Abstract
Primary antibody deficiencies (PAD) are the most prevalent group of primary immunodeficiencies (PID) in adults and immunoglobulin replacement therapy (IRT) is the mainstay therapy to improve clinical outcomes. IRT is, however, expensive and, in minor PAD, clear recommendations concerning IRT are lacking. We conducted a retrospective real‐life study to assess the effectiveness of low‐dose IRT in minor PAD on 143 patients fulfilling European Society for Immunodeficiencies (ESID) diagnostic criteria for immunoglobulin (Ig)G subclass deficiency (IgGSD) or unclassified antibody deficiency (UAD). All patients were treated with intravenous low‐dose IRT (0.14 ± 0.06 g/kg/month). Immunoglobulin (Ig) classes and IgG subclasses were measured at baseline and after 1 year of IRT. The annual rate of total infections, upper respiratory tract infections (URTI), lower respiratory tract infections (LRTI) and hospitalizations was measured at baseline and after 1 and 2 years of IRT. After 1 year of IRT significant improvement was demonstrated in: (a) serum IgG (787.9 ± 229.3 versus 929.1 ± 206.7 mg/dl; p < 0.0001); (b) serum IgG subclasses (IgG1 = 351.4 ± 109.9 versus 464.3 ± 124.1, p < 0.0001; IgG2 = 259.1 ± 140 versus 330.6 ± 124.9, p < 0.0001; IgG3 = 50.2 ± 26.7 versus 55.6 ± 28.9 mg/dl, p < 0.002); (c) annual rate of total infections (5.75 ± 3.87 versus 2.13 ± 1.74, p < 0.0001), URTI (1.48 ± 3.15 versus 0.69 ± 1.27; p < 0.005), LRTI (3.89 ± 3.52 versus 1.29 ± 1.37; p < 0.0001) and hospitalizations (0.37 ± 0.77 versus 0.15 ± 0.5; p < 0.0002). The improvement persisted after 2 years of IRT. No significant improvement in URTI annual rate was noted in UAD and in patients with bronchiectasis. In conclusion, low‐dose IRT can improve clinical outcomes in UAD and IgGSD patients, providing a potential economical advantage over the standard IRT dose.
Keywords: IgG subclass deficiency, low‐dose intravenous immunoglobulins, primary antibody deficiencies, unclassified hypogammaglobulinemia
In our retrospective real‐life study we assessed the effectiveness of low‐dose intravenous immunoglobulin replacement therapy (IRT) on 143 patients fulfilling ESID diagnostic criteria for IgG subclass deficiency (IgGSD) or Unclassified Antibody Deficiency (UAD). After one year of low‐dose IRT significant improvement was demonstrated in: a) serum IgG; b) Serum IgG subclasses; c) annual rate of total infections, upper and lower respiratory tract infections and hospitalizations. The improvement persisted after two years of IRT. In conclusion, low‐dose IRT can improve clinical outcomes in UAD and IgGSD patients, with significant cost reduction in comparison to the standard IRT dose.

INTRODUCTION
Primary antibody deficiencies (PAD) are a wide group of primary immunodeficiencies (PID) characterized by low serum levels of one or more immunoglobulin (Ig) class and/or one or more IgG subclass [1]. From a clinical viewpoint, most patients present with recurrent upper respiratory tract infections (URTI) and lower respiratory tract infections (LRTI) resulting in anatomical injury involving the respiratory tract with the development of bronchiectasis [2].
Among severe PADs, an important group is represented by common variable immunodeficiency (CVID), characterized by recurrent respiratory, gastrointestinal and urinary tract infections and heavy impairment of quality of life (QoL) [3]. The prevalence of CVID is estimated to occur in approximately 1:25000 of the population [4]. Milder PADs have been observed, however, in many patient cohorts, such as IgG subclass deficiency (IgGSD) or unclassified antibody deficiency (UAD) [5]. IgGSD is characterized by the reduction of one or more IgG subclass with normal total serum IgG levels, and can be associated with specific polysaccharides antibody deficiency. UAD encompasses a wide group of patients showing more severe serum Ig reduction without fulfilling CVID or other PID criteria. Guidelines for treatment of IgGSD and UAD are lacking, even if prophylactic antibiotic treatment and immunoglobulin replacement therapy (IRT) have been used [6, 7]. Milder PADs have been less extensively studied; however, their prevalence in patients with obstructive lung disease and bronchiectasis is probably underestimated and carries a significant burden of disease [5, 8, 9]. In obstructive lung disease, PADs are correlated with frequent respiratory exacerbations, which are considered a strong risk factor for accelerated lung function decline and worse prognosis [8]. Some epidemiological data report that affecting approximately 23% of patients with difficult‐to‐treat chronic rhinosinusitis and approximately 13% of patients with recurrent rhinosinusitis are affected by PAD [8].
IRT is the standard therapy for PAD. IRT is effective in reducing the recurrence and the severity of infections, therefore reducing hospitalizations and mortality while improving patients’ quality of life [10, 11], and resulting in a cost‐effective therapy [11]. IRT, however, is strongly advised only in some primary immunodeficiencies such as congenital agammaglobulinemia, common variable immunodeficiency (CVID), hyper‐IgM syndrome, severe combined immunodeficiencies and Wiskott–Aldrich syndrome [12]. A summary of guidelines on the management of PAD with IRT suggested to achieve trough IgG serum levels of at least 5 g/dl and ideally 6.5–10 g/dl [13]; however, universally protective trough IgG serum levels in PAD patients remain undefined [14]. Although some case–series report clinical efficacy of this treatment have been published [11, 12, 15, 16, 17, 18, 19], IRT in patients with IgGSD and UAD is not universally advised. In this study, we retrospectively reviewed clinical outcomes such as the reduction of infections in a large cohort of patients with IgGSD or UAD treated with IRT.
METHODS
For this retrospective study, patients fulfilling the European Society for Immunodeficiencies (ESID) criteria for IgGSD and UAD [20] were selected from the clinical cohort of PAD patients referred to our immunology clinic from January 2006 to December 2017. According to the diagnostic criteria proposed by ESID, secondary causes of hypogammaglobulinemia and T cell defects were ruled out. The patients were symptomatic from recurrent infections and therefore had been treated with IRT, using locally available preparations (Privigen© and Venital©) administered once every 4 weeks. All patients were visited monthly before IRT administration with a follow‐up duration of at least 2 years. All enrolled patients had chest tomography (CT) imaging at baseline; chest CT were reviewed by an experienced thoracic radiologist to evaluate for the presence of bronchiectases. Patients with isolated IgA or IgM reduction were not enrolled into this study. All patients underwent pneumococcal vaccine (Prevenar©), but pneumococcal vaccine response was not assessed according to the ESID criteria used for diagnosis before IRT, which did not require vaccine response assessment.
Demographic and laboratory data and medical therapy were retrieved from clinical records. Ig classes (Cobas 8000®; Roche Diagnostic, Basel, Switzerland) and IgG subclasses (radial immunodiffusion; BindaridTM Kits, The Binding Site, Birmingham, UK) serum levels were measured at baseline and after 1 year of IRT.
We chose, as clinical outcomes, the annual rate of total infections, URTI, LRTI and hospitalizations. The URTI, LRTI and other infections were always confirmed by a physician. Hospitalization was defined as admission to a hospital ward for an acute infection. The comorbidities were summarized in a total score using the age‐adjusted Charlson comorbidity index (ACCI) [21]. The procedures followed in the study were approved by the Local Ethical Committee (protocol RF‐2013‐02358775).
Statistical analysis
Statistical analysis was performed using Python version 3.8.0 (Anaconda distribution). P‐values lower than 0.05 were considered statistically significant.
RESULTS
Patients’ characteristics
A cohort of 143 patients was retrospectively recruited for the study; in the overall population, 85 (59.4%) patients had a diagnosis of IgGSD, while 58 (40.6%) had a diagnosis of UAD. The patients were mainly female (100 of 143, 69.9%), with a mean age at diagnosis of 60.9 ± 14 years; IgGSD patients were diagnosed in a younger age than UAD patients [57.4 ± 14.1 versus 66.1 ± 12.3; p = not significant (NS)]. A relevant smoking exposure (former smoker = 91 of 143, 63.6%) and a significant comorbidity burden (AACI = 3.9 ± 2.4) were present. In the study cohort, IRT was administered monthly at a mean dose of 0.14 ± 0.06 g/kg; IgGSD patients received a slightly lower dose than UAD patients (IgGSD = 0.13 ± 0.06 g/kg; UAD = 0.15 ± 0.07 g/kg; p < 0.05). In the entire study population, the rate of total infectious events in the year before starting IRT was 5.7 ± 3.9, consistent with the rate in the previous years.
IgG, IgA, IgM and IgG subclasses serum levels are listed in Table 1 for both groups, in addition to demographic features and main comorbidities.
TABLE 1.
Demographic and clinical features at baseline in the study cohort
| All (n = 143) | IgGSD (n = 85) | UAD (n = 58) | p‐value | |
|---|---|---|---|---|
| Male | 43 (30.1) | 24 (28.3) | 19 (32.8) | 0.69 |
| Age at diagnosis (years) | 60.9 ± 14 | 57.4 ± 14.1 | 66.1 ± 12.3 | <0.001 |
| Smoker | ||||
| No | 61 (42.7) | 40 (47.1) | 21 (36.2) | 0.26 |
| Former | 82 (57.3) | 45 (52.9) | 37 (63.8) | 0.26 |
| ACCI | 3.9 ± 2.4 | 3.6 ± 2.5 | 4.3 ± 2.2 | 0.08 |
| Serum Ig (mg/dl) | ||||
| IgG | 787.9 ± 229.3 | 929.4 ± 173.7 | 580.4 ± 113.6 | <0.001 |
| IgA | 171.7 ± 94.4 | 205.5 ± 96.6 | 122 ± 65.8 | <0.001 |
| IgM | 113.3 ± 75.4 | 122.5 ± 67.5 | 99.7 ± 84.4 | 0.07 |
| IgG1 | 351.4 ± 109.9 | 377.4 ± 113.9 | 313.2 ± 92.1 | <0.001 |
| IgG2 | 259.1 ± 140 | 310.3 ± 141.3 | 183.9 ± 98.6 | <0.001 |
| IgG3 | 50.2 ± 26.7 | 56.1 ± 27.9 | 41.5 ± 22.2 | <0.001 |
| IgG4 | 26.2 ± 25.9 | 33.3 ± 26.3 | 15.9 ± 21.4 | <0.001 |
| Chronic lung disease | 80 (55.9) | 54 (63.5) | 26 (44.8) | 0.06 |
| Asthma | 54 (37.8) | 37 (43.5) | 17 (29.3) | 0.2 |
| COPD | 26 (18.1) | 17 (20) | 9 (15.5) | 0.45 |
| Monthly i.v. Ig dose (g/kg) | 0.14 ± 0.06 | 0.13 ± 0.06 | 0.15 ± 0.07 | 0.04 |
| Bronchiectasis | 69 (48.2) | 45 (52.9) | 24 (41.4) | 0.23 |
| Malignancies | 14 (9.8) | 9 (10.6) | 5 (8.6) | 0.33 |
| Solid | 12 (8.4) | 7 (8.2) | 5 (8.6) | 0.45 |
| Haematological | 2 (1.4) | 2 (2.4) | 0 (0) | 0.63 |
| Total infections | 5.7 ± 3.9 | 6.1 ± 4.6 | 5.2 ± 3.4 | 0.14 |
| LRTI | 3.9 ± 3.5 | 4 ± 3.9 | 3.7 ± 2.9 | 0.57 |
| URTI | 1.5 ± 3.1 | 1.6 ± 3.4 | 1.2 ± 2.7 | 0.45 |
| Hospitalizations | 0.4 ± 0.8 | 0.29 ± 0.7 | 0.48 ± 0.8 | 0.15 |
Abbreviations: ACCI = age‐adjusted Charlson comorbidity index; COPD = chronic obstructive lung disease; Ig = immunoglobulin; IgGSD = IgG subclass deficiency; LRTI = lower respiratory tract infections; UAD = unclassified antibody deficiency; URTI = upper respiratory tract infections.
Note: Data presented as mean ± standard deviation (SD) or n (%).
IRT is able to significantly increase IgG and IgG subclasses serum levels
In comparison to baseline levels, IRT was able to significantly increase trough IgG (787.9 ± 229.3 versus 929.1 ± 206.7 mg/dl; p < 0.0001) and IgG subclasses (IgG1 = 351.4 ± 109.9 versus 464.3 ± 124.1, p < 0.0001; IgG2 = 259.1 ± 140 versus 330.6 ± 124.9, p < 0.0001; IgG3 = 50.2 ± 26.7 versus 55.6 ± 28.9 mg/dl, p < 0.002) serum levels in the whole cohort. As expected, IgA, IgM and IgG4 serum levels did not show significant modifications, as blood donors derived Ig do not contain significant amount of these Ig classes (Figure 1). IRT was effective in ameliorating trough IgG and IgG subclasses serum levels in both (UAD and IgGSD) groups of the study cohort (Supporting information, Tables S1 and S2s).
FIGURE 1.
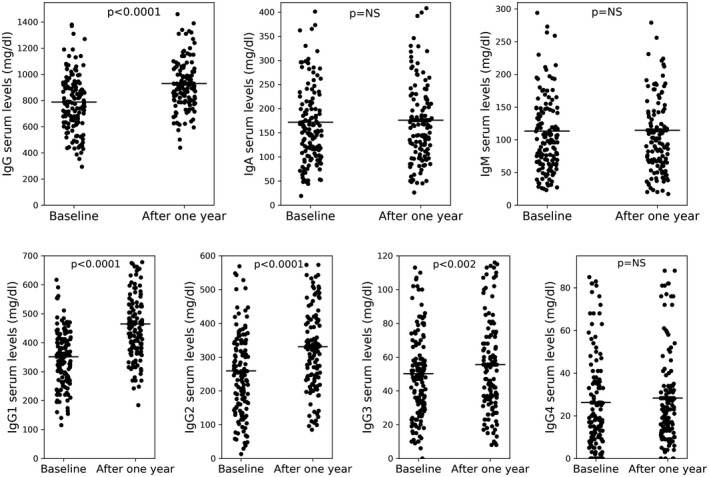
Immunoglobulin (Ig) classes and IgG subclasses in the study cohort at baseline and 1 year after immunoglobulin replacement therapy (IRT)
IRT is able to reduce respiratory infections and hospitalizations
We then evaluated the treatment efficacy of IRT on clinical outcomes such as the total number of infections, URTI, LRTI and hospitalizations. The annual rate of total infections dropped significantly from 5.75 ± 3.87 to 2.13 ± 1.74 (p < 0.0001) after 1 year of IRT; the improvement was also confirmed by analyzing the annual rate of URTI (from 1.48 ± 3.15 to 0.69 ± 1.27; p < 0.005) and the annual rate of LRTI (from 3.89 ± 3.52 to 1.29 ± 1.37; p < 0.0001), which represent the most important types of infections in this patient groups. The hospitalization annual rate, although small in our group, was also significantly reduced (from 0.37 ± 0.77 to 0.15 ± 0.5; p < 0.0002) (see Figure 2).
FIGURE 2.
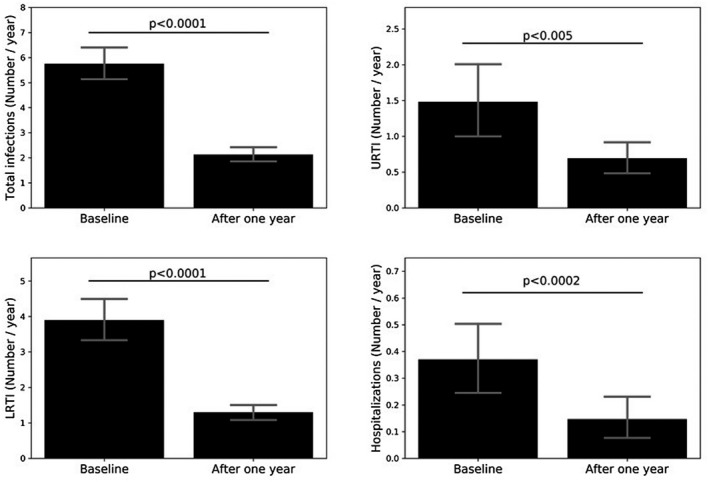
Total infections, upper respiratory tract infections (URTI), lower respiratory tract infections (LTRI) and hospitalizations in the study cohort at baseline and 1 year after immunoglobulin replacement therapy (IRT)
The improvement in the clinical outcomes was confirmed during the second year of treatment. No significant differences between the first and the second year of therapy were found comparing the number of total infections, URTI, LRTI and hospitalizations.
IRT is effective in both UAD and IgGSD patients
When we analyzed the clinical outcomes after 1 year of treatment in both IgGSD and UAD patients IRT demonstrated similar efficacy, except for URTI. The annual rate of total infections in IgGSD dropped significantly from 6.14 ± 4.09 to 2.16 ± 1.76 (p < 0.0001) as well as the annual rate of URTI (1.65 ± 3.44 versus 0.68 ± 1.23; p < 0.02), LRTI (4.03 ± 3.89 versus 1.38 ± 1.45; p < 0.0001) and of the hospitalizations (0.29 ± 0.72 versus 0.12 ± 0.5; p < 0.02). The same results were observed in patients affected by UAD in total infections (5.17 ± 3.43 versus 2.07 ± 1.74; p < 0.0001), LRTI (3.69 ± 2.91 versus 1.17 ± 1.26; p < 0.0001) and hospitalizations (0.48 ± 0.84 versus 0.19 ± 0.51; p < 0.004) but not in the number of URTI (1.24 ± 2.69 versus 0.71 ± 1.34; p = NS).
Patients affected by bronchiectasis
Considering the clinical importance of bronchiectasis as comorbidity in PAD patients, we conducted a subgroup analysis. There were no significant demographic differences between patients with (69; 48.2%) and without bronchiectasis (74; 51.8%), as shown in Table 2. Although the number of total infections and hospitalizations were not different at baseline between patients with and without bronchiectasis, the presence of bronchiectasis, in both IgGSD and UAD, was associated with a clinical picture characterized by significantly more frequent LRTI rate and lower URTI rate (Table 2). IRT was effective in correcting antibody deficiency in patients with bronchiectasis, regardless of the clinical diagnosis (UAD or IgGSD; Supporting information, Table S3).
TABLE 2.
Baseline demographic and clinical features in patients with and without bronchiectasis
| Patients with bronchiectasis | Patients without bronchiectasis | p‐value | |
|---|---|---|---|
| Male | 19 (27.5) | 24 (32.4) | 0.65 |
| Age at diagnosis (years; mean ± SD) | 61.9 ± 12.2 | 60 ± 15.5 | 0.4 |
| Smoker | |||
| No | 33 (47.8) | 28 (59.6) | 0.3 |
| Former | 36 (52.3) | 46 (40.4) | 0.3 |
| AACI (mean ± SD) | 3.8 ± 2.1 | 4 ± 2.6 | 0.69 |
| Serum Ig (mg/dl) | |||
| IgG | 813 ± 251.7 | 764.4 ± 205.3 | 0.21 |
| IgA | 179.8 ± 92.4 | 164 ± 96.2 | 0.32 |
| IgM | 122.7 ± 67.4 | 104.4 ± 81.6 | 0.15 |
| IgG1 | 351.2 ± 126.1 | 351.5 ± 93.2 | 0.19 |
| IgG2 | 265.6 ± 144 | 253 ± 136.8 | 0.59 |
| IgG3 | 51.8 ± 26.6 | 48.7 ± 26.9 | 0.48 |
| IgG4 | 28.4 ± 28.5 | 24.3 ± 23.1 | 0.34 |
| Monthly Ig dose (g/kg; mean ± SD) | 0.15 ± 0.07 | 0.13 ± 0.05 | 0.08 |
| Total infections (mean ± SD) | 5.7 ± 3.7 | 5.8 ± 4 | 0.8 |
| LRTI (mean ± SD) | 4.6 ± 3.7 | 3.2 ± 3.2 | 0.01 |
| URTI (mean ± SD) | 0.7 ± 3 | 2.2 ± 3.8 | 0.005 |
| Hospitalizations (mean ± SD) | 0.38 ± 0.77 | 0.36 ± 0.79 | 0.93 |
Abbreviations: ACCI = age‐adjusted Charlson comorbidity index; Ig = immunoglobulins; LRTI = lower respiratory tract infections; URTI = upper respiratory tract infections.
Note: Data presented as mean ± standard deviation (SD) or n (%).
Considering the treatment effect in this important patient subgroup, significant improvement after 1 year of IRT was demonstrated in number of total infections (5.7 ± 3.7 versus 2.3 ± 1.7; p < 0.0001), LRTI (4.6 ± 3.7 versus 1.7 ± 1.5; p < 0.0001) and hospitalizations (0.38 ± 0.77 versus 0.09 ± 0.44; p < 0.0003). but not in the number of URTI (0.72 ± 1.96 versus 0.38 ± 0.67; p = NS). Even after therapy, patients with bronchiectasis showed a higher rate of LRTI (1.7 ± 1.5 versus 0.9 ± 1.1; p < 0.0007) and a lower rate of URTI (0.38 ± 0.67 versus 0.99 ± 1.6; p < 0.005) in comparison to patients without bronchiectasis (Figure 3).
FIGURE 3.
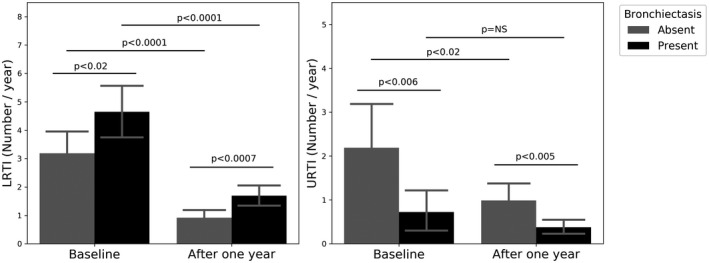
Lower and upper respiratory tract infections at baseline and after 1 year of immunoglobulin replacement therapy (IRT) in patients with and without bronchiectasis. URTI = upper respiratory tract infections; LRTI = lower respiratory tract infections
DISCUSSION
Our real‐life study, although retrospective and monocentric, suggests a significant beneficial clinical effect of IRT in terms of reduction of both URTI and, of utmost importance, LRTI in patients suffering from UAD and IgGSD. More importantly, this protective effect seems to be present using low‐dose IRT, corresponding to less than half the standard dose (400 mg/kg) commonly advised for CVID patients. These therapeutic results are important, considering that IRT dose is not pivotal for the efficacy of therapy; IgG trough levels, in combination with respiratory tract infection reduction, is indeed recommended as an efficacy end‐point [14]. However, anti‐pneumococcal vaccination could account, at least in part, for the reduction of infections observed in the study cohort. The study was not designed to assess the separate contribution of anti‐pneumococcal vaccination and IRT in the reduction of infections of patients with IgGSD or UAD, so further studies with a randomized, placebo controlled design are needed to clarify this correlation and to validate the treatment with low‐dose IRT in UAD and IgGSD patients.
The reduction of LRTI is critical, taking into account that, regardless of PAD type, similarly high rates of infections and bronchiectasis are observed [22]. It has been clearly shown that, in all PID patients, major LRTI such as pneumonia are the most frequent clinical manifestations leading to structural lung injury such as bronchiectasis [4, 23]. Moreover, the presence of bronchiectasis is associated with recurrent LRTI that hasten the formation of bronchiectasis, creating a vicious circle harmful to the patient in the long term. To more clearly define the role of low‐dose IRT in the treatment of PAD, it would be interesting to also evaluate its efficacy in CVID patients with or without bronchiectasis.
Early diagnosis is crucial not only in CVID and other major PID, but also in UAD and IgGSD, and may allow consideration of the initiation of IRT. In particular, in IgGSD, the normal total IgG serum levels, despite the recurrence of respiratory infections, can delay the diagnosis increasing the risk of development of comorbidities such as bronchiectasis leading to chronic obstructive lung disease. One of the biggest concerns regarding IRT in these patients is the economic burden of the treatment, although several studies attest the efficacy and cost effectiveness of IRT [24, 25, 26]. Indeed, as has been shown in CVID patients, the overall costs are reduced after diagnosis due to appropriate management [26]. In conclusion, in our case series of UAD and IgGSD patients, low‐dose IRT seems to be able to correct the antibody defect and, more importantly, to significantly reduce the frequency of respiratory tract infections and hospitalizations.
Some strengths of our study include the huge number of patients, the real‐life nature, the wide follow‐up duration and the monocentric design, which reduces evaluation bias. However, the retrospective nature of the study can be a drawback, as well as the lack of protein and polysaccharide vaccine response assessment, which was not performed because ESID diagnostic criteria for IgGSD and UAD did not require vaccine response assessment at the time of diagnosis of the patients in our cohort.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Emanuele Vivarelli and Andrea Matucci equally contributed to the study concept and design, data acquisition and analysis and writing of the manuscript. All authors participated in the critical revision and final approval of the manuscript.
Supporting information
Table S1‐S3
ACKNOWLEDGEMENTS
This study was not supported by funding.
Vivarelli E, Matucci A, Bormioli S, Parronchi P, Liotta F, Cosmi L, et al. Effectiveness of low‐dose intravenous immunoglobulin therapy in minor primary antibody deficiencies: A 2‐year real‐life experience. Clin Exp Immunol. 2021;205:346–353. 10.1111/cei.13629
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author EV on reasonable request.
REFERENCES
- 1.Min Q, Meng X, Wang JY. Primary antibody deficiencies. Adv Exp Med Biol. 2020;1254:117–44. [DOI] [PubMed] [Google Scholar]
- 2.Wall LA, Wisner EL, Gipson KS, Sorensen RU. Bronchiectasis in primary antibody deficiencies: a multidisciplinary approach. Front Immunol. 2020;31:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann U, Routes JM, Soler‐Palacín P, Jolles S. The lung in primary immunodeficiencies: new concepts in infection and inflammation. Front Immunol. 2018;8:1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odnoletkova I, Kindle G, Quinti I, Grimbacher B, Knerr V, Gathmann B, et al.; Plasma Protein Therapeutics Association (PPTA) Taskforce . The burden of common variable immunodeficiency disorders: a retrospective analysis of the European Society for Immunodeficiency (ESID) registry data. Orphanet J Rare Dis. 2018;13:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger M, Geng B, Cameron DW, Murphy LM, Schulman ES. Primary immune deficiency diseases as unrecognized causes of chronic respiratory disease. Respir Med. 2017;132:181–188. [DOI] [PubMed] [Google Scholar]
- 6.Gelfand EW, Ochs HD, Shearer WT. Controversies in IgG replacement therapy in patients with antibody deficiency diseases. J Allergy Clin Immunol. 2013;131:1001–5. [DOI] [PubMed] [Google Scholar]
- 7.Smits BM, Kleine Budde I, de Vries E , Ten Berge IJM, Bredius RGM, van Deuren M , et al. Immunoglobulin replacement therapy versus antibiotic prophylaxis as treatment for incomplete primary antibody deficiency. J Clin Immunol. 2020. doi: 10.1007/s10875-020-00841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwitzguébel AJ, Jandus P, Lacroix JS, Seebach JD, Harr T. Immunoglobulin deficiency in patients with chronic rhinosinusitis: systematic review of the literature and meta‐analysis. J Allergy Clin Immunol. 2015;136:1523–31. [DOI] [PubMed] [Google Scholar]
- 9.McCullagh BN, Comellas AP, Ballas ZK, Newell JD Jr, Zimmerman MB, Azar AE. Antibody deficiency in patients with frequent exacerbations of chronic obstructive pulmonary disease (COPD). PLOS ONE. 2017;12:e0172437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orange JS, Hossny EM, Weiler CR, Ballow M, Berger M, Bonilla FA, et al.; Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology . Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117:S525–53. [DOI] [PubMed] [Google Scholar]
- 11.Shehata N, Palda V, Bowen T, Haddad E, Issekutz TB, Mazer B, et al. The use of immunoglobulin therapy for patients with primary immune deficiency: an evidence‐based practice guideline. Transfus Med Rev. 2010;24:S28–50. 10.1016/j.tmrv.2009.09.011. PMID: 19962579. [DOI] [PubMed] [Google Scholar]
- 12.Modell V, Gee B, Lewis DB, Orange JS, Roifman CM, Routes JM, et al. Global study of primary immunodeficiency diseases (PI) – diagnosis, treatment, and economic impact: an updated report from the Jeffrey Modell Foundation. Immunol Res. 2011;51:61–70. [DOI] [PubMed] [Google Scholar]
- 13.Roifman CM, Berger M, Notarangelo LD. Management of primary antibody deficiency with replacement therapy: summary of guidelines. Immunol Allergy Clin North Am. 2008;28:875‐6. [DOI] [PubMed] [Google Scholar]
- 14.Quinti I, Soresina A, Guerra A, Rondelli R, Spadaro G, Agostini C, et al. IPINet Investigators. Effectiveness of immunoglobulin replacement therapy on clinical outcome in patients with primary antibody deficiencies: results from a multicenter prospective cohort study. J Clin Immunol. 2011;31:315–22. [DOI] [PubMed] [Google Scholar]
- 15.Wasserman RL. Personalized therapy: immunoglobulin replacement for antibody deficiency. Immunol Allergy Clin North Am. 2019;39:95–111. [DOI] [PubMed] [Google Scholar]
- 16.Olinder‐Nielsen AM, Granert C, Forsberg P, Friman V, Vietorisz A, Björkander J. Immunoglobulin prophylaxis in 350 adults with IgG subclass deficiency and recurrent respiratory tract infections: a long‐term follow‐up. Scand J Infect Dis. 2007;39:44–50. [DOI] [PubMed] [Google Scholar]
- 17.Abdou NI, Greenwell CA, Mehta R, Narra M, Hester JD, Halsey JF. Efficacy of intravenous gammaglobulin for immunoglobulin G subclass and/or antibody deficiency in adults. Int Arch Allergy Immunol. 2009;149:267–74. [DOI] [PubMed] [Google Scholar]
- 18.Abrahamian F, Agrawal S, Gupta S. Immunological and clinical profile of adult patients with selective immunoglobulin subclass deficiency: response to intravenous immunoglobulin therapy. Clin Exp Immunol. 2010;159:344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khokar A, Gupta S. Clinical and immunological features of 78 adult patients with primary selective IgG subclass deficiencies. Arch Immunol Ther Exp. 2019;67:325–34. [DOI] [PubMed] [Google Scholar]
- 20.Seidel MG, Kindle G, Gathmann B, Quinti I, Buckland M, van Montfrans J, et al.; ESID Registry Working Party and collaborators . The European Society for Immunodeficiencies (ESID) Registry Working Definitions for the Clinical Diagnosis of Inborn Errors of Immunity. J Allergy Clin Immunol Pract. 2019;7:1763–70. [DOI] [PubMed] [Google Scholar]
- 21.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. [DOI] [PubMed] [Google Scholar]
- 22.Shin JJ, Liauw D, Siddiqui S, Lee J, Chung EJ, Steele R, et al. Immunological and clinical phenotyping in primary antibody deficiencies: a growing disease spectrum. J Clin Immunol. 2020;40:592‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reisi M, Azizi G, Kiaee F, Masiha F, Shirzadi R, Momen T, et al. Evaluation of pulmonary complications in patients with primary immunodeficiency disorders. Eur Ann Allergy Clin Immunol. 2017;49:122–8. [PubMed] [Google Scholar]
- 24.Modell V, Quinn J, Ginsberg G, Gladue R, Orange J, Modell F. Modeling strategy to identify patients with primary immunodeficiency utilizing risk management and outcome measurement. Immunol Res. 2017;65:713–20. [DOI] [PubMed] [Google Scholar]
- 25.Vaughan LJ. Managing cost of care and healthcare utilization in patients using immunoglobulin agents. Am J Manag Care. 2019;25:S105–S111. [PubMed] [Google Scholar]
- 26.Sadeghi B, Abolhassani H, Naseri A, Rezaei N, Aghamohammadi A. Economic burden of common variable immunodeficiency: annual cost of disease. Expert Rev Clin Immunol. 2015;11:681–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S3
Data Availability Statement
The data that support the findings of this study are available from the corresponding author EV on reasonable request.


