Abstract
Objectives: We would like to clarify the imaging findings of the main tumor that may omit the requirement for lymph node dissection in clinical IA (cIA) lung adenocarcinoma.
Methods: A total of 336 patients with cIA lung adenocarcinomas with normal preoperative carcinoembryonic antigen (CEA) who underwent surgical resection were analyzed. We investigated the association between various computed tomography (CT) imaging findings or the maximum standardized uptake value (SUVmax) of fluorodeoxyglucose-position emission tomography (FDG-PET) and lymph node metastasis. The maximum tumor diameter was calculated from the CT images using both the lung window setting (LD) and mediastinal window setting (MD). The diameter of the solid component (CD) was defined as consolidation diameter in lung window setting. The solid component ratio (C/T) was defined as CD/LD.
Results: SUVmax, MD, and C/T were independent factors related to lymph node metastasis, but CD was not (p = 0.38). The conditions required for the positive predictive value (PPV) to reach 100% were 10.6 mm for MD, 12.5 mm for CD, and 0.55 for C/T. SUVmax did not reach 100%.
Conclusions: In cIA lung adenocarcinoma with CEA in the normal range, we found that it may be possible for lymph node dissection to be omitted by MD, CD, and C/T.
Keywords: lung adenocarcinoma, lymph node metastasis, cIA, mediastinal window setting, invasive size
Introduction
Lobectomy and lymph node dissection are considered the standard curative surgery for lung cancer.1) Although the therapeutic significance of lymph node dissection in the treatment of lung cancer remains controversial, it is believed that the role of resection to determine the stage of disease is significant when considering multidisciplinary treatment.2–4) However, if it is known preoperatively that there is no lymph node metastasis, neither dissection nor sampling is required for either therapeutic or staging purposes. Furthermore, the surgical invasiveness can be alleviated by omitting lymph node dissection and sampling.
Advances in diagnostic imaging, especially in computed tomography (CT), have made it possible to predict the degree of invasion and progression of lung adenocarcinoma with considerable accuracy from imaging findings of the main lesion.5–12) In particular, pathological invasion in adenocarcinoma has been defined, and a new T classification was proposed. The new pT classification excludes the lepidic area, which is considered to be the non-invasive region, from the tumor diameter. As a result, the diameter of consolidation, which excludes the so-called ground glass attenuation (GGA) area, is also used as the tumor diameter in diagnostic imaging.13,14) The cT classification reflects the degree of pathological invasiveness much more than before, but it still needs to be devised in terms of selecting pN0 cases.
In our previous study, we reported that the tumor size determined by the mediastinal window setting (MD) was highly associated with pathological invasive size (IS), and was strongly associated with lymph node metastases and pathological invasiveness in lung adenocarcinoma.5,8–9,12) Moreover, it has been shown that fluorodeoxyglucose-position emission tomography (FDG-PET) findings in the main lesion are associated with lymph node metastasis.10) Furthermore, we have revealed that carcinoembryonic antigen (CEA) was a significant independent factor associated with invasiveness and lymph node metastasis of cIA and early-stage lung adenocarcinoma.11,12,15)
Based on these findings, we sought to determine simple and reliable imaging findings that can select patients with cIA adenocarcinoma with normal CEA, in whom lymph node dissection can be omitted.
Materials and Methods
Institutional Review Board approval was obtained from Teikyo University School of Medicine (17035), and the need for informed consent was waived as only de-identified information was used for analysis.
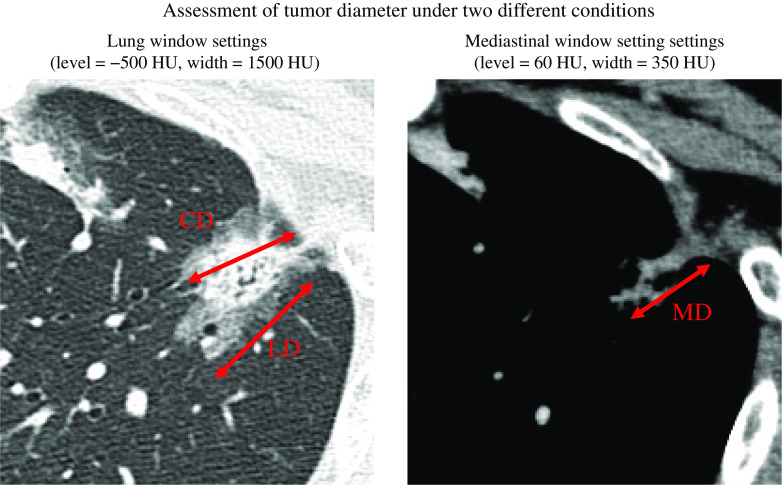
Between January 2015 and December 2017, 533 patients with cIA lung adenocarcinoma underwent surgical resection at the Aichi Cancer Hospital. In all patients, preoperative staging was assessed using chest CT, abdominal CT or ultrasonography, brain CT or magnetic resonance imaging, and FDG-PET CT. The clinical mediastinal and hilar lymph node status was deemed positive if the chest CT findings revealed that the shorter axis was >1.0 cm. The status of the mediastinal, hilar, and interlobar nodes was assessed according to the 8th TNM classification for lung cancer.14,15) All tumors were examined using CT with thin section (1.0–2.0 mm) conditions on digital image data. Thin-section chest CT images, FDG-PET CT images, and clinicopathological findings were examined. FDG-PET-CT images were acquired using a dedicated PET/CT scanner (Biograph 40; SIEMENS Healthcare Japan Co., Tokyo, Japan) in the same facility (Nagoya Radiological Diagnosis Institute), and the maximum standardized uptake value (SUVmax) was used for analysis. The tumor dimension was evaluated under two different CT imaging conditions: lung window settings (level = −500 Hounsfield unit [HU], width = 1500 HU) and mediastinal window settings (level = 60, width = 350 HU) (Fig. 1). The CT images were evaluated for the maximum tumor dimension using the lung window settings (LD) and MD, and evaluated for the tumor maximum diameter of the solid component (consolidation:CD) of the tumor in lung window settings. The solid component ratio (C/T) was defined as maximum tumor diameter using CD/LD.
Fig. 1. Tumor diameter assessed under two different conditions. CD: maximal consolidation diameter evaluated with lung window setting; HU: Hounsfield unit; MD: maximal diameter evaluated with mediastinal window setting.
Each diameter was determined with consent of three specialists (YS, NS, and HK) of general thoracic surgery at the conference. We excluded 81 cases with high CEA and 116 cases with insufficient preoperative imaging modality; thus, 336 cases were used for final analysis.
The patient records were examined for age, sex, preoperative nodal status, and various imaging findings (CD, MD, C/T, and SUVmax), and the patient characteristics are summarized in Table 1. The pathological invasive size was evaluated by hematoxylin and eosin (H&E) stain and elastic stain, including invasion to lymphatic vessels (ly), vascular vessels (v), and the pleura (pl); ly, v, or pl was used as indicator of pathological invasiveness.
Table 1. Patient characteristics.
| Age (years) | 29–84 (median, 66) |
| Sex (male/female) | 150/186 |
| cT | |
| Tis/T1mi/T1a/T1b/T1c | 21/36/82/118/79 |
| Pathological IS | 6–35 (median, 12.0) mm |
| CD | 0–30 (median, 13.0) mm |
| MD | 0–29 (median, 8.0) mm |
| p-N | |
| N0/N1/N2/Nx | 246/13/9/68 |
| Lymph node dissection or sampling | |
| Yes/others | 268/68 |
CD: Lung window setting consolidation diameter; IS: invasive size; MD: mediastinal window setting diameter
Statistical analysis
Receiver operating characteristic (ROC) and area under the curve (AUC) analyses were applied for imaging and pathological variables. The correlation between each imaging variable and pathological invasive size was examined. Factors related to lymph node metastasis and invasiveness were analyzed by logistic regression multivariate analyses. Statistical analyses were performed by JMP 13 (SAS Institute Inc., Cary, NC, USA), and a p value <0.05 was considered statistically significant.
Results
AUC between lymph node metastasis or invasiveness and imaging variables
As an indicator of histological invasiveness, the AUC was highest in MD (correlation coefficient, 0.79–0.85), followed by C/T (0.75–0.80), CD (0.75–0.81), and SUV (0.65–0.68); in particular, MD was comparable to IS (0.73–0.85). The AUC, as an index of lymph node metastasis, was highest in MD (0.84), and similar in C/T (0.79), CD (0.79), and SUV (0.80); in particular, MD was higher than invasive size (0.80). The correlation with invasive size was highest in MD (0.76), followed by CD (0.73), C/T (0.54), and SUVmax (0.27), while SUV and C/T were not highly correlated (Table 2).
Table 2. AUC by ROC and correlation coefficient between variables and invasive size.
| Variables | AUC ly (n = 336) |
AUC v (n = 336) |
AUC pl (n = 336) |
AUC ly, v, or pl (n = 336) |
AUC LN (n = 268) |
Correlation with invasive size |
|---|---|---|---|---|---|---|
| SUVmax | 0.67 | 0.65 | 0.69 | 0.68 | 0.80 | 0.27 (p <0.001) |
| CD | 0.76 | 0.77 | 0.75 | 0.81 | 0.79 | 0.73 (p <0.001) |
| MD | 0.83 | 0.81 | 0.79 | 0.85 | 0.84 | 0.76 (p <0.001) |
| C/T | 0.77 | 0.79 | 0.75 | 0.80 | 0.79 | 0.54 (p <0.001) |
| Invasive size | 0.83 | 0.80 | 0.73 | 0.85 | 0.80 | N.A |
AUC: area under an ROC curve; C/T: CD/T; CD: maximal consolidation diameter evaluated with lung window setting; T: total tumor diameter evaluated with lung window setting; LN: lymph node metastasis; ly: lymph vessel invasion; MD: maximal diameter evaluated with mediastinal window setting; pl: pleural invasion; ROC: receiver operating characteristic; SUVmax: maximum standardized uptake value; v: vascular invasion
Multivariate analyses between lymph node metastasis or invasiveness and imaging variables
SUVmax, MD, and C/T were independent factors related to lymph node metastasis, while CD was not an independent factor (p = 0.38). In relation to pathological invasiveness (ly/v/pl), MD and C/T were significantly associated with pathological invasiveness, but SUVmax and CD were not (Table 3).
Table 3. Multivariate logistic regression analysis.
| Variables | LN metastasis (n = 268) | Invasiveness (ly/v/pl) (n = 336) | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| SUVmax | 1.06 | 1.01–1.11 | 0.03 | 0.96 | 0.88–1.05 | 0.20 |
| CD | 0.91 | 0.74–1.12 | 0.38 | 1.04 | 0.97–1.12 | 0.30 |
| MD | 1.23 | 1.01–1.50 | 0.02 | 0.84 | 0.76–0.90 | <0.001 |
| C/T | 1.05 | 1.01–1.09 | 0.01 | 0.98 | 0.97–0.99 | 0.006 |
C/T: CD/T; CD: maximal consolidation diameter evaluated with lung window setting; T: total tumor diameter evaluated with lung window setting; CI: confidence interval; LN: lymph node; ly: lymph vessel invasion; MD: maximal diameter evaluated with mediastinal window setting, OR: odds ratio; pl: pleural invasion; SUVmax: maximum standardized uptake value; v: vascular invasion
Imaging conditions for N0 case selection: conditions for PPV to reach 100%, number of compatible patients, and sensitivity
The conditions for reaching 100% positive predictive value (PPV) were 12.5 mm for CD, 10.6 mm for MD, and 0.55 for C/T. The SUVmax did not reach 100% PPV, even if the cut-off value was set to 0, the PPV was 98.2% (110/112). Of the 246 pathological N0 (pN0) cases, the number of patients that could be selected under the above conditions was 116 for CD (sensitivity, 47.2%), 141 for MD (sensitivity, 57.3%), and 91 for C/T (sensitivity, 37.0%). When pN0 was selected by cT, Tis, T1mi, and T1a were applicable (same result as CD 10 mm or less), and 95 pN0 cases were selected (sensitivity, 38.2%) (Table 4).
Table 4. Examination of lymph node metastasis negative cases among lymph node dissection.
| Variables | Cut-off | No. patients | Sensitivity |
|---|---|---|---|
| cT | Tis/T1mi/T1a (CD <10 mm) | 95 | 38.2% |
| CD | 12.5 mm | 116 | 47.2 % |
| MD | 10.6 mm | 141 | 57.3 % |
| C/T | 0.55 | 91 | 37.0% |
| SUVmax | NA | NA | NA |
100% PPV cutoff value for lymph node metastasis. Total number of patients = 249.
C/T: CD/T; CD: maximal consolidation diameter evaluated with lung window setting; T: total tumor diameter evaluated with lung window setting; MD: maximal diameter evaluated with mediastinal window setting; NA: not available, because the variable did not reach PPV 100%; SUVmax: maximum standardized uptake value; PPV: positive predictive value
Discussion
With the development of diagnostic imaging, the ability to detect and treat lung cancer early has increased. With regard to surgery, a rational change in treatment is required as the number of early detection cases increases. Limited surgery, including segmental resection and wedge resection, selective lymph node dissection, and omission of dissection represent such rational surgical changes. In the field of lung cancer surgery, determining reasonable indication criteria for each surgical procedure is as important as developing and establishing surgical techniques. The new cT classification improves the consistency between the degree of pathological invasiveness and diagnostic imaging (especially CT). The recent use of the consolidation diameter evaluated with LD settings has allowed a more accurate reflection of the extent of invasion than the previous cT classification.13,14) In other words, the new cT is a method of measuring the degree of tumor progression by CD; however, it remains to be seen how accurately the CD-based diagnostic imaging predicts lymph node metastasis.
In the case of cIA adenocarcinoma with normal CEA, MD, C/T, and SUVmax were revealed as independent imaging findings associated with lymph node metastasis. In contrast, when examined with these four factors, CD was not an independent factor.
With regard to invasiveness, MD and C/T were found to be independent imaging factors, but SUV and CD were not. Since MD and C/T reflect the invasiveness of the tumor well, they are also good indicators of lymph node metastasis. MD had the highest correlation coefficient (0.76) with pathological invasive size among the four factors, which is consistent with it also reflecting the degree of histological invasiveness well. In contrast, the correlation coefficient of C/T with invasive size was 0.54, which was not considered to be high.
These results suggest that MD is the most accurate and rational method of selecting cases without lymph node metastasis among conventional diagnostic imaging methods. In addition, MD was associated with pathological invasive size and histological invasiveness more than other imaging modalities. Furthermore, it was revealed that lymph node metastasis does not occur in cIA adenocarcinoma with normal CEA when the MD is 10.6 mm or less (sensitivity, 57.3%). Similarly, it was also found that lymph node metastasis does not occur if the CD is 12.5 mm or less, or when the C/T is 0.55 or less (sensitivity, 47.2% and 37.0%, respectively). Regarding SUVmax, PPV reached only 98.2% even if the value was set to 0. It may be difficult to determine the indication for omitting lymph node dissection based only on the SUVmax of the main lesion.
Preoperative diagnosis of lymph node metastasis, especially with imaging, is important for developing a therapeutic strategy for primary lung cancer. However, it is difficult to accurately stage lymph node metastasis in lung cancer patients by preoperative imaging, such as MRI and CT.16) Recently, positron emission tomography (PET-CT), which uses both CT (for morphological evaluation) and PET (for the evaluation of tumor activity), has been used to evaluate metastatic lesions in lymph nodes. Studies have shown that PET-CT is useful for the evaluation of mediastinal lymph nodes, but it has not been demonstrated to be able to accurately extract pN0. Some lymph node metastases, such as micrometastases, do not give an accurate metastasis evaluation no matter how closely the degree of swelling of the lymph nodes, or the uptake of FDG into the lymph nodes, are examined.17–19) We have reported that both the imaging findings (MD, solid components of tumor) of the tumor and CEA values are highly correlated with the degree of tumor invasiveness, which allows more accurate preoperative evaluation of lymph node metastasis in cIA.5–8,20) CD is a rational tumor size measurement method that reflects the degree of adenocarcinoma, and was adopted in the 8th edition WHO classification.14) However, MD may be more suitable to determine the degree of invasion and the correlation with the invasive size.8–10) Although CD reflects the extent of tumor invasion much better than the total tumor diameter determined by the LD, it is often difficult to determine the boundary between consolidation and GGO, which is easier with the MD.
The cT category, reflecting pathological features, was finally proposed in the newly revised eighth edition of TNM classification.14,15) The cT category basically reflects a new pathological classification that takes into account the degree of fibrosis and scar formation, as well as the mode of extension to the alveolar structures in adenocarcinoma.21,22) In the cT category, the pathological invasive diameter is recognized as the consolidation diameter based on lung field conditions in CT imaging. It can be said that the cT category reflects tumor progression and malignancy more than the previous classification based on simple lung field conditions14,15); therefore, the correlation between invasive size and diagnostic imaging findings is very important. In contrast, since C/T and SUVmax do not specifically measure invasive size, it is not unexpected that the correlation coefficient is low. Furthermore, not only MD, but also the AUC of MD has a higher correlation coefficient with invasive size than CD. The difference between the CD and MD diameters is apparently caused only by the difference in window width and window level, despite the fact that they have the same digital data. Further studies are needed to determine which image rendering conditions (window width, window level) are most suitable for expressing the degree of invasiveness and lymph node metastasis.
CEA is an independent prognostic factor that is significantly associated with lymph node metastasis in early lung adenocarcinoma.11,12,15) In addition, among small lung adenocarcinomas, it has been reported that high levels of serum CEA are highly associated with non-lepidic predominant adenocarcinoma types (papillary, acinar, solid with mucin, etc.), which are considered to be more aggressive.7) Since this study excluded cases with high CEA, it may be necessary to consider the exclusion of some non-lepidic types that are particularly likely to have lymph node metastases, in which that lymph node metastasis could occur even with MD <10.6 mm and CD <12.5 mm.
FDG-PET is a useful imaging modality to evaluate tumor invasiveness and activity; however, it was found that it is difficult to search for the possibility of lymph node metastasis from the findings of the main lesion in cIA, especially for the absence of lymph node metastasis. Even with an SUVmax of 0 (no accumulation), lymph node metastasis was observed at a low frequency.
This study had a limitation in that it included a small number of retrospective studies. In addition, since it was limited to the CEA normal group, poor prognosis cIA groups may be omitted to some extent.
Conclusion
In cIA lung adenocarcinoma with CEA in the normal range, it was suggested that lymph node dissection could be omitted by cutting off 12.5 mm for CD, 10.6 mm for MD, and 0.55 for C/T. Sensitivity was highest in the order of MD, CD, and C/T.
Acknowledgment
We wish to thank ENAGO (https://www.enago.jp/) for editing a draft of this manuscript.
Disclosure Statement
All authors have no conflict of interest.
References
- 1).Cahan WG. Radical lobectomy. J Thorac Cardiovasc Surg 1960; 39: 555– 72. [PubMed] [Google Scholar]
- 2).Izbicki JR, Passlick B, Pantel K, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: results of a prospective randomized trial. Ann Surg 1998; 227: 138– 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Wright G, Manser RL, Byrnes G, et al. Surgery for non-small cell lung cancer: systematic review and meta-analysis of randomised controlled trials. Thorax 2006; 61: 597– 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011; 141: 662– 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Sakao Y, Nakazono T, Tomimitsu S, et al. Lung adenocarcinoma can be subtyped according to tumor dimension by computed tomography mediastinal-window setting. Additional size criteria for clinical T1 adenocarcinoma. Eur J Cardiothorac Surg 2004; 26: 1211– 5. [DOI] [PubMed] [Google Scholar]
- 6).Nakazono T, Sakao Y, Yamaguchi K, et al. Subtypes of peripheral adenocarcinoma of the lung: differentiation by thin-section CT [published correction appears in Eur Radiol 2006 May; 16(5): 1185]. Eur Radiol 2005; 15: 1563– 68. [DOI] [PubMed] [Google Scholar]
- 7).Sakao Y, Miyamoto H, Oh S, et al. The impact of cigarette smoking on prognosis in small adenocarcinomas of the lung: the association between histologic subtype and smoking status. J Thorac Oncol 2008; 3: 958– 62. [DOI] [PubMed] [Google Scholar]
- 8).Sakao Y, Kuroda H, Mun M, et al. Prognostic significance of tumor size of small lung adenocarcinomas evaluated with mediastinal window settings on computed tomography. PLoS One 2014; 9: e110305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Sakakura N, Inaba Y, Yatabe Y, et al. Estimation of the pathological invasive size of pulmonary adenocarcinoma using high-resolution computed tomography of the chest: a consideration based on lung and mediastinal window settings. Lung Cancer 2016; 95: 51– 6. [DOI] [PubMed] [Google Scholar]
- 10).Kuroda H, Mori S, Tanaka H, et al. Prognostic significance of combined radiologic imaging modalities for prognosis of clinical IA adenocarcinomas. Oncotarget 2018; 9: 10745– 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Sakao Y, Sakuragi T, Natsuaki M, et al. Clinicopathological analysis of prognostic factors in clinical IA peripheral adenocarcinoma of the lung. Ann Thorac Surg 2003; 75: 1113– 7. [DOI] [PubMed] [Google Scholar]
- 12).Sakao Y, Nakazono T, Sakuragi T, et al. Predictive factors for survival in surgically resected clinical IA peripheral adenocarcinoma of the lung. Ann Thorac Surg 2004; 77: 1157– 61; discussion 1161-2. [DOI] [PubMed] [Google Scholar]
- 13).Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2016; 11: 1204– 23. [DOI] [PubMed] [Google Scholar]
- 14).Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011; 6: 244– 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Sakao Y, Tomimitsu S, Takeda Y, et al. Carcinoembryonic antigen as a predictive factor for postoperative tumor relapse in early-stage lung adenocarcinoma. Eur J Cardiothorac Surg 2004; 25: 520– 2. [DOI] [PubMed] [Google Scholar]
- 16).Glazer GM, Gross BH, Aisen AM, et al. Imaging of the pulmonary hilum: a prospective comparative study in patients with lung cancer. AJR Am J Roentgenol 1985; 145: 245– 8. [DOI] [PubMed] [Google Scholar]
- 17).Nomori H, Watanabe K, Ohtsuka T, et al. The size of metastatic foci and lymph nodes yielding false-negative and false-positive lymph node staging with positron emission tomography in patients with lung cancer. J Thorac Cardiovasc Surg 2004; 127: 1087– 92. [DOI] [PubMed] [Google Scholar]
- 18).Pak K, Park S, Cheon GJ, et al. Update on nodal staging in non-small cell lung cancer with integrated positron emission tomography/computed tomography: a meta-analysis. Ann Nucl Med 2015; 29: 409– 19. [DOI] [PubMed] [Google Scholar]
- 19).Dejima H, Kuroda H, Oya Y, et al. Evaluation of lobar lymph node metastasis in non-small cell lung carcinoma using modified total lesion glycolysis. J Thorac Dis 2018; 10: 6932– 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Sakao Y, Miyamoto H, Sakuraba M, et al. Prognostic significance of a histologic subtype in small adenocarcinoma of the lung: the impact of nonbronchioloalveolar carcinoma components. Ann Thorac Surg 2007; 83: 209– 14. [DOI] [PubMed] [Google Scholar]
- 21).Shimosato Y, Suzuki A, Hashimoto T, et al. Prognostic implications of fibrotic focus (scar) in small peripheral lung cancers. Am J Surg Pathol 1980; 4: 365– 73. [DOI] [PubMed] [Google Scholar]
- 22).Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995; 75: 2844– 52. [DOI] [PubMed] [Google Scholar]