Abstract
Detailed methods for imaging calcium activity in single cells within the ventral posteromedial thalamic nucleus of the rat was completed for the first time in these studies. These methods also detail the procedure to image calcium activity in individual GABAergic neurons within the reticular thalamic nucleus using GAD1-Cre rats. This activity was measured in freely behaving rats allowing for recording of activity from GABA neurons during behavioral testing. Key methods for imaging success are:
-
•
Calcium activity in the lateral thalamic region is inhibited by isoflurane anesthesia and GCAMP florescent cells are often not observed when mounting the baseplate.
-
•
If no cells are observed when mounting the baseplate then place the lens at 300 micrometers or focus on a blood vessel if present.
-
•
Depending on the virus, a one microliter infusion could be needed to produce a one millimeter field of GCAMP positive cells for imaging with a lens having a one millimeter diameter.
Keywords: GCAMP, Reticular thalamus, ventral posteromedial nucleus
Specifications table
| Subject Area | Neuroscience |
| More specific subject area | Calcium imaging of individual neurons within the thalamus of freely behaving animals |
| Method name | Miniscope lens placement and calcium imaging in rat thalamus |
| Name and reference of original method | Resendez SL, Jennings JH, Ung RL, Namboodiri VM, Zhou ZC, Otis JM, Nomura H, McHenry JA, Kosyk O, Stuber GD (2016), Visualization of cortical, subcortical and deep brain neural circuit dynamics during naturalistic mammalian behavior with head-mounted microscopes and chronically implanted lenses. Nat Protoc 11:566-597. [2] |
| Resource availability |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Animal husbandry
-
1)
This study was carried out in accordance with the recommendations of Institutional Animal Care and Use Committee Guidebook and Texas A&M University College of Dentistry Institutional Animal Care and Use Committee. The animal protocol was approved by the Texas A&M University College of Dentistry Institutional Animal Care and Use Committee.
-
2)
Transgenic male (300 g) (Rat Resource and Research Center strain LE-Tg(Gad1-iCre)3Ottc, RRRC#: 00751, developed by Brandon Harvey and Jim Pickel were kept on a 14:10 light/dark cycle.
Surgery Prep
-
1)
Prepare surgical equipment Fig. 1
-
2)
Place in anesthesia box
-
3)
Maintain high flow (5 L/min) and 5% isoflurane until rat is anesthetized
-
4)
Trim head with clippers, wipe area with sterile gauze
-
5)
Place in anesthesia box and maintain a high flow (5 L/min) until rat is anesthetized
-
6)
Clean ear bars and mouth apparatus with sterile 0.9% saline solution
-
7)
Make sure ear bars and mouth apparatus is completely dry before mounting rat (helps reduce ear infection)
-
8)
Connect oxygen monitor, run Physio Suite (Kent Scientific Corporation, Torrington, CT), and place mask for application of isoflurane.
-
9)
Place rat in ear bars
-
10)
Make sure head doesn't move laterally
-
11)
Attach gas mask and head securely to mouthpiece
-
12)
Maintain anesthesia with 1–2 L/min air flow and 1–2% isoflurane
-
13)
Place lubricant on eyes with sterile cotton swab to prevent drying (Puralube vet ointment, Dechra Veterinary Products, Overland Park, KS)
-
14)
Make incision with scalpel #15 blade from between eyes to 5 mm behind lambda
-
15)
Move skin out of the surgical site by placing bull dog clamps (Fig. 2)
-
16)
Clean area by scraping the bone with a scalpel blade
Fig. 1.
Surgical equipment preparation.
Fig. 2.
Surgical placement of screws.
Important-you do not want any of this soft tissue to go inside the holes used for the screws as it will weaken the attachment of the screws
-
1)
Clean area with sterile 0.9% saline solution, letting saline sit on skull for 30–60 s before removal helps with bleeding.
-
2)
Dab lightly with sterile cotton gauze to remove saline, repeat rinsing and removal until clean and dry.
Screw placement and skull preparation
-
1)
Use #2 round burr to create rough surface on skull for attachment of dental cement.
-
2)
Place support screws (#0–80 x 1/8″ Round Head Slotted Machine Screw)(Granger Catalog #2AY40) to support lens on skull plate (Fig. 2). The first time you perform a surgery you may need to mark the injection and lens placement location until you have idea of best screw placement.
-
3)
Important: place screw a minimum of 5 mm distance from the lens placement site to allow for correct seating of the lens (Fig. 3).
-
4)
Drill hole for the placement of 4 screws in a rectangular pattern as vertical as possible without puncturing the dura (Fig. 2). Fewer screws may be functional and the experimenter can test in their animals.
-
5)
Turn in screw no more than two turns. This prevents the inserted screw into the surface of the brain and cutting the dura.
-
6)
Clean area with sterile 0.9% saline solution letting saline sit on skull for 30–60 s before removal helps with bleeding. Dab up solution, wiping or rubbing solution will often induce more bleeding.
Fig. 3.
Marking location of injection opening on skull.
Flat skull and measurements process
-
1)
Mount a Hamilton syringe (Neuros model #7001, Hamilton Reno NV) to the stereotaxic instrument.
-
2)
Check make sure needle is vertical. We use index card (Fig. 4).
-
3)
Take a reading on the Bregma suture
-
4)
Take a reading on the Lambda suture
-
5)
Want the Bregma and Lambda measurements to differ by no more than 0.2 mm in depth
-
6)
Adjust tilt of skull and repeat as necessary to obtain the flat skull.
Fig. 4.
Adjustment of injection needle to ensure a 90 degree angle.
Injection Burr Holes
-
1)
Ensure the skull is clean and dry. If bleeding or blood is present cover area with sterile 0.9% saline solution, letting saline sit on skull for 30–60 s before removal helps with bleeding. Repeat as necessary.
-
2)
Place needle apparatus at proper coordinates, lightly mark needle placement on skull with round burr. (Fig. 3)
-
3)
For the ventral posteromedial thalamic nucleus we used coordinates AP=3.7, midline=3.0, and depth=6.0 mm from Bregma and for the reticular thalamic nucleus we used AP=3.7, midline=3.5, depth=6.0 mm from Bregma [1].
-
4)
Increase depth of hole in the skull then recheck by dropping needle to hole on skull, repeat periodically as you create burr hole.
-
5)
Repeat for all injection sites
-
6)
Important: In the case of the craniotomy produced for lens insertion, widen to accommodate the injection and lens to be place on-center of hole. The injection needle and lens should be centered on the circular craniotomy (Fig. 5). This will allow for proper targeting of the GCAMP cells for imaging.
-
7)
Try to not puncture dura (if possible, it will be punctured once injection needle is pushed through). There will likely be some bleeding. If bleeding is profuse dab up some of pooling blood with cotton swab but do not touch needle or apparatus.
Fig. 5.
Placement of lens after injection.
Virus loading into needle
-
1)
Rinse and clean Hamilton syringe by rinsing 5 times with sterile 0.9% saline. Filling and expelling solution.
-
2)
Clean needle with cotton swabs wetted with sterile 0.9% saline
-
3)
Pipette 4 microliter droplet virus solution into cap of 1.5 ml centrifuge tube
-
4)
Get needle halfway into droplet and centered
-
5)
Draw up 0.5–1 microliter with syringe pump (Stoelting model # 780310 s, Wood Dale IL) (Fig. 6)
-
6)
Withdrawal 0.5–1 microliter in 30 s
-
7)
Pipette leftover virus from cap back into tube, cap tube and place on ice
-
8)
We performed a test of this procedure by infusing the reticular thalamic nucleus with 0.5 microliter of pAAV1.Syn.Flex.GCAMP7f.WPRE.SV40 (Addgene, Watertown, MA)
Fig. 6.
Hamilton syringe and syringe pump on stereotaxic holder.
Injection of virus
-
1)
Place Hamilton syringe pump with stereotaxic holder back on stereotactic apparatus
-
2)
Move needle on center of desired burr hole.
-
3)
Important: If the needle is not on center then you have moved the needle during filling procedure by touching a surface, recenter needle.
-
4)
Slowly lower to specified depth
-
5)
Lower needle at rate of 0.6 mm every minute
-
6)
Set syringe pump to infuse at rate of 50 nanoliters per minute
-
7)
After infusion is complete wait 10 min before withdrawal of needle
-
8)
Raise needle at rate of 0.6- millimeters every minute
-
9)
Clean needle with cotton swabs wetted with sterile 0.9% saline
Lens implant
-
1)
Clean caniotomy and skull with cotton swabs wetted with sterile 0.9% saline, dabbing and not rubbing or twisting.
-
2)
For persistent bleeding let a pool of 0.9% saline rest for 30 s to 1 min and then dab dry
-
3)
Use air to dry skull bone
-
4)
Load lens (9.0 mm length by 1.0 mm diameter, Inscopix, Palo Alto, CA) into stereotactic apparatus (Fig. 5), Use tweezers that have silicone covering to prevent scratching the lens while handling (Fig. 1 bottom panel)
-
5)
Take depth measurement at Bregma with the lens
-
6)
Calculate specific depth of lens placement. This will be 0.2 mm above the injection site. In our studies the coordinate for depth was 5.8 mm. Histological examination should be performed to determine proper placement.
-
7)
Move lens to be in the center of desired burr hole
-
8)
Place sterile normal saline in and around burr hole
-
9)
Drop lens at a rate of 0.6 mm every minute until the specific depth is reached
Bonding process
-
1)
Chill porcelain disk for mixing dental cement (Metabond, Parkell Inc. Edgewood, NY)
-
2)
1 scoop + 3 drops quick base + 1 drop catalyst
-
3)
Mix with wooden tip from cotton swab
-
4)
Shave wooden end of tip to a flattened shape which will be used as a spatula to place dental cement (Fig. 7 and 8)
-
5)
Apply cement gently, try to use capillary action to apply. Ensure that some cement contacts the implanted screws.
-
6)
Important: ensure no dental cement gets on the lens holder near the connection with the lens or you will not be able to separate the holder from the implanted lens. (Fig. 7)
-
7)
Let harden 20 min
-
8)
Remove lens implant apparatus
-
9)
Add more dental cement around lens after removing apparatus to bond lens in place and attach lens to supporting screws (Fig. 8)
-
10)
Let harden for 20 min
-
11)
Place a 3 mm square of Parafilm on top of lens (Fig. 9)
-
12)
Cover lens with a KWIK-SIL silicone low viscosity (World Precision Instruments, Sarasota, FL) (Fig. 10)
-
13)
The screw placement procedure could be moved to this step. You would bond the lens to the skull first. Second, place the screws and then third, bond the lens to the screws using a second batch of Metabond mixture.
Fig. 7.
Cement lens to skull with lens in holder.
Fig. 8.
Cement lens to screws after holder is removes.
Fig. 9.
place parafilm over top of lens.
Fig. 10.
Cover lens with protectant silicone.
Finish and close
-
1)
Close via sutures or superglue any excess skin around surgical site
-
2)
Inject with pain medication
-
3)
Place rat in recovery cage on heating pad
-
4)
Monitor until awake
-
5)
Place in cage without small particulate bedding (blotting paper or paper towels are appropriate) and place a gel food cup in cage.
-
6)
We recommend individual housing
Mounting baseplate for miniscope
-
1)
Four to six weeks after lens placement the baseplate for the miniscope will be secured
-
2)
Place in anesthesia box
-
3)
Maintain a high flow (5 L/min) and 5% isoflurane until rat is anesthetized
-
4)
Clean ear bars and mouth apparatus with sterile 0.9% saline solution
-
5)
Make sure ear bars and mouth apparatus is completely dry before mounting rat
-
6)
Connect oxygen monitor and place in gas mask as needed while mounting
-
7)
Place rat in ear bars.
-
8)
Make sure head doesn't move laterally
-
9)
Attach gas mask and head securely to mouthpiece
-
10)
Maintain anesthesia with 1–2 L/min air flow and 1–2% isoflurane
-
11)
Important: Score the baseplate with grooves using the burr to assist in dental cement attachment (Fig. 11).
-
12)
Place baseplate (Inscopix) on InVoke miniscope (Inscopix) (Fig. 12)
-
13)
Create mound or mushroom of dental cement around the lens for an attachment surface for the baseplate. Wait at least 30 min for the cement to harden. Or you can stop here and complete the procedure the next day. At this point a lot of cement is needed and we use a cheaper dental cement (Stoelting) (Fig. 13).
-
14)
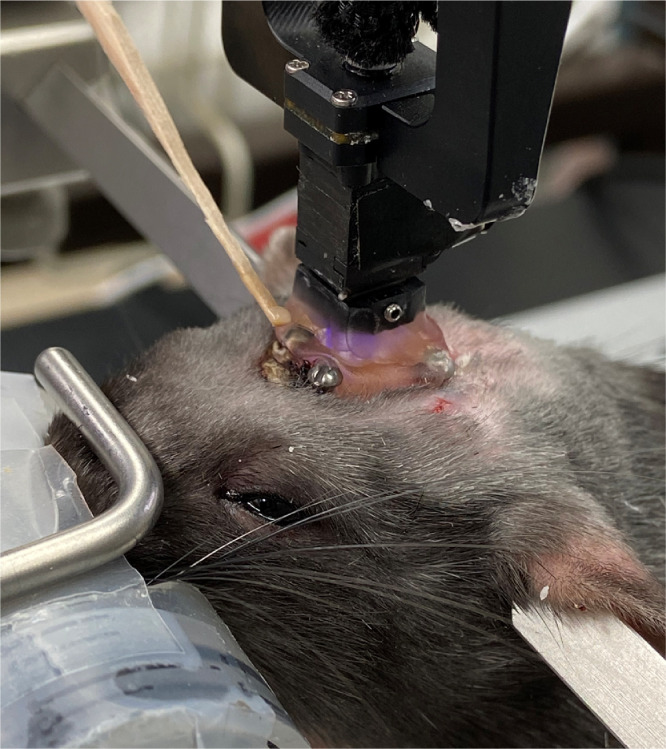
Move miniscope over implanted lens with several millimeters separation between the objective lens of the miniscope and the implanted lens. Ensure the lens of the minscope and the implanted lens are parallel, manipulate the head of the animal to obtain a flat skull and move the lens holder to obtain a parallel orientation (Fig. 14).
-
15)
Start the Inscopix imaging software and turn on miniscope. Move miniscope so that lenses touch and you see the lenses are clean and parallel with a clear ring in the image (Fig. 15).
-
16)
Raise the miniscope to focus on any blood vessels. Slowly move miniscope up and down to image flashing cells. Important: under anesthesia often no visible flashing in the thalamus is observed but once the animal is awake the flashing cells are visible. In the event no flashing cells are observed focus lens on blood vessel (Fig. 16) or simply mount scope 290 micrometers above the implanted lens. The newer Inscopix miniscopes have a focusing feature that allows for fine adjustment.
-
17)
Use Metabond to attach baseplate to the dental cement mushroom surrounding the lens (Fig. 17).
-
18)
Wait at least 20 min to harden. View image with miniscope to determine that the focal plane has not changed . If the focus has changed you can use burr to remove dental cement and reattach baseplate.
-
19)
Remove miniscope and place baseplate cover on baseplate (Fig. 18).
-
20)
Place rat in recovery cage on heating pad
-
21)
Monitor until awake
-
22)
Place in cage
-
23)
We recommend individual housing
Fig. 11.
Baseplate preparation for attachement.
Fig. 12.
nVoke miniscope in stereotaxic holder imaging implanted lens.
Fig. 13.
Use dental cement to create a region for miniscope baseplate to attach.
Fig. 14.
Miniscope objective lens and implanted lens parallel.
Fig. 15.
View from miniscope when objective lens from miniscope and implanted lens are parallel and touching.
Fig. 16.
View from miniscope of a blood vessel in focus.
Fig. 17.
Attachement of baseplate to the skull.
Fig. 18.
Cover for baseplate to protect lens.
Miniscope and imaging procedures
-
1)
An InVoke microscope from Inscopix was placed within the bracket and images were captured and processed with Inscopix software. Inscopix Data Processing software was used to identify individual cells and events were detected using a threshold factor of 10.
-
2)
Animal behavior was recorded simultaneously using VLC media player software (VideoLAN) and a Microsoft LifeCam (Microsoft, Redmond, WA). Recordings were exported in the MJPEG format in Shotcut open-source software for importing into Inscopix Data Processing software (Fig. 20).
Fig. 20.
Video from Inscopix Data Processing software for a female rat. Upper left corner is the calcium image of cells in the reticular thalamic nucleus in a behaving rat. Each colored circular region outlines a cell that had calcium activity. Signal was enhanced by calculating the delta F over F. In the upper right is a video of the behavior of the animal during testing. The animal was placed in the chamber for the first 5 min without testing and then during the next 5 min the rat was tested by poking the animal with a 60-gram filament every 15 s. The bottom panel shows the traces for three individual cells. A dot over the spike on the trace indicates where the software marked that peak as an event using a threshold set at 10.
Behavioral testing
-
1)
Place Escape/Avoidance Paradigm (PEAP) testing was performed during the morning of the light phase. To accomplish this, the rats were placed in a 30 cm X 30 cm X 30 cm acrylic box. The box has four walls and floor with the top of the box open and half the box is covered in black cloth on the outside of the acrylic. The rat was poked with a 60-gram filament every 15 s
Immuno-fluorescent staining
-
1)
Rats were injected with 100 mg/kg ketamine and 10 mg/kg xylazine. After injection the animals were perfused with 9% sucrose followed by 4% paraformaldehyde.
-
2)
Fixed tissues were stored in 25% sucrose, frozen, cryo-sectioned and the 32 µm sections placed on Histobond slides (VWR international, Radnor, PA).
-
3)
The tissue was post-fixed for 5 min in 4% paraformaldehyde, rinsed and then blocked for 2 h at room temperature with a PBS solution containing 5% normal goat serum (Sigma-Aldrich, St. Louis, MO) and 0.3% Triton-X 100.
-
4)
The slides were then incubated in a primary GAD67 antibody (Millipore clone 1G10.2, MAB5406) at a 1:500 dilution solution overnight at 4 °C. The primary antibody was diluted with PBS, 5% BSA and 0.3% Triton X-100.
-
5)
After incubation in primary antibody the slides were then rinsed three times in PBS and Triton-X 100 for a total of 45 min and placed for 2 h in secondary goat anti-mouse 568 antibody (Invitrogen, Carlsbad, CA) at a 1:500 dilution with PBS, 5% BSA and 0.3% Triton X-100.
-
6)
After rinsing the slides three times in PBS and 0.3% Triton X-100 for a total of 45 min, the slides were mounted with Fluoromount-G mounting medium containing Hoechst 33342 stain (Electron Microscopy Sciences, Hatfield, PA).
-
7)
The fluorescent signal was imaged (Fig. 19) using a Nikon fluorescent microscope, NIS-Elements imaging software and a Photometrics CoolSnap K4 CCD camera (Roper Scientific, Inc, Duluth, GA). Controls eliminating the primary antibody showed no signal (data not shown).
Fig. 19.
Sagittal section of rat brain after infusion of GCAMP7f into the reticular thalamic nucleus. GAD1-Cre rats were infused with AAV1 containing a Cre dependent GCAMP7f construct. A lens was placed above the injection site. After six weeks the GCAMP signal was recorded. After sacrifice the brain section was stained with an antibody to GAD1 (GAD67, red) and counterstained with Hoechst 33342. Panel A shows the placement of the lens on a stereotaxic atlas image (Paxinos and Watson). Panel B is a low magnification image showing GCAMP positive cells in the reticular thalamic nucleus (Rt). GAD 67 positive stained cells are shown in red. A low magnification image of three GCAMP positive cells is shown in panels C and D. The GAD 67 staining is shown in panel E and the Hoechst 33342 stained nuclei are shown in panel F. Arrows point to the same three cells in panels C through F. Ventral posteromedial nucleus (VPM), ventral posterolateral nucleus (VPL). Bar in panel B is 200 micrometers and the bar in panel C is 50 micrometers.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
References
- 1.Paxinos G., Watson C. Sixth Edition Amsterdam. The Netherlands: Elsevier Inc; 2007. The rat brain in stereotaxic coordinates. [Google Scholar]
- 2.Resendez S.L., Jennings J.H., Ung R.L., Namboodiri V.M., Zhou Z.C., Otis J.M., Nomura H., McHenry J.A., Kosyk O., Stuber G.D. Visualization of cortical, subcortical and deep brain neural circuit dynamics during naturalistic mammalian behavior with head-mounted microscopes and chronically implanted lenses. Nat Protoc. 2016;11:566–597. doi: 10.1038/nprot.2016.021. [DOI] [PMC free article] [PubMed] [Google Scholar]