Abstract
The blood-brain barrier (BBB) is the major obstacle for brain drug delivery and limits the treatment options for central nervous system diseases. To circumvent the BBB, we introduce focused ultrasound-mediated intranasal brain drug delivery (FUSIN). FUSIN utilizes the nasal route for direct nose-to-brain drug administration, bypassing the BBB and minimizing systemic exposure to the major organs, such as heart, lung, liver, and kidney [1]. It also uses transcranial ultrasound energy focused at a targeted brain region to induce microbubble cavitation, enhancing the transport of intranasally administered agents at the FUS-targeted brain location. FUSIN is unique because it can achieve noninvasive and localized brain drug delivery with minimized systemic toxicity to other major organs. The goal of this paper is to provide a detailed protocol for FUSIN delivery to the mouse brain.
-
•
FUSIN delivery utilizes the nose-to-brain pathway for brain drug delivery.
-
•
FUSIN utilizes FUS combined with microbubble to significantly enhance the delivery efficiency of intranasally administered drugs to the FUS targeted brain regions.
-
•
FUSIN achieves efficient brain delivery with minimized systemic exposure in the major organs.
Keywords: Focused ultrasound, Microbubbles, Intranasal administration, Blood-brain barrier, Brain drug delivery
Graphical abstract
Specifications table
| Subject Area: | Neuroscience |
| More specific subject area: | Brain drug delivery |
| Method name: | Focused ultrasound-mediated intranasal brain drug delivery technique (FUSIN) |
| Name and reference of original method: |
H. Chen, C.C. Chen, C. Acosta, S.Y. Wu, T. Sun, E.E. Konofagou, A new brain drug delivery strategy: Focused ultrasound-enhanced intranasal drug delivery, PLoS One. 9 (2014) e108880.https://doi.org/10.1371/journal.pone.0108880. H. Chen, G.Z.X. Yang, H. Getachew, C. Acosta, C. Sierra Sánchez, E.E. Konofagou, Focused ultrasound-enhanced intranasal brain delivery of brain-derived neurotrophic factor, Sci. Rep. 6 (2016) 28599.https://doi.org/10.1038/srep28599. D. Ye, X. Zhang, Y. Yue, R. Raliya, P. Biswas, S. Taylor, Y. Tai, J.B. Rubin, Y. Liu, H. Chen, Focused ultrasound combined with microbubble-mediated intranasal delivery of gold nanoclusters to the brain, J. Control. Release. 286 (2018) 145153.https://doi.org/10.1016/j.jconrel.2018.07.020. R. Ji, M. Smith, Y. Niimi, M.E. Karakatsani, M.F. Murillo, V. Jackson-Lewis, S. Przedborski, E.E. Konofagou, Focused ultrasound enhanced intranasal delivery of brain derived neurotrophic factor produces neurorestorative effects in a Parkinson’s disease mouse model, Sci. Rep. 9 (2019) 19402.https://doi.org/10.1038/s41598-019-55294-5. |
| Resource availability |
Method details
Materials and equipment
Intranasal delivery
-
•
A small pillow for mouse head support is made by rolling a 2 × 2 gauze square and tape it to form a pillow.
-
•
Pipettor (e.g., P20).
-
•
Pipette tips.
-
•
Timer.
-
•
The drug/agent solution to be administered to the mouse through IN [e.g., Near-infrared fluorescent dye-labeled bovine serum albumin (800CW-BSA)].
-
•
Anesthesia machine (Mobile Anesthesia System, Harvard Apparatus) with anesthesia chamber and nose cone.
FUS treatment
-
•
Adult mice [e.g., Cr.NIH (Swiss), 6–8 weeks, ~25 g body weight]
-
•
Microbubbles: There are commercially available microbubbles, for example, Definity (Lantheus Medical Imaging) and Optison (GE Healthcare). Microbubbles can also be made in-house [2], [3].
-
•
Ultrasound gel (Aquasonic 100, Parker Labs).
-
•
Hair removal cream (Veet gel hair removal cream, Amazon).
-
•
Small animal hair clipper (Wahl BravMini Professional Cordless Clipper Kit, Kent Scientific).
-
•
Ophthalmic ointment.
-
•
0.9% saline.
-
•
Absorbent gauze pads.
-
•
Alcohol pads.
-
•
Hand warmers for warming up the mouse tails for tail vein injection (HotHands Hand Warmer, Amazon).
-
•
Electrical heating pad.
-
•
Surgical tape.
-
•
Hamilton syringe (Model 1825, Hamilton, Fisher Scientific).
-
•
1 mL disposable plastic syringes with 27 G × 1/2″ needles for injecting saline and microbubbles through a tail vein catheter.
-
•
Polyethylene tubing (I.D. 0.38 mm) to connect the 27 G × 1/2″ needles to the Hamilton syringe and the 1 mL plastic syringe.
-
•
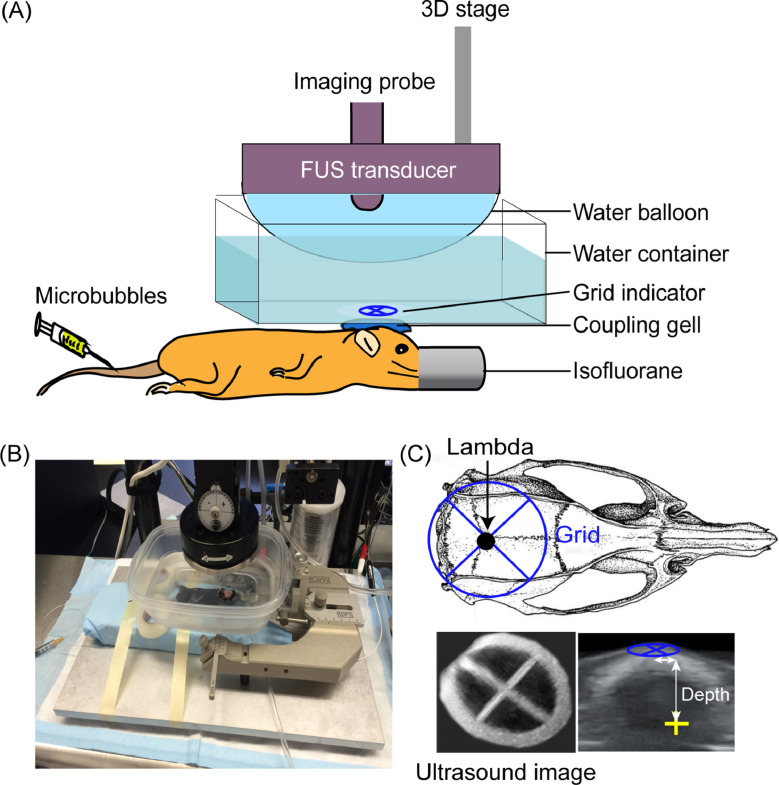
FUS system: FUS transducer, function generator, power amplifier, 3D stage, degassing unit, and software control (e.g., VIFU 2000, Alpinion US Inc., Bothell, WA, USA). The FUS transducer is attached to a silicone membrane and filled with degassed and distilled water, forming a water balloon to provide acoustic coupling to the FUS transducer (Fig. 2A, 2B).
-
•
An ultrasound imaging system (e.g., EQ12R, Alpinion, Seoul, Korea) or an MRI scanner for image-guided FUS targeting to a specific brain location.
-
•
A small plastic water container (200 mL) with a circular opening (diameter = 2 cm) at the bottom (Fig. 2A, 2B). The opening is sealed with an acoustically and optically transparent membrane (Tegaderm, 3 M, St. Paul, MN, USA).
-
•
A stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA).
Fig. 2.
FUS setup. (A) Illustration and (B) picture of the FUS setup. (C) FUS targeting at a specific brain location with the assistance of a grid. The grid is placed on top of the mouse head with the center of the grid aligned visually with the lambda on the skull, which can be visualized through the scalp. B-mode images of the grid were obtained and reconstructed in 3D to identify the grid cross point. The specific brain location is targeted based on its stereotactic coordinates in X, Y, and Z in reference to the lambda according to the mouse brain atl as [8].
Methods
Intranasal administration
-
•
Anesthetize the mouse. We normally use isoflurane for animal anesthesia. The mouse is first placed in an anesthetic induction chamber with 2% isoflurane, then moved to a nose cone that delivers anesthetic gas at 1–2% isoflurane to maintain the anesthetization. The mouse nose is kept in the nose cone and released when administering the desired delivery agent to the nose.
-
•
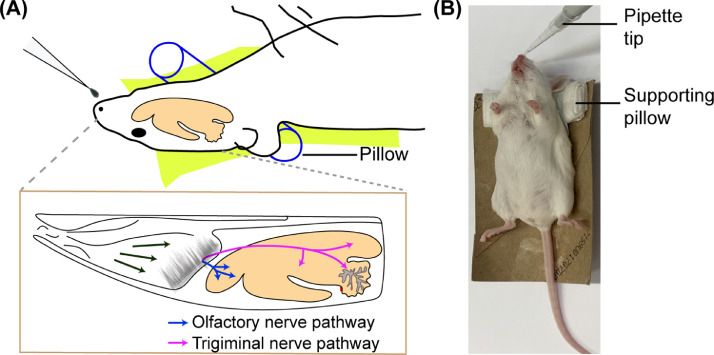
Place the mouse supine on a holder with a small pillow under its neck (Fig. 1). The pillow ensures that the mouse's neck remains flat and that the airway is not constricted. When placing the mouse neck on the pillow, the mouse head is naturally lying on the pillow, which is around 10°−30° to the table. Place a heating pad under the holder.
-
•
Prepare the delivery agent (e.g., 800CW-BSA, dextran, nanoparticles, etc.) to be administered intranasally. Place it nearby, and store in ice if necessary.
-
•
Set the volume of the P20 pipettor to 3 µL. This volume can form a drop size suitable for delivering to adult mice weighing between 18 g and 35 g. For newborn mice, the volume should be no more than 1 µL. For rats, the volume should be no more than 12 µL [4].The volume must be controlled to avoid choking the animal.
-
•
Draw 3 µL solution of delivery agent into a pipette tip, move the mouse from the nose cone and place the pipette tip near the mouse's nose at a certain angle (~45°) from the table. This angle can be adjusted based on the solution's viscosity to form a liquid drop at the end of the pipette tip without causing the drop to fall before the animal inhales it. Slowly form a round drop at the end of the pipette tip by pipetting the dosing solution slowly. Lower the drop until it is close enough to one side of the mouse nostril and let the mouse inhale the drop on its own. Allowing the mouse to inhale the drop increases the chance of the delivery agent solution to reach the olfactory mucosa, located at the back of the nose (Fig. 1A).
-
•
Once the mouse inhales the drop, insert the mouse nose back to the nose cone and wait at least 2 mins before repeating this procedure for the other side of the nostril. It is important to wait at least 2 mins between drops to allow the drops to soak into the nasal epithelium thoroughly regardless of the drug concentration of the drops. Waiting less than 2 mins could cause the solution to build up in the mouse's nasal cavity and cause choking.The waiting time can be extended to 5 mins if the nose cavities are full of fluid.
-
•
Repeat the previous two steps until the mouse has received 8 drops, totaling 24 µL of delivery agent solution. This total volume of delivery agent is sufficient for small-large mice (18 – 35 g). For newborn mice, a smaller total volume (8 µL) and droplet size (1 µL) is recommended to be used to accommodate a smaller nasal cavity, with 15–20 s waiting period between each drop [5]. For rats, a larger total volume (48 µL) can be administered, with 12 µL drop size each to alternate nares and a 5 min waiting period between each drop [4].
-
•
During this whole process, monitor mouse breathing carefully by watching the movement of the chest wall and adjusting the anesthesia level accordingly. Make sure the mouse is breathing smoothly and consistently during the whole IN delivery process. After the last drop, move forward to the focused ultrasound treatment described below.
Fig. 1.
(A) Illustration and (B) picture of IN administration. The mouse is placed at the supine position with its neck supported by a pillow.
Focused ultrasound treatment
-
•
Prepare the FUS system. The FUS system consists of a FUS transducer, function generator, and power amplifier. The design of the FUS system can vary, but the basic requirement is able to generate ultrasound pulses with controllable parameters, such as pulse amplitude, pulse length, pulse repetition frequency, and total duration. The FUS transducer normally has a frequency of 1.5 MHz for mice experiments. The FUS transducer is normally connected to a 3D stage to adjust the position of the transducer. The whole system can be controlled by customized code in MATLAB or other programs.
-
•
Prepare the microbubbles. Definity microbubbles can be used in this study, so as other microbubbles. For using definity, one needs to follow the user instruction carefully when using Definity. Take the Definity vial from the 4 °C refrigerator. Only when it is ready for tail vein injection of the microbubble, active the vial via mechanical agitation using a VialMix (Lantheus Medical Imaging) shaker for a pre-set time of 45 s. As microbubbles are easy to be ruptured, effort should be made to handle the microbubbles carefully. For example, use a large gage (18 or 20 G) needle to draw the microbubbles from its vial and gently hand agitate the syringe to evenly distribute the microbubbles.
-
•
Transfer the mouse from the IN-delivery stage to the ultrasound treatment stage. Place the mouse on a heated support in the prone position. The mouse head is immobilized by the stereotaxic frame with two ear bars and one biting bar. Apply ophthalmic ointment in each eye to protect the cornea from desiccation or other injuries. The limbs of the mouse are fixed with surgical tape on the support.
-
•
Remove the fur of the mouse from head to neck using a hair clipper. Apply hair removal cream and clean it after 2–4 mins with gauze and alcohol wipe. Make sure that no fur is left on the head and that the scalp remains intact. Maintain caution when applying the hair removal cream, as leaving the hair removal cream too long can burn the mice scalp.
-
•
Place a tail vein catheter to provide an easy route for microbubble injection. Warm the tail with a hand warmer if necessary, wipe the tail with an alcohol wipe, and insert a 27 G × 1/2″ needle to the tail vein with another end of the needle connected to a tube. The tube is first connected to a 1 mL disposable plastic syringe filled with saline. Inject 30 µL saline to the mouse tail vein to make sure the tail vein injection is successful. If successful, secure the catheter using surgical tape.
-
•
Place a water container on the mouse head and coupled with degassed ultrasound gel. The bottom of the water container has a window sealed with Tegaderm. The FUS transducer is attached to a water balloon filled with degassed water. The water balloon is immersed in the water container to provide acoustic coupling.
-
•
FUS transducer targeting at a specific brain location can be achieved in different ways. Previous studies have shown that FUSIN can be used for spatially targeted delivery of agents to the caudate putamen [6], [7]. This study aims to achieve successful agent delivery to the brainstem. In this study, the FUS system is integrated with an ultrasound imaging system. Specifically, an ultrasound imaging probe (L8–17, working frequency 8–17 MHz, center frequency 12 MHz, Alpinion, Seoul, Korea) is inserted into the FUS transducer center opening and co-axially aligned with the FUS transducer (Fig. 2A). The FUS targeting is performed with the assistance of a metal grid, which is visible on the ultrasound images. The grid is positioned in the water container on top of the mouse head with the crossing point aligned with lambda, an anatomic landmark on the skull, and visible through the mouse intact skin on the head. The crossing point of the grid is identified based on B-mode imaging of the grid (Fig. 2C). For the targeting of a specific brain location, the transducer is moved in X, Y, and Z directions according to the coordinates of the targeted brain location in reference to the lambda determined based on the mouse brain atlas [8]. For example, to target the right brainstem in this study, the FUS transducer is moved according to the stereotactic locations of the right brainstem in reference to lambda: 0.0 mm in Y and −1.5 mm in X. The depth of the FUS focus is adjusted to be 4.00 mm from the skull as measured from the B-mode images of the mouse head (Fig. 2C). Magnetic resonance imaging (MRI)-guided FUS system can also be used to increase the precision and accuracy of targeting, especially for treating the brain tumor [9], [10].
-
•
Microbubble injection: The dose of the Definity used is 1 × or 10 × of the clinical dose (10 µL/kg). Use the Hamilton to draw the microbubble solution. Then, replace the 1 mL saline syringe in previous step with the Hamilton syringe. Carefully inject the microbubbles slowly to the tail vein, change back to the saline syringe, and inject saline to flush the tail vein catheter.
-
•
FUS sonication: Immediately following microbubbles injection, turn on the FUS sonication with defined pressure, pulse length, pulse repetition frequency, and total duration. The FUS transducer in our study had a center frequency of 1.5 MHz, a focal depth of 60 mm, an aperture of 60 mm, a circular central opening of 38 mm, and was driven by a built-in signal generator. The pressure amplitudes and beam dimensions of the FUS transducer were calibrated using a needle hydrophone (Onda, CA, USA) in a degassed water tank before the experiment. The pressures reported here were the measured hydrophone peak negative pressures with corrections for mouse skull attenuation by 18% [11]. The full width at half maximum (FWHM) of the axial and lateral pressure profiles was 6.04 mm and 0.62 mm, respectively. The parameters used in our study are normally peak-negative pressure = 0.54 MPa, pulse length = 6.7 ms, pulse repetition frequency = 5 Hz, and duration = 1 min. During FUS sonication, the ultrasound imaging system can be used in passive listening mode for passive cavitation imaging.
-
•
Gently remove the tail vein catheter and apply pressure with gauze until the blood stops from flowing out from the puncture site. For the acute study, the animal is sacrificed by transcranial perfusion. For survival studies, the remove the mouse from the FUS treatment stage to a warm heating pad for recovery. Return the mouse to its cage once the mice wakes up.
Methods validation
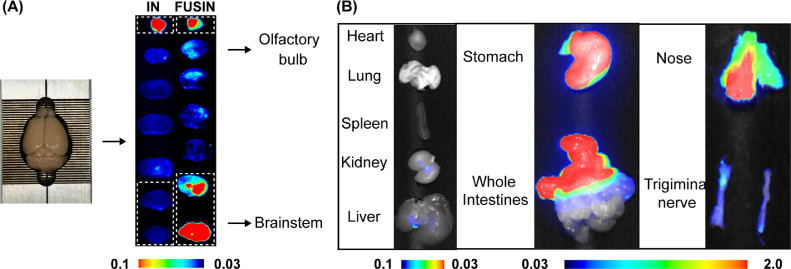
FUSIN delivery outcome evaluation: Depending on the agents used in the FUSIN delivery studies, the FUSIN delivery outcome can be evaluated with different approaches. For example, we used near-infrared fluorescent dye-labeled bovine serum albumin (800CW-BSA) as our model agent in this study and assessed the brain delivery and biodistribution of the agent using Pearl Imaging System (LI-COR Biosciences, Lincoln, NE). After perfusion, the mouse brains were excised and sliced into 2-mm coronal sections using a brain matrix (RBM-2000C; ASI Instruments, Inc., Warren, MI, USA). The nose, trigeminal nerve, heart, lung, spleen, kidney, liver, stomach, and intestines were harvested to study the biodistribution of 800CW-BSA. The brain slices (Fig. 3A) and all organs were examined by the Pearl Imaging System using the 800 nm channel for 800CW-BSA (Fig. 3B). This finding is consistent with our previous study, which has found that the concentration of IN-administered radiolabeled and fluorescence-labeled gold nanoclusters was relatively low in all major organs except the stomach and intestines [1], [12]. Those accumulations in the stomach and intestine could be excreted through feces, leading to minimal systemic toxicity [13]. Moreover, our previous study also compared the systemic distribution associated with FUSIN, IN and IV delivery of the gold nanoparticles. The systemic exposure of the gold nanoparticles by IN delivery was one-magnitude lower than that by IV in major organs except the stomach and intestines. However, there is no significant difference between FUSIN and IN regarding the systemic biodistribution of the gold nanoparticles [1], [12].
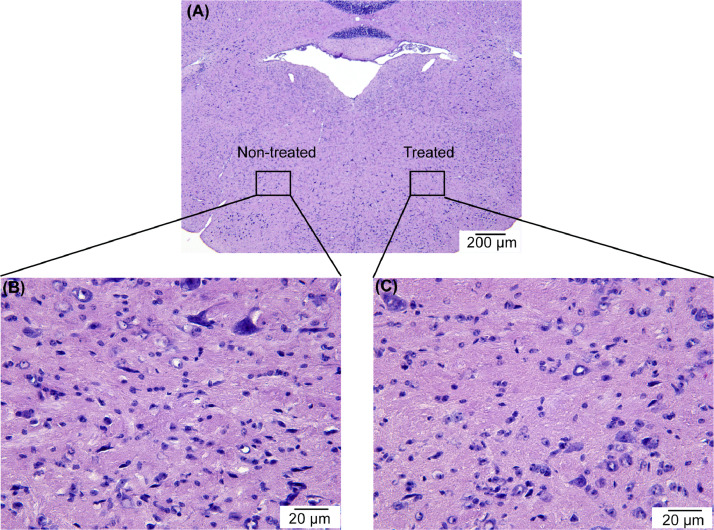
Fig. 4.
Representative histological examination of the treated side and non-treated side of the mouse brainstem showing that FUSIN treatment did not induce hemorrhage or neuron damage.
Fig. 3.
FUSIN outcome assessment. (A) Fluorescence imaging showed the spatial distribution of 800CW-BSA in the brain after IN delivery and FUSIN delivery targeting the brainstem. The high fluorescence intensity observed at the olfactory bulb confirmed IN-administered 800CW-BSA transport to the brain along the olfactory pathway. (B) Fluorescence images of major organs, nose, and trigeminal nerve showed the biodistribution of 800CW-BSA delivered by FUSIN.
Our previous study showed that FUSIN delivery of nanoparticles did not induce any change or damage to the nasal tissue, trigeminal nerves, or the FUS-targeted brainstem and caudate putamen [7], [12], [14]. Shown below is the representative hematoxylin and eosin (H&E) staining of the FUS-treated brainstem at 0.70 MPa, which is the highest tested acoustic pressure level (0.70 MPa) used in our FUSIN delivery studies. No hemorrhage or neuron damage was observed in all the H&E-stained mouse brain slices, confirming that the FUS parameters used in FUSIN study are safe at the histological level.
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) Grants R01EB027223, R01EB030102 and R01MH116981. It was also partially supported by the Charlie Teo Foundation and Little Legs Foundation. The authors would like to thank Yimei Yue for assisting with the animal experiment, Dr. Jingyi Luan and Dr. Srikanth Singamaneni for providing the 800CW-BSA.
Contributor Information
Dezhuang Ye, Email: dezhuang.ye@wustl.edu.
Hong Chen, Email: hongchen@wustl.edu.
References
- 1.Ye D., Zhuang X., Yue Y., Taylor S., Tai Y.C., Rubin J.B., Liu Y., Chen H. Comparison of focused ultrasound-mediated intranasal delivery and focused ultrasound-induced blood-brain barrier disruption in the delivery of gold nanoclusters to the brainstem. IEEE Int. Ultrason. Symp. IUS. 2018–Janua. 2018:5–8. [Google Scholar]
- 2.Feshitan J.a., Chen C.C., Kwan J.J., Borden M.a. Microbubble size isolation by differential centrifugation. J. Colloid Interface Sci. 2009;329:316–324. doi: 10.1016/j.jcis.2008.09.066. [DOI] [PubMed] [Google Scholar]
- 3.Wang S., Samiotaki G., Olumolade O., a Feshitan J., Konofagou E.E. Microbubble type and distribution dependence of focused ultrasound-induced blood-brain barrier opening. Ultrasound Med. Biol. 2014;40:130–137. doi: 10.1016/j.ultrasmedbio.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar N.N., Lochhead J.J., Pizzo M.E., Nehra G., Boroumand S., Greene G., Thorne R.G. Delivery of immunoglobulin G antibodies to the rat nervous system following intranasal administration: distribution, dose-response, and mechanisms of delivery. J. Control. Release. 2018;286:467–484. doi: 10.1016/j.jconrel.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H., Dai C.L., Gu J.H., Peng S., Li J., Yu Q., Iqbal K., Liu F., Gong C.X. Intranasal administration of insulin reduces chronic behavioral abnormality and neuronal apoptosis induced by general anesthesia in neonatal mice. Front. Neurosci. 2019;13:1–13. doi: 10.3389/fnins.2019.00706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H., Chen C.C., Acosta C., Wu S.Y., Sun T., Konofagou E.E. A new brain drug delivery strategy: focused ultrasound-enhanced intranasal drug delivery. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0108880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H., Yang G.Z.X., Getachew H., Acosta C., Sierra Sánchez C., Konofagou E.E. Focused ultrasound-enhanced intranasal brain delivery of brain-derived neurotrophic factor. Sci. Rep. 2016;6:28599. doi: 10.1038/srep28599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.K.B.J. Franklin, G. Paxinos, The mouse brain in stereotaxic coordinates, 2007.
- 9.McDannold N., Vykhodtseva N., Hynynen K. Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood-brain barrier disruption. Ultrasound Med. Biol. 2008;34:930–937. doi: 10.1016/j.ultrasmedbio.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDannold N., Vykhodtseva N., Hynynen K. Blood-brain barrier disruption induced by focused ultrasound and circulating preformed microbubbles appears to be characterized by the mechanical index. Ultrasound Med. Biol. 2008;34:834–840. doi: 10.1016/j.ultrasmedbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi J.J., Pernot M., Small S.A., Konofagou E.E. Noninvasive, transcranial and localized opening of the blood-brain barrier using focused ultrasound in mice. Ultrasound Med. Biol. 2007;33:95–104. doi: 10.1016/j.ultrasmedbio.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Ye D., Zhang X., Yue Y., Raliya R., Biswas P., Taylor S., Tai Y., Rubin J.B., Liu Y., Chen H. Focused ultrasound combined with microbubble-mediated intranasal delivery of gold nanoclusters to the brain. J. Control. Release. 2018;286:145–153. doi: 10.1016/j.jconrel.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talegaonkar S., Mishra P.R. Intranasal delivery : an approach to bypass the blood brain barrier. Indian J. Pharmacol. 2004;36:140–147. [Google Scholar]
- 14.Ye D., Luan J., Pang H., Yang Y., Nazeri A., Rubin J.B., Chen H. Characterization of focused ultrasound-mediated brainstem delivery of intranasally administered agents. J. Control. Release. 2020;328:276–285. doi: 10.1016/j.jconrel.2020.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]