Abstract
Background and aims:
The IM-UNITI study and long-term extension (LTE) evaluated the long-term efficacy, safety, and immunogenicity of subcutaneous ustekinumab maintenance therapy in patients with Crohn’s disease (CD). Here we report final results of IM-UNITI LTE through 5 years.
Methods:
Patients completing safety and efficacy evaluations at Week 44 of the maintenance study were eligible to participate in the LTE and continue the treatment they were receiving. Unblinding occurred after completion of maintenance study analyses (August 2015), and patients receiving placebo were discontinued. No dose adjustment occurred in the LTE. Efficacy assessments were conducted every 12 weeks until unblinding and at dosing visits thereafter through Week 252. Serum ustekinumab concentrations and anti-drug antibodies (ADAs) were evaluated through Weeks 252 and 272, respectively.
Results:
Using an intent-to-treat analysis of all patients randomized to ustekinumab at maintenance baseline, 34.4% of patients in the q8w group and 28.7% in the q12w group were in clinical remission at Week 252. Corresponding remission rates among patients who entered the LTE were 54.9% and 45.2%. Overall, adverse event rates (per 100 patient-years) from maintenance Week 0 through the final visit were generally similar in the placebo and combined ustekinumab groups for all adverse events (440.3 vs. 327.6), serious adverse events (19.3 vs. 17.5), infections (99.8 vs. 93.8), and serious infections (3.9 vs. 3.4). Serum ustekinumab concentrations were maintained throughout the LTE. ADAs occurred in 5.8% of patients who received ustekinumab during induction and maintenance and continued in the LTE.
Conclusion:
Patients receiving subcutaneous ustekinumab maintained clinical remission through 5 years. No new safety signals were observed. ClinicalTrials.gov number NCT01369355
Keywords: Ustekinumab, Crohn’s disease, long-term extension
INTRODUCTION
Crohn’s disease (CD) is a chronic, progressive inflammatory disorder frequently requiring long-term therapy to control disease activity.1 Many current maintenance therapies for CD have important limitations including inadequate efficacy and undesirable adverse events (AEs). Although corticosteroids are commonly used to induce remission,2 long-term use is associated with toxicity, resistance, dependence, and increased risk of mortality.3 Immunosuppressants are often used for maintenance treatment, however thiopurines are associated with increased risk of skin cancer and lymphoma and susceptibility to viral infection.4 Methotrexate can cause liver toxicity5 and acute pneumonitis.6 Although tumor necrosis factor (TNF) antagonists are generally safe and effective for long-term treatment, patients frequently lose response, often through development of anti-drug antibodies (ADAs).7 Moreover, rates of serious infection such as bacterial pneumonia and tuberculosis are increased with TNF antagonists. Clinical experience with newer biologic therapies like ustekinumab is not as extensive as for TNF antagonists. Long-term safety and effectiveness studies are needed to fully assess the therapeutic index of these agents.
The IM-UNITI study and long-term extension (LTE) evaluated subcutaneous (SC) ustekinumab maintenance treatment in patients with CD who had received intravenous (IV) ustekinumab induction treatment.8 Previous publications showed that ustekinumab maintenance treatment was safe and effective through 3 years with low ADA rates.9,10 Among patients who responded to IV ustekinumab induction and were randomized in the maintenance study, 43.0% of patients in the SC ustekinumab every 8 weeks (q8w) group and 38.0% of patients in the SC ustekinumab every 12 weeks (q12w) group were in clinical remission at Week 152.10
Here, we report the final results of the IM-UNITI LTE based upon data accrued through 5 years, representing the longest duration of anti-interleukin (IL)12/23 treatment reported to date in patients with inflammatory bowel disease (IBD).
METHODS
Study Design and Endpoints
The CD ustekinumab program included two randomized double-blind, placebo-controlled, 8-week, IV induction studies (UNITI-1/UNITI-2), a SC maintenance study through Week 44 (IM-UNITI), and an LTE through Week 272. Detailed study designs, as well as results through Week 156, have been previously published.8–10 Briefly, patients who responded to ustekinumab IV induction were randomized to SC placebo or ustekinumab (90 mg q8w or q12w) maintenance (Supplemental Figure 1). Patients who did not initially respond to ustekinumab or placebo IV induction were given a single dose of SC or IV ustekinumab, respectively, 8 weeks after induction (Week 0 of IM-UNITI). If these patients were subsequently in response 8 weeks later, they continued to receive SC ustekinumab maintenance (90 mg q8w or q12w, respectively) as part of the nonrandomized population. Patients responding to placebo IV induction were also included in the nonrandomized population and continued to receive placebo maintenance. Patients who completed safety and efficacy evaluations at Week 44 and, in the opinion of the investigator, might benefit from continued treatment, had the opportunity to participate in the LTE and continue the treatment they were receiving at Week 44. Unblinding occurred after Week 44 analyses were completed (August 2015). At this time, patients still receiving SC placebo were discontinued from the study, and patients receiving ustekinumab continued their current dosage in an open-label fashion. Between Week 8–32, patients with loss of response (CDAI score ≥220 points and a ≥100-point increase from the Week 0 score) could have a single dose adjustment (Supplemental Figure 1). No dose adjustment occurred in the LTE.
In the LTE, efficacy assessments were conducted every 12 weeks until unblinding, and then at q8w or q12w dosing visits. A full Crohn’s Disease Activity Index [CDAI] score was collected at these visits.11 Clinical remission was defined as a CDAI score of <150 points, and clinical response was defined as a reduction from induction Week 0 in the CDAI score of ≥100 points [or a CDAI score of <150]. Corticosteroid-free remission was defined as a CDAI score of <150 points in a patient who was not receiving corticosteroids in the 7 days prior to the visit.
Analyses of serum ustekinumab concentrations and ADAs were evaluated through Week 252 and Week 272 respectively, using the validated drug-tolerant electrochemiluminescent immunoassay method on the Meso Scale Discovery (MSD®) platform (Gaithersburg, Maryland, United States of America) described previously.10 Serum ustekinumab concentrations were assessed over time during the LTE. Comparisons of serum ustekinumab concentrations between dose regimens were based on trough concentrations at common dosing visits and were included in the analysis for data points between Weeks 96 and 252.
Statistical Analyses
Efficacy data through 5 years are presented using a few methods. First, an intent-to-treat analysis on all originally randomized patients from Week 0 of the maintenance study. In this analysis, patients with missing data at an analysis time point, including those who discontinued or did not enter the LTE, were considered to be not in clinical remission regardless of their CDAI score. In addition, patients who met prespecified treatment failure criteria prior to an analysis time point were considered to be not in clinical remission at all subsequent time points regardless of their CDAI score. Treatment failure criteria were: having CD-related surgery due to lack of efficacy of study agent (excluding minor procedures such as drainage of superficial abscess or seton placement), discontinuation of study agent due to lack of efficacy or due to an AE of worsening CD, loss of clinical response (from Week 8–32), or initiation of or increase in dose of corticosteroids or immunosuppressants (prior to Week 44 only).
Additionally, analyses were conducted that focused solely on randomized patients in the maintenance study who continued in the LTE. In the nonresponder imputation analysis, patients with missing data or who met treatment failure rules were considered to be nonresponders or not in clinical remission regardless of their CDAI score. Treatment failure criteria in this analysis were: having CD-related surgery due to lack of efficacy of study agent (excluding minor procedure such as drainage of superficial abscess or seton placement) or discontinuation of study agent due to lack of efficacy or due to an AE indicated of worsening CD prior to the designated analysis timepoint.
An observed case analysis on randomized patients in the maintenance study who continued in the LTE was also conducted, in which neither missing data nor treatment failure rules were applied. Finally, a modified observed case analysis of randomized patients in the maintenance study who continued in the LTE was also conducted, where treatment failure rules were applied. Treatment failure criteria in this analysis were: CD-related surgery due to lack of efficacy of study agent (excluding minor procedure such as drainage of superficial abscess or seton placement) or discontinuation of study agent due to lack of efficacy or due to an AE of worsening CD prior to the designated analysis timepoint.
Safety was evaluated by summarizing AEs, serious AEs, infections, serious infections, and malignancies per 100 patient-years in all patients entering the LTE and in randomized patients in the LTE who had received at ≥1 dose of study agent.
All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS
Patient disposition
As previously reported,8–10 397 patients who responded 8 weeks after IV ustekinumab induction were randomized to SC maintenance treatment with placebo, ustekinumab 90 mg q12w, or ustekinumab 90 mg q8w. After completing IM-UNITI Week 44 primary endpoint, 298 of the randomized patients entered the LTE (Supplemental Figure 2). Table 1 summarizes patient characteristics, including CDAI scores, Inflammatory Bowel Disease Questionnaire (IBDQ) scores, C-reactive protein (CRP) levels, clinical remission rates, and corticosteroid and immunosupressant use at maintenance baseline for all randomized patients and at Week 44 for randomized patients who entered the LTE. Of patients who entered the LTE, 77.4% receiving ustekinumab q12w, 84.1% receiving q8w, and 63.4% who had previously dose adjusted (prior to Week 44) were in clinical remission at Week 44 (Table 1).
Table 1.
Summary of patient characteristics of randomized patients in the maintenance study at maintenance baseline (Week 0) and of randomized patients entering the LTE at Week 44
| Patient characteristics at maintenance baseline (Week 0) for all randomized patients | |||
|
| |||
| Placeboa | Ustekinumab 90 mg q12w | Ustekinumab 90 mg q8w | |
|
| |||
| N | 133 | 132 | 132 |
| Median CDAI | 135.0 | 134.0 | 127.0 |
| Median IBDQ | 167.0 | 172.0 | 176.5 |
| Median CRP (mg/L) | 4.28 | 5.16 | 4.46 |
| Patients in clinical remission at maintenance baseline. | |||
| N | 80 (60.2%) | 80 (60.6%) | 80 (60.6%) |
| Patients receiving corticosteroids at maintenance | 51 | 46 | 53 |
| baseline (P.Eq dose; excluding budesonide) (mg/day) | |||
| Mean (SD) | 18.3 (11.01) | 18.7 (11.48) | 18.8 (10.37) |
| Immunosupressants | 47 (35.3%) | 52 (39.4%) | 44 (33.3%) |
| 6-MP/AZA | 38 (28.6%) | 44 (33.3%) | 31 (23.5%) |
| MTX | 9 (6.8%) | 8 (6.1%) | 13 (9.8%) |
|
| |||
| Patient characteristics at Week 44 for randomized patients entering the LTE | |||
|
| |||
| Ustekinumab 90 mg q12w | Ustekinumab 90 mg q8w | Prior dose adjustment b | |
|
| |||
| N | 84 | 82 | 71 |
| Median CDAI | 95.5 | 70.5 | 130.0 |
| Median IBDQ | 189.0 | 185.5 | 171.0 |
| Median CRP | 3.5 | 3.7 | 4.0 |
| Patients in clinical remission | 65 (77.4%) | 69 (84.1%) | 45 (63.4%) |
| Patients receiving corticosteroids at maintenance | |||
| baseline (including budesonide) | 34/84 (40.5%) | 34/82 (41.5%) | 38/71 (53.5%) |
| Patients receiving corticosteroids at maintenance | |||
| baseline (P.Eq dose; excluding budesonide) (mg/day) | 25/84 (29.8%) | 26/82 (31.7%) | 30/71 (42.3%) |
| Mean (SD) | 15.7 (11.26) | 15.2 (9.01) | 18.6 (10.08) |
| Immunomodulatory drugs | 32 (38.1%) | 26 (31.7%) | 22 (31.0%) |
| 6-MP/AZA | 27 (32.1%) | 20 (24.4%) | 18 (25.4%) |
| MTX | 5 (6.0%) | 6 (7.3%) | 4 (5.6%) |
6-MP, 6- methyl prednisone, AZA, azathioprine, CDAI, Crohn’s Disease Activity Index; CRP, C-reactive protein; IBDQ, Inflammatory Bowel Disease Questionnaire; IV, intravenous; LTE, long-term extension; MTX, methotrexate; P.Eq, prednisone equivalent, q12w, every 12 weeks; q8w, every 8 weeks; SC, subcutaneous; SD, standard deviation
Patients who were in clinical response to ustekinumab IV induction dosing and were randomized to placebo SC on entry into this maintenance study. Patients who were in clinical response to ustekinumab IV induction dosing, were randomized to receive study drugs on entry into this maintenance study, and did not meet loss of response criteria from Week 8 through Week 32.
Patients who were in clinical response to ustekinumab induction dosing, were randomized, met loss of clinical response criteria from Week 8 through Week 32, and initiated ustekinumab 90 mg SC q8w (for patients randomized to receive placebo SC or ustekinumab 90 mg SC q12w on entry into this maintenance study) or continue ustekinumab 90 mg SC q8w (for patients randomized to receive ustekinumab 90 mg SC q8w on entry into this maintenance study) in this maintenance study.
Among patients who were randomized to ustekinumab maintenance and entered the LTE, approximately half (124/237; 52.3%) completed dosing through 5 years. The most common reasons for discontinuation were AEs, lack of efficacy, and withdrawal of consent (Table 2). Rates of discontinuation were similar between individual ustekinumab SC dose groups (46.4% in the q12w group and 41.5% in q8w). Patients receiving SC placebo in the LTE discontinued in August 2015 after study unblinding and completion of Week 44 analyses. Because of this, patients in the placebo group have variable lengths of follow-up in the LTE. Of the 131 patients who were randomized to placebo at maintenance baseline, data were available through Week 44 for 72.5% (95/131) of these patients, through Week 92 for 43.5 % (57/131), through Week 164 for 20.6% (27/131), and through Week 252 for 13.0% (17/131).
Table 2.
Number of randomized patients who discontinued study agent during the LTE (through final Week 252 dosing visit)
| Placebo SCa | Ustekinumab 90 mg q12wa | Ustekinumab 90 mg q8wa | Prior dose adjustmentb | |
|---|---|---|---|---|
|
| ||||
| N | 61 | 84 | 82 | 71 |
| Patients who discontinued, N | 61 (100%) | 39 (46.4%) | 34 (41.5%) | 40 (56.3%) |
| Reason for discontinuation | ||||
| Adverse events | 7 (11.5%) | 14 (16.7%) | 10 (12.2%) | 13 (18.3%) |
| Due to a worsening of CD | 3/7 (42.9%) | 10/14 (71.4%) | 5/10 (50.0%) | 6/13 (46.2%) |
| Lack of efficacy | 5 (8.2%) | 7 (8.3%) | 4 (4.9%) | 9 (12.7%) |
| Protocol violation | 0 | 1 (1.2%) | 1 (1.2%) | 1 (1.4%) |
| Study terminated by sponsor | 0 | 0 | 0 | 0 |
| Physician decision | 0 | 1 (1.2%) | 3 (3.7%) | 1 (1.4%) |
| Lost to follow-up | 1 (1.6%) | 2 (2.4%) | 1 (1.2%) | 1 (1.4%) |
| Withdraw of consent for administration of study agent | 4 (6.6%) | 12 (14.3%) | 11 (13.4%) | 11 (15.5%) |
| Death | 0 | 1 (1.2%) | 0 | 2 (2.8%) |
| Placebo patients discontinued due to study unblinding (after Week 44 analysis complete) | 44 (72.1%) | NA | NA | NA |
CD, Crohn’s disease; IV, intravenous; NA, not applicable; LTE, long-term extension; q12w, every 12 weeks; q8w, every 8 weeks; SC, subcutaneous
Patients who were in clinical response to ustekinumab IV induction dosing, were randomized in the maintenance study, and did not meet loss of response criteria from Week 8 through Week 32.
Patients who were in clinical response to ustekinumab induction dosing, were randomized in the maintenance study, met loss of clinical response criteria from Week 8 through Week 32, and initiated ustekinumab 90 mg SC q8w (for patients initially randomized to placebo SC or ustekinumab 90 mg SC q12w) or continued ustekinumab 90 mg SC q8w (for patients initially randomized to ustekinumab 90 mg SC q8w).
Overall, 16.8% (50/298) of randomized patients who entered the LTE met treatment failure criteria and were considered to be not in clinical response or remission at subsequent time points through Week 252, including 1 (0.3%) with a CD-related surgery due to lack of efficacy of study agent (drainage with seton placement), 25 (8.4%) who discontinued study agent due to a lack of efficacy, and 24 (8.1%) who discontinued study agent due to an AE of worsening CD.
Intent-to-treat analysis of all patients originally randomized in the maintenance study
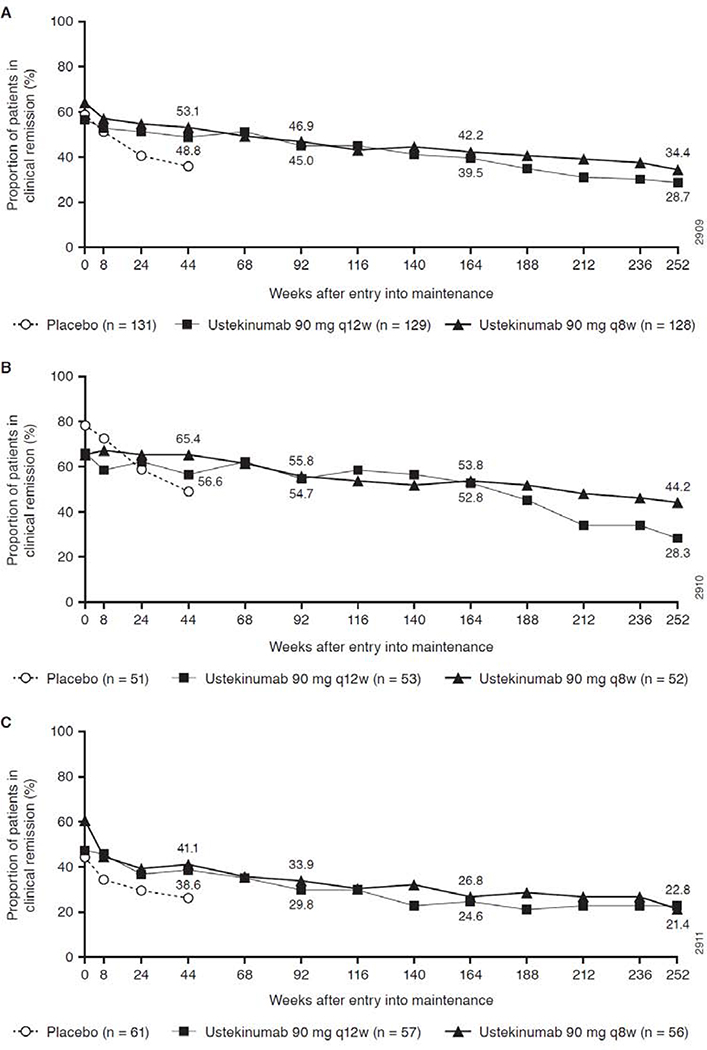
Figure 1 shows clinical remission rates over the full 5 years of treatment, using an intent-to-treat analysis from Week 0 of IM-UNITI for all originally randomized induction responders, applying missing data rules (including patients who did not enter the LTE or discontinued) and treatment failure rules (carrying forward treatment failures that occurred any time during the study including before Week 44). At Week 252, 28.7% in the q12w group and 34.4% in the q8w group were in clinical remission (Figure 1A). Among TNF antagonist-naïve patients, remission rates were 28.3% for the q12w group and 44.2% for q8w (Figure 1B). Remission rates after 5 years for the TNF antagonist-failure subset were 22.8% and 21.4%, respectively; Figure 1C, however, durability within both subsets was similarly consistent over time. Specifically, the proportion of patients in remission in each dose group decreased by approximately 5% each year from years 1 through 5.
Figure 1.
Clinical remission over time through Week 252 (intent-to treat analysis a,b) for (A) all patients randomized in the maintenance study, (B) TNF antagonist-naïve patients, and (C) TNF-antagonist failure patients
Randomized patients who entered the LTE
Nonresponder imputation analysis in the LTE
Among randomized patients who entered the LTE at Week 44, rates of clinical remission at Week 252 were 45.2% in the q12w group and 54.9% in the q8w group (Table 3). Clinical remission rates over time from Weeks 44–252 are presented in Supplemental Figure 3. Of the patients in clinical remission at Week 252, 89.5% (34/38) and 93.3% (42/45), respectively, were also not receiving corticosteroids (ie, corticosteroid-free remission) at Week 252.
Table 3.
Efficacy assessments at Week 252 among randomized patients who entered the LTE
| Ustekinumab |
||||
|---|---|---|---|---|
| 90mg SC q12wa | 90 mg SC q8w |
|||
| 90 mg SC q8wa | Previous dose adjustmentb | Combined | ||
|
| ||||
| N | 84 | 82 | 71 | 153 |
| Clinical remissionc, n/N (%) | 38/84 (45.2%) | 45/82 (54.9%) | 31/71 (43.7%) | 76/153 (49.7%) |
| Clinical remission among TNF antagonist-naïve patientsc, n/N (%) | 15/38 (39.5%) | 23/39 (59.0%) | 16/28 (57.1%) | 39/67 (58.2%) |
| Clinical remission in TNF antagonist failure patientsc, n/N (%) | 13/32 (40.6%) | 12/27 (44.4%) | 11/32 (34.4%) | 23/59 (39.0%) |
| Clinical remission and not receiving corticosteroids at Week 252c,d n/N (%) | 34/84 (40.5%) | 42/82 (51.2%) | 25/71 (35.2%) | 67/153 (43.8%) |
| Clinical remission and not receiving corticosteroids at Week 252 among patients in remission at Week 252 n/N | 34/38 (89.5%) | 42/45 (93.3%) | 25/31 (80.6%) | 67/76 (88.2%) |
| Clinical responsec, n/N (%) | 45/84 (53.6%) | 47/82 (57.3%) | 33/71 (46.5%) | 80/153 (52.3%) |
| CDAI | ||||
| Mean (SD) change from maintenance baseline | −10.1 (112.18) | −13.6 (107.65) | 6.4 (126.54) | −4.3 (116.83) |
| CRP | ||||
| Mean (SD) change from maintenance baseline | 1.67 (14.845) | −1.11 (12.784) | 3.78 (23.605) | 1.16 (18.699) |
| Normalized CRPe at Week 252 | 23/61 (37.7%) | 20/56 (35.7%) | 22/56 (39.3%) | 42/112 (37.5%) |
| Concomitant CD medications | ||||
| Patients not receiving corticosteroids at Week 252 among those receiving corticosteroids at maintenance baselined | 25/34 (73.5%) | 22/34 (64.7%) | 19/38 (50.0%) | 41/72 (56.9%) |
| Patients receiving ustekinumab monotherapy without immunomosupressants at Week 252 | 32/48 (66.7%) | 39/54 (72.2%) | 27/40 (67.5%) | 66/94 (70.2%) |
CD, Crohn’s disease; CDAI, Crohn’s disease activity index; CRP, c-reactive protein; IV, intravenous; LTE, long-term extension q12w, every 12 weeks; q8w, every 8 weeks; SD, standard deviation; TNF, tumor necrosis factor
Patients who were in clinical response to ustekinumab IV induction dosing, were randomized in the maintenance study, and did not meet loss of response criteria from Week 8 through Week 32.
Patients who were in clinical response to ustekinumab induction dosing, were randomized in the maintenance study, met loss of clinical response criteria from Week 8 through Week 32, and initiated ustekinumab 90 mg SC q8w (for patients initially randomized to placebo SC or ustekinumab 90 mg SC q12w) or continued ustekinumab 90 mg SC q8w (for patients initially randomized to ustekinumab 90 mg SC q8w).
Patients who had a Crohn’s disease-related surgery due to lack of efficacy of study agent (with the exception of minor procedures such as drainage of an superficial abscess or seton placement), discontinuation of study agent due to lack of efficacy or due to an adverse event indicated to be of worsening Crohn’s disease prior to the designated analysis timepoint are considered not to be in clinical remission/response, regardless of their CDAI score. Patients who had insufficient data at the designated analysis timepoint were considered not to be in clinical remission/response.
Patients who had a missing value in corticosteroids use at designated analysis timepoint had their last value carried forward
Number and percentage of patients with normalized CRP at Week 252 among those with abnormal CRP at induction baseline.
Abnormal CRP is defined as CRP value >3 mg/L.
At maintenance baseline, 40.5% (34/84) of patients in the q12w group and 41.5% (34/82) of patients in the q8w group were receiving oral corticosteroids (Table 1). Of these patients, 73.5% (25/34) in the q12w group and 64.7% (22/34) of patients in the q8w group were no longer receiving corticosteroids at Week 252 (Table 3). The mean daily prednisone equivalent dose for patients receiving corticosteroids at maintenance baseline was 15.7 mg/day for those in the q12w group and 15.2 mg/day for those in the q8w group. The average dose was reduced at Week 44 to 4.3 and 5.3 mg/day in the q12w and q8w groups, respectively. These decreases were maintained through Week 252 (4.5 and 3.6 mg/day, respectively; Supplemental Figure 4). In the LTE, 11 patients (4.6%) receiving ustekinumab had CD-related surgery.
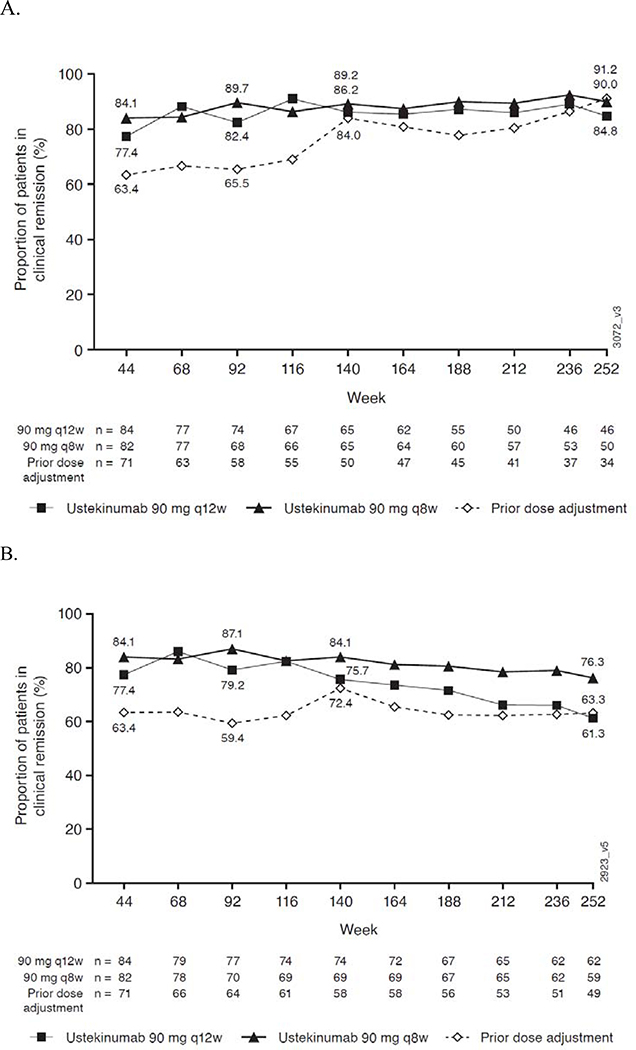
Observed case
Figure 2 shows the remission rates among patients who had data available at the assessment timepoint (observed case analysis). Of the patients who had data at Week 252, 84.8% (39/46) in the q12w group, 90.0% (45/50) in the q8w group, and 91.2% (31/34) in the prior dose adjustment group were in clinical remission at Week 252. In the modified observed case analysis (treatment failure rules applied), remission rates were 61.3% (38/62), 76.3% (45/59), and 63.3% (31/49), respectively.
Figure 2.
Observed case analysisa,b (A) and modified observed case analysisa,b,d (B) of clinical remission for randomized patients who entered the LTE
Week 16 Induction Responders
Week 16 induction responders were patients who did not respond 8 weeks after IV ustekinumab induction, but subsequently were in response at Week 16 (after the first 90mg SC injection at Week 8). These patients continued to receive q8w treatment throughout the maintenance study and LTE as part of the nonrandomized population. Remission rates at Week 252 in these patients were similar to the randomized population, with 43.8% (88/201) in remission at Week 252.
Fistulas
Of the 567 ustekinumab-treated patients from the randomized and nonrandomized populations who entered the LTE, 61 had ≥1 open and draining fistula at induction baseline. At Week 252, 24/31 (77.4%) of these patients with data available were in fistula response (defined as a ≥50% reduction in number of draining fistulas).
Safety
From maintenance Week 0 through the final safety visit, patients in the combined (randomized and nonrandomized) ustekinumab group had 2267.6 total patient-years of follow-up, with 856.1 patient-years in the q12w group and 1411.4 patient-years in the q8w group and an average duration of follow-up of 209.0 weeks and 207.3 weeks, respectively (Table 4). Overall, safety events (per 100 patient-years) were generally similar in the placebo and combined ustekinumab groups for all AEs (440.3 vs. 327.6), serious AEs (19.3 vs. 17.5), infections (99.8 vs. 93.8), and serious infections (3.9 vs. 3.4, respectively).
Table 4.
Summary of events per 100 patient-years from Week 0 through Week 252; all patients and randomized patients entering the LTE
|
|
||||||||
| All patients entering the LTE | ||||||||
|
| ||||||||
| Placeboa | Ustekinumab 90 mg q12wb | Ustekinumab 90 mg q8wc | Combined ustekinumab | |||||
|
| ||||||||
| N | 151 | 213 | 354 | 567 | ||||
| Average duration of follow-up (weeks) | 105.2 | 209.0 | 207.3 | 208.0 | ||||
| Total patient-years of follow-up | 305.5 | 856.1 | 1411.4 | 2267.6 | ||||
| Deaths | 0 | 2 | 4 | 6 | ||||
| Number of events per 100 patient -years of follow-up (95% CI)e | ||||||||
| Adverse events | 440.3 (417.1,464.5) | 303.2 (291.7,315.1) | 342.3 (332.8,352.1) | 327.6 (320.2,335.1) | ||||
| Serious adverse events | 19.3 (14.7,24.9) | 18.2 (15.5,21.3) | 17.0 (14.9,19.3) | 17.5 (15.8,19.3) | ||||
| Infectionsd | 99.8 (88.9,111.7) | 91.7 (85.4,98.3) | 95.2 (90.1,100.4) | 93.8 (89.9,97.9) | ||||
| Serious infectionsd | 3.9 (2.0,6.9) | 4.7 (3.3,6.4) | 2.7 (1.9,3.7) | 3.4 (2.7,4.3) | ||||
| Malignancies | 1.70 | 1.19 | 0.99 | 1.06 | ||||
|
| ||||||||
| Randomized patients entering the LTE | ||||||||
|
| ||||||||
| Ustekinumab |
||||||||
| 90 mg SC q8w |
||||||||
| On 90 mg SC q8w due to dose adjustment prior to Week 44 | ||||||||
|
|
||||||||
| Placebo SCf.g | Ustekinumab 90 mg q12wf.g | Ustekinumab 90 mg q8wf.g | 90 mg SC q8w → 90 mg SC q8wh | placebo SC → 90 mg SC q8wh | 90 mg SC q12w → 90 mg SC q8wh | All 90 mg SC q8w | All ustekinumab | |
|
| ||||||||
| N | 96 | 103 | 99 | 17 | 35 | 19 | 153 | 237 |
| Average duration of follow-up (weeks) | 71.7 | 175.3 | 181.7 | 170.1 | 183.0 | 195.4 | 202.6 | 206.9 |
| Total patient-years of follow-up | 132.4 | 347.3 | 345.9 | 55.6 | 123.2 | 71.4 | 596.0 | 943.2 |
| Deaths | 0 | 1 | 0 | 0 | 2 | 0 | 2 | 3 |
| Number of events per 100 patient -years of follow-up | ||||||||
| Adverse events | 474.44 | 279.34 | 301.57 | 352.44 | 353.88 | 423.04 | 331.71 | 312.44 |
| Serious adverse events | 24.18 | 12.38 | 11.85 | 23.38 | 12.99 | 16.81 | 13.76 | 13.25 |
| Infectionsd | 108.79 | 84.95 | 77.20 | 98.90 | 92.53 | 96.66 | 84.73 | 84.82 |
| Serious infectionsd | 7.55 | 4.03 | 3.18 | 3.60 | 1.62 | 1.40 | 2.68 | 3.18 |
| Malignancies | 1.48 | 1.11 | 0 | 0 | 1.88 | 4.90 | 1.03 | 1.06 |
|
|
|
|||||||
CI, Confidence interval; IV, intravenous; LTE, long-term extension; q12w, every 12 weeks; q8w, every 8 weeks; SC, subcutaneous
Includes: 1) Patients who were in clinical response to ustekinumab IV induction dosing, were randomized and received placebo SC on entry into this maintenance study, and did not meet loss of response criteria from Week 8 through Week 32; 2) Patients who were in clinical response to placebo IV induction dosing and received placebo SC on entry into this maintenance study.
Includes: 1) Patients who were in clinical response to ustekinumab IV induction dosing, were randomized and received ustekinumab 90 mg SC q12w, and did not meet loss of response criteria from Week 8 through Week 32; 2)Patients who were not in clinical response to placebo IV induction dosing, received ustekinumab 130 mg IV at Week 0, achieved clinical response at Week 8, and initiated ustekinumab 90 mg SC q12w.
Includes: 1)Patients who were in clinical response to ustekinumab IV induction dosing, were randomized on entry into this maintenance study, received ustekinumab 90 mg SC q8w, or met loss of response criteria from Week 8 through Week 32 and received ustekinumab 90 mg SC q8w thereafter; 2)Patients who were not in clinical response to ustekinumab IV induction dosing, received ustekinumab 90 mg SC at Week 0, achieved clinical response at Week 8, and initiated ustekinumab 90 mg SC q8w.
Infections as assessed by the investigator.
Confidence intervals based on an exact method, assuming that the observed number of events follows a Poisson distribution.
Includes: Patient who were in clinical response to ustekinumab IV induction dosing and were randomized to receive study drug on entry to the maintenance study.
Includes data up to the time of meeting loss of response criteria for dose adjustment (occurred from Week 8 through Week 32).
Includes data from the time of meeting loss of response criteria for dose adjustment (occurred from Week 8 through Week 32) onward.
From Week 44 through the final safety visit, 4.53 and 3.19 treatment-emergent serious infections per 100 patient-years occurred in the placebo and combined ustekinumab groups, respectively (Supplement Table 1). Serious infections occurring on ustekinumab at a rate of ≥0.1 per 100 in the full LTE included: anal abscess, pneumonia, cellulitis, diverticulitis, gastroenteritis, abdominal abscess, perirectal abscess, pyelonephritis, sepsis, and cholecystitis. (Supplemental Table 1). Only one case of active TB was reported through Week 96.9
As previously reported,9, 10 6 deaths occurred between Weeks 0 and 156 (sudden death [ustekinumab q8w] and cardiopulmonary arrest [ustekinumab q12w], suicide [ustekinumab q8w], end-stage renal disease [ustekinumab q8w], acute myocardial infarction [ustekinumab q12w], and septic shock [ustekinumab q8w]; Table 4). No additional deaths were reported after Week 156.
From Week 0 through Week 252, among all patients entering the LTE, rates of malignancies (events per 100 patient-years) were 1.70 in the placebo and 1.06 in the combined ustekinumab group (Table 4). Four malignancies (excluding non-melanoma skin cancer [NMSC]) were previously reported through Week 156: testicular seminoma, adenocarcinoma of the small intestine, and chronic myeloid leukemia in patients receiving ustekinumab and papillary thyroid cancer in a placebo-treated patient.10 Six additional malignancies (excluding NMSC) were reported between Weeks 156 and 272. One intraocular melanoma and one renal cell carcinoma occurred in patients on q12w, and the events on q8w were endometrial adenocarcinoma, lentigo maligna melanoma, lobular breast cancer in situ, and pancreatic carcinoma. No lymphomas were observed throughout the LTE.
Pharmacokinetics and Immunogenicity
Serum ustekinumab concentrations were maintained during the LTE at levels similar to those observed during the maintenance study, with steady-state levels in patients receiving q8w dosing approximately 3-fold greater than those in patients receiving q12w dosing. In the patients randomized to q8w, median serum steady-state trough ustekinumab concentrations ranged from 2.04 to 2.37 μg/mL between Weeks 8–44 and from 2.18 to 3.13 μg/mL between Weeks 44–252. In patients randomized to q12w who did not receive dose adjustment, median serum steady-state trough ustekinumab concentrations ranged from 0.62 to 0.76 μg/mL between Weeks 8–44 and from 0.71 to 0.88 μg/mL between Weeks 44–252.
Among 532 patients who received ustekinumab during induction and maintenance and continued on ustekinumab in the LTE, the incidence of ADAs remained low, with 5.8% of patients (31/532) testing positive at one or more visits through 5 years. Nearly half of these patients (14/31, 45%) were positive at only one visit and negative at all other visits. Of patients who received ustekinumab q8w and q12w, 5.0% and 7.0%, respectively, had antibodies ≥1 visit. The incidence of antibodies to ustekinumab was low, regardless of concomitant immunosuppressive therapy use. Among the 181 patients in the q8w and q12w groups who were receiving immunosuppressants at Week 44, 14 patients (7.7%) developed antibodies at any time during the study, compared to 17 of 351 patients (4.8%) not receiving immunosuppressants. In patients randomized to ustekinumab, the proportion of patients in clinical remission at Week 252 was 46.7% (7/15) for those who had ADAs detected at any time during the study and 48.2% (107/222) for those who did not have ADAs detected at any time during the study.
DISCUSSION
This study presents efficacy and safety data from 5 years of ustekinumab treatment, the longest treatment experience with an antibody to IL-12/23 in IBD patients thus far reported. Results show that long-term SC ustekinumab therapy was well tolerated and effective at maintaining clinical remission through 5 years in patients who were TNF antagonist-naïve, as well as those who did not respond to, had lost response to, or were intolerant of TNF antagonists.
We evaluated the treatment effect of ustekinumab maintenance in all patients who had responded to IV ustekinumab induction and were randomized to SC maintenance therapy, following these patients throughout the 1-year maintenance study and 4-year LTE. In the intent-to-treat analyses, patients who discontinued, received dose adjustment, or met prespecified treatment failure criteria were considered not to be in clinical remission at subsequent timepoints. Even with this conservative analysis approach, 28.7% of patients in the q12w and 34.4% in the q8w group were in clinical remission at 5 years (Week 252). Notably, this analysis shows that among patients who were TNF antagonist-naïve, close to half (44%) of those who responded to IV ustekinumab induction were in remission 5 years later on SC q8w maintenance.
Throughout the LTE, the proportion of patients in clinical remission decreased by only approximately 5% each year and was primarily driven by patient discontinuation. The most common reasons for discontinuation were AEs (including those related to CD), lack of efficacy, and withdrawal of consent. However, many discontinuations were unrelated to efficacy. Nearly one-third of the patients who discontinued withdrew consent, highlighting the difficulty of maintaining patient enrollment in a long-term clinical trial. Importantly, through 5 years, approximately half of the patients who entered the LTE continued through the final dosing visit, providing a robust dataset. As shown in the observed case analysis, among patients who continued through the final efficacy visit at Week 252, 84.8% of the patients in the q12w and 90.0% in the q8w group were in clinical remission.
Ustekinumab q12w and q8w doses generally performed similarly during the LTE for most clinical measures. The q8w dose appeared to have numerically greater percentages of patients in clinical remission than the q12w, particularly in the last year of the LTE among TNF antagonist-naïve patients. However, the study was not designed or powered to evaluate differences between individual ustekinumab treatment regimens.
Corticosteroid-free remission rates were high in both groups at Week 252, and the majority of patient who in remission at Week 252 were not receiving corticosteroids. Additionally, the majority of patients who were receiving corticosteroids at maintenance baseline and entered the LTE were able to taper off corticosteroids successfully and were not receiving them at Week 252. Previous analysis of IM-UNITI data through 3 years did not show an increase in efficacy or impact on ADAs with concurrent immunosuppressant use, suggesting that concomitant use of immunosuppressants with ustekinumab is not necessary.10 Approximately 70% of patients were receiving ustekinumab monotherapy (ie, not receiving immunosuppressants) at Week 252.
Pharmacokinetic results showed that long-term SC ustekinumab dosing during the LTE maintained serum ustekinumab concentrations at levels similar to those observed during the maintenance study. Patients receiving q8w dosing consistently had approximately 3-fold greater steady-state serum ustekinumab levels compared with those receiving q12w dosing.
Consistent with previous reports from this study 9,10 and long-term studies of ustekinumab in psoriatic conditions,12, 13 ADA rates remained low through the LTE and did not appear to have clinical relevance. This is in contrast to TNF antagonist agents, in which immunogenicity has been shown to lead to treatment failure.14 Concomitant immunosuppressant therapy may be required to reduce immunogenicity of these agents. In our study, ADA formation rates were not higher in patients receiving ustekinumab monotherapy than those receiving concomitant immunosuppressants.
Data from this study confirm that the safety profile of ustekinumab in CD through 5 years remains consistent with the established safety profile of ustekinumab in psoriasis and psoriatic arthritis. Through 5 years of IM-UNITI, rates of safety events (including AEs, serious AEs, infection, serious infections, and AEs leading to discontinuation) were comparable between ustekinumab- and placebo-treated patients. Previous studies have indicated that the use of anti-TNF agents carries the risk of TB infection in IBD patients.15 However, there was no evidence for increased risk for opportunistic infections or TB in IM-UNITI maintenance and LTE. Additionally, through 5 years of ustekinumab maintenance treatment in this study, there was no evidence that ustekinumab increased the risk of death, anaphylactic and delayed hypersensitivity (serum-like sickness) reactions, or malignancy.
In conclusion, through 5 years of cumulative data from UNITI/IM-UNITI trials, ustekinumab 90 mg SC q12w and q8w safely maintained clinical response and remission in patients with CD.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND:
Data from the IM-UNITI trial showed that ustekinumab was safe and effective in patients with Crohn’s disease though 3 years.
FINDINGS:
5 years of cumulative data from IM-UNITI showed that ustekinumab maintained clinical response and remission in Crohn’s disease and confirmed the established safety profile of ustekinumab in psoriatic diseases.
IMPLICATIONS FOR PATIENT CARE:
Data from this study suggest that concomitant use of immunosuppressants with ustekinumab is not necessary. At 5 years, the majority of patients in remission were not receiving corticosteroids.
ACKNOWLEDGMENTS
This manuscript is dedicated to Professor Paul Rutgeerts who passed away in September 2020. Prof. Rutgeerts was a leading clinician, scientist, and mentor in the IBD field. He was also instrumental in the development and execution of the UNITI and IM-UNITI trials, authored previous publications from these studies, and contributed to early drafts of this paper before his passing.
We thank the patients, investigators, and study personnel who made the IM-UNITI study possible. Under the direction of the authors and in accordance with Good Publication Practices, Kirsten Schuck Gross of Janssen Scientific Affairs, LLC provided writing and editorial assistance.
Disclosures:
William Sandborn reports research grants from Abbvie, Abivax, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Genentech, Gilead Sciences, Glaxo Smith Kline, Janssen, Lilly, Pfizer, Prometheus Biosciences, Seres Therapeutics, Shire, Takeda, Theravance Biopharma; consulting fees from Abbvie, Abivax, Admirx, Alfasigma, Alimentiv (Robarts Clinical Trials, owned by Health Academic Research Trust [HART]), Alivio Therapeutics, Allakos, Amgen, Applied Molecular Transport, Arena Pharmaceuticals, Bausch Health (Salix), Beigene, Bellatrix Pharmaceuticals, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Meyers Squibb, Celgene, Celltrion, Cellularity, Cosmo Pharmaceuticals, Escalier Biosciences, Equillium, Forbion, Genentech/Roche, Gilead Sciences, Glenmark Pharmaceuticals, Gossamer Bio, Immunic (Vital Therapies), Index Pharmaceuticals, Intact Therapeutics, Janssen, Kyverna Therapeutics, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pandion Therapeutics, Pfizer, Progenity, Prometheus Biosciences, Protagonists Therapeutics, Provention Bio, Reistone Biopharma, Seres Therapeutics, Shanghai Pharma Biotherapeutics, Shire, Shoreline Biosciences, Sublimity Therapeutics, Surrozen, Takeda, Theravance Biopharma, Thetis Pharmaceuticals, Tillotts Pharma, UCB, Vendata Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals, Vivreon Biosciences, Zealand Pharma; and stock or stock options from Allakos, BeiGene, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences, Progenity, Shoreline Biosciences, Ventyx Biosciences, Vimalan Biosciences. Spouse: Iveric Bio - consultant, stock options; Progenity - stock; Oppilan Pharma - consultant, stock options; Prometheus Biosciences - employee, stock options; Ventyx Biosciences – stock options; Vimalan Biosciences – stock options.
Bruce Sands reports personal fees from AbbVie, personal fees from Amgen, personal fees from AstraZeneca, personal fees from Bristol-Myers Squibb, grants and personal fees from Celgene, personal fees and non-financial support from Janssen Research & Development, LLC., personal fees and non-financial support from MedImmune, grants, personal fees and non-financial support from Takeda, personal fees from Akros Pharma, personal fees from Arena Pharmaceuticals, personal fees from Boehringer-Ingelheim, personal fees from Forward Pharma, personal fees from Immune Pharmaceuticals, personal fees and non-financial support from Lilly, personal fees from Shire, personal fees from Synergy Pharmaceuticals, personal fees from Theravance Biopharma R&D, personal fees from TiGenix, personal fees from TopiVert Pharma, personal fees from Receptos, personal fees from Allergan, personal fees from EnGene, personal fees from Target PharmaSolutions, personal fees from Lycera, personal fees from Lyndra, personal fees from Ironwood Pharmaceuticals, personal fees from Salix, personal fees from Vivelix Pharmaceuticals, personal fees from UCB, personal fees from Oppilan Pharmaceuticals,personal fees from Gilead, personal fees from Rheos Medicines, personal fees from Seres Therapeutics, personal fees from 4D Pharma, personal fees from Capella Bioscience, personal fees from Otsuka, personal fees from Ferring, personal fees from Protagonist Therapeutics, personal fees from Palatin Technologies, grants, personal fees and non-financial support from Pfizer, personal fees from Hoffman-La Roche, personal fees from Prometheus Laboratories.
Stephen Hanauer received grant support and fees for consulting, lectures, and serving on an advisory board from Janssen, Abbvie, and Takeda; consulting and or advisory board fees from Pfizer, Receptos, Novartis, Gilead, Boehringer-Ingelheim, Seres Therapeutics, Ferring, Bristol-MyersSquibb, Amgen, Genentech, Merck, Samsung Bioepis, Protagonist, Salix, TiGenix, Allergan, Celgene, Vhsquared; grant support from Abbvie, Janssen, Pfizer, Celgene, Boehringer-Ingelheim, Takeda, Gilead
Stephan Targan received consulting fees from: Robarts Clinical Trials, Prometheus Biosciences and owns stock in Prometheus Biosciences.
Subrata Ghosh is a member of steering committees of Janssen, Abbvie, Boehringer Ingelheim, Gilead, Celgene, BMS; has received speaker honorarium from Abbvie, Takeda, Janssen, Ferring, Pfizer, Celltrion; and served on advisory committees of Janssen, Takeda, Abbvie, Eli Lilly, Pfizer, Gilead, Galapagos, Roche.
Willem JS de Villiers reports receiving consulting fees from Janssen.
Jean-Frederic Colombel reports receiving personal fees from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Celltrion, Enterome, Ferring, Genen- tech, Janssen–Johnson & Johnson, MedImmune, Merck, Pfizer, Protagonist, Second Genome, Seres, Shire, Takeda, Theradiag, and PPM Services, receiving grant support from AbbVie, Janssen–Johnson & Johnson, and Takeda, and holding stock options in Genfit and Intestinal Biotech Development.
Brian G Feagan received grant/research support from AbbVie Inc., Amgen Inc., AstraZeneca/MedImmune Ltd., Atlantic Pharmaceuticals Ltd., Boehringer-Ingelheim, Celgene Corporation, Celltech, Genentech Inc/Hoffmann-La Roche Ltd., Gilead Sciences Inc., GlaxoSmithKline (GSK), Janssen Research & Development LLC., Pfizer Inc., Receptos Inc. / Celgene International, Sanofi, Santarus Inc., Takeda Development Center Americas Inc., Tillotts Pharma AG, UCB; a consultant for Abbott/AbbVie, AdMIRx Inc., Akebia Therapeutics, Allergan, Amgen, Applied Molecular Transport Inc., Aptevo Therapeutics, Asta Pharma, Astra Zeneca, Atlantic Pharma, Avir Pharma, Biogen Idec, BioMx Israel, Boehringer-Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharma, Roche/Genentech, Galapagos, Galen/Atlantica, GiCare Pharma, Gilead, Gossamer Pharma, GSK, Inception IBD Inc, Intact Therapeutics, JnJ/Janssen, Kyowa Kakko Kirin Co Ltd., Lexicon, Lilly, Lycera BioTech, Merck, Mesoblast Pharma, Millennium, Nestles, Nextbiotix, Novonordisk, ParImmune, Parvus Therapeutics Inc., Pfizer, Prometheus Therapeutics and Diagnostics, Progenity, Protagonist, Qu Biologics, Rebiotix, Receptos, Salix Pharma, Shire, Sienna Biologics, Sigmoid Pharma, Sterna Biologicals, Synergy Pharma Inc., Takeda, Teva Pharma, TiGenix, Tillotts, UCB Pharma, Vertex Pharma, Vivelix Pharma, VHsquared Ltd., Zyngenia; a member of the speakers bureau for Abbott/AbbVie, JnJ/Janssen, Lilly, Takeda, Tillotts, UCB Pharma; a member of the scientific advisory board for Abbott/AbbVie, Allergan, Amgen, Astra Zeneca, Atlantic Pharma, Avaxia Biologics Inc., Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Centocor Inc., Elan/Biogen, Galapagos, Genentech/Roche, JnJ/Janssen, Merck, Nestles, Novartis, Novonordisk, Pfizer, Prometheus Laboratories, Protagonist, Salix Pharma, Sterna Biologicals, Takeda, Teva, TiGenix, Tillotts Pharma AG, UCB Pharma; and Senior Scientific Officer – Robarts Clinical Trials Inc.
Rory Rebuck, Yuhua Wang, Bin Zou, Omoniyi J Adedokun, and John P. Lynch are employees of Janssen Research & Development, LLC. and own stock/stock options.
Christopher Gasink is an employee of Janssen Scientific Affairs, LLC. and owns stock/stock options.
FUNDING: Funding for this study was provided by Janssen Research & Development, LLC. William Sandborn is supported in part by NIDDK-funded San Diego Digestive Diseases Research Center (P30 DK120515).
Abbreviations list:
- ADA
anti-drug antibodies
- AE
adverse event
- CD
Crohn’s disease
- CDAI
Crohn’s disease activity index
- CI
confidence interval
- CRP
c-reactive protein
- IBDQ
Inflammatory Bowel Disease Questionnaire
- IL
interleukin
- IV
intravenous
- LTE
long-term extension
- MSD
Meso Scale Discovery
- NMSC
non-melanoma skin cancer
- PBO
placebo
- Q8w
every 8 weeks
- Q12w
every 12 weeks
- SC
subcutaneous
- TB
tuberculosis
- TNF
tumor necrosis factor
- UST
ustekinumab
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Peyrin-Biroulet L, Loftus EV Jr., Colombel JF, et al. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol 2010;105:289–297. [DOI] [PubMed] [Google Scholar]
- 2.Roda G, Chien Ng S, Kotze PG, et al. Crohn’s disease. Nat Rev Dis Primers 2020;6:22. [DOI] [PubMed] [Google Scholar]
- 3.D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet 2008;371:660–667. [DOI] [PubMed] [Google Scholar]
- 4.Goel RM, Blaker P, Mentzer A, et al. Optimizing the use of thiopurines in inflammatory bowel disease. Ther Adv Chronic Dis 2015;6:138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domenech E, Manosa M, Navarro M, et al. Long-term methotrexate for Crohn’s disease: safety and efficacy in clinical practice. J Clin Gastroenterol 2008;42:395–399. [DOI] [PubMed] [Google Scholar]
- 6.Conway R, Low C, Coughlan RJ, et al. Methotrexate and lung disease in rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheumatol 2014;66:803–812. [DOI] [PubMed] [Google Scholar]
- 7.McLean LP, Shea-Donohue T, Cross RK. Vedolizumab for the treatment of ulcerative colitis and Crohn’s disease. Immunotherapy 2012;4:883–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016;375:1946–1960. [DOI] [PubMed] [Google Scholar]
- 9.Sandborn WJ, Rutgeerts P, Gasink C, et al. Long-term efficacy and safety of ustekinumab for Crohn’s disease through the second year of therapy. Aliment Pharmacol Ther 2018;48:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanauer SB, Sandborn WJ, Feagan BG, et al. IM-UNITI: Three-year efficacy, safety, and immunogenicity of ustekinumab treatment of Crohn’s disease. J Crohns Colitis 2020;14:23–32. [DOI] [PubMed] [Google Scholar]
- 11.Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn’s Disease Activity Index. Gastroenterology 1976; 70:439–444. [PubMed] [Google Scholar]
- 12.Langley RG, Lebwohl M, Krueger GG, et al. Long-term efficacy and safety of ustekinumab, with and without dosing adjustment, in patients with moderate-to-severe psoriasis: results from the PHOENIX 2 study through 5 years of follow-up. Br J Dermatol 2015; 172:1371–1383. [DOI] [PubMed] [Google Scholar]
- 13.Kimball AB, Papp KA, Wasfi Y, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol 2013;27:1535–1545. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy NA, Heap GA, Green HD, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol 2019;4:341–353. [DOI] [PubMed] [Google Scholar]
- 15.Lee JW, Choi CH, Park JH, et al. Clinical features of active tuberculosis that developed during anti-tumor necrosis factor therapy in patients with inflammatory bowel disease. Intest Res 2016;14:146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.