relative to white, as has been reported in other population-based studies [31–33]. Similar to previously published work [23–26], we found that NPDR and diabetes severity, represented in this study as higher DCSI score, were associated with increased risk of dry AMD development.
Several limitations should be considered when interpreting the results of this study. The analysis may have identified associations, but causal inferences about the effect of metformin on dAMD are difficult to discern. Importantly, insurance claims data analysis utilizes ICD-9 and ICD-10 coding to determine diagnoses. While the study design was strict in selecting codes for each diagnosis and excluded ICD codes for diagnoses that might be confused for AMD, diagnoses were unable to be verified through medical record review and coding accuracy cannot be guaranteed. Claims data also do not include information on smoking status, an established risk factor for AMD [27, 34–35]. In addition, though our analysis adjusted for relevant medical comorbidities, we did not include medications used to treat these conditions as covariates and therefore may not have accounted for drug-drug interactions. Also, using pharmacy fill records to determine metformin dose exposure does not appropriately reflect how much of the medication the patient actually ingests and metformin use prior to the lookback period cannot be captured in the cumulative dose analysis. Lastly, results from this study using data from a single insurance company may not be generalizable to populations covered by a different insurance provider or those who are uninsured.
In summary, this study, based on medical claims from a large US insurer, demonstrates small, conflicting associations between metformin exposure and the development of dAMD. Based on these results, there is not sufficient evidence to suggest that metformin has a meaningful impact on the development of dAMD. Further studies are needed to clarify the role of metabolism-altering systemic medications like metformin on AMD pathogenesis.
Supplementary Material
Funding:
National Institutes of Health K23 Award (1K23EY025729 - 01); University of Pennsylvania Core Grant for Vision Research (2P30EY001583). Additional funding was provided by Research to Prevent Blindness, Karen & Herbert Lotman Fund for Macular Vision Research Foundation, and the Paul and Evanina Mackall Foundation.
Footnotes
Declaration of Conflicting of interests: Thomas J. Wubben participated in the Allergan (Irvine, CA) Fostering Innovative Retina Stars of Tomorrow (FIRST) program. Cagri G. Besirli consults for and has equity in iRenix Medical, and receives royalties from iRenix Medical and ONL therapeutics. None of these interests relate directly to the material presented in the manuscript.
References
- 1.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106–e116. [DOI] [PubMed] [Google Scholar]
- 2.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. NEJM 2008;358:2606–2617. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Chou C-F, Klein BEK, et al. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol 2011;129:75–80. [DOI] [PubMed] [Google Scholar]
- 4.Ferrington DA, Ebeling MC, Kapphahn RJ, et al. Altered bioenergetics and enhanced resistance to oxidative stress in human retinal pigment epithelial cells from donors with age-related macular degeneration. Redox Biol 2017;13:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanus J, Anderson C, Wang S. RPE necroptosis in response to oxidative stress and in AMD. Ageing Res Rev 2015;24:286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambert NG, ElShelmani H, Singh MK, et al. Risk factors and biomarkers of age-related macular degeneration. Prog Retin Eye Res 2016;54:64–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Handa JT, Bowes Rickman C, Dick AD, et al. A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat Commun 2019;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia 2017;60:1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu L, Ash JD. The role of AMPK pathway in neuroprotection. Adv Exp Med Biol 2016;854:425–30. [DOI] [PubMed] [Google Scholar]
- 10.Qi B, Hu L, Zhu L, et al. Metformin attenuates cognitive impairments in hypoxia-ischemia neonatal rats via improving remyelination. Cell Mol Neurobiol 2017;37:1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Zhu J, Liu K, Huang K, et al. Metformin improves neurologic outcome via AMP-activated protein kinase-mediated autophagy activation in a rat model of cardiac arrest and resuscitation. J Am Heart Assoc 2018;7(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Bonet E, Buxó M, Cuyàs E, et al. Neoadjuvant metformin added to systemic therapy decreases the proliferative capacity of residual breast cancer. J Clin Med 2019;8(12):2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saka Herrán C, Jané-Salas E, Estrugo Devesa A, et al. Protective effects of metformin, statins and anti-inflammatory drugs on head and neck cancer: A systematic review. Oral Oncol 2018;85:68–81. [DOI] [PubMed] [Google Scholar]
- 14.Nesti L, Natali A. Metformin effects on the heart and the cardiovascular system: A review of experimental and clinical data. Nutr Metab Cardiovasc Dis 2017;27:657–669. [DOI] [PubMed] [Google Scholar]
- 15.Lin H-C, Stein JD, Nan B, et al. Association of geroprotective effects of metformin and risk of open-angle glaucoma in persons with diabetes mellitus. JAMA Ophthalmol 2015;133:915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu L, Kong L, Wang J, et al. Stimulation of AMPK prevents degeneration of photoreceptors and the retinal pigment epithelium. Proc Natl Acad Sci USA 2018;115:10475–10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown EE, Ball JD, Chen Z, et al. The common antidiabetic drug metformin reduces odds of developing age-related macular degeneration. Invest Ophthalmol Vis Sci 2019;60:1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y-Y, Shen Y-C, Lai Y-J, et al. Association between metformin and a lower risk of age-related macular degeneration in patients with type 2 diabetes. J Ophthalmol 2019;2019:1649156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart JM, Lamy R, Wu F, et al. Relationship between oral metformin use and age-related macular degeneration. Ophthalmol Retina. Epub ahead of print 7 June 2020. DOI: 10.1016/j.oret.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee H, Jeon HL, Park SJ, et al. Effect of statins, metformin, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers on age-related macular degeneration. Yonsei Med J 2019;60:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ClinicalTrials.gov. Metformin for the Minimization of Geographic Atrophy Progression in Patients with AMD (METforMIN). Available from: https://clinicaltrials.gov/ct2/show/NCT02684578; Updated January 29, 2019. Accessed January 26, 2020.
- 22.Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care 2008;14:15–23. [PMC free article] [PubMed] [Google Scholar]
- 23.Vassilev ZP, Ruigómez A, Soriano-Gabarró M, et al. Diabetes, cardiovascular morbidity, and risk of age-related macular degeneration in a primary care population. Invest Ophthalmol Vis Sci 2015;56:1585–1592. [DOI] [PubMed] [Google Scholar]
- 24.He M-S, Chang F-L, Lin H-Z, et al. The association between diabetes and age-related macular degeneration among the elderly in Taiwan. Diabetes Care 2018;41:2202–2211. [DOI] [PubMed] [Google Scholar]
- 25.Hahn P, Acquah K, Cousins SW, et al. Ten-year incidence of age-related macular degeneration according to diabetic retinopathy classification among Medicare beneficiaries. Retina 2013;33:911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topouzis F, Anastasopoulos E, Augood C, et al. Association of diabetes with age-related macular degeneration in the EUREYE study. Br J Ophthalmol 2009;93:1037–1041. [DOI] [PubMed] [Google Scholar]
- 27.Klein R, Deng Y, Klein BEK, et al. Cardiovascular disease, its risk factors and treatment, and age-related macular degeneration: Women’s Health Initiative Sight Exam ancillary study. Am J Ophthalmol 2007;143:473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joachim N, Mitchell P, Burlutsky G, et al. The incidence and progression of age-related macular degeneration over 15 years: The Blue Mountains Eye Study. Ophthalmology 2015;122:2482–2489. [DOI] [PubMed] [Google Scholar]
- 29.Klein R, Klein BEK, Linton KLP. Prevalence of age-related maculopathy: The Beaver Dam Eye Study. Ophthalmology 1992;99:933–943. [DOI] [PubMed] [Google Scholar]
- 30.Vingerling JR, Dielemans I, Hofman A, et al. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology 1995;102:205–210. [DOI] [PubMed] [Google Scholar]
- 31.Klein R, Klein BEK, Knudtson MD, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the Multi-Ethnic Study of Atherosclerosis. Ophthalmology 2006;113:373–380. [DOI] [PubMed] [Google Scholar]
- 32.Friedman DS, Katz J, Bressler NM, et al. Racial differences in the prevalence of age-related macular degeneration: the Baltimore Eye Survey. Ophthalmology 1999;106:1049–1055. [DOI] [PubMed] [Google Scholar]
- 33.Vanderbeek BL, Zacks DN, Talwar N, et al. Racial differences in age-related macular degeneration rates in the United States: a longitudinal analysis of a managed care network. Am J Ophthalmol 2011;152:273–282.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cruickshanks KJ, Klein R, Klein BE, et al. Sunlight and the 5-year incidence of early age-related maculopathy: the Beaver Dam Eye Study. Arch Ophthalmol 2001;119:246–250. [PubMed] [Google Scholar]
- 35.Klein R, Klein BE, Moss SE. Relation of smoking to the incidence of age-related maculopathy. The Beaver Dam Eye Study. Am J Epidemiol 1998;147:103–110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


