Abstract
Nitrite and nitrate are present in many foods. Nitrate can be converted into nitrite in human body. Nitrite can react with secondary amines to form secondary amines and with thiols to form nitrosothiols. Some nitrosamines are cancers suspect. Because of their importance in terms of human health, research on these compounds is still topical and the use of a rapid and reproducible method for determination and quantification of these compounds is necessary.
This article presents a method to study the chemical reactivity of nitrite in meat products through the analysis of non-volatile nitrosamines and nitrosothiols based on:
-
•
A specific alkaline and heat extraction of nitro-compounds followed by deprotenization by ultrafiltration
-
•
NO detection by the Griess reaction
-
•
NO released from S-NO and N-NO bonds by UV light followed by a specific cleavage of S-NO bonds with HgCl2
This method, validated on cured meat products, could be developed in the same way on all products containing nitrite and nitrate and leading to the formation of nitroso-compounds. The limit of detection for these compounds are of the order of the micromole per liter.
Keywords: Nitrite, Nitrate, Nitrosamine, Nitrosothiol, Differential spectroscopy
Graphical abstract
Specifications table
| Subject Area | Biochemistry, Genetics and Molecular Biology |
| More specific subject area | Chemistry of food products |
| Method name | Biochemical characterization of nitroso compounds in food products |
| Name and reference of original method |
Ionescu, A., Zara, M., Aprodu, I., Vasile, A., & Carac, G. (2006). Monitoring des nitrites et nitrates residules des produits de viande salée avec le teste nitrite merckoquant. Scientific Study & Research, 7(4), 745–755. Breider, F., & von Gunten, U. (2017). Quantification of Total N-Nitrosamine Concentrations in Aqueous Samples via UV-Photolysis and Chemiluminescence Detection of Nitric Oxide. Analytical Chemistry, 89(3), 1574–1582. Gaston, B. (1999). Nitric oxide and thiol groups. Biochimica et Biophysica Acta, 1411, 323–333. |
| Resource availability |
Reagents: Griess reagent kit for nitrite and nitrate assays (Ref: 23,479–1KT-F) from Sigma Aldrich (Saint Louis, USA). VivaspinⓇ 2 system (PES membrane with a cut-off of 5 kDa) (ref: VS0212) and the syringe filters with 0.22 µm regenerated cellulose membranes (Ref: 17,761) from Sartorius (Göttingen, Germany). |
*Method details
The day before the assay, pure water, NaOH 2% and HCl 7.4% should be prepared and then stored at 4 °C.
The test tubes for the determinations of nitrites, nitrates and nitrosamines must be placed at −80 °C the day before the assay. Thus, as soon as the samples are placed in the tube, all reactions are stopped.
The test tubes for the dosage of nitrosothiols must be placed at 4 °C the day before the dosage. Once the samples are placed in these tubes, they are then used at room temperature, so it is not necessary to place them at −80 °C.
Sample preparation and nitrite and nitrate extraction
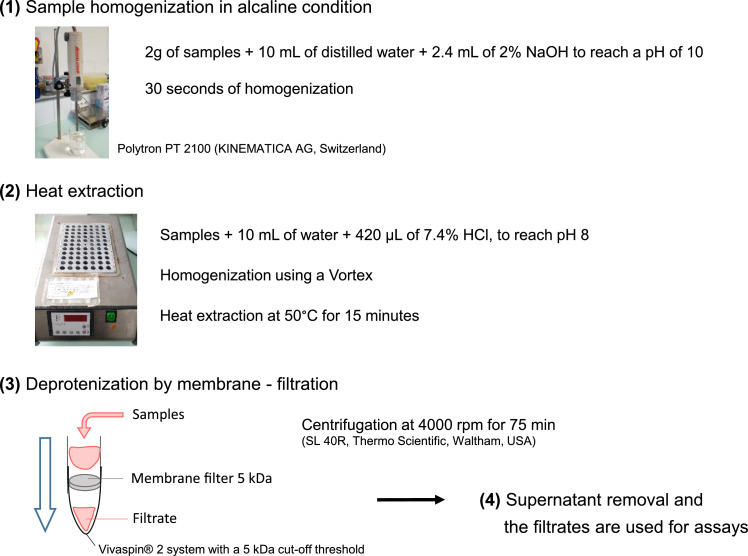
Nitrite and nitrate were extracted in cured meat samples using a method adapted from Ionescu et al. [5], and represented in Fig. 1.
Fig. 1.
Nitrite and nitrate extraction procedure.
Meat cured meat samples (2 g) were homogenized with a Polytron PT 2100 (KINEMATICA AG, Switzerland) for 30 s in 10 mL of water and 2.4 mL of 2% NaOH.
Then, the volume and pH was adjusted for subsequent steps by adding 10 mL of water and 420 µL of 7.4% HCl, to reach a final pH of 8.
The samples were homogenized with a vortex and incubated at 50 °C in a dry-bath for 15 min for the heat extraction.
Finally, the samples were deproteinized by centrifugation with a VivaspinⓇ 2 system with a 5 kDa cut-off threshold. The centrifugation was performed at 4000 rpm, at 16 °C for 75 min, with an SL 40R centrifuge (Thermo Scientific, Waltham, USA).
The supernatants were eliminated and the filtrates were used for assays described above by differential spectroscopy.
Determination of nitrite and nitrate ion contents
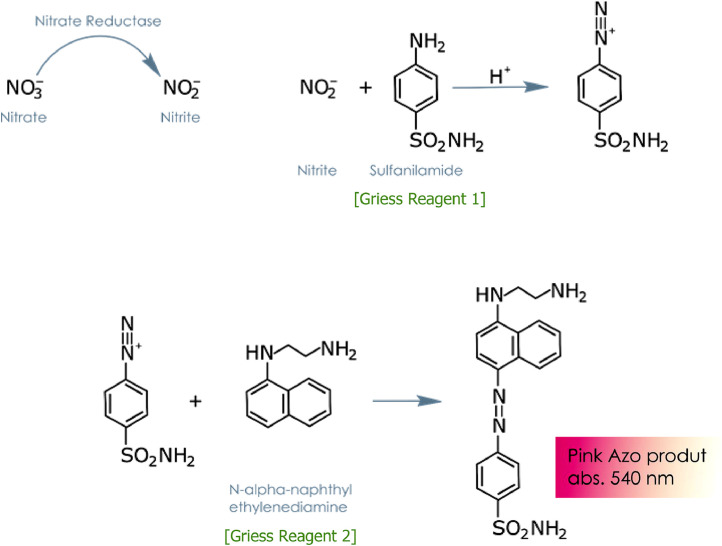
Nitrite and nitrate ion contents were then determined in the filtrates using the Griess reaction colorimetric assay kit (Ref. 23,479–1KT-F, Sigma-Aldrich) (Fig. 2).
Fig. 2.
Chemistry of the Griess Reagents.
The nitrite range was prepared from 0 µM to 100 µM, with the standard solution and the buffer from the kit (Fig. 3).
Fig. 3.
Nitrite range from 0 µM to 100 µM.
The Griess reagent 1 was added to the samples then shaked 5 min at 25 °C and 800 rpm, in the dark. Then Griess reagent 2 was added, then shaked 10 min at 25 °C and 800 rpm, in the dark.
The nitrate range was prepared from 0 to 100 µM with the standard solution and the buffer from the kit.
The nitrate reductase enzyme and cofactor were added to the samples, then shaked for 2 h at 25 °C at 800 rpm, in the dark.
The Griess reagent 1 was added to the samples then shaked 5 min at 25 °C and 800 rpm, in the dark.
Then Griess reagent 2 was added, then shaked 10 min at 25 °C and 800 rpm, in the dark.
The absorbance was measured on a microplate at 540 nm on a MULTISKAN SPECTRUM spectrophotometer (Thermo Scientific, Waltham, USA). The results were expressed in µM.
Determination of nitroso-compounds
Nitroso-compounds were determined by the Griess method with an associated specific cleavage of N-NO and S-NO bonds (Fig. 4).
Fig. 4.
Nitroso-compounds determination by differential spectroscopy.
Determination of nitrosothiol content
The filtrates (1 mL) prepared in section 1 were saturated with HgCl2 (10 mg) to specifically cleave the S-NO bonds of nitrosothiols [4]. The samples were filtered on syringe filters with 0.22 µm regenerated cellulose membrane (Ref: 17,761). Nitrite ion content was measured using the Griess reaction as described in Section 2, and the difference between this measure and the level of nitrite ions initially present in the sample (Section 1) gave the nitrosothiol content [4]. The results were expressed in µM.
Determination of nitrosamine content
Principe
UV irradiation cleaves the S-NO bonds of nitrosothiols and the N-NO bonds of nitrosamines and nitrosamides [1,4], giving a mixture of nitrite and nitrate. C-nitroso and O-nitroso containing compounds, oximes, nitramines, and N-oxide are stable under UV light and do not interfere in the assay [1]. Nitrosylheme iron does not interfere in the assays because of the withdrawal of nitroso-myoglobin during Vivaspin centrifugation. If released, nitrosyl iron (Fe-NO) is rapidly transformed, in oxidative medium, into more stable Fe [(OH)ONOO−]2− [3] with a UV resistant O-NO bond.
Method
The filtrates prepared in section 1 were irradiated with a UV lamp (model LF 215.S, 254 nm, 2 × 15 W) from Uvitec (Cambridge, England) with a sample distance with source of d = 2 cm (Fig. 5).
Fig. 5.
UV lamp.
Irradiation kinetics were sampled at 1, 15, 30, 60 and 120 min of exposure. The content of both nitrite and nitrate was measured using the Griess reaction. The rate of evaporation should be taken into account when calculating the nitroso compound content by weighing sample tubes before and after irradiation.
The concentration of [NO2− + NO3−] was plotted versus the inverse of time and extrapolation to 1/t = 0, obtained by fitting a second-degree polynomial function, giving the maximum rate of nitrite + nitrate released. An example is given in Fig. 6 where ham was cooked at 220 °C during 6 min. This measure gave a NO content corresponding to the sum of the nitrosothiol, non-volatile nitrosamine and nitrosamide contents.
Fig. 6.
Kinetic of total NO release (nitrosothiols + nitrosamines) during UV irradiation.
The subtraction of nitrosothiol content, obtained as described in section 3, gave the non-volatile nitrosamine and the nitrosamide contents (Apparent Total N-Nitroso Compounds, ATNC). Nevertheless, nitrosamides decompose rapidly and, contrary to nitrosamines, they do not accumulate in food products, so their level is generally not detectable [2]. The results were expressed in µM. The assays are carried out in triplicates.
Acknowledgments
None.
Determination of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Breider F., von Gunten U. Quantification of total N-nitrosamine concentrations in aqueous samples via UV-photolysis and chemiluminescence detection of nitric oxide. Anal. Chem. 2017;89(3):1574–1582. doi: 10.1021/acs.analchem.6b03595. [DOI] [PubMed] [Google Scholar]
- 2.Chow Y.L., Dhaliwal S.S., Polo J. Nitrosamide carcinogenesis: nitrosation of amide linkages and facile decomposition of resulting nitrosamides. IARC Sci. Publ. 1984;57:317–325. [PubMed] [Google Scholar]
- 3.Fotiou S., Fotiou D., Deliconstantinos G. Formation of heme-iron complexes with nitric oxide (NO) and peroxynitrite (ONOO-) after ultraviolet radiation as a protective mechanism in rat skin. In Vivo. 2009;23:281–286. [PubMed] [Google Scholar]
- 4.Gaston B. Nitric oxide and thiol groups. Biochim. Biophys. Acta. 1999;1411:323–333. doi: 10.1016/s0005-2728(99)00023-7. [DOI] [PubMed] [Google Scholar]
- 5.Ionescu A., Zara M., Aprodu I., Vasile A., Carac G. Monitoring des nitrites et nitrates residuels des produits de viande salée avec le teste nitrite merckoquant. Sci. Study Res. 2006;7(4):745–755. [Google Scholar]