Introduction
Diabetes in children and adolescents is now acknowledged to be a complex disorder with heterogeneity in its etiology, pathogenesis, clinical presentation, and outcomes. The majority of pediatric diabetes is classified into one of two broad categories according to physiologic framework established by the American Diabetes Association (ADA) in 1997:1 type 1 diabetes (T1D), an absolute deficiency of insulin usually due to autoimmune destruction of the beta cells, and type 2 diabetes (T2D), a combination of insulin resistance and relative insulin deficiency.2,3 Until recently, diabetes diagnosed in children and adolescents was almost entirely considered to be T1D, but T2D has recently emerged among youth with obesity and in high risk ethnic populations.4,5 Although there are other forms of diabetes that affect youth, including genetic defects of beta-cell function, genetic defects of insulin action, and a variety of secondary forms of diabetes3,6, this chapter focuses on the most common forms of pediatric diabetes: T1D and T2D in youth.
Presentation and Classifications of Diabetes in Youth
Categories of diabetes in youth are distinguished by characteristic clinical presentations, summarized in Table 1.
Table 1:
Classical Presentation of Diabetes in Youth
| Type 1 diabetes | T2D | Monogenic diabetes | |
|---|---|---|---|
| Age at onset or presentation | Bimodal; Age 4–6 years, Age 10–14 years | Post-pubertal | Before age 25 years |
| Autoantibodies | Present | Absent | Absent |
| Weight status | Usually normal weight, increasing prevalence of overweight or obesity | Overweight or obesity common (>90%) | Usually normal weight, increasing prevalence of overweight or obesity |
| Insulin resistance | Less common | Present | Absent |
| Risk of Diabetic Ketoacidosis | High | Low | Low |
| Endogenous insulin production (C-peptide level) | Low | Detectable | Detectable |
| Family history of diabetes | Infrequent (10–15%) | Frequent (90%) | Frequent, usually multiple generations |
Autoantibodies include for GADA, IA-2A, ZnT8-A Monogenic diabetes includes neonatal diabetes and mitochondrial diabetes arising from single-gene mutations in HNF1-alpha, HNF4-alpha or glucokinase. Adapted from Shah AS, Nadeau KJ. The changing face of paediatric diabetes. Diabetologia. 2020 Apr;63(4):683–691; with permission.
Type 1 diabetes
T1D is caused by immune-mediated β-cell destruction leading to insulin deficiency. Symptoms appear when approximately 90% of pancreatic β-cells are destroyed,7 are usually rapid in onset, and include polyuria, polydipsia, weight loss, abdominal symptoms, headaches, and ketoacidosis. Insulin is necessary for survival.8 The autoimmune destruction of the β-cells is mediated by T-cells and accompanied by the formation of autoantibodies such as those against the 65KD isoform of glutamic acid decarboxylase (GADA), those against the Zinc Transporter 8 (Zn-T8A), insulinoma-associated-2 antibodies (IA-2), insulin autoantibodies (IAA), and islet cell autoantibodies (ICA). These antibodies are present prior to the appearance of clinical disease and predict disease development.9–11 The presence of each antibody at diagnosis varies with age of onset, race/ethnicity and sex,12–14 but one or more autoantibodies are typically present at diagnosis with T1D in 80–90% of affected children.8
Type 2 diabetes
The current view is that T2D in youth is primarily characterized by insulin resistance detected at the level of skeletal muscle, liver, and adipose tissues with a failure of β-cell compensation and a relative insulin deficiency,.8 However, the exact sequence of metabolic changes leading to dysglycemia and ultimately youth-onset T2D remains unknown. For example, increasing evidence support the possibility, at least in subsets of individuals at risk of T2D, that hyper-responsiveness of the islet β-cell to a hostile environment drives hyperinsulinemia; this may be the ‘upstream’ culprit of excessive weight gain, insulin resistance, subsequent β-cell failure and the development of T2D .{Nolan, 2019 #1499}{Malone, 2019 #1500} While the extent to which children progress through stages of obesity, insulin resistance, and glucose intolerance to T2D is not fully understood, it appears that the pathway to disease and the clinical evolution of the disease, once diagnosed, are much shorter and less predictable in children than in adults, comprising a different and more aggressive pathophysiologic process.15 For example, the TODAY trial found that youth with T2D showed a more rapid decline in insulin sensitivity and beta cell function compared to adults, with decreased response to metformin and rosiglitazone treatment.16
Pediatric patients with T2D are overweight or obese (BMI ≥85th percentile for age and sex), and comorbidities such as hypertension and dyslipidemia can be present at diagnosis. Often there is a strong family history in first- and second-degree family members. Weight loss at diagnosis is less common than in T1D, and acanthosis nigricans is frequently identified on physical examination. Patients usually present with evidence of residual β-cell function, although no standardized cut-offs exist for insulin or C-peptide levels. These patients typically lack evidence of autoimmunity. Ketosis is less common than in T1D as individuals with T2D usually produce enough insulin secretion to prevent lipolysis. Insulin may or may not be required at diagnosis or for long-term treatment of hyperglycemia, but insulin is typically not required for survival.8
Epidemiology
Type 1 diabetes
Approximately 98,200 children under the age of 15 years and 128,900 youth under the age of 20 years are estimated to develop T1D annually worldwide.17 The incidence and prevalence of T1D varies greatly between different countries, and within countries, between different ethnic populations (Figure 1).18,19 An increase in incidence of T1D has been observed globally in recent decades, with a disproportionately greater increase in those under the age of 5 years20,21 and in developing countries or those undergoing recent economic transition.20,22 There is also variation in temporal trends in global incidence of T1D in youth, with evidence for a plateau in incidence in some countries in recent years, as well as cyclical trends.18 Most recent data on incidence per 100,000 population per year showed that Finland (62.3), Sweden (43.2) and Kuwait (41.7) have the highest incidence rates of T1D among youth 0–14 years.23 In Asia, the incidence of T1D is very low; for example ~2 per 100,000 person-years in Japan;24 3.1 per 100,000 in China;25 and ~5 per 100,000 in Taiwan.26 In the United States, nationally-representative data from the SEARCH for Diabetes in Youth study showed that the incidence of T1D is increasing,5 with the highest rates of increase among Hispanic white youth compared to non-Hispanic white youth (4.2% vs. 1.2%).5
Figure 1: Age-sex standardized incidence rates (per 100,000 population per annum) of T1D in children and adolescents aged 0–14 years.
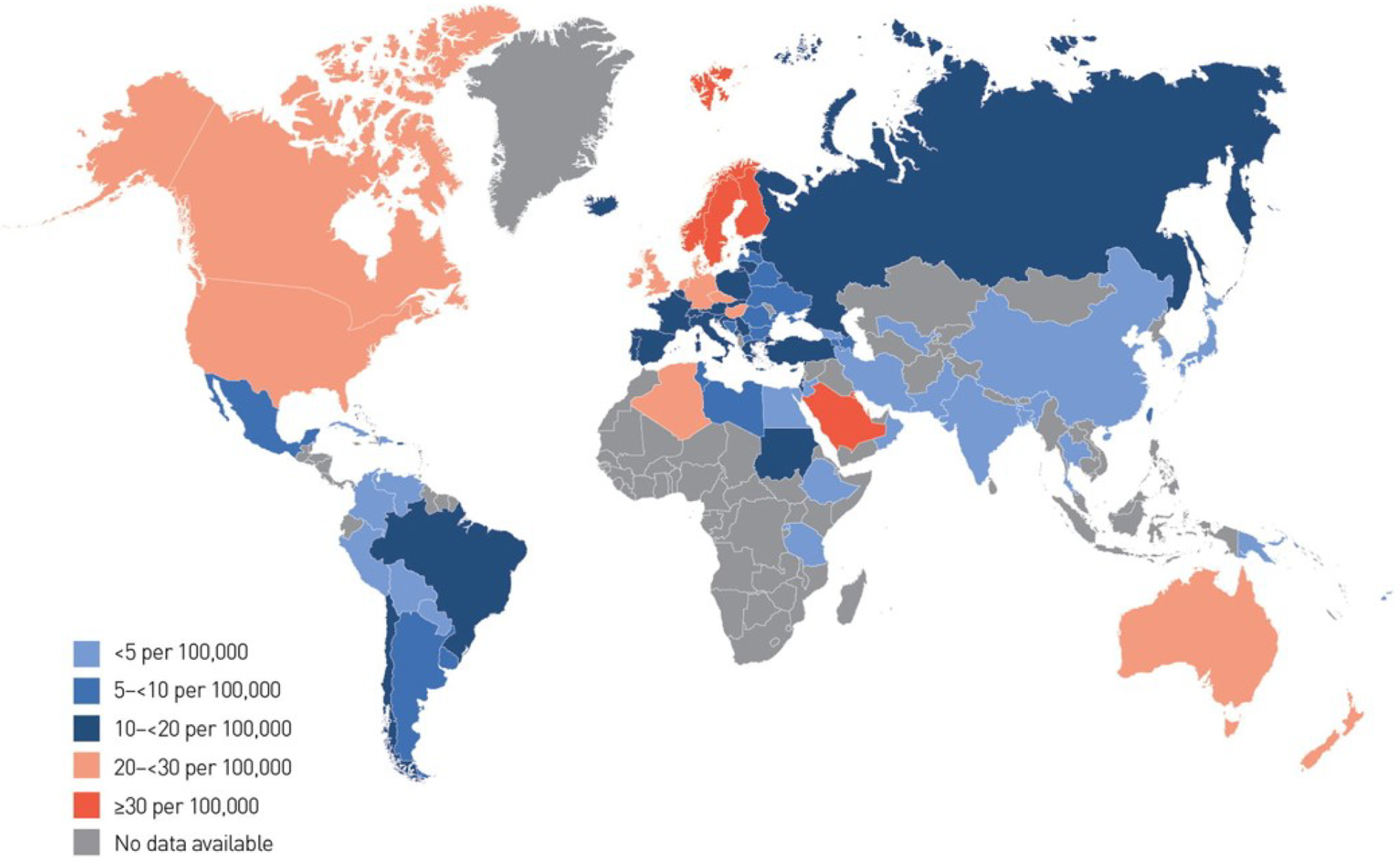
Estimates are directly standardized where possible, with countries shaded according to their rate.
From Patterson CC, Karuranga S, Salpea P, et al. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Diabetes Atlas. Diabetes research and clinical practice. 2019;157:107842; with permission.
Data on the incidence of T1D in low- and middle-income countries (LMIC) are limited. Available estimates are highly variable across the globe but suggest a temporal increase since 1990, particularly among younger age ranges (i.e. 0–4 years).27 In particular, data are sparse from African and South East Asian countries; the need for enhanced epidemiologic surveys and research in these and other LMIC has been emphasized.17,27
By way of prevalence, there are an estimated 600,900 children under 15 years and 1,110,1000 youth under 20 years living with T1D worldwide.17 Over a quarter of the prevalent cases occur in Europe, followed by 20% on the North American continent.17 In most western countries, T1D accounts for over 90% of childhood and adolescent diabetes, while across the lifespan, T1D accounts for 5–10% of individuals with diabetes.18 The International Diabetes Federation offers an interactive resource which displays T1D estimates in children and adolescents and offers data for download, including incidence and prevalence estimates from countries worldwide.23,28 Within the US, the prevalence of T1D was shown to be highest among white youth and lowest in American Indian youth, with prevalence rates of 2.55 per 1000 (95% CI, 2.48–2.62) versus 0.35 per 1000 (95% CI, 0.26–0.47), respectively.29
Looking ahead, the EURODIAB study projected that between 2005 and 2020, the number of new cases with T1D in European children aged less than 5 years will double, and the prevalence in those aged ≤14 years old will increase by 70%.30 In the U.S alone, projections based on SEARCH data suggest that number of youth with T1D age < 20 years may increase by 23% by year 2050, even if incidence of T1D remains stable, with even greater increases is incidence continues to rise.31 As above, one challenge to estimating global incidence and prevalence rates is that nationwide, population-based prospective registries are typically only conducted in well-resourced countries, limiting representation from lower resources settings.17
Type 2 diabetes
Compared to childhood T1D, population based epidemiological data are more limited18; therefore, the burden of T2D in youth is more difficult to estimate. Estimations are further challenged by the relative rarity and the limited standard clinical and epidemiological definitions.19 Despite this, evidence from the past 20 years strongly suggests that T2D is becoming more common32 particularly in high-risk indigenous populations.33,34
Epidemiologic estimates of the incidence of T2D in children and adolescents have ranged from 1–51/1000.4 Increasing incidence rates for T2D in pediatric patients have been reported in the US, Canada, Japan, Austria, United Kingdom and Germany.35–37 In the US, the SEARCH study, one of the few population-based studies of childhood T2D that exist, estimated that the annual number of newly diagnosed youth with T2D is approximately 3,700.38 Other studies estimate that T2D may now account for 20% to 50% of new-onset diabetes cases in specific pediatric populations within the U.S.39,40
Although worldwide incidence T2D in children and adolescents vary substantially among countries, age categories and ethnic groups,41 incidence appears to be consistently highest youth of nonwhite origin and other indigenous populations.5,42 Within the U.S., the SEARCH study has provided comprehensive estimates of incidence of pediatric T2D in all major racial ethnic groups, showing that in 2002–2005, the rates of T2D (per 100,000 per year) were the highest among American Indian youth (20.5 and 35.3 for ages 10–14 and 15–19 years, respectively), followed by African-American (20.8 and 17.0 respectively), Asian and Pacific Islanders (11.6 and 12.6) and Hispanic youth (11.2 and 12.0), and were low (3.3 and 4.1) among non-Hispanic Whites.38 Of note, the highest incidence rates of (screen-detected) T2D in youth were reported by the Pima Indian study: 330 per 100,000 per year.43 Data from LMIC are extremely limited, particularly with regard to African, South American, and Asian countries.41
Figure 2 summarizes global prevalence estimates of T2D in youth. Overall, prevalence patterns mirror incidence patterns, where T2D appears to affect disproportionately minority racial/ethnic groups, especially African Americans, South and East Asians, Pacific Island Natives, and American Indians/First Nation peoples.44–49 In the US, SEARCH estimated that 19,147 children/youth in the U.S. had T2D in 2009, with the highest prevalence among American Indian and African American youth.50 Gaps in prevalence data to represent LMIC mirror those for the incidence data, where estimates from these countries remain limited to date.41
Figure 2: Global Burden of T2D in youth.
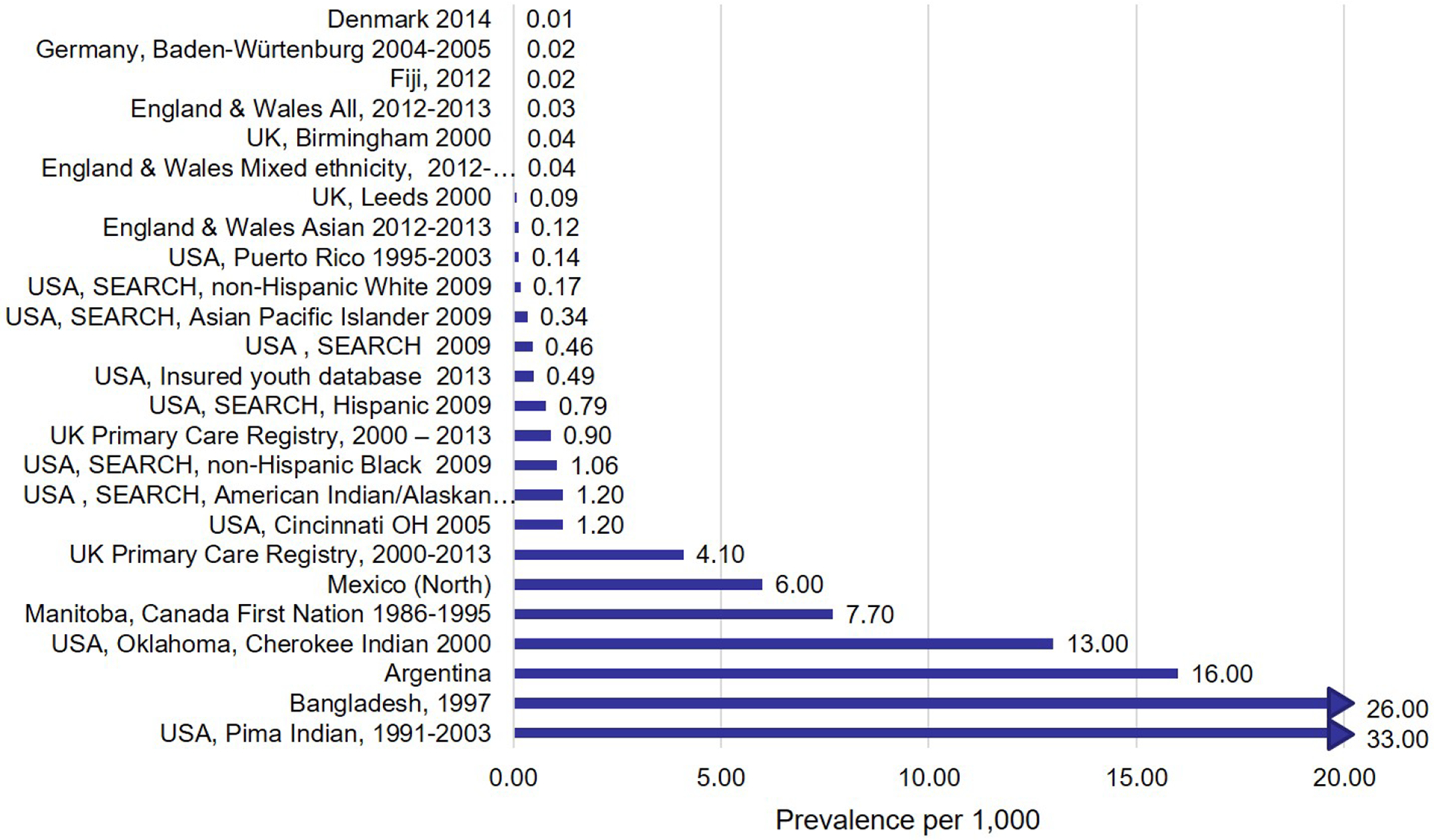
.
SEARCH data projects that by year 2050, even at current incidence rates, the number of youth with T2D may increase by almost 50%, while if the incidence of T2D increases, there may be more than a fourfold increase in the number of youth with T2D.31 Worldwide, with increasing levels of obesity and physical inactivity among children and adolescents in many countries, T2D in childhood and adolescence has the potential to become a global public health issue.23
Etiology and Risk Factors
Type 1 diabetes
It is accepted that the etiology of T1D is multifactorial; however, the specific roles for the immune system, genetic susceptibility, and environmental factors in the pathogenic processes underlying T1D remain unclear. Diabetes-associated autoantibodies, which are serological markers of β-cell autoimmunity, include GAD, IA2, IAA and ZnT8.51 Genetic susceptibility plays a large role in T1D with the HLA genotypes (DR and DQ genes) explaining approximately 40–50% of T1D risk.52 The remaining genetic risk for T1D can be attributed to the other non-HLA genes or loci that are involved in, or contribute to, immune regulation in the pancreatic β-cells.53,54 However, studies exploring potential temporal changes in the frequency and/or distribution of HLA genotypes associated with T1D susceptibility found a decreasing frequency of high-risk HLA genotypes over time in individuals diagnosed with T1D,55–61 which may suggest an increasing role for environmental factors in etiology.7
Several environmental factors have been implicated in the disease etiology and are thought to operate through a variety of mechanisms, including triggering an autoimmune response, overloading the β-cells and promoting apoptosis, or, as proposed more recently, through alterations in the intestinal microbiome (Table 2). While the exact environmental triggers (infective, nutritional and/or chemical) which initiate pancreatic β-cell destruction remain largely unknown, the process usually begins months to years before the manifestation of clinical symptoms.62–64 Notably, the “hygiene hypothesis” proposes that the decreasing early life exposure to infectious agents in Westernized societies has led to an impairment in the maturation of the immune system, thus permitting an increased occurrence of immune-mediated disorders including T1D.65 The accelerator66 and the overload hypotheses67 both propose that environmental risk factors prevalent in contemporary societies may accelerate the onset of T1D to affect younger children by increasing the demand for insulin production and thus overloading the β-cells. Novel insights may soon be available from longitudinal, collaborative efforts such as The Environmental Determinants of Diabetes in the Young (TEDDY) study, that follow children at risk for T1D from birth onwards.68
Table 2: Risk factors and key evidence for the development of T1D and T2D in youth; information.
| Diabetes Type | Risk Factor | Summary of evidence |
|---|---|---|
| Type 1 diabetes | Genetics | Genetic susceptibility is determined by multiple genes. HLA genotype confers approximately 30–50% of risk. The remaining genetic risk for T1D can be attributed to the other non-HLA genes or loci identified that contribute model to small effects on disease risk. |
| Viral infections, childhood immunizations, ad early life immune exposure to infectious agents | Viral infections may trigger autoimmunity and accelerate the autoimmune destruction of β-cells in genetically susceptible individuals, including enterovirus infection during pregnancy, infancy, childhood, and adulthood, although prospective data remains mixed. Data for other viruses are limited. Large population-based studies have found no associations between childhood immunizations and the development of T1D. | |
| Early life diet | Breastfeeding and early exposure to cows’ milk have been extensively studied, suggesting both a protective effect of breastfeeding, as well as little or no association. Positive associations between early exposure to solid foods, such as cereals or gluten-containing foods, and risk of T1D have been reported. Overfeeding early in life might lead to accelerated weight gain, resulting in β-cell overload and failure. | |
| Early life growth | US and European cohort studies have shown that children who later developed T1D have faster growth trajectories or weight gain in the first years of life. Some studies have demonstrated an inverse association between age at T1D diagnosis and childhood BMI. | |
| Type 2 diabetes | Genetics | There is often strong family history among affected youth, specific genetic factors have yet to be deterministically identified. |
| Obesity, diet, and physical activity | As in adults,124–128 the recent rise in T2D in youth is believed to have paralleled the increasing prevalence of overweight worldwide. Obesity is linked to increased fast food or consumption of high sugar/high fat diets, with concurrent decrease in physical activity levels. | |
| Socioeconomic status | As in adults, youth with T2D are more likely to be from lower socioeconomic backgrounds, which may reflect higher prevalence of obesity. | |
| Early life factors | There is a U- or J-shaped relationship between birth weight and adult obesity and metabolic disease, demonstrating that both a nutritionally limited or excessive in utero environment can lead to postnatal obesity and T2D later in life. Exposure to maternal diabetes in utero is a significant risk factor for obesity, impaired glucose tolerance and T2D in youth. However, breastfeeding appears to be protective against later development obesity and T2D. | |
| Endocrine Disrupting chemicals | There is moderate evidence for a relationship between exposure to dichlorodiphenyldichloroethylene (p,p′-DDE) and diabetes development.129 Data from humans on other EDCs, such as bisphenol A, phthalates and perfluorinated chemicals, are limited, as are studies among youth specifically. |
Adapted from Dabelea D, Hamman RF, Knowler WC. Chapter 15: Diabetes in Youth. In: Cowie CC, Casagrande SS, Menke A et al., eds. Diabetes in America. 3rd ed. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (US); 2018; with permission.
Type 2 diabetes
The etiology of T2D includes contribution by genetic and physiologic components, lifestyle factors such as excess energy intake, insufficient physical activity, and increased sedentary behavior;4 the resulting pathogenesis of T2D is variable between individuals.69 The current view is that peripheral insulin resistance is a key feature that occurs early in the disease course, and initially is compensated by increased insulin secretion reflected in hyperinsulinemia.69 Sustained hyperglycemia over time results in beta cell exhaustion and declining insulin secretion and glucose toxicity. However, T2D is a heterogeneous disease, as recently reported by studies of adult-onset diabetes from Scandinavia, where distinct pathophysiologic subgroups were identified by cluster analysis,{Ahlqvist, 2018 #1501} including one characterized by obesity, severe hyperinsulinemia and insulin resistance and another by severe insulin-deficiency. Similar research in youth-onset T2D is, at best, limited, but urgently needed, to build foundational knowledge regarding the sequence of metabolic abnormalities leading to obesity, dysglycemia and T2D, which is the necessary first step for the development of efficient, targeted and precise prevention and treatment approaches.
Although many studies show a strong family history among affected youth, with 45–80% having at least one parent with diabetes and 74–100% having a first or second degree relative with T2D,33 there are very limited data on genes associated with early-onset T2D.70 However, family history does not always imply a genetic cause, as factors such as similar environmental influences within families and the effects of the intrauterine environment on the offspring also demand consideration, as described in Table 2.
Management of Diabetes in Youth
There are comprehensive pediatric- and adolescent-specific guidelines for care.15 As in adults, the management of both T1D and T2D is centered around keeping blood glucose levels in near-normal ranges to delay or prevent the development of cardiovascular disease risk factors and diabetes-related complications.15 Medical standards of care also emphasize individualized care considering patient factors and preferences in the selection of clinical goals and approaches, with special attention to integrating culturally-sensitive and developmentally-appropriate self-management education and tools.15 It is recommended that a new diagnosis of diabetes in youth prompt screening for comorbidities, including celiac disease and thyroid disease among youth with T1D and cardiovascular disease risk factors or components of metabolic syndrome among youth with T2D.15 Youth should also undergo screening for subclinical or early complications of diabetes,15,71 with prompt intervention upon positive findings,72 including psychosocial well-being.15,73
In this age range, diabetes care providers must work closely with youth and their families to foster supportive home and school74 environments for self-management.15 Additionally, towards the end of adolescence, pediatric care providers should work with adult care providers to facilitate the transition from pediatric to adult care, which typically occurs in early adulthood and represents a critical period for other major life transitions and shifting responsibilities of diabetes care.15
Type 1 diabetes
T1D self-management regime includes monitoring blood glucose, dosing insulin, measuring and regulating carbohydrates, and responding to episodes of hypoglycemia with appropriate intake of rapid-acting carbohydrate.75 Blood glucose monitoring can be accomplished with frequent blood glucose checks or use of newly-developed continuous glucose monitoring systems (CGM),76 which have shown considerable uptake in recent years particularly among pediatric populations.77 In a departure from previous years, the updated 2021 American Diabetes Association Standards of Care now recommend that CGM be considered in all children and adolescents with T1D given evidence for an association between CGM use, adherence, and clinical outcomes.78
For insulin replacement, care guidelines recommend intensive insulin therapy that consists of multiple-dose insulin injections (3–4 injections/day of basal and prandial insulin) or insulin pump therapy.15,79 In recent years, a number of novel diabetes devices and technologies have emerged,80 including sensor-augmented pump therapy ranging from low-glucose suspend, to predictive low-glucose suspend, to hybrid closed loop systems81 these technologies have shown benefit in pediatric and adolescent populations with regards to improved glycemic control with decreased hypogycemia.82–86 It is likely that these technologic advances in the diabetes treatment landscape, and particularly the fully-automated insulin delivery systems, will transform day-to-day diabetes management in coming years by decreasing the burden of glucose monitoring and insulin dosing.
Optimal nutrition and monitoring of carbohydrate intake is an important component of the recommended treatment plan for youth with diabetes,15,76 although literature on specific diets for T1D, including low and very low carbohydrate diets, is mixed and lacking in rigorous, randomized studies. It is also recommended that youth and adolescents with T1D engage in regular physical activity after receiving appropriate education on blood glucose management strategies to avoid exercise-related glucose disturbances.15,87 Finally, non-insulin adjuvants have recently been evaluated in combination with insulin to improve glycemic control in the setting of T1D, including amylin analogues, metformin, sodium–glucose cotransporter-2 inhibitors, and glucagon-like peptide-1 receptor agonists.88
Specific to the management of pediatric T1D versus the majority of adult cases is the careful individualization of HbA1c targets to avoid hypoglycemia, which may be more common in this age range due to erratic patterns in exercise and food intake. In generally, an HbA1c goal of <7% is appropriate for many children, but less stringent targets (i.e. HbA1c <7.5) are appropriate for those with frequent hypoglycemia, hypoglycemia unawareness, inability to access or use blood glucose monitoring technology regularly. In general, lower targets may be appropriate for youth if they do not lead to more frequent hypoglycemia or during the honeymoon period shortly after diagnosis.15
Given the rising burden of childhood diabetes in LMIC, recent clinical practice guidelines have provided additional references for diabetes care in low-resource settings. There are unique challenges to diabetes management in these settings, including high out-of-pocket expenses which may be cost-prohibitive, limited availability of insulin and other diabetes supplies, suboptimal home management equipment, food scarcity, and social deprivation and discrimination or stigma.89 International programs such as Life for a Child, Changing Diabetes in Children, and Insulin for Life have focused on providing critical resources for diabetes management to youth with T1D in LMIC. Other efforts have developed framework for T1D management including insulin therapy, blood glucose monitoring HbA1c testing complications screening, diabetes education, and multidisciplinary team care according to the tiers of resources that are available.90
Type 2 diabetes
As with T1D, comprehensive diabetes self-management education and support is central to providing clinical care among new-onset T2D in childhood as well as longstanding disease.15 Long-term weight management is also central to the management of pediatric T2D, which can be achieved through healthy diet and regular physical activity, which comprise first-line approaches in this pouation.15,91 As in youth without diabetes, best-practices for weight management emphasize selection of weight-oriented strategies that are tailored to the individual child and family, including dietary goals, physical activity, the home environment, and other self-management behaviors.92 Compared to T1D, where all youth must initiate insulin replacement therapy as soon as possible following diagnosis, pharmacologic management of T2D varies according to clinical features and includes metformin, liraglutide, and insulin treatment.15,91,93 Care providers should refer to the algorithm depicted in the most recent ADA consensus guidelines for an approach to new-onset diabetes in youth with overweight and obesity.15 Evidence for best pharmacologic practices remain limited and has focused on metformin. Metabolic surgery is indicated for the treatment of T2D in adolescents with BMI >35 kg/m2, uncontrolled glycemia, and/or significant comorbidities after treatment with other lifestyle and pharmacologic interventions.15 Limited evidence suggests benefits associated with bariatric surgery94 but more randomized control trials are need, particularly among adolescents and young adults with T2D.15 In addition, there may be a role for technological advances such as CGM, insulin pumps, and automated insulin delivery systems, particularly among advanced T2D requiring aggressive glycemic control; the need for these treatment modalities will likely vary case-by-case.
Outcomes and Complications of Diabetes
Glycemic Control
Youth with diabetes show variable degrees of suboptimal glycemic control as measured by HbA1c, which can vary considerably after onset of disease through puberty and into early adulthood. In the US-based SEARCH study, 17% of type 1 youth had HbA1c ≥ 9.5%), as did 27% of type 2 youth.95 Poor glycemic control was associated with increasing age, as well as longer duration of for both diabetes types.95 Over the course of development, data from youth with T1D demonstrate elevated HbA1c levels that peak to >9.0% in 17-year-olds and remain elevated >8.0% until a mean age of 30 years.96 Poorer glycemic control during early adulthood or from childhood to young adulthood has been attributed to a lack of continuity in diabetes-related clinical care as well as changes in self-care as children and adolescents with T1D grow into adulthood. Longitudinal data from pediatric T2D are limited.97–99 Unfortunately, there is a substantial body of evidence of health inequity that affects glycemic control outcomes, including significant race-based disparities.100,101 A number of sociodemographic risk-factors including lower socioeconomic status, lower parental educational attainment, less parental involvement in diabetes management, and impaired family dynamics have also been identified.
While some evidence suggest that glycemic control may be improving over the past one to three decades in the US, Europe, and Australia,102–104 other studies have shown no change across 19 countries105 and even increases in mean HbA1c in the most recent US-based clinical T1D Exchange Registry.106 Altogether, these data suggest that HbA1c remains unacceptably high among youth, which increases the risk for complications as they age into adulthood, discussed below.
In general, clinical outcomes are known to be consistently worse among LMIC, particularly where essential resources and diabetes supplies may be limited.90,107,108 These differences are most starkly represented by higher mortality rates compared to well-resourced countries,109 but data also suggest poorer glycemic control and more frequent complications.17,110,90
Complications
Sustained hyperglycemia in diabetes is linked to the development of chronic complications of the disease, which represent the major source of morbidity and mortality. The benefits of intensive insulin therapy for the prevention of long-term microvascular and microvascular complications of T1D were demonstrated by the Diabetes Control and Complications Trial (DCCT),111,112 with persistent benefit over 30 years later.113,114 In youth and adolescents, multiple studies have corroborated that the risk for these outcomes is associated with glycemic control.19
Data on both acute and chronic complications among youth with T1D and have been comprehensively reviewed and discussed extensively elsewhere,19 including microvascular complications (i.e. diabetic retinopathy, nephropathy, peripheral neuropathy, and cardiac autonomic neuropathy) and cardiovascular disease risk factors or markers of subclinical cardiovascular disease (i.e. hypertension, dyslipidemia, obesity and insulin resistance, arterial stiffness, carotid intima medial thickness, and coronary artery calcification). In short, diabetes diagnosed in childhood and adolescence remains a leading cause of nephropathy, retinopathy, neuropathy, and coronary and peripheral vascular disease later in life; it is now recognized that these complications emerge early in disease duration114 and often co-occur.115,116 Figure 3 shows a subset of major diabetes complications in the US youth and adolescents by diabetes type.
Figure 3: Age-adjusted prevalence of complications in youth-onset diabetes by type.
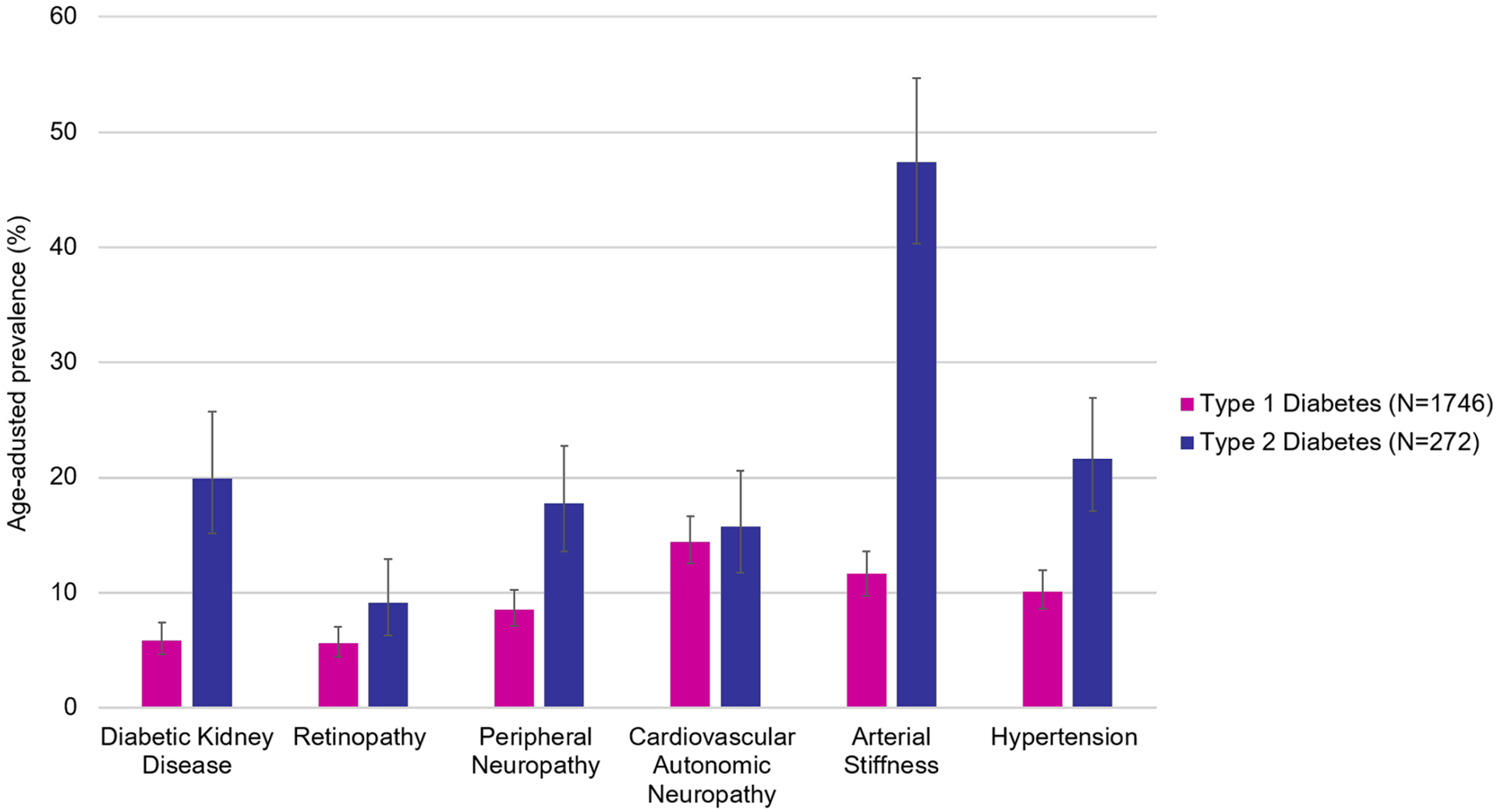
Data from Dabelea D, Stafford JM, Mayer-Davis EJ, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. Jama. 2017;317(8):825–835.
In children and adolescents with T1D, acute complications such as diabetic ketoacidosis (DKA) and hypoglycemia are more common than chronic complications and carry a greater risk of morbidity and mortality, particularly severe hypoglycemia.117 More recently, early and subclinical cardiovascular disease has emerged as a major clinical concern as the prevalence of overweight and obesity grows within this patient population.118 Although less common than in T1D, youth with T2D can also present in DKA, with reported frequencies from 8–29%.19 Youth with T2D appear to have consistently and significantly higher prevalence of comorbidities than their peers with T1D (Figure 3).114 As above, the prevalence of complications seem to be higher among LMIC, owing to suboptimal glycemic control resulting from inadequate or unstable resources for diabetes management.90 Finally, emerging data from the COVID-19 pandemic suggest that infection with SARS-CoV-2 virus may be associated with an increase in diabetic ketoacidosis in both adult and pediatric populations,119–121 although more studies are needed to untangle to mechanistic framework for this association.
Mortality
Mortality rates are approximately 2–5 times higher for youth with T1D compared to the general population, secondary to acute complications;117 these data have been reviewed and discussed19 Mortality rates appear to be higher for females than males and for non-white versus white youth, although overall rates may be improving over time.19 An analysis by World Bank income groups from the IDF Diabetes Atlas showed that although the majority of T1D cases occur in the high-income and upper-middle-income countries, the majority of deaths are in the low-income and lower-middle-income countries.17 Data on mortality among youth with T2D are comparatively quite limited but appear to be similarly inflated 2–3 times higher than the general population and related to length of disease duration.19
Challenges and Opportunities
There exist opportunities to better characterize diabetes in youth and transform knowledge to evidence-based interventions to prevent and diagnose the disease earlier or improve management and outcomes to prevent complications. First, more robust data are needed on the epidemiology of T1D and T2D in youth across the globe, particularly in lower resource settings. The initiation of sustainable surveillance systems for pediatric diabetes will be maximally informative if they are designed as population-based rather than clinic-based efforts and incorporate tools to differentiate childhood T1D from T2D. By way of etiology and risk factors, data from novel, large-scale prospective efforts such as the TEDDY Study are expected to yield insights into whether or how recent changes in exposures to such risk factors may be responsible for the steady increase in T1D worldwide.68 Longitudinal pre-birth cohort studies may yield insights into T2D in particular.19 Finally, a growing number of genetic data may also help to elucidate high risk markers to identity at-risk youth for both types of diabetes screening. As the field continues to inform on the role of environmental exposures, their biologic signatures ad pathways, the causal associations or mechanisms that are identified may represent robust targets for novel interventions to prevent or delay the onset of childhood diabetes.
In the meantime, new treatment options, including diabetes devices for T1D and non-insulin pharmacologic options for T2D, are currently under study among youth and adolescents and offer potential to reduce the incidence and prevalence of complications. It is critical that changes to treatment based on these new data are made accessible and equitable to youth around the world, including those in low-resource or underserved locations.
Changes in epidemiology underscore that as there are more youth are diagnosed with T1D or T2D, particularly early in life, these populations will have a longer duration of exposure to an altered metabolic milieu, increasing the risk of acute complications, chronic micro- and macrovascular complications and other comorbidities. In particular, the development of elevated levels of cardiovascular risk factors and pre-clinical cardiovascular disease among youth with diabetes and the potential future impact on morbidity and mortality pose special challenges. Contemporary large scale translational and epidemiological studies in youth with diabetes to understand these outcomes are lacking, primarily due to the lack of common standardized protocols and validated surrogate endpoints that can be compared across studies. Future studies are needed urgently, in addition to the development and validation of the effects of prevention programs on the development and burden of complications and mortality.
There exist significant health disparities in pediatric diabetes, made apparent from global mortality differences in lower-middle-income countries versus upper-middle-income countries, as well as racial/ethnic differences within the specific countries. Interventions to address disparities will be central to improving outcomes among children and adolescents with diabetes within specific geographic populations and across the globe, requiring collaboration from multiple clinical and community stakeholders to be effective.
Finally, from a global healthcare system perspective, the increasing prevalence of diabetes in youth, coupled with the need for high-quality disease management may place a large demand on healthcare costs.8,122 While increasing understanding of the multifactorial etiology of childhood diabetes and its complications will hopefully translate into improved strategies for care or prevention of these disease, the future impact of childhood diabetes should not be underestimated; for this reason, the conduction and translation of high-quality research in this field should be prioritized to meet and address the clinical and public health challenges related to the growing population of youth with diabetes.
Summary.
The incidence and prevalence of T1D and T2D are rising worldwide, along with a significant burden of complications, which appear to emerge earlier than previously believed. More population-based, prospective cohorts are needed to accurately characterize incidence, prevalence and trends in pediatric diabetes burden, short- and long-term morbidity and mortality across the world.
New data may yield insights into etiologic risk factors for both T1D and T2D; both diseases are multifactorial with contribution from genetics as well as environmental or lifestyle factors.
New treatment modalities are emerging, particularly for T1D, which may aid in glycemic management and decrease risk for acute and chronic complications of diabetes.
Clinics Care Points.
Diabetes in youth necessitates early and aggressive intervention to prevent morbidity and mortality associated with both acute and chronic complications. Research is underway to identify and offer approaches to intervene on risk factors for the disease and complications.
The clinical care of diabetes must be tailored to an individual child or adolescent, including goals and modalities of treatment, such that it is sustainable, developmentally appropriate, and culturally sensitive. Ensuring that individuals have access to vital diabetes supplies is essentially, particularly in low resource settings common in LMIC.
Care must also be considered in the context of where the child spends time (i.e. home, school), integrate other individuals who will share diabetes responsibility or have influence on key behavioral aspects of management, and anticipate the transition from pediatric to adult care providers in early adulthood.
Key Points.
Although diabetes in youth was previously thought to be primarily type 1 diabetes, the incidence of type 2 diabetes in rising among youth and has now become a major form of pediatric diabetes.
The incidence of both type 1 and type 2 diabetes is rising, with disproportional increases among non-white youth in the United States. Incidence estimates from low- and middle-income countries are based on limited data, but suggest increasing incidence worldwide.
While the etiology of pediatric diabetes is complex, prospective cohort studies may soon reveal novel insights into genetic and environmental risk factors for both type 1 and type 2 diabetes, as well as differences in pathophysiology.
Management of diabetes in youth is centered around maintaining glycemic control to prevent acute and chronic complications with rapidly emerging new technologic and pharmacologic treatment modalities.
Diabetes in youth is a serious disease, and tight glycemic control is difficult to attain over childhood and into young adulthood. Unfortunately, diabetes diagnosed in childhood and adolescence remains a leading cause of leading cause of nephropathy, retinopathy, neuropathy, and coronary and peripheral vascular disease later in life; it is now recognized that these complications emerge early in disease course.
Synopsis.
Diabetes is a common disease among pediatric populations in the US and worldwide. The incidence of type 1 and type 2 diabetes is rising, with disproportional increases in racial/ethnic subpopulations. As the prevalence of obesity continue to rise, type 2 diabetes now represents a major form of pediatric diabetes. Management of diabetes in youth centers on maintaining glycemic control to prevent acute and chronic complications. This chapter summarizes the epidemiology, etiology, management, and complications of type 1 and type 2 diabetes in youth, as well as future directions and opportunities.
Disclosure statement:
ARK is supported by the National Institute of Diabetes And Digestive and Kidney Diseases of the National Institutes of Health under Award Number F30DK113728. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. ARK has received financial support from Novo Nordisk for travel to present data in 2019.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Association AD. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–1197. [DOI] [PubMed] [Google Scholar]
- 2.Association AD. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S14. [DOI] [PubMed] [Google Scholar]
- 3.Mayer-Davis EJ, Kahkoska AR, Jefferies C, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatric diabetes. 2018;19:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pulgaron ER, Delamater AM. Obesity and type 2 diabetes in children: epidemiology and treatment. Current diabetes reports. 2014;14(8):508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. New England Journal of Medicine. 2017;376(15):1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hattersley AT, Greeley SA, Polak M, et al. ISPAD Clinical Practice Consensus Guidelines 2018: The diagnosis and management of monogenic diabetes in children and adolescents. 2018. [DOI] [PubMed]
- 7.Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes care. 2015;38(10):1964–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Association AD. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2013;36(Supplement 1):S67–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker JM, Barriga KJ, Yu L, et al. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab. 2004;89(8):3896–3902. [DOI] [PubMed] [Google Scholar]
- 10.Wasserfall CH, Atkinson MA. Autoantibody markers for the diagnosis and prediction of type 1 diabetes. Autoimmun Rev. 2006;5(6):424–428. [DOI] [PubMed] [Google Scholar]
- 11.Yu L, Boulware DC, Beam CA, et al. Zinc transporter-8 autoantibodies improve prediction of type 1 diabetes in relatives positive for the standard biochemical autoantibodies. Diabetes Care. 2012;35(6):1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Autoantibodies in Diabetes. Diabetes. 2005;54(suppl_2):S52–S61. [DOI] [PubMed] [Google Scholar]
- 13.Graham J, Hagopian WA, Kockum I, et al. Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes. 2002;51(5):1346–1355. [DOI] [PubMed] [Google Scholar]
- 14.Vermeulen I, Weets I, Asanghanwa M, et al. Contribution of Antibodies Against IA-2 and Zinc Transporter 8 to Classification of Diabetes Diagnosed Under 40 Years of Age. Diabetes Care. 2011;34(8):1760–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Association AD. 13. Children and Adolescents: Standards of Medical Care in Diabetes- 2020. Diabetes Care. 2020;43(Supplement 1):S163–S182. [DOI] [PubMed] [Google Scholar]
- 16.Narasimhan S, Weinstock RS. Youth-onset type 2 diabetes mellitus: lessons learned from the TODAY study. Paper presented at: Mayo Clinic Proceedings; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patterson CC, Karuranga S, Salpea P, et al. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Diabetes Atlas. Diabetes research and clinical practice. 2019;157:107842. [DOI] [PubMed] [Google Scholar]
- 18.Mayer-Davis EJ, Kahkoska AR, Jefferies C, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatric diabetes. 2018;19:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dabelea D, Hamman RF, Knowler WC. Diabetes in Youth. In: Cowie CC, Casagrande SS, Menke A, Cissell MA, Eberhardt MS, Meigs JB, Gregg EW, Knowler WC, Barrett-Connor E, Becker DJ, Brancati FL, Boyko EJ, Herman WH, Howard BV, Narayan KMV, Rewers M, Fradkin JE, . Diabetes in America. 3rd ed. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (US); 2018August. CHAPTER 15. PMID: 33651555.. [PubMed] [Google Scholar]
- 20.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G, Group ES. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–2033. [DOI] [PubMed] [Google Scholar]
- 21.Gyurus EK, Patterson C, Soltesz G. Twenty-one years of prospective incidence of childhood type 1 diabetes in Hungary--the rising trend continues (or peaks and highlands?). Pediatric Diabetes. 2012;13(1):21–25. [DOI] [PubMed] [Google Scholar]
- 22.Sipetic S, Maksimovic J, Vlajinac H, et al. Rising incidence of type 1 diabetes in Belgrade children aged 0–14 years in the period from 1982 to 2005. Journal of endocrinological investigation. 2013;36(5):307–312. [DOI] [PubMed] [Google Scholar]
- 23.Federation. ID, IDF Diabetes Atlas teB, https://www.diabetesatlas.org BAa.
- 24.Tajima N, Morimoto A. Epidemiology of childhood diabetes mellitus in Japan. Pediatr Endocrinol Rev. 2012;10 Suppl1:44–50. [PubMed] [Google Scholar]
- 25.Zhao Z, Sun C, Wang C, et al. Rapidly rising incidence of childhood type 1 diabetes in Chinese population: epidemiology in Shanghai during 1997–2011. Acta Diabetol. 2014. [DOI] [PubMed] [Google Scholar]
- 26.Lin WH, Wang MC, Wang WM, et al. Incidence of and mortality from Type I diabetes in Taiwan from 1999 through 2010: a nationwide cohort study. PLoS One. 2014;9(1):e86172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adeloye D, Chan KY, Thorley N, et al. Global and regional estimates of the morbidity due to type I diabetes among children aged 0–4 years: a systematic review and analysis. Journal of global health. 2018;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Federation ID. Diabetes Data Portal IDF Diabetes Atlas; 9th Edition2019. [Google Scholar]
- 29.Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. Jama. 2014;311(17):1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G, Group atES. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–2033. [DOI] [PubMed] [Google Scholar]
- 31.Imperatore G, Boyle JP, Thompson TJ, et al. Projections of Type 1 and Type 2 Diabetes Burden in the U.S. Population Aged <20 Years Through 2050: Dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35(12):2515–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeitler P, Fu J, Tandon N, et al. Type 2 diabetes in the child and adolescent. Pediatr Diabetes. 2014;in press. [DOI] [PubMed] [Google Scholar]
- 33.Dabelea D, Pettitt DJ, Jones KL, Arslanian SA. Type 2 diabetes mellitus in minority children and adolescents. An emerging problem. Endocrinol Metab Clin North Am. 1999;28(4):709–729. [DOI] [PubMed] [Google Scholar]
- 34.Kitagawa T, Mano T, Fujita H. The epidemiology of childhood diabetes mellitus in Tokyo metropolitan area. Tohoku J exp Med. 1983;141 Suppl:171–9:171–179. [DOI] [PubMed] [Google Scholar]
- 35.Reinehr T 2 diabetes mellitus in children and adolescents. World journal of diabetes. 2013;4(6):270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nature reviews endocrinology. 2012;8(4):228. [DOI] [PubMed] [Google Scholar]
- 37.Cizza G, Brown R, Rothe K. Rising incidence and challenges of childhood diabetes. A mini review. Journal of endocrinological investigation. 2012;35(5):541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Group TWGftSfDiYS. Incidence of Diabetes in Youth in the United States. JAMA: The Journal of the American Medical Association. 2007;297(24):2716–2724. [DOI] [PubMed] [Google Scholar]
- 39.Hannon TS, Rao G, Arslanian SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics. 2005;116(2):473–480. [DOI] [PubMed] [Google Scholar]
- 40.Program DiCAWGotNDE. An Update on Type 2 Diabetes in Youth From the National Diabetes Education Program. Pediatrics. 2004;114(1):259–263. [DOI] [PubMed] [Google Scholar]
- 41.Farsani SF, Van Der Aa M, Van Der Vorst M, Knibbe C, De Boer A. Global trends in the incidence and prevalence of type 2 diabetes in children and adolescents: a systematic review and evaluation of methodological approaches. Diabetologia. 2013;56(7):1471–1488. [DOI] [PubMed] [Google Scholar]
- 42.Fagot-Campagna A, Pettitt D, Engelgau M, et al. Wilн liamson DF, Narayan KM: Type 2 diabetes among North American children and adн olescents: an epidemiologic review and a public health perspective. J Peн diatr. 2000;136:664–672. [DOI] [PubMed] [Google Scholar]
- 43.Pavkov ME, Hanson RL, Knowler WC, Bennett PH, Krakoff J, Nelson RG. Changing patterns of type 2 diabetes incidence among Pima Indians. Diabetes Care. 2007;30(7):1758–1763. [DOI] [PubMed] [Google Scholar]
- 44.Savage PJ, Bennett PH, Senter RG, Miller M. High prevalence of diabetes in young Pima Indians: evidence of phenotypic variation in a genetically isolated population. Diabetes. 1979;28(10):937–942. [DOI] [PubMed] [Google Scholar]
- 45.Dabelea D, Hanson RL, Bennett PH, Roumain J, Knowler WC, Pettitt DJ. Increasing prevalence of Type II diabetes in American Indian children. Diabetologia. 1998;41(8):904–910. [DOI] [PubMed] [Google Scholar]
- 46.Dean H NIDDM-Y in First Nation children in Canada. Clin Pediatr (Phila). 1998;37(2):89–96. [DOI] [PubMed] [Google Scholar]
- 47.Scott CR, Smith JM, Cradock MM, Pihoker C. Characteristics of youth-onset noninsulin-dependent diabetes mellitus and insulin-dependent diabetes mellitus at diagnosis. Pediatrics. 1997;100(1):84–91. [DOI] [PubMed] [Google Scholar]
- 48.Neufeld ND, Raffel LJ, Landon C, Chen YD, Vadheim CM. Early presentation of type 2 diabetes in Mexican-American youth. Diabetes Care. 1998;21(1):80–86. [DOI] [PubMed] [Google Scholar]
- 49.Macaluso CJ, Bauer UE, Deeb LC, et al. Type 2 diabetes mellitus among Florida children and adolescents, 1994 through 1998. Public Health Rep. 2002;117(4):373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamman RF, Pettitt D, Dabelea D, et al. Estimates of the Burden of Diabetes in United States Youth in 2009. Diabetes. 2012;61(Suppl 1):A355. [Google Scholar]
- 51.Watkins RA, Evans-Molina C, Blum JS, Dimeglio LA. Established and emerging biomarkers for the prediction of type 1 diabetes: a systematic review. Transl Res. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pociot F, McDermott MF. Genetics of type 1 diabetes mellitus. Genes Immun. 2002;3(5):235–249. [DOI] [PubMed] [Google Scholar]
- 53.Sepe V, Loviselli A, Bottazzo GF. Genetics of type 1A diabetes. N Engl J Med. 2009;361(2):211. [DOI] [PubMed] [Google Scholar]
- 54.Barrett JC, Clayton DG, Concannon P, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nature genetics. 2009;41(6):703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gillespie KM, Bain SC, Barnett AH, et al. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet. 2004;364(9446):1699–1700. [DOI] [PubMed] [Google Scholar]
- 56.Vehik K, Hamman RF, Lezotte D, et al. Trends in high-risk HLA susceptibility genes among Colorado youth with type 1 diabetes. Diabetes Care. 2008;31(7):1392–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fourlanos S, Varney MD, Tait BD, et al. The rising incidence of type 1 diabetes is accounted for by cases with lower-risk human leukocyte antigen genotypes. Diabetes Care. 2008;31(8):1546–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hermann R, Knip M, Veijola R, et al. Temporal changes in the frequencies of HLA genotypes in patients with Type 1 diabetes--indication of an increased environmental pressure? Diabetologia. 2003;46(3):420–425. [DOI] [PubMed] [Google Scholar]
- 59.Hermann R, Knip M, Veijola R, et al. Temporal changes in the frequencies of HLA genotypes in patients with Type 1 diabetes--indication of an increased environmental pressure? Diabetologia. 2003;46(3):420–425. [DOI] [PubMed] [Google Scholar]
- 60.Gillespie KM, Bain SC, Barnett AH, et al. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet. 2004;364(9446):1699–1700. [DOI] [PubMed] [Google Scholar]
- 61.Fourlanos S, Varney MD, Tait BD, et al. The rising incidence of type 1 diabetes is accounted for by cases with lower-risk human leukocyte antigen genotypes. Diabetes Care. 2008;31(8):1546–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verge CF, Gianani R, Kawasaki E, et al. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996;45(7):926–933. [DOI] [PubMed] [Google Scholar]
- 63.Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial--Type 1. Diabetes Care. 2005;28(5):1068–1076. [DOI] [PubMed] [Google Scholar]
- 64.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309(23):2473–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gale EA. A missing link in the hygiene hypothesis? Diabetologia. 2002;45(4):588–594. [DOI] [PubMed] [Google Scholar]
- 66.Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between Type I and Type II diabetes. Diabetologia. 2001;44(7):914–922. [DOI] [PubMed] [Google Scholar]
- 67.Dahlquist G Can we slow the rising incidence of childhood-onset autoimmune diabetes? The overload hypothesis. Diabetologia. 2006;49(1):20–24. [DOI] [PubMed] [Google Scholar]
- 68.Group TS. The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatric diabetes. 2007;8(5):286–298. [DOI] [PubMed] [Google Scholar]
- 69.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. The Lancet. 2014;383(9922):1068–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dabelea D, Dolan LM, D’Agostino R Jr., et al. Association testing of TCF7L2 polymorphisms with type 2 diabetes in multi-ethnic youth. Diabetologia. 2010;54(3):535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahmud FH, Elbarbary NS, Fröhlich-Reiterer E, et al. ISPAD Clinical Practice Consensus Guidelines 2018: other complications and associated conditions in children and adolescents with type 1 diabetes. Pediatric diabetes. 2018;19(Suppl 27):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donaghue KC, Marcovecchio ML, Wadwa RP, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Microvascular and macrovascular complications in children and adolescents. Pediatric diabetes. 2018;19:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Delamater AM, de Wit M, McDarby V, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Psychological care of children and adolescents with type 1 diabetes. Pediatric diabetes. 2018;19:237–249. [DOI] [PubMed] [Google Scholar]
- 74.Goss P, Middlehurst A, Acerini C, et al. ISPAD Position statement on type 1 diabetes in schools. Pediatric diabetes. 2018;19(7):1338–1341. [DOI] [PubMed] [Google Scholar]
- 75.Hood KK, Peterson CM, Rohan JM, Drotar D. Association between adherence and glycemic control in pediatric type 1 diabetes: a meta-analysis. Pediatrics. 2009;124(6):e1171–1179. [DOI] [PubMed] [Google Scholar]
- 76.Association AD. Professional Practice Committee: Standards of Medical Care in Diabetes—2018. Am Diabetes Assoc; 2018. [DOI] [PubMed] [Google Scholar]
- 77.DeSalvo DJ, Miller KM, Hermann JM, et al. Continuous Glucose Monitoring (CGM) and Glycemic Control Among Youth with Type 1 Diabetes (T1D): International comparison from the T1D Exchange and DPV Initiative. Pediatric Diabetes. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.13. Children and Adolescents: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(Supplement 1):S180–S199. [DOI] [PubMed] [Google Scholar]
- 79.Danne T, Phillip M, Buckingham BA, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Insulin treatment in children and adolescents with diabetes. Pediatric diabetes. 2018;19:115–135. [DOI] [PubMed] [Google Scholar]
- 80.Sherr JL, Tauschmann M, Battelino T, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Diabetes technologies. Pediatric diabetes. 2018;19:302–325. [DOI] [PubMed] [Google Scholar]
- 81.Forlenza GP, Messer LH, Maahs DM, Cherñavvsky DR. Artificial pancreas in pediatrics. The Artificial Pancreas: Elsevier; 2019:237–259. [Google Scholar]
- 82.Haidar A, Legault L, Matteau-Pelletier L, et al. Outpatient overnight glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or conventional insulin pump therapy in children and adolescents with type 1 diabetes: an open-label, randomised controlled trial. The lancet Diabetes & endocrinology. 2015;3(8):595–604. [DOI] [PubMed] [Google Scholar]
- 83.Russell SJ, Hillard MA, Balliro C, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. The lancet Diabetes & endocrinology. 2016;4(3):233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.El-Khatib FH, Balliro C, Hillard MA, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. The Lancet. 2017;389(10067):369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, et al. Safety evaluation of the MiniMed 670G system in children 7–13 years of age with type 1 diabetes. Diabetes technology & therapeutics. 2019;21(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Breton MD, Kanapka LG, Beck RW, et al. A Randomized Trial of Closed-Loop Control in Children with Type 1 Diabetes. New England Journal of Medicine. 2020;383(9):836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adolfsson P, Riddell MC, Taplin CE, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Exercise in children and adolescents with diabetes. Pediatric diabetes. 2018;19:205–226. [DOI] [PubMed] [Google Scholar]
- 88.Harris K, Boland C, Meade L, Battise D. Adjunctive therapy for glucose control in patients with type 1 diabetes. Diabetes, metabolic syndrome and obesity: targets and therapy. 2018;11:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Codner E, Acerini CL, Craig ME, Hofer SE, Maahs DM. ISPAD clinical practice consensus guidelines 2018: limited care guidance appendix. Pediatric diabetes. 2018;19:328–338. [DOI] [PubMed] [Google Scholar]
- 90.Ogle GD, von Oettingen JE, Middlehurst AC, Hanas R, Orchard TJ. Levels of type 1 diabetes care in children and adolescents for countries at varying resource levels. Pediatric diabetes. 2019;20(1):93–98. [DOI] [PubMed] [Google Scholar]
- 91.Zeitler P, Arslanian S, Fu J, et al. ISPAD clinical practice consensus guidelines 2018: type 2 diabetes mellitus in youth. Pediatric diabetes. 2018;19:28–46. [DOI] [PubMed] [Google Scholar]
- 92.Spear BA, Barlow SE, Ervin C, et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Supplement 4):S254–S288. [DOI] [PubMed] [Google Scholar]
- 93.Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and management of youth-onset type 2 diabetes: a position statement by the American Diabetes Association. Diabetes Care. 2018;41(12):2648–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Inge TH, Courcoulas AP, Jenkins TM, et al. Weight loss and health status 3 years after bariatric surgery in adolescents. New England Journal of Medicine. 2016;374(2):113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Petitti DB, Klingensmith GJ, Bell RA, et al. Glycemic control in youth with diabetes: the SEARCH for diabetes in Youth Study. J Pediatr. 2009;155(5):668–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38(6):971–978. [DOI] [PubMed] [Google Scholar]
- 97.Moore SM, Hackworth NJ, Hamilton VE, Northam EP, Cameron FJ. Adolescents with type 1 diabetes: parental perceptions of child health and family functioning and their relationship to adolescent metabolic control. Health and quality of life outcomes. 2013;11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lawrence JM, Standiford DA, Loots B, et al. Prevalence and correlates of depressed mood among youth with diabetes: the SEARCH for Diabetes in Youth study. Pediatrics. 2006;117(4):1348–1358. [DOI] [PubMed] [Google Scholar]
- 99.Pyatak EA, Sequeira P, Peters AL, Montoya L, Weigensberg MJ. Disclosure of psychosocial stressors affecting diabetes care among uninsured young adults with Type 1 diabetes. Diabetic medicine : a journal of the British Diabetic Association. 2013;30(9):1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Petitti DB, Klingensmith GJ, Bell RA, et al. Glycemic control in youth with diabetes: the SEARCH for diabetes in Youth Study. The Journal of pediatrics. 2009;155(5):668–672. e663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kahkoska AR, Shay CM, Crandell J, et al. Association of race and ethnicity with glycemic control and hemoglobin A1c levels in youth with type 1 diabetes. JAMA network open. 2018;1(5):e181851–e181851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Svoren BM, Volkening LK, Butler DA, Moreland EC, Anderson BJ, Laffel LM. Temporal trends in the treatment of pediatric type 1 diabetes and impact on acute outcomes. J Pediatr. 2007;150(3):279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rosenbauer J, Dost A, Karges B, et al. Improved Metabolic Control in Children and Adolescents With Type 1 Diabetes: A trend analysis using prospective multicenter data from Germany and Austria. Diabetes Care. 2012;35(1):80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bulsara MK, Holman CD, Davis EA, Jones TW. The impact of a decade of changing treatment on rates of severe hypoglycemia in a population-based cohort of children with type 1 diabetes. Diabetes Care. 2004;27(10):2293–2298. [DOI] [PubMed] [Google Scholar]
- 105.de Beaufort CE, Swift PG, Skinner CT, et al. Continuing stability of center differences in pediatric diabetes care: do advances in diabetes treatment improve outcome? The Hvidoere Study Group on Childhood Diabetes. Diabetes Care. 2007;30(9):2245–2250. [DOI] [PubMed] [Google Scholar]
- 106.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes technology & therapeutics. 2019;21(2):66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ogle G, Kim H, Middlehurst A, Silink M, Jenkins A. Financial costs for families of children with type 1 diabetes in lower-income countries. Diabetic Medicine. 2016;33(6):820–826. [DOI] [PubMed] [Google Scholar]
- 108.Sidibé A, Traore H, Liman-Ali I, Dembele M, Traore A, Cisse I. Juvenile diabetes in Mali. A 8 year prospective follow up. Rev Fr d’Endocrinologie Clin Nutr Metab [Internet]. 1999;40(6):513–521. [Google Scholar]
- 109.Duarte Gómez E, Gregory GA, Castrati Nostas M, Middlehurst AC, Jenkins AJ, Ogle GD. Incidence and mortality rates and clinical characteristics of type 1 diabetes among children and young adults in Cochabamba, Bolivia. Journal of diabetes research. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pacaud D, Lemay JF, Richmond E, et al. Contribution of SWEET to improve paediatric diabetes care in developing countries. Pediatric diabetes. 2016;17:46–52. [DOI] [PubMed] [Google Scholar]
- 111.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group.NEnglJ Med. 1993;329(14):977–986. [DOI] [PubMed] [Google Scholar]
- 112.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Orchard TJ, Nathan DM, Zinman B, et al. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. Jama. 2015;313(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dabelea D, Stafford JM, Mayer-Davis EJ, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. Jama. 2017;317(8):825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sauder KA, Stafford JM, Mayer-Davis EJ, et al. Co-occurrence of early diabetes-related complications in adolescents and young adults with type 1 diabetes: an observational cohort study. The Lancet Child & Adolescent Health. 2019;3(1):35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kahkoska AR, Nguyen CT, Adair LA, et al. Longitudinal phenotypes of type 1 diabetes in youth based on weight and glycemia and their association with complications. The Journal of Clinical Endocrinology & Metabolism. 2019;104(12):6003–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dahlquist G, Kallen B. Mortality in childhood-onset type 1 diabetes: a population-based study. Diabetes Care. 2005;28(10):2384–2387. [DOI] [PubMed] [Google Scholar]
- 118.Corbin KD, Driscoll KA, Pratley RE, et al. Obesity in type 1 diabetes: pathophysiology, clinical impact, and mechanisms. Endocrine reviews. 2018;39(5):629–663. [DOI] [PubMed] [Google Scholar]
- 119.Kamrath C, Mönkemöller K, Biester T, et al. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID-19 pandemic in Germany. Jama. 2020;324(8):801–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Palermo NE, Sadhu AR, McDonnell ME. Diabetic ketoacidosis in COVID-19: unique concerns and considerations. The Journal of Clinical Endocrinology & Metabolism. 2020;105(8):2819–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reddy PK, Kuchay MS, Mehta Y, Mishra SK. Diabetic ketoacidosis precipitated by COVID-19: a report of two cases and review of literature. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020;14(5):1459–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Herman WH. The Economic Costs of Diabetes: Is It Time for a New Treatment Paradigm? Diabetes Care. 2013;36(4):775–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shah AS, Nadeau KJ. The changing face of paediatric diabetes. Diabetologia. 2020:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ford ES, Williamson DF, Liu SM. Weight change and diabetes incidence - findings from a national cohort of US adults. Am J Epidemiol. 1997;146(3):214–222. [DOI] [PubMed] [Google Scholar]
- 125.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122(7):481–486. [DOI] [PubMed] [Google Scholar]
- 126.Cassano PA, Rosner B, Vokonas PS, Weiss ST. Obesity and body fat distribution in relation to the incidence of non-insulin-dependent diabetes mellitus: A prospective cohort study of men in the Normative Aging Study. Am J Epidemiol. 1992;136:1474–1486. [DOI] [PubMed] [Google Scholar]
- 127.Hazuda HP, Mitchell BD, Haffner SM, Stern MP. Obesity in Mexican American subgroups: findings from the San Antonio Heart Study. Am J Clin Nutr. 1991;53:1529S–1534S. [DOI] [PubMed] [Google Scholar]
- 128.Feskens EJ, Kromhout D. Effects of body fat and its development over a ten-year period on glucose tolerance in euglycaemic men: the Zutphen Study. Int J Epidemiol. 1989;18:368–373. [DOI] [PubMed] [Google Scholar]
- 129.Lind PM, Lind L. Endocrine-disrupting chemicals and risk of diabetes: an evidence-based review. Diabetologia. 2018;61(7):1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]


