Graphical Abstract
Background
It is not uncommon for paradoxical results in science to be unappreciated. Perturbations in the orbit of the planet Mercury with no observation of a nearby planet were paradoxical under Newtonian mechanics and later explained by the theory of relativity (1). The reduction in the incidence of scurvy with the ingestion of citrus fruit was ignored for many years under the dominant theory that putrefaction caused scurvy (2–4). As noted by the philosopher Willam James (5), ‘Round about the accredited and orderly facts of every science there ever floats a sort of dust-cloud of exceptional observations, … when they come as mere marvels and oddities rather than as things of serious moment, one neglects or denies them with the best of scientific consciences.’ Rather than neglecting paradoxical results, researches should leverage them to make progress.
Paradoxes under the somatic mutation theory
The dominant somatic mutation theory (SMT) of tumorigenesis says that accumulated mutations in a founder cell result in a tumor with those mutations. In recent years, there has been a growing appreciation of paradoxical results that challenge SMT (6–10). Some of the most notable paradoxical results under SMT are the following.
(i) A chemical not known to damage genes causes cancer (11), a phenomenon not possible under SMT.
(ii) Filter implants with small pore sizes induced sarcomas while filter implants with large pore sizes did not (12), a result with no obvious SMT explanation.
(iii) In two experiments involving deletions of genes in transgenic mice (13,14), the tumors lacked the deletion, contradicting the SMT requirement that the inducing mutation appear in the tumor.
(iv) Childhood neuroblastoma can regress to benign ganglioneuroma or fibrosis (15), a phenomenon that should not occur under SMT which assumes mutations are permanent.
(v) The experiment of Maffini et al. (16) in which a carcinogen applied to stromal tissue yielded cancer in epithelial tissue, contradicting the SMT implication that a mutation in stromal tissue would only lead to a tumor in the stromal tissue.
(vi) Infection caused by parasitic flatworms are strongly associated with bladder cancer (17), an epidemiological observation with no clear SMT explanation.
(vii) Injection of Scharlach R dye and olive oil into rabbit years yielded tissue alterations similar to cancer that were later replaced by normal tissue (18), violating the SMT requirement that mutations yield permanent changes.
Alternative theories of tumorigenesis
There are over 20 published theories of tumorigenesis besides SMT (7). (Here the term ‘theory’ encompasses the names of theories called ‘hypothesis’ or ‘paradigm’.) Some alternative theories to SMT focus only a single paradoxical result. The following alternative theories of tumorigenesis can explain multiple paradoxical results:
(i) The tissue organization field theory postulates that carcinogenesis occurs at the tissue level, the default state of all cells is proliferation, and abnormal interactions between the mesenchyme/stroma and the parenchyma of a morphogenic field lead to tumors (9,10,19).
(ii) The detached pericyte hypothesis (7,20) postulates the following events in tumorigenesis. A carcinogen or chronic inflammation causes pericytes to detach from blood vessel cell walls. Some detached pericytes form myofibroblasts which alter the extracellular matrix (ECM). Other detached pericytes develop into mesenchymal stem cells that adhere to the altered ECM. The altered ECM blocks normal regulatory signals causing the adhered mesenchymal stem cells to develop into a tumor.
(iii) The Brücher–Jamall paradigm (8) postulates the following events in carcinogenesis. A pathogenic stimulus leads to chronic inflammation, fibrosis and changes in the cellular microenvironment which lead to a pre-cancerous niche. The pre-cancerous niche triggers a chronic stress escape strategy whose failure to resolve causes normal cells to transition to cancer cells.
(iv) The cell reversal theory (21) postulates that a perturbation on the cell or its microenvironment reverses differentiated cells into a non-differentiated stem-like state, that, without the strict control mechanisms of a normal stem cell niche, initiates a tumor.
These four theories share the common theme that tumors arise from the disruption of regulatory controls due to alterations in surrounding tissue, and genetic instability is a byproduct of tumorigenesis. The theories differ in the postulated mechanism for how the alterations in the surrounding tissue lead to tumor development. It is important not to combine theories (e.g. SMT and tissue organization field theory) into a hybrid theory with special cases to explain each challenge to SMT. A vague theory that is difficult to disprove has little value (22).
Alternative explanations of evidence supporting SMT
To be credible, alternative hypotheses of tumorigenesis need to explain evidence supporting SMT. Perhaps the strongest evidence supporting SMT are experiments showing that mice with induced mutations develop cancer, suggesting that mutations act directly on the tissue that develops into a tumor. However, a plausible alternative explanation (under the detached pericyte hypothesis) is that mutations alter the ECM, which indirectly leads to a tumor. Another plausible explanation (under an expanded view of the tissue organization field theory) is that mutations alter the morphogenic field, which indirectly leads to a tumor. Support for this view comes from transgenic mouse studies where the tumor cells lacked the inducing mutation (13,14).
Other strong evidence supporting SMT are hereditary cancers. The hereditary disease Xeroderma pigmentosum involves defects in DNA nucleotide excision repair that protects against sunlight DNA damage. The fact that Xeroderma pigmentosum also confers a 1000-fold increase in susceptibility to skin cancer (23) strongly supports SMT. However, Cockayne syndrome also involves defects in the DNA nucleotide excision repair that protects against sunlight DNA damage, but with normal skin cancer risk (23). One possible non-SMT explanation (under the detached pericyte hypothesis) is that Xeroderma pigmentosum is associated with actinic keratosis (24) and there is no evidence of actinic keratosis in patients with Cockayne syndrome (25). Actinic keratosis may be a marker for changes in ECM (7).
The case for a diversified tumorigenesis research strategy
There is a need for a diversified tumorigenesis research strategy which continues SMT research but also studies paradoxical results in the framework of alternative theories of tumorigenesis. To understand the value of a diversified tumorigenesis research strategy, consider the following analogy involving the search for buried treated treasure hidden on an island. Searchers for the treasure (understanding tumorigenesis) can either follow a well-known treasure map A (SMT) or a less well-known treasure map B (alternative theories of tumorigenesis, each corresponding to a different location on the map) (26). Paradigm instability says that the more you dig at the map A location without finding treasure, (i) the more you want to continue digging at the map A location because you think you are close to the treasure, and (ii) the more you want to start some digging at the map B locations because of doubts about the correctness of map A. The diversification strategy recommends splitting efforts between digging based on map A (studies guided by the SMT) and digging on the same island based on map B (studies guided by alternative theories of tumorigenesis).
Consider a recent challenge to SMT, namely the large accumulation of genetic mutations in healthy aging tissue. A standard research strategy involves digging at the map A location to ‘better delineate’ genetic mutations in healthy aging tissue versus cancer (27). A diversified research strategy involves additional digging at the various map B locations to also experimentally investigate the alternative theories of tumorigenesis, which do not postulate driver mutations.
A cancer paradox initiative
A cancer paradox initiative would support researchers studying paradoxical findings under SMT. For example, investigators may try to replicate the 1915 experiment involving the injection of Scharlach R and olive oil into rabbit years that yielded cancer-like changes that regressed (18). Investigators could study changes in the stroma cells as well as changes the epithelial tissue over time. They could look for changes in ECM, alterations in morphogenic fields, detached pericytes or evidence of differentiated cells reversing to an undifferentiated state.
As a prerequisite to investigating cancer paradoxes, investigators could also investigate specific aspects of alternative theories of tumorigenesis. For example, a proposed experiment to investigate the detached pericyte hypothesis involved randomizing lineage-tracing mice to a carcinogen or no carcinogen and then looking for evidence of pericyte detachment, migration and transformation.
Potential implications for cancer prevention
Alternative theories of tumorigenesis that arise from the cancer paradox initiative could have important implications for cancer prevention. For example, to reduce cancer incidence, the detached pericyte hypothesis and the Brücher–Jamall paradigm would suggest a greater focus on chemoprevention agents to reduce fibrosis. In fact, recent experimental evidence suggests that the reason metformin, a drug primarily used to lower glucose levels, reduces cancer incidence is that it reduces fibrosis (28). A better understanding of tumorigenesis could lead to more focused targets for chemoprevention.
Conclusion
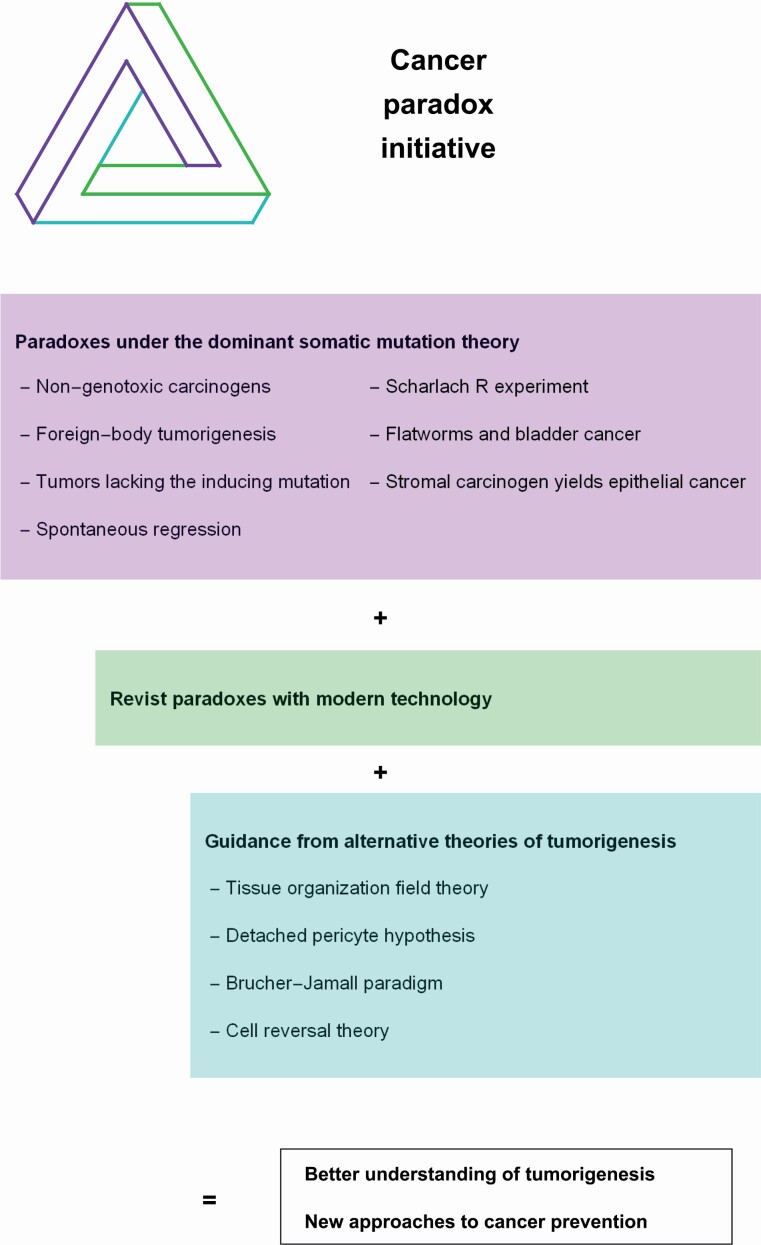
This article makes the case for private or government funding of a cancer paradox initiative. The three rotational parts of the ‘impossible’ Penrose triangle (29,30) in the graphical abstract symbolize the three components of a cancer paradox initiative. The first component consists of the various experimental and observational paradoxes under the SMT. Besides the seven paradoxes listed here, there are many additional paradoxes (31–34). These paradoxes indicate serious problems with the SMT that suggest opportunities for new research. The second component of the cancer paradox initiative is revisiting the paradoxes using modern technology. For example, investigators may wish to revisit some puzzling experiments using live imaging visualization, which had been used to study melanoma initiation (35). The third component stresses the importance of guiding investigations based on alternative theories of tumorigenesis. Simply collecting new facts is insufficient. As Henri Poincare (36) elegantly wrote ‘Science is built up of facts, as a house is built of stones; but an accumulation of facts is no more a science than a heap of stones is a house.’ Investigators need a theoretical framework to understand experimental results and make predictions that can be tested. Importantly, a cancer paradox initiative will not only improve scientific understanding of tumorigenesis but would likely lead to new strategies for cancer prevention.
Acknowledgements
The opinions presented should not be viewed as official opinions or positions of the National Cancer Institute.
Funding
S.G.B. is an employee of the National Cancer Institute.
Conflict of Interest Statement: None declared.
References
- 1.Tatarewicz, J. (1997) Rise and eclipse of an elusive planet. Nature, 388, 435. [Google Scholar]
- 2.Raizman, N. (2004) Scurvy: how a surgeon, a mariner, and a gentleman solved the greatest medical mystery of the age of sail. J. Clin. Invest., 114, 1690. [Google Scholar]
- 3.Tansey, T. (2016) Medical research: Mariners’ malady. Nature, 540, 338. [Google Scholar]
- 4.Harrison, M. (2013) Scurvy on sea and land: political economy and natural history, c. 1780–c. 1850. J. Marit. Res., 15, 7–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James, W. (1890) The hidden self. Scribners, 7, 361–373. [Google Scholar]
- 6.Baker, S.G. (2015) A cancer theory kerfuffle can lead to new lines of research. J. Natl. Cancer Inst., 107, dju405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker, S.G. (2018) The detached pericyte hypothesis: a novel explanation for many puzzling aspects of tumorigenesis. Org. J. Biol. Sci., 2, 25–41. [Google Scholar]
- 8.Brücher, B.L., et al. (2014) Epistemology of the origin of cancer: a new paradigm. BMC Cancer, 14, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonnenschein, C., et al. (2020) Over a century of cancer research: inconvenient truths and promising leads. PLoS Biol., 18, e3000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soto, A.M., et al. (2011) The tissue organization field theory of cancer: a testable replacement for the somatic mutation theory. Bioessays, 33, 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mally, A., et al. (2002) Non-genotoxic carcinogens: early effects on gap junctions, cell proliferation and apoptosis in the rat. Toxicology, 180, 233–248. [DOI] [PubMed] [Google Scholar]
- 12.Karp, R.D., et al. (1973) Tumorigenesis by Millipore filters in mice: histology and ultrastructure of tissue reactions as related to pore size. J. Natl. Cancer Inst., 51, 1275–1285. [DOI] [PubMed] [Google Scholar]
- 13.Raaijmakers, M.H., et al. (2010) Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature, 464, 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu, B., et al. (2012) Multifocal epithelial tumors and field cancerization from loss of mesenchymal CSL signaling. Cell, 149, 1207–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas, D., et al. (1988) Complete pathologic maturation and regression of stage IVS neuroblastoma without treatment. Cancer, 62, 818–825. [DOI] [PubMed] [Google Scholar]
- 16.Maffini, M.V., et al. (2004) The stroma as a crucial target in rat mammary gland carcinogenesis. J. Cell Sci., 117, 1495–1502. [DOI] [PubMed] [Google Scholar]
- 17.Palumbo, E. (2007) Association between schistosomiasis and cancer: a review. Infect. Dis. Clin. Pract. 15, 145–148. [Google Scholar]
- 18.Bullock, F.D., et al. (1915) A study of the Scharlach R reaction and of allied forms of epithelial proliferation. J. Med. Res., 33, 53–92.13. [PMC free article] [PubMed] [Google Scholar]
- 19.Sonnenschein, C., et al. (2016) Carcinogenesis explained within the context of a theory of organisms. Prog. Biophys. Mol. Biol., 122, 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker, S.G. (2020) Rethinking carcinogenesis: the detached pericyte hypothesis. Med. Hypotheses, 144, 110056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carvalho, J. (2020) Cell reversal from a differentiated to a stem-like state at cancer initiation. Front. Oncol., 10, 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feymann, R. (1964) Richard Feynman on the Scientific Method (1964), from the Character of Physical Law. Messenger Lectures Cornell University. https://www.youtube.com/watch?v=0KmimDq4cSU (21 June 2021, date last accessed) [Google Scholar]
- 23.Kraemer, K.H., et al. (2007) Xeroderma pigmentosum, trichothiodystrophy and Cockayne syndrome: a complex genotype-phenotype relationship. Neuroscience, 145, 1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuchs, A., et al. (2007) The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol. Surg., 33, 1099–1101. [DOI] [PubMed] [Google Scholar]
- 25.Nance, M.A., et al. (1992) Cockayne syndrome: review of 140 cases. Am. J. Med. Genet., 42, 68–84. [DOI] [PubMed] [Google Scholar]
- 26.Baker, S.G., et al. (2010) Research on early-stage carcinogenesis: are we approaching paradigm instability? J. Clin. Oncol., 28, 3215–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiala, C., et al. (2020) Mutations in normal tissues—some diagnostic and clinical implications. BMC Med., 18, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shankaraiah, R.C., et al. (2019) Metformin prevents liver tumourigenesis by attenuating fibrosis in a transgenic mouse model of hepatocellular carcinoma. Oncogene, 38, 7035–7045. [DOI] [PubMed] [Google Scholar]
- 29.Penrose, R., et al. (1958) Impossible objects: a special type of visual illusion. Br. J. Psychol., 49, 31–33. [DOI] [PubMed] [Google Scholar]
- 30.Tunyan, K. (2020) Possible adventures in impossible figures. Steam J., 4, 10. [Google Scholar]
- 31.Baker, S.G., et al. (2007) Paradoxes in carcinogenesis: new opportunities for research directions. BMC Cancer, 7, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker, S.G. (2012) Paradoxes in carcinogenesis should spur new avenues of research: an historical perspective. Disrupt. Sci. Technol., 1, 100–107. [Google Scholar]
- 33.Baker, S.G. (2012) Paradox-driven cancer research. Disrupt. Sci. Technol., 1, 143–148. [Google Scholar]
- 34.Baker, S.G. (2014) Recognizing paradigm instability in theories of carcinogenesis. BJMMR, 4, 1149–1163. [Google Scholar]
- 35.Kaufman, C.K., et al. (2016) A zebrafish melanoma model reveals emergence of neural crest identity during melanoma initiation. Science, 351, aad2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poincare, H. (1902) Science and Hypothesis. Walter Scott Publishing, London, UK. [Google Scholar]