Abstract
In recent years, many new treatments for relapsed/refractory (R/R) multiple myeloma (MM) have improved patient prognosis, but the prognosis of patients with extramedullary MM is still particularly poor. Therefore, more efficacious therapies and novel strategies are urgently needed for these patients. The aim of this study was to observe and compare the efficacy and safety of humanized anti-B cell maturation antigen (anti-BCMA) chimeric antigen receptor (CAR) T cell therapy in R/R MM patients with and without extramedullary disease. Seven R/R MM patients with extramedullary disease and 13 without extramedullary disease received humanized anti-BCMA CAR T cell therapy. The overall response rate was not different between patients with and without extramedullary disease. There was no difference in the progression-free survival (PFS) or overall survival (OS) rates between the two groups at 180 days, but the PFS and OS rates in patients with extramedullary disease were lower at 360 days than those in patients without extramedullary disease. Although some patients with extramedullary disease experienced further disease progression, their M protein level did not increase. We did not see this change trend of M protein in patients without extramedullary disease. However, this was not observed in patients without extramedullary disease. Among patients who responded to CAR T cell therapy, the grades of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxic syndrome (ICANS) were much higher among patients with extramedullary disease. In summary, R/R MM patients with extramedullary disease could benefit from humanized anti-BCMA CAR T cell therapy in the short term, although the CRS and ICANS grades were much higher in patients with extramedullary disease. Therefore, anti-BCMA CAR T cell therapy allows for a remission time for R/R MM patients with extramedullary disease, which could be maintained by bridging hematopoietic stem cell transplantation, radiotherapy, and other therapies.
Clinical Trial Registration
http://www.chictr.org.cn/index.aspx, identifiers ChiCTR1800017051 and ChiCTR2000033925.
Keywords: anti-B cell maturation antigen chimeric antigen receptor T, multiple myeloma, relapsed, refractory, extramedullary disease, efficacy
Introduction
Multiple myeloma (MM) is a malignant disease characterized by monoclonal proliferation of bone marrow (BM) plasma cells. Although effective treatment, such as proteasome inhibitors, immunomodulatory agents, monoclonal antibodies, and autologous hematopoietic stem cell transplantation (auto-HSCT), can significantly improve patient prognosis (1–4), the prognosis of patients with relapsed/refractory (R/R) MM is poor (5, 6). Extramedullary MM (EMM) is a rare type of MM in which MM cells escape the BM, become independent of the BM microenvironment, and invade other organs, resulting in extramedullary disease. Any organ can be infiltrated, including the skin, soft tissues, liver, spleen, kidney, lymph nodes, breast, pleura, and central nervous system (7, 8). The molecular mechanisms underlying EMM development have not yet been defined. The prognosis of EMM is different from that of MM without extramedullary disease (9). EMM is associated with an increased risk and poor prognosis, and many treatments, including ASCT, have failed to improve patient outcomes in most studies (10). In recent years, many new treatments for R/R MM have improved patient prognosis, but the prognosis of patients with EMM is still particularly poor (11–15). Therefore, more efficacious therapies and novel strategies are urgently needed for EMM.
Anti-B cell maturation antigen (anti-BCMA) is specifically expressed in the cells of almost all MM patients (16). Anti-BCMA chimeric antigen receptor (CAR) T cell therapy had satisfactory efficacy and prolonged the survival time of R/R MM patients in early clinical trials (17–20). However, there are limited data on anti-BCMA CAR T cell therapy in patients with EMM. Whether anti-BCMA CAR T cell therapy could improve the outcomes of R/R MM patients with extramedullary disease needs to be further explored.
Patients and Methods
Patients
Twenty R/R MM patients were enrolled in a clinical trial of humanized anti-BCMA CAR T cell therapy (ChiCTR1800017051 and ChiCTR2000033925) between January 2019 and October 2020. These 20 R/R MM patients included seven patients with at least one assessable extramedullary lesion and 13 patients without any assessable extramedullary disease. The extramedullary disease in the seven patients was classified as either extramedullary-extraosseous (EM-E) or extramedullary-bone related (EM-B). None of the seven patients had solitary extramedullary/bone plasmacytoma.
Preparation and Administration of Humanized Anti-BCMA CAR T Cell Therapy
Peripheral blood mononuclear cells were collected from R/R MM patients and isolated by Ficoll density gradient centrifugation. CD3+ T cells were selected using CD3 microbeads (Miltenyi Biotec, Inc., Cambridge, MA, USA), stimulated by anti-CD3/anti-CD28 mAb-coated Human T-Expander beads (Cat. no. 11141D, Thermo Fisher Scientific, Inc., Waltham, MA, USA), and cultured in X-Vivo 15 medium (Lonza Group, Ltd., Basel, Switzerland) supplemented with 250 IU/ml interleukin (IL)-2 (Proleukin, Novartis International AG, Basel, Switzerland). CD3+ T cells (3 × 106) were transduced with lentiviral supernatant from 293T cells transfected with anti-BCMA CAR plasmid (20 µg, lenti-BCMA-2rd-CAR, Shanghai Genbase Biotechnology Co., Ltd. Shanghai, China) at a multiplicity of infection of 0.5 and cultured in media containing IL-2 (250 U/ml). On the 12th to 15th days of cultivation, transduction efficiencies of anti-BCMA CAR were analyzed by flow cytometry (FCM) (BD Biosciences, San Jose, CA, USA).
All patients received lymphodepleting chemotherapy with fludarabine (30 mg/m2) and cyclophosphamide (400 mg/m2) from day −4 to day −2. Autologous anti-BCMA CAR T cells were infused on day 0 (2 × 106 cells/kg) in all patients.
Evaluation Criteria for Diagnostic and Therapeutic Efficacy
The diagnosis of EMM, clinical response, and disease progression after humanized anti-BCMA CAR T cell therapy were assessed according to the International Myeloma Working Group Guidelines uniform response criteria for MM (21). Follow-up was performed from the date of humanized anti-BCMA CAR T cell infusion until the cutoff date or until the patient died. The clinical responses included stringent complete response (sCR), complete response (CR), very good partial response (VGPR), partial response (PR), minimal response, stable disease (SD), and progressive disease. In our study, we assessed the objective response rate (ORR), overall survival (OS), and progression-free survival (PFS). The ORR was defined as the proportion of patients who achieved sCR, CR, VGPR, or PR.
Assessable extramedullary disease was detected using computed tomography. MM cells in the pleural effusion and cerebrospinal fluid were detected using FCM. During humanized anti-BCMA CAR T cell therapy administration, the proportions of anti-BCMA CAR T cells in the peripheral blood were assessed by FCM on days 0, 4, 7, 14, 28, and 60 after infusion of anti-BCMA CAR T cells. Proportions of anti-BCMA CAR T cells in the pleural effusion and cerebrospinal fluid were also observed by FCM.
Adverse Events (AEs) of Humanized Anti-BCMA CAR T Cell Therapy
AEs of humanized anti-BCMA CAR T cell therapy were also assessed. The cytokine release syndrome (CRS) grade was determined according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.03 (22). Immune effector cell-associated neurotoxicity syndrome (ICANS) was used to assess neurotoxicity (23). Cytokines, including IL-6, IL-2R, and tumor necrosis factor-α (TNF-α) were assessed on days 0, 7, 14, 28, and 60 by enzyme-linked immunosorbent assay.
Statistical Analysis
Data are expressed as the mean ± SE. The probabilities of PFS and OS were estimated using the Kaplan-Meier method and compared with the log-rank test. All statistical analyses were performed using GraphPad Prism 8 and SPSS 17.0. Statistical significance was set at P < 0.05.
Results
Patient Characteristics
The characteristics of all R/R MM patients enrolled in this clinical trial are listed in Table 1. BCMA was expressed on MM cells in all the R/R MM patients when they were enrolled in our study (Table 1). The expressions of BCMA in MM cells by FCM at the time of enrollment are shown in Supplementary Figure 1. The only difference between the two groups was the presence of extramedullary disease (Table 2). In our study, two patients in the EMM group had prior anti-BCMA CAR-T cell therapy. These two patients received murine anti-BCMA CAR-T cell therapy before, but they did not benefit from this time treatment. So they were enrolled in this humanized anti-BCMA CAR-T cell therapy. In the seven R/R MM patients with extramedullary disease, the mean time from diagnosis of extramedullary disease to enrolment in this clinical trial was 9.57 ± 3.08 months. All the patients had BCMA expression in MM cells at the time of enrolment. The median follow-up times were 7.3 months (range, 2–12 months) in patients with extramedullary disease and 12.5 months (3–27 months) in patients without extramedullary disease.
Table 1.
Baseline characteristics of the R/R multiple myeloma patients with or without extramedullary disease.
| Pt | Sex | Age (years) | KPS | Subtype | ISS stage | MM cells in the BM, % | Bone lesions | Extramedullary disease | Time to diagnosis of extramedullary disease (months) | High-risk cytogenetic lesions | Lines of prior therapy | Previous BCMA CAR T cell therapy (Murine BCMA CAR T) | Previous auto-HSCT | BCMA expression in MM cells (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| With extramedullary disease | ||||||||||||||
| 1 | Male | 57 | 90 | κ light chain | III | 86.0% | Yes | EM-B/EM-E | 5 | t(4;14), t(14;16), Del(17p) | 14 | No | Yes | 92.53 |
| 2 | Female | 71 | 100 | IgG-κ | II | 55.3% | Yes | EM-B/EM-E | 14 | Del(17p),t(4;14) | 10 | Yes | No | 94.39 |
| 3 | Male | 59 | 100 | κ light chain | III | 17.0% | Yes | EM-B/EM-E | 9 | t(14,16) | 7 | Yes | No | 81.82 |
| 4 | Male | 73 | 90 | IgD-λ | II | 23.04% | Yes | EM-E | 3 | None | 12 | No | No | 94.39 |
| 5 | Female | 38 | 90 | κ light chain | II | 70.44% | Yes | EM-B/EM-E | 6 | None | 17 | No | Yes | 93.18 |
| 6 | Male | 56 | 80 | IgG-κ | II | 32.26% | Yes | EM-B/EM-E | 5 | None | 10 | No | No | 80.26 |
| 7 | Female | 58 | 90 | IgG-λ | III | 88.12% | Yes | EM-E | 12 | None | 30 | No | No | 93.68 |
| Without extramedullary disease | ||||||||||||||
| 1 | Female | 70 | 100 | IgG-κ | II | 32.56% | Yes | - | - | Del(17p), t(4;14) | 10 | No | No | 94.76 |
| 2 | Male | 58 | 100 | κ light chain | III | 41.75% | Yes | - | - | t(14,16) | 7 | No | No | 92.57 |
| 3 | Male | 63 | 80 | IgG-κ | III | 35.62% | Yes | - | - | t(14,16) | 5 | No | Yes | 97.84 |
| 4 | Male | 66 | 100 | IgA-κ | II | 19.2% | Yes | - | - | t(14;16), Del(17p) | 16 | No | No | 90.99 |
| 5 | Female | 55 | 90 | IgG-λ | II | 44.28% | No | - | - | None | 7 | No | No | 90.41 |
| 6 | Female | 52 | 90 | IgA-λ | II | 20.72% | Yes | - | - | None | 9 | No | No | 82.93 |
| 7 | Female | 72 | 100 | Nonsecretory | II | 28.98% | Yes | - | - | None | 6 | No | No | 95.70 |
| 8 | Female | 77 | 90 | IgD-κ | III | 66.8% | Yes | - | - | None | 13 | No | No | 97.06 |
| 9 | Female | 42 | 100 | IgG-κ | III | 22.14% | Yes | - | - | t(14;16) | 5 | No | No | 96.24 |
| 10 | Female | 45 | 90 | IgG-κ | I | 19.44% | Yes | - | - | None | 5 | No | No | 82.73 |
| 11 | Female | 46 | 90 | IgG-κ | III | 22.23% | Yes | - | - | None | 7 | No | No | 88.83 |
| 12 | Male | 54 | 90 | IgG-κ | III | 17.5% | Yes | - | - | None | 8 | No | No | 92.31 |
| 13 | Male | 54 | 80 | IgG-λ | II | 14.8% | Yes | - | - | t(14;16) | 9 | No | Yes | 88.16 |
Pt, Patient; KPS, Karnofsky performance scale; ISS, international staging system; MM, multiple myeloma; BM, bone marrow; BMCA, B cell maturation antigen; CAR, chimeric antigen receptor; auto-HSCT, autologous haematopoietic stem cell transplantation; EM-B, extramedullary-bone related; EM-E, extramedullary-extraosseou.
Extramedullary-extraosseous (EM-E): Hematogenous dissemination leads to soft tissue tumors at the anatomical site far from the bone.
Extramedullary-bone related (EM-B): Breaks through the bone cortex and only invade the surrounding soft tissues.
Table 2.
To compare the baseline characteristics of the R/R multiple myeloma patients with or without extramedullary disease.
| With extramedullary disease (n = 7) | Without extramedullary disease (n = 13) | P | |
|---|---|---|---|
| Sex, male | 4 (57.1%) | 8 (61.5%) | >0.99 |
| Age, years | 58 (38-73) | 58 (42-77) | 0.964 |
| KPS >80 | 7 (100%) | 13 (100%) | 0.796 |
| Subtype, %κ light chain | 42.9% | 7.7% | 0.101 |
| ISS stage, I-II:III (n) | 4:7 | 7:6 | 0.444 |
| MM cells in the BM, %, mean (range) | 36.6 (7.8-86.0) | 14.8 (1.8-66.7) | 0.059 |
| Bone lesions | 7 (100%) | 11 (84.6%) | 0.521 |
| Extramedullary MM | 7 (100%) | 0 (0%) | <0.0001 |
| Extramedullary-extraosseous | 6 (85.7%) | 0 (0%) | 0.0002 |
| Extramedullary-bone related | 5 (71.5%) | 0 (0%) | 0.001 |
| High-risk cytogenetic lesions | 3 (42.9%) | 6 (46.2%) | >0.99 |
| Lines of prior therapy, mean (range) | 12 (7-30) | 7 (5-16) | 0.085 |
| 2 (28.6%) | 0 (0%) | 0.111 | |
| Auto-HSCT before BCMA CAR T therapy | 2 (28.6%) | 2 (15.4%) | 0.587 |
Data are presented as n (%) unless otherwise indicated.
ISS, international staging system; MM, multiple myeloma; BM, bone marrow; auto-HSCT, autologous haematopoietic stem cell therapy; CAR, chimeric antigen receptor.
In bold: The only difference between the two groups was the presence of extramedullary disease (P < 0.05).
Transduction Efficiency, Amplification Efficiency, and Infusion Dose of Humanized Anti-BCMA CAR T Cells
The median humanized anti-BCMA CAR transduction efficiencies were 38.74 ± 9.18% in the extramedullary disease group and 42.26 ± 10.41% in the no extramedullary disease group (P = 0.826). After 12 to 15 days of culture, the median humanized anti-BCMA CAR T cell numbers were 5.87 ± 2.77 × 106 cells/kg and 5.01 ± 1.94 × 106 cells/kg in the extramedullary disease and no extramedullary disease groups, respectively (P = 0.799). The patients received anti-BCMA CAR T cell doses of 2.21 ± 0.39 × 106 cells/kg and 2.06 ± 0.44 × 106 cells/kg, respectively, on day 0 (P = 0.825). Manufactured products and phenotypical analysis of the final formulation per patient are in Supplementary Table 1.
Clinical Response and Survival
The median response times to achieve the best effect were 2.7 months (1–6 months) in the extramedullary disease group and 2.3 months (1–4 months) in the no extramedullary disease group. There was no difference in the median response time between the two groups (P = 0.967).
Five patients in the extramedullary disease group (Ptwith 1, 2, 5, 6, and 7) (71.4%) experienced further disease progression and died of their primary disease. Only three patients in the no extramedullary disease group (Ptwithout 4, 6, and 8) (3/13, 23.1%) experienced disease progression and died of their primary disease. Ptwithout 2 also experienced further disease progression, but he was still alive at the cutoff date (Figure 1A). When the disease progress again in all the patients, the anti-BCMA expressions in myeloma cells was positive in Ptwith 2,5,7 and Ptwithout 2,4, whereas it was negative in Ptwith 1,6 and Ptwithout 6,8.
Figure 1.
Clinical responses of the humanized anti-BCMA CAR-T cell therapy. (A) Ptwith 1,2,5,6,7 (5/7, 71.4%) and Ptwithout 4,6,8 (3/13, 23.1%) developed disease progression again and died of their primary disease. (B) There was no difference in ORR between patients with and without extramedullary disease when they achieved the best effect (P=0.876). (C) M protein levels increased again in Ptwith 5,7 when their disease progressed again, but it did not increase when the disease of Ptwith 1,2,6 progressed again. (D) All the patients with disease progression were accompanied by an increase of M protein level.
After this anti-BCMA CAR-T cell therapy, the ORR in all the R/R MM patients was 80% (16/20). In all the seven patients with extramedullary disease, five patients had an ORR reaction to this therapy (71.43%, 5/7), Of the seven patients with extramedullary disease, five responded to CAR T cell therapy, including three patients who achieved sCR/CR, one patient who achieved VGPR, and one patient who achieved PR. The other two patients achieved SD only. In the patients without extramedullary disease, 11 patients had an ORR reaction to this therapy (84.61%, 11/13), Of the patients without extramedullary disease, 11 patients responded to CAR T cell therapy, including six patients who achieved sCR/CR, two patients who achieved VGPR, and three patients who achieved PR. The other two patients achieved SD only (Figure 1B). There was no difference in the ORR between patients with and without extramedullary disease when they achieved the best effect (P = 0.876). Only one patient without extramedullary disease (Ptwithout 11) received auto-HSCT 3 months after anti-BCMA CAR T-cell therapy.
The M protein levels in the peripheral blood were assessed following anti-BCMA CAR T-cell infusion. In the seven patients with extramedullary disease, the level of M protein increased again in Ptwith 5 and 7 after disease progression (Figures 1C, D ). However, there was no increase in the M protein levels of Ptwith 1 and 2 after disease progression. All patients without extramedullary disease who experienced disease progression had an increase in M protein levels (Figure 1D). We also assessed the anti-BCMA expression in MM cells among patients with disease progression. We observed anti-BCMA expression in the MM cells of Ptwith 2, 5, and 7 and Ptwithout 2 and 4 but not those of Ptwith 1 and 6 and Ptwithout 6 and 8.
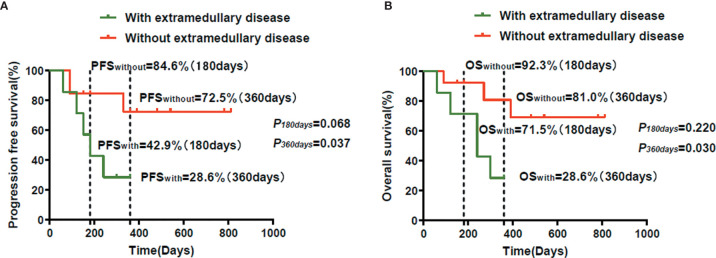
Kaplan-Meier analysis showed that there was no difference in the PFS rate between patients with extramedullary disease (42.9%) and those without extramedullary disease (84.6%) at 180 days (P = 0.068). However, the PFS rate of patients with extramedullary disease (28.6%) was lower than that of patients without extramedullary disease (72.5%) at 360 days (P = 0.037) (Figure 2A). There was no difference in the OS rate between patients with extramedullary disease (71.5%) and those without extramedullary disease (92.3%) at 180 days (P = 0.220). However, the OS rate of patients with extramedullary disease (28.6%) was lower than that of patients without extramedullary disease (81.0%) at 360 days (P = 0.030) (Figure 2B).
Figure 2.
The survival observation of the humanized anti-BCMA CAR-T cell therapy. (A) The PFS in patients with extramedullary disease was lower than that of the patients without extramedullary disease at 360 days (P=0.037). There was no difference of the PFS in the two groups at 180 days (P=0.068). (B) The OS in patients with extramedullary disease was lower than that of the patients without extramedullary disease at 360 days (P=0.030). There was no difference of the OS in the two groups at 180 days (P=0.220).
In our study, the mean OS among patients with extramedullary disease was 17.29 ± 4.95 months.
Efficacy of Humanized Anti-BCMA CAR T Cell Therapy in Patients With EMM
We evaluated the efficacy of humanized anti-BCMA CAR T-cell therapy in the seven patients with extramedullary disease, including the efficacy for extramedullary lesions. At the beginning of the study, Ptwith 4 had skin lesions with no extramedullary bone-related disease in the adjacent bone, Ptwith 7 had myeloma cells infiltrating the pleural effusion and central nervous system MM, and Ptwith 5 had a number of extramedullary bone-related lesions. The four remaining patients all had at least one extramedullary soft tissue lesion far from the bone.
Although the M protein in the peripheral blood of Ptwith 6 disappeared after treatment, as did myeloma cells in the BM, he did not achieve sCR/CR as of the cutoff date because he had extramedullary lesions that did not disappear after humanized anti-BCMA CAR T cell therapy. The skin lesion far from the bone of Ptwith 4 disappeared after anti-BCMA CAR T cell therapy. The myeloma cells in the cerebrospinal fluid of Ptwith 7 disappeared after therapy. The soft tissue masses in the remaining four patients with extramedullary disease disappeared after anti-BCMA CAR T cell therapy (Figure 3).
Figure 3.
The efficacy to the extramedullary disease. The skin lesion far from bone of Ptwith 4 disappeared after anti-BCMA CAR-T cell therapy. Myeloma cells in cerebrospinal fluid of Ptwith 7 disappeared after therapy. Soft tissue masses disappeared after this therapy.
The Proportions of Humanized Anti-BCMA CAR T Cells
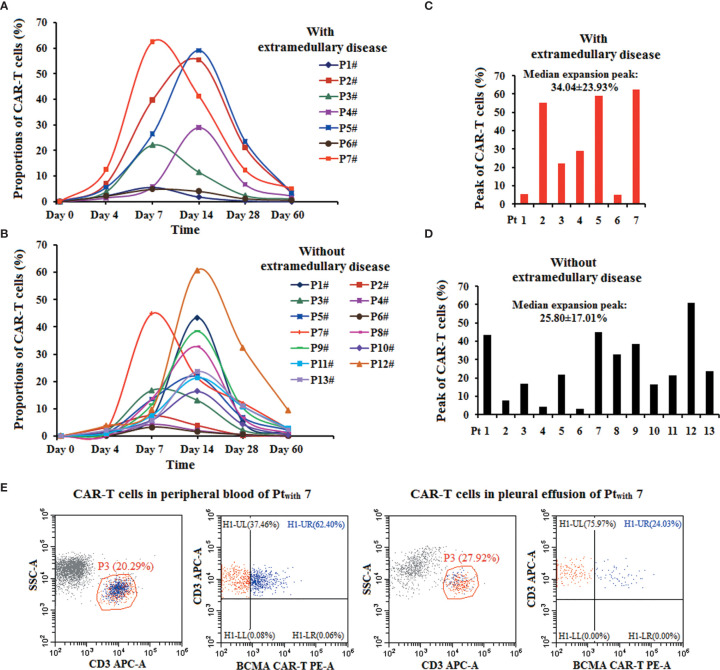
The proportion of CAR T cells was assessed on days 0, 4, 7, 14, 28, and 60 post-infusion. There was no difference in the median expansion peak of the humanized anti-BCMA CAR T cells in the peripheral blood of patients with extramedullary disease (34.04 ± 23.93%) and those without extramedullary disease (25.80 ± 17.01%) (P = 0.438) (Figures 4A–D). The highest anti-BCMA CAR T cell expression in the peripheral blood occurred in Ptwith 7 (62.4%), and the level of anti-BCMA CAR T cells in her pleural effusion was 24.03% (Figure 4E).
Figure 4.
The proportions of the anti-BCMA CAR-T cells in the therapy. (A, B). The proportions of anti-BCMA-CAR T cells in CD3+ T cells in peripheral blood in the two group of patients. (C, D). There was no difference in the median expansion peak of the anti-BCMA CAR-T cells in CD3+ T cells in peripheral blood in the two group of patients (P= 0.438). (E). In Ptwith 7, the highest anti-BCMA CAR-T cell expression in peripheral blood was 62.4%, the level of anti-BCMA CAR-T cells in her pleural effusion was 24.03%.
AEs and Serum Levels
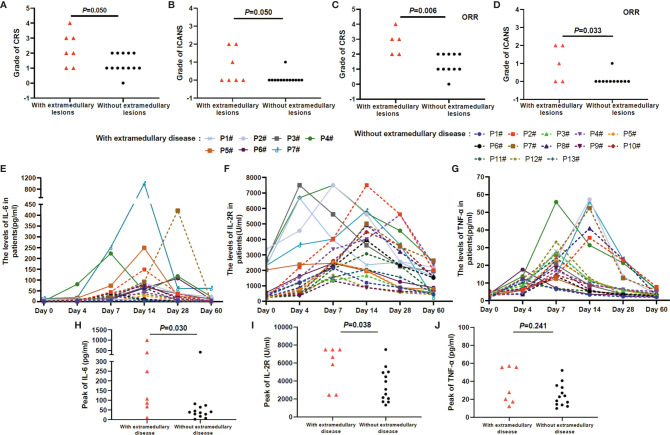
Ptwith 7 was diagnosed with grade 4 CRS, Ptwith 2 and 5 were diagnosed with grade 3 CRS, and the remaining patients in both groups were diagnosed with grades 0 to 2 CRS. Ptwith 2 and 7 were diagnosed with grade 2 ICANS, Ptwith 2 and Ptwithout 8 were diagnosed with grade 1 ICANS, and the remaining patients in both groups were diagnosed with grade 0 ICANS. The proportions of the grades of CRS and ICANS did not differ between the two groups (P CRS = 0.050 and P ICANS = 0.050) (Figures 5A, B). However, when including only patients who responded to CAR T cell therapy, the grades of CRS and ICANS in patients with extramedullary disease were significantly higher than those in patients without extramedullary disease (P CRS = 0.006 and P ICANS = 0.033) (Figures 5C, D). No patient died of any grade CRS or ICANS during therapy. CRS grade >3 was more frequent in patients with extramedullary disease than in those without extramedullary disease (P = 0.031) (Table 3).
Figure 5.
AEs and the serum levels in the anti-BCMA-CAR T cell therapy. (A, B) The grades of CRS and ICANS had no different in patients with and without extramedullary disease (P CRS = 0.050 and P ICANS = 0.050). (C, D) In patients obtained an ORR reaction, the grades of CRS and ICANS in patients with extramedullary disease was higher than that of in patients without extramedullary disease (P CRS =0.006 and P ICANS = 0.033). (E–G) The serum levels of IL-6, IL-2R, and TNF-a peaked 4 to 7 days after the infusion of anti-BCMA CAR T cells and declined 12 to 21 days. (H, I) IL-6 and IL-2R levels were higher in patients with extramedullary disease group than that of in patients without extramedullary disease group (P IL-6 = 0.030 and P IL-2R = 0.038). (J) There was no different in the TNF-a level between the two groups (P TNF-a = 0.241).
Table 3.
Adverse events in the anti-BCMA-CAR T cell therapy.
| With extramedullary disease (n=7) | Without extramedullary disease (n=13) | P values | |
|---|---|---|---|
| CRS | |||
| Grade 0-2 | 4 (57.1%) | 13 (100.0%) | |
| Grade ≥3 | 3 (42.9%) | 0 (0%) | P=0.031 |
| ICANS | |||
| Grade 0-2 | 7 (100.0%) | 13 (100.0%) | |
| Grade≥3 | 0 | 0 | - |
| Coagulopathy | |||
| Grade 0-2 | 5 (71.4%) | 12 (92.3%) | |
| Grade≥3 | 2 (28.6%) | 1 (7.6%) | P=0.270 |
| Gastrointestinal | |||
| Grade 0-2 | 6 (85.7%) | 13 (100.0%) | |
| Grade≥3 | 1 (14.3%) | 0 | P=0.350 |
| Creatinine increased | |||
| Grade 0-2 | 7 (100.0%) | 13 (100.0%) | |
| Grade≥3 | 0 | 0 | - |
| Transaminase increases | |||
| Grade 0-2 | 5 (71.4%) | 13 (100.0%) | |
| Grade≥3 | 2 (28.6%) | 0 | P=0.111 |
| Cardiopulmonary | |||
| Grade 0-2 | 6 (85.7%) | 13 (100.0%) | |
| Grade≥3 | 1 (14.3%) | 0 | P=0.350 |
| Hematological toxicity | |||
| Leukopenia | |||
| Grade 0-2 | 4 (57.1%) | 11 (84.6%) | |
| Grade≥3 | 3 (42.9%) | 2 (15.4%) | P=0.290 |
| Anemia | |||
| Grade 0-2 | 5 (71.4%) | 10 (76.9%) | |
| Grade≥3 | 2 (28.6%) | 3 (23.1%) | P=1.000 |
| Thrombocytopenia | |||
| Grade 0-2 | 3 (42.9%) | 11 (84.6%) | |
| Grade≥3 | 4 (57.1%) | 2 (15.4%) | P=0.122 |
Data are presented as n (%).
CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxic syndrome.
In bold: CRS grade >3 was more frequent in patients with extramedullary disease than in those without extramedullary disease (P = 0.031).
The cytokine levels of IL-6, IL-2R, and TNF-α changes during humanized anti-BCMA CAR T-cell therapy (Figures 5E–G). In all of the patients, the serum levels of IL-6, IL-2R, and TNF-α peaked 4 to 7 days after the infusion of anti-BCMA CAR T cells and declined 12 to 21 days after infusion. The serum levels of IL-6 and IL-2R were higher in patients with extramedullary disease than in those without extramedullary disease (P IL-6 = 0.030 and P IL-2R = 0.038) (Figures 5H, I). However, there was no difference in the serum level of TNF-α between patients with and without extramedullary disease (P TNF-α = 0.241) (Figure 5J).
Patients developed fever with or without chills, headache, fatigue, nausea, reduced appetite, edema, tachycardia, and other symptoms during humanized anti-BCMA CAR T cell therapy (Table 3). All of the patients recovered 14 to 28 days after the infusion of anti-BCMA CAR T cells. Patients also experienced grades 1 to 4 hematological toxicities in the course of anti-BCMA CAR T-cell therapy. These toxicities occurred 5 to 8 days after infusion of anti-BCMA CAR T cells, and patients recovered 14 to 60 days after infusion. The patients received tocilizumab, methylprednisolone, antipyretic drugs, and symptomatic treatment for AEs. Three patients with extramedullary disease and two patients without extramedullary disease who presented with grade 3 hematological toxicity were diagnosed with gram-negative bacterial infections, which were cured by anti-infective therapy. No patient was diagnosed with invasive fungal disease, and none of the patients died of infections.
Discussion
Seven R/R MM patients with assessable extramedullary disease and 13 R/R MM patients without extramedullary disease were enrolled in this clinical trial of humanized anti-BCMA CAR T-cell therapy. The process of observation after anti-BCMA CAR T cell therapy was comprehensive rather than just observing the changes in M protein levels. Although the grades of CRS and ICANS were much higher in patients with extramedullary disease, the ORR was not different in patients with and without extramedullary disease, and there was no difference between the two groups in the PFS and OS rates at 180 days. These results suggest that R/R MM patients with extramedullary disease who respond to anti-BCMA CAR T cell therapy can use this time as a bridge to other treatments, such as HSCT, radiotherapy, and other therapies.
MM is a malignant B-cell disease associated with organ damage, including renal failure, anemia, bone damage, and hypercalcemia (21, 24). EMM is defined as the proliferation of malignant plasma cells outside the BM. The EMM incidence is approximately 10% to 15% in all R/R MM patients (25, 26). Primary EMM occurs in approximately 4% to 16% of MM patients at diagnosis, and secondary EMM occurs in approximately 6% to 20% of MM patients during disease progression (27). There are two types of EMM. EM-E occurs when hematogenous dissemination leads to soft tissue tumors at a site far from the bone, and EM-B involves MM cells breaking through the bone cortex and invading only the surrounding soft tissues (9, 28–31). EM-E occurs in approximately 3% of MM patients who experience relapse, whereas EM-B occurs in 6%-34% of patients (30, 32). In our study, 7 of 20 R/R MM patients had at least one assessable extramedullary disease. Although in previous studies, the incidence of EM-B was higher than that of EM-E, the incidence of EM-E was higher in our study. This might be because some EM-B patients received radiotherapy and were ineligible for this humanized anti-BCMA CAR T cell clinical trial.
There are currently no guidelines regarding treatment for EMM. The outcomes of patients with EMM are different from those of MM without extramedullary disease, even those who receive the same therapy (9). Therefore, the prognosis of primary and secondary EMM is very poor (30). A study on the different prognoses of EMM patients showed that the OS of patients with EM-E (5 months) was poorer than that of patients with EM-B (12 months) (32). In another retrospective series on secondary EMM, the OS were 13.6 months for EM-E and 39.8 months for EM-B (33). In our study, the mean OS of patients with EMM was 17.29 ± 4.95 months. Whether humanized anti-BCMA CAR T-cell therapy has a better efficacy in R/R MM patients with extramedullary disease and prolongs the OS requires further exploration. A recent retrospective study showed that auto-HSCT imparted a survival benefit for patients with both EM-E and EM-B (33). The median PFS from diagnosis was 49 months in EMM patients who received auto-HSCT and 28.1 months in EMM patients who did not receive auto-HSCT. However, few other studies have shown that auto-HSCT could overcome the poor prognosis of EMM. Most studies have consistently shown that the prognosis for EMM is very poor, even among patients who undergo auto-HSCT (30, 34, 35). In our study, two patients (Ptwith 1 and 5) who received auto-HSCT did not benefit from this therapy.
BCMA is a highly selective target for CAR T-cell therapy in patients with R/R MM (36). This therapy has emerged as a novel therapeutic approach with the potential for long-term disease control in R/R MM patients (17–20, 37). In particular, humanized anti-BCMA CAR T cell therapy, which has been approved by the FDA (37), had an ORR of 85% in 33 R/R MM patients. This included 15 patients (45%) who achieved CR. However, 6 of the 15 patients who had CR relapsed. Hematologic toxicity and CRS were the most common AEs. However, very few studies have focused on the evaluation of anti-BCMA CAR T-cell therapy in R/R MM patients with EM-E or EM-B. In Brudno’s study of anti-BCMA CAR T cell therapy, one R/R MM patient with an abdominal mass was reported to be responsive to this therapy (38). Another study reported a clinical trial of a biepitope-targeting CAR against BCMA (LCAR-B38M), in which five R/R MM patients with extramedullary infiltration experienced tumor disappearance after CAR T cell therapy. One R/R MM patient with EM-B showed absence of extramedullary disease and malignant pleural effusion after anti-BCMA CAR T cell therapy in another study (18).
Although there was no difference in the PFS and OS rates in R/R MM patients with and without extramedullary disease at 180 days, the PFS and OS rates in patients with extramedullary disease were lower than those in patients without extramedullary disease at 360 days. Therefore, the long-term efficacy of anti-BCMA CAR T cell therapy in R/R MM patients with extramedullary disease remains unsatisfactory. It is whether these patients benefit from radiotherapy or HSCT before further disease progression. We need to explore this issue further. Another interesting finding is that some patients with extramedullary disease experienced further disease progression, but the level of M protein did not increase. However, this phenomenon was not observed in R/R MM patients without extramedullary disease. In the process of observation after their anti-BCMA CAR-T cell therapy, the examination to R/R MM patients with extramedullary disease should be comprehensive, rather than just observing the changes of M protein levels.
CAR T cell therapy can produce potentially life-threatening toxicities, such as CRS and neurotoxicity (23, 39–41). Close monitoring of the side effects of CAR T cell therapy can help control such complications with prompt and appropriate treatment (42–46). Although there was no difference in the median expansion peak of the humanized anti-BCMA CAR T cells in the two patient groups in our study, the grades of CRS and ICANS in patients with extramedullary disease were higher than those in patients without extramedullary disease among patients who responded to therapy. Further, the serum IL-6 was much higher in patients with extramedullary disease. These results might be related to the tumor burden in patients with extramedullary disease. Therefore, attention should be paid to AEs in R/R MM patients with extramedullary disease receiving anti-BCMA CAR T cell therapy.
The efficacy and safety of humanized anti-BCMA CAR T cell therapy in R/R MM patients with extramedullary disease needs to be explored in the future. In particular, anti-BCMA CAR T cell therapy is used in combination with additional therapies to improve efficacy. We might be able to improve the outcomes of R/R MM patients with extramedullary disease who respond to anti-BCMA CAR T cell therapy by bridging to transplantation or radiotherapy before further disease progression.
Conclusion
The monitoring to R/R MM patients with extramedullary disease after the anti-BCMA CAR-T cell therapy should be comprehensive. Although the grades of CRS and ICANS were much higher in patients with extramedullary disease, the ORR was not different in patients with or without extramedullary disease, and there was no difference in the PFS and OS rates between the two groups at 180 days. These results indicate an opportunity for R/R MM patients with extramedullary disease who respond to anti-BCMA CAR T-cell therapy to bridge to other treatments.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of the Department of Hematology, Tianjin First Center Hospital (Tianjin, China) (Approved No. of ethic committee: 2015002X and 2020N028KY). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
Concept and design: QD and YW. Drafted or revised the manuscript: HD, ML, TY, HZ, XW, and RC. Acquisition of data: HD, JY, and JL. Analysis and interpretation of data: HD and QD. Writing, review, and/or revision of manuscript: HD. Study supervision: QD. All authors contributed to the article and approved the submitted version.
Conflict of Interest
Author JY was employed by the company Shanghai Genbase Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all our patients for their participation in our clinical trials. We thank the Shanghai Genbase Biotechnology Co., Ltd. for providing us with anti-BCMA CAR-T cells.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.720571/full#supplementary-material
References
- 1.Spencer A, Lentzsch S, Weisel K, Avet-Loiseau H, Mark TM, Spicka I, et al. Daratumumab Plus Bortezomib and Dexamethasone Versus Bortezomib and Dexamethasone in Relapsed or Refractory Multiple Myeloma: Updated Analysis of CASTOR. Haematologica (2018) 103(12):2079–87. 10.3324/haematol.2018.194118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, et al. Daratumumab Plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N Engl J Med (2018) 378(6):518–28. 10.1056/NEJMoa1714678 [DOI] [PubMed] [Google Scholar]
- 3.Dimopoulos M, Weisel K, van de Donk N, Ramasamy K, Gamberi B, Streetly M, et al. Pomalidomide Plus Low-Dose Dexamethasone in Patients With Relapsed/Refractory Multiple Myeloma and Renal Impairment: Results From a Phase II Trial. J Clin Oncol (2018) 36(20):2035–43. 10.1200/JCO.2017.76.1742 [DOI] [PubMed] [Google Scholar]
- 4.Avet-Loiseau H, Bahlis NJ, Chng WJ, Masszi T, Viterbo L, Pour L, et al. Ixazomib Significantly Prolongs Progression-Free Survival in High-Risk Relapsed/Refractory Myeloma Patients. Blood (2017) 130(24):2610–8. 10.1182/blood-2017-06-791228 [DOI] [PubMed] [Google Scholar]
- 5.Moreau P. How I Treat Myeloma With New Agents. Blood (2017) 130(13):1507–13. 10.1182/blood-2017-05-743203 [DOI] [PubMed] [Google Scholar]
- 6.Lonial S, Boise LH, Kaufman J. How I Treat High-Risk Myeloma. Blood (2015) 126(13):1536–43. 10.1182/blood-2015-06-653261 [DOI] [PubMed] [Google Scholar]
- 7.Gagelmann N, Eikema DJ, Iacobelli S, Koster L, Nahi H, Stoppa AM, et al. Impact of Extramedullary Disease in Patients With Newly Diagnosed Multiple Myeloma Undergoing Autologous Stem Cell Transplantation: A Study From the Chronic Malignancies Working Party of the EBMT. Haematologica (2018) 103(5):890–7. 10.3324/haematol.2017.178434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinstock M, Ghobrial IM. Extramedullary Multiple Myeloma. Leuk Lymphoma (2013) 54(6):1135–41. 10.3109/10428194.2012.740562 [DOI] [PubMed] [Google Scholar]
- 9.Usmani SZ, Heuck C, Mitchell A, Szymonifka J, Nair B, Hoering A, et al. Extramedullary Disease Portends Poor Prognosis in Multiple Myeloma and Is Over-Represented in High-Risk Disease Even in the Era of Novel Agents. Haematologica (2012) 97(11):1761–7. 10.3324/haematol.2012.065698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhutani M, Foureau DM, Atrash S, Voorhees PM, Usmani SZ. Extramedullary Multiple Myeloma. Leukemia (2020) 34(1):1–20. 10.1038/s41375-019-0660-0 [DOI] [PubMed] [Google Scholar]
- 11.Smith EL, Mailankody S, Staehr M, Wang X, Senechal B, Purdon TJ, et al. BCMA-Targeted CAR T-Cell Therapy Plus Radiotherapy for the Treatment of Refractory Myeloma Reveals Potential Synergy. Cancer Immunol Res (2019) 7(7):1047–53. 10.1158/2326-6066.CIR-18-0551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nijhof IS, van de Donk N, Zweegman S, Lokhorst HM. Current and New Therapeutic Strategies for Relapsed and Refractory Multiple Myeloma: An Update. Drugs (2018) 78(1):19–37. 10.1007/s40265-017-0841-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh A, Mailankody S, Giralt SA, Landgren CO, Smith EL, Brentjens RJ. CAR T Cell Therapy for Multiple Myeloma: Where are We Now and Where are We Headed? Leuk Lymphoma (2018) 59(9):2056–67. 10.1080/10428194.2017.1393668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonneveld P. Management of Multiple Myeloma in the Relapsed/Refractory Patient. Hematol Am Soc Hematol Educ Program (2017) 2017(1):508–17. 10.1182/asheducation-2017.1.508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latif A, Kapoor V, Sipra Q, Malik SU, Bilal J, Bin Riaz I, et al. Disease Milestones Through Bibliometric Analysis of the Top 100 Cited Articles in Multiple Myeloma. Cureus (2018) 10(4):e2438. 10.7759/cureus.2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosen N. Chimeric Antigen Receptor T-Cell Therapy for Multiple Myeloma. Cancers (2019) 11(12):2024. 10.3390/cancers11122024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-Cell Therapy Bb2121 in Relapsed or Refractory Multiple Myeloma. N Engl J Med (2019) 380(18):1726–37. 10.1056/NEJMoa1817226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, et al. B Cell Maturation Antigen-Specific CAR T Cells are Clinically Active in Multiple Myeloma. J Clin Invest (2019) 129(6):2210–21. 10.1172/JCI126397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adaniya SPS, Cohen AD, Garfall AL. Chimeric Antigen Receptor T Cell Immunotherapy for Multiple Myeloma: A Review of Current Data and Potential Clinical Applications. Am J Hematol (2019) 94:S28–33. 10.1002/ajh.25428 [DOI] [PubMed] [Google Scholar]
- 20.Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, et al. T Cells Expressing an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Multiple Myeloma. Blood (2016) 128(13):1688–700. 10.1182/blood-2016-04-711903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group Updated Criteria for the Diagnosis of Multiple Myeloma. Lancet Oncol (2014) 15(12):e538–48. 10.1016/S1470-2045(14)70442-5 [DOI] [PubMed] [Google Scholar]
- 22.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current Concepts in the Diagnosis and Management of Cytokine Release Syndrome. Blood (2014) 124(2):188–95. 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated With Immune Effector Cells. Biol Blood Marrow Tr (2019) 25(4):625–38. 10.1016/j.bbmt.2018.12.758 [DOI] [PubMed] [Google Scholar]
- 24.Rollig C, Knop S, Bornhauser M. Multiple Myeloma. Lancet (2015) 385(9983):2197–208. 10.1016/S0140-6736(14)60493-1 [DOI] [PubMed] [Google Scholar]
- 25.Terpos E, Rezvani K, Basu S, Milne AE, Rose PE, Scott GL, et al. Plasmacytoma Relapses in the Absence of Systemic Progression Post-High-Dose Therapy for Multiple Myeloma. Eur J Haematol (2005) 75(5):376–83. 10.1111/j.1600-0609.2005.00531.x [DOI] [PubMed] [Google Scholar]
- 26.Alegre A, Granda A, Martinez-Chamorro C, Diaz-Mediavilla J, Martinez R, Garcia-Larana J, et al. Different Patterns of Relapse After Autologous Peripheral Blood Stem Cell Transplantation in Multiple Myeloma: Clinical Results of 280 Cases From the Spanish Registry. Haematologica (2002) 87(6):609–14. 10.3324/%x [DOI] [PubMed] [Google Scholar]
- 27.Oriol A. Multiple Myeloma With Extramedullary Disease. Adv Ther (2011) 28(Suppl 7):1–6. 10.1007/s12325-011-0079-0 [DOI] [PubMed] [Google Scholar]
- 28.Touzeau C, Moreau P. How I Treat Extramedullary Myeloma. Blood (2016) 127(8):971–6. 10.1182/blood-2015-07-635383 [DOI] [PubMed] [Google Scholar]
- 29.Blade J, Fernandez de Larrea C, Rosinol L, Cibeira MT, Jimenez R, Powles R. Soft-Tissue Plasmacytomas in Multiple Myeloma: Incidence, Mechanisms of Extramedullary Spread, and Treatment Approach. J Clin Oncol (2011) 29(28):3805–12. 10.1200/JCO.2011.34.9290 [DOI] [PubMed] [Google Scholar]
- 30.Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, Presenting Features and Outcome of Extramedullary Disease in Multiple Myeloma: A Longitudinal Study on 1003 Consecutive Patients. Ann Oncol (2010) 21(2):325–30. 10.1093/annonc/mdp329 [DOI] [PubMed] [Google Scholar]
- 31.Vande Broek I, Vanderkerken K, Van Camp B, Van Riet I. Extravasation and Homing Mechanisms in Multiple Myeloma. Clin Exp Metastasis (2008) 25(4):325–34. 10.1007/s10585-007-9108-4 [DOI] [PubMed] [Google Scholar]
- 32.Pour L, Sevcikova S, Greslikova H, Kupska R, Majkova P, Zahradova L, et al. Soft-Tissue Extramedullary Multiple Myeloma Prognosis Is Significantly Worse in Comparison to Bone-Related Extramedullary Relapse. Haematologica (2014) 99(2):360–4. 10.3324/haematol.2013.094409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beksac M, Seval GC, Kanellias N, Coriu D, Rosinol L, Ozet G, et al. A Real World Multicenter Retrospective Study on Extramedullary Disease From Balkan Myeloma Study Group and Barcelona University: Analysis of Parameters That Improve Outcome. Haematologica (2020) 105(1):201–8. 10.3324/haematol.2019.219295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minnema MC, van de Donk NW, Zweegman S, Hegenbart U, Schonland S, Raymakers R, et al. Extramedullary Relapses After Allogeneic Non-Myeloablative Stem Cell Transplantation in Multiple Myeloma Patients do Not Negatively Affect Treatment Outcome. Bone Marrow Transplant (2008) 41(9):779–84. 10.1038/sj.bmt.1705982 [DOI] [PubMed] [Google Scholar]
- 35.Perez-Simon JA, Sureda A, Fernandez-Aviles F, Sampol A, Cabrera JR, Caballero D, et al. Reduced-Intensity Conditioning Allogeneic Transplantation Is Associated With a High Incidence of Extramedullary Relapses in Multiple Myeloma Patients. Leukemia (2006) 20(3):542–5. 10.1038/sj.leu.2404085 [DOI] [PubMed] [Google Scholar]
- 36.Tai YT, Anderson KC. Targeting B-Cell Maturation Antigen in Multiple Myeloma. Immunotherapy (2015) 7(11):1187–99. 10.2217/imt.15.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho SF, Anderson KC, Tai YT. BCMA CAR T-Cell Therapy Arrives for Multiple Myeloma: A Reality. Ann Transl Med (2018) 6(Suppl 2):S93. 10.21037/atm.2018.11.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brudno JN, Maric I, Hartman SD, Rose JJ, Wang M, Lam N, et al. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J Clin Oncol (2018) 36(22):2267–80. 10.1200/JCO.2018.77.8084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brudno JN, Kochenderfer JN. Toxicities of Chimeric Antigen Receptor T Cells: Recognition and Management. Blood (2016) 127(26):3321–30. 10.1182/blood-2016-04-703751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu D, Zhao J. Cytokine Release Syndrome: Grading, Modeling, and New Therapy. J Hematol Oncol (2018) 11(1):121. 10.1186/s13045-018-0653-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z, Han W. Biomarkers of Cytokine Release Syndrome and Neurotoxicity Related to CAR-T Cell Therapy. Biomark Res (2018) 6:4. 10.1186/s40364-018-0116-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and Toxicity Management of 19-28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Sci Transl Med (2014) 6(224):224ra25. 10.1126/scitranslmed.3008226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neill L, Rees J, Roddie C. Neurotoxicity-CAR T-Cell Therapy: What the Neurologist Needs to Know. Pract Neurol (2020) 20(4):285–93. 10.1136/practneurol-2020-002550 [DOI] [PubMed] [Google Scholar]
- 44.Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric Antigen Receptor T-Cell Therapy - Assessment and Management of Toxicities. Nat Rev Clin Oncol (2018) 15(1):47–62. 10.1038/nrclinonc.2017.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mei H, Jiang H, Wu Y, Guo T, Xia L, Jin R, et al. Neurological Toxicities and Coagulation Disorders in the Cytokine Release Syndrome During CAR-T Therapy. Br J Haematol (2018) 181(5):689–92. 10.1111/bjh.14680 [DOI] [PubMed] [Google Scholar]
- 46.Brudno JN, Kochenderfer JN. Recent Advances in CAR T-Cell Toxicity: Mechanisms, Manifestations and Management. Blood Rev (2019) 34:45–55. 10.1016/j.blre.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.