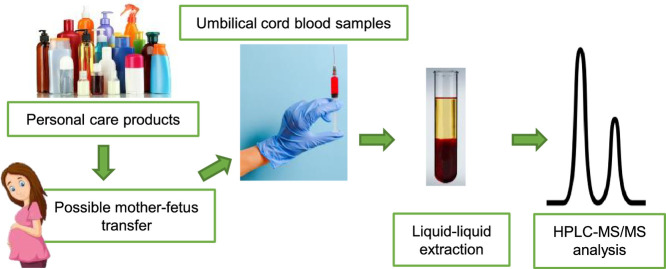
Abstract
UV filters and parabens are compounds used in large quantities in modern societies and have become ubiquitous in the environment. They are considered compounds of emerging concern due to the unwanted effects they cause in the environment and their bioaccumulation potential in humans. Considering their endocrine disrupting activity and their so far unknown effects in newborns, a continuous monitoring of these substances is required. In this work, we developed and validated a new sensitive methodology for the analysis of 8 UV filters and metabolites, and 4 parabens in umbilical cord blood samples. The method consisted of a liquid-liquid extraction and phase separation by freezing. Then, the organic extract was further analyzed at alkaline pH using liquid chromatography coupled to tandem-mass spectrometry (LC-MS/MS) using a QqLIT hybrid mass spectrometer as analyzer. The low limits of detection achieved (0.01–0.42 ng/mL) allowed the reliable simultaneous quantification of UV filters and parabens in this complex biological matrix.
-
•
Simple, fast and sensitive analysis of UV filters and parabens in cord blood samples.
-
•
First simultaneous analysis of UV filters and parabens in cord blood.
-
•
Allows the evaluation of perinatal transfer of UV filters and parabens from the mother to the fetus.
Keywords: Umbilical cord blood, Parabens, Sunscreens, Benzophenones, HPLC, Tandem mass spectrometry
Graphical abstract
Specifications table
| Subject Area: | Biochemistry, genetics and molecular biology |
| More specific subject area: | Analytical chemistry, CECs, human health, environment |
| Method name: | UV filters and parabens determination in umbilical cord blood |
| Name and reference of original method: | This analytical method is based on the method described in Kolatorova, L., Vitku, J., Hampl, R., Adamcova, K., Skodova, T., Simkova, M., Parizek, A., Starka, L., & Duskova, M. (2018). Exposure to bisphenols and parabens during pregnancy and relations to steroid changes. Environ. Res., 163, 115–122. https://doi.org/10.1016/j.envres.2018.01.031 |
| Resource availability: | NA |
Method details
Background
The industrial production and the use of personal care products (PCPs) have increased in recent years. Among these compounds are UV filters and parabens, which are extensively used as sunscreens and as preservatives, respectively. They are present in cosmetics, sunscreens, lotions, hygiene products, but also in foodstuff, plastics, rubbers, and textiles [9]. These compounds are considered contaminants of emerging concern (CECs) for the negative effects they can cause in the environment, including their potential for bioaccumulation [5,6] and biomagnification through the food web [3]. Their bioaccumulation in humans has also been reported [8,12,15,19,20]. This fact combined with their endocrine disrupting activity [1,2,10,13,14] make a regular monitoring of their occurrence necessary. This is even more important in crucial stages of life like pregnancy [4], where the exposure of the unborn to these substances might have short- and long-term consequences in the development of the fetuses. Similar works are reported for the analysis of benzophenones or parabens in cord blood [7,12,[16], [17], [18]]. However, most of these methods use a solid-phase extraction [7,16,18] implying time-consuming and tedious steps, in addition to other steps such as long incubation, and derivatization. Kruse et al. used a laborious method, including an incubation of 3 h. This method was developed to detect benzophenone derivatives in serum (from the mothers and the fetuses) but all the cord blood samples analyzed were below the method limit of detection. Recently Song et al. presented a simpler method, however, it included time-consuming steps (incubation of 12 h and shaking for 60 min). Despite that, only benzophenone-type compounds could be determined. This work describes a sensitive method for the simultaneous analysis of UV filters and paraben preservatives in umbilical cord blood in order to achieve a better understanding of the bioaccumulation of these compounds and their maternal transfer. To this end, a method used for the analysis of bisphenols and parabens [11] was adapted and significantly simplified for the simultaneous analysis of eight benzophenone-type UV filters and metabolites, and four parabens in human cord blood.
Chemicals and reagents
Table 1 lists the selected compounds. The UV filters avobenzone (AVO), benzophenone-2 (BP2), benzophenone-4 (BP4), benzophenone-3 (BP3) and their main metabolites namely benzophenone-1 (BP1), 4-hydroxybenzophenone (4HB), 4,4′-dihydroxybenzophenone (4DHB), and 2,2′-dihydroxy-4-methoxybenzophenone (DHMB, BP8), and the paraben preservatives methyl paraben (MePB), propyl paraben (PrPB), benzyl paraben (BePB), and butyl paraben (BuPB) were purchased from Sigma Aldrich (Darmstadt, Germany). The isotopically labelled compounds 2‑hydroxy-4‑methoxy-2′,3′,4′,5′,6′-d5 (BP3-d5), benzyl paraben-d4 (BePB-d4), and 5-(2,5-dimethylphenoxy)−2,2-bis(trideuteriomethyl)pentanoic acid (Gemfibrozil-d6) were purchased from CDN isotopes (Quebec, Canada). Water and methanol (MeOH) of high performance liquid chromatography (HPLC) grade were obtained from J.T. Backer (Deventer, The Netherlands) and the nitrogen (99.995% purity) was supplied by Air Liquide (Barcelona, Spain). Formic acid (HCOOH) and ammonium acetate (AcNH4) were from Merck (Darmstadt, Germany). For the extraction process methyl tert‑butyl ether (MTBE), sodium chloride (NaCl), sodium hydrogen-carbonate (NaHCO3) from Sigma Aldrich, and ammonium formate (NH4HCO2) from Fisher Scientific (Fair Lawn, New Jersey, EEUU) were used.
Table 1.
Target compounds name, acronym, family, CAS number, molecular mass, chemical structure and log octanol-water partition coefficient.
| Compound | Other names | Family | CAS number | Molecular mass (g/mol) | Structure | log kow |
|---|---|---|---|---|---|---|
| Benzophenone-3 (BP3) | Oxybenzone; 2-Hydroxy-4-methoxybenzophenone | Benzophenones | 131-57-7 | 228.24 | 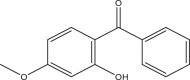 |
3.79 |
| Benzophenone-1 (BP1) | 2,4-Dihhydroxybenzophenone | Benzophenones | 131-56-6 | 214.22 | 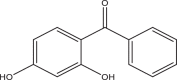 |
3.15 |
| 4-Hydroxybenzophenone (4HB) | – | Benzophenones | 1137-42-4 | 193.18 | 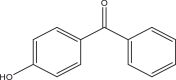 |
2.92 |
| 4,4′-Dihydroxybenzophenone (DHB) | – | Benzophenones | 611-99-4 | 214.22 | 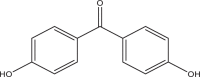 |
2.19 |
| 2,2′-Dihydroxy-4-methoxybenzophenone (DHMB, BP8) | Benzophenone-8; Dioxybenzone | Benzophenones | 131-53-3 | 244.25 | 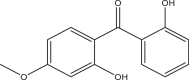 |
3.82 |
| Benzophenone-2 (BP2) | 2,2′,4,4′-Tetrahydroxybenzophenone | Benzophenones | 131-55-5 | 246.22 | 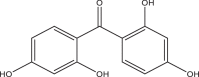 |
2.78 |
| Benzophenone-4 (BP4) | 5-benzoyl-4‑hydroxy-2-methoxybenzene sulfonic acid; HMBS; Sulisobenzone | Benzophenones | 4065-45-6 | 308.31 | 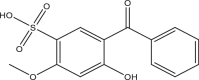 |
0.88 |
| Avobenzone (AVO) | 1-(4‑tert-butylphenyl)−3-(4-methoxyphenyl)propane-1,3-dione | Benzophenones | 70,356-09-1 | 31,017 | 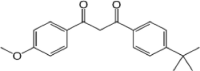 |
4.51 |
| Methyl paraben (MePB) | Methyl 4-hydroxybenzoate | Parabens | 99-76-3 | 152 | 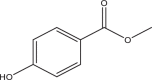 |
2 |
| Propyl paraben (PrPB) | Propyl 4-hydroxybenzoate | Parabens | 94-13-3 | 180.2 | 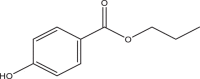 |
2.98 |
| Butyl paraben (BuPB) | Butyl 4-hydroxybenzoate | Parabens | 94-26-8 | 194.23 | 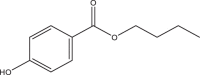 |
3.47 |
| Benzyl paraben (BePB) | Benzyl 4-hydroxybenzoate | Parabens | 94-18-8 | 228.24 | 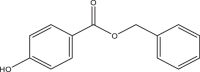 |
3.7 |
A mix of isotopically labelled internal standards containing BP3-d5, BePB-d4 and gemfibrozil-d6 was prepared in MeOH with the appropriate volume of the standard stock solutions at a concentration of 200 ng/mL, and was stored at -20º C.
Ethical aspects
Cord blood samples were provided by the Sant Joan de Déu Hospital (Barcelona, Spain), and were donated voluntarily by the mothers, who were asked to sign an informed consent to participate in the study, well before delivery. The present study was approved by the Ethics Review Board of the University of Barcelona and Sant Joan de Déu Hospital. All the data compiled were saved following the current regulation on Protection of Personal Data and guarantee of digital rights (Ley Orgánica 3/2018).
Sampling and sample extraction
Umbilical cord blood samples were collected in metal-free serum tubes (to have the serum component of the blood) after direct extraction by venipuncture from the umbilical cords obtained immediately after delivery. The biological samples were stored at Sant Joan de Déu Hospital following the Spanish Law of Biomedical Investigation of 2007 (Law 14/2007) until shipment via urgent courier to the IDAEA-CSIC laboratories for analysis. All samples were received in perfect conditions and correctly codified, and were preserved frozen until analysis.
Samples were centrifuged at 3500 rpm during 5 min to remove cell devris and the serum was collected with a Pasteur pipette for further analysis. Then, 500 µL of each serum sample were spiked with 100 µL of the mix of internal standards solution and 500 µL of a physiological solution of NaCl previously prepared with MeOH (0.137 M). Isolation of the target analytes was carried out by liquid-liquid extraction adding 2 mL of MTBE. The mix was vigorously shaken and the samples were frozen until the organic and aqueous phases were separated. The organic phases were transferred with a Pasteur pipette to 2 mL HPLC-vials and further evaporated until almost dryness under a gentle current of nitrogen. Then, 0.5 mL of NaHCO3 (100 mM) were added up to pH 10.5 and the samples were incubated at 60 ºC for 5 min. Further, the samples were evaporated again under a stream of nitrogen until near dryness and then, 0.3 mL of the buffer NH4HCO2 (10 mM) and 0.3 mL of MeOH were added to dilute the samples up to 1:1 (v:v) proportion. Finally, the extracts were brought to dryness and further reconstituted with 1 mL of MeOH. The extracts were stored at -20ºC until HPLC-MS/MS analysis.
Instrumental analysis
The chromatographic separation of the compounds was performed in a Hibar PurosherⓇ STARⓇ HR R-18 (50 mm × 2.0 mm, 5 μm) column using a Symbiosis™ Pico instrument from Spark Holland (Emmen, The Netherlands). Detection was carried out in a 4000 Q TRAP™ hybrid quadrupole-linear ion trap mass spectrometer from Applied Biosystems-Sciex (Foster City, CA, USA). Mobile phases consisted of MeOH and H2O 0.1% HCOOH in positive ionization mode determination, and MeOH and H2O 5 mM AcNH4 in negative ionization mode, respectively. The detailed gradient profiles are shown in Tables 2 and 3. The injection volume was set up to 20 µL. Electrospray ionization in positive (ESI+) and negative (ESI-) modes were selected. Tandem-mass spectrometry detection (MS/MS) was performed under selected reaction monitoring (SRM) mode for improved sensitivity and selectivity. The two most intense transitions were selected and used for the quantification (most intense, 1st transition) and confirmation (second most intense, 2nd transition) of each compound. The principal parameters of the developed HPLC-MS/MS method, including chromatographic retention time (tR), selected transitions and ionization parameters are compiled in Table 4. Analytical standards, reagent blank samples, and quality control solutions were included in each analysis batch together with the serum extracts. The Analyst v. 1.4.2 software package (Applied Biosystems) was used for acquisition and data analysis processing
Table 2.
Mobile phases used in positive mode, with its gradient flow and time.
| Positive ionization | |||
|---|---|---|---|
| Time (min) | % Mobile Phase A* | % Mobile Phase B^ | Flow (mL/min) |
| 0 | 95 | 5 | 0.3 |
| 7 | 25 | 75 | 0.3 |
| 10 | 0 | 100 | 0.3 |
| 15 | 0 | 100 | 0.3 |
| 17 | 95 | 5 | 0.3 |
| 23 | 95 | 5 | 0.3 |
A: H2O 0,1% in HCOOH;.
B: MeOH 0,1% in HCOOH.
Table 3.
Mobile phases used in negative mode, with its gradient flow and time.
| Negative ionization | |||
|---|---|---|---|
| Time (min) | % Mobile Phase A* | % Mobile Phase B^ | Flow (mL/min) |
| 0 | 95 | 5 | 0.3 |
| 3 | 50 | 50 | 0.3 |
| 6 | 10 | 90 | 0.3 |
| 13 | 0 | 100 | 0.3 |
| 17 | 0 | 100 | 0.3 |
| 18 | 95 | 5 | 0.3 |
| 20 | 95 | 5 | 0.3 |
A: H2O 5 mM AcNH4.
B: MeOH 5 mM AcNH4.
Table 4.
Retention time (tR), selected MS/MS transitions, internal standard (IS) used, and ionization parameters for each compound. (-) for those analyzed in negative ionization mode.
| Compound | tR | 1st transition | DP (V) | CE (eV) | CxP (eV) | 2nd transition | DP (V) | CE (eV) | CxP (eV) | IS |
|---|---|---|---|---|---|---|---|---|---|---|
| BP3 | 12.12 | 229>151 | 40 | 25 | 12 | 229>105 | 40 | 27 | 16 | BP3-d5 |
| BP1 | 11.39 | 215>137 | 40 | 27 | 10 | 215>105 | 40 | 29 | 6 | BP3-d5 |
| 4HB | 11.36 | 199>121 | 40 | 25 | 8 | 199>105 | 40 | 27 | 8 | BP3-d5 |
| 4DHB | 10.41 | 215>121 | 45 | 27 | 8 | 215>93 | 45 | 45 | 6 | BP3-d5 |
| DHMB | 11.93 | 245>121 | 43 | 29 | 8 | 245>151 | 43 | 27 | 12 | BP3-d5 |
| BP2 | 10.89 | 247>137 | 46 | 25 | 8 | 247>109 | 46 | 45 | 8 | BP3-d5 |
| BP4 (-) | 8.42 | 307>227 | -50 | -34 | -15 | 307>211 | -70 | -40 | -9 | Gemfibrozil-d6 |
| AVO | 13.04 | 311>135 | 40 | 25 | 15 | 311>161 | 40 | 25 | 15 | BP3-d5 |
| BePB (-) | 9.5 | 227>92 | -65 | -26 | -9 | 227>136 | -65 | -22 | -1 | BePB-d4 |
| BuPB (-) | 9.54 | 193>137 | -55 | -22 | -5 | 193>92 | -55 | -34 | -13 | BePB-d4 |
| PrPB (-) | 9.22 | 179>92 | -60 | -30 | -13 | 179>137 | -60 | -24 | -5 | BePB-d4 |
| MePB (-) | 8.5 | 151 > 92 | -45 | -28 | -7 | 151>136 | -45 | -20 | -9 | BePB-d4 |
DP: Declustering potential (V); CE: Collision energy (eV); CxP: Collision cell exit potencial (eV);
Quality assurance and quality control
One of the most common problems in trace analysis is background contamination. Therefore, procedural blanks were processed and analyzed. The procedural blanks were prepared using 500 µL of HPLC water and submitted to all the steps in the sample analysis. No quantifiable peaks of the target analytes were measured, as shown in Figs. 1 and 2, where the peak area of the spiked samples at 5 ng/mL are notably higher than those of the blanks. Furthermore, all the glass material was cleaned with MeOH and acetone and dried at 400 ºC overnight before use. Quality controls (mix of standards at known concentrations) were randomly measured along the samples’ analysis sequence to ensure a reliable determination. The tR of the compounds were compared at a tolerance of 2.5% maximum, and the relative ion intensities of the two SRM transitions (1st transition / 2nd transition) were compared at a tolerance level below 15% with those of the standards. The target compounds were identified following EU normative (Commission Decision 2002/657/EC). Isotopically labelled standards for each family of compounds were used to overcome potential matrix effects and thus, for proper quantification. The calibration curves were built through ten mix standard solutions at 1, 3, 5, 10, 30, 50, 100, 300, 500 and 700 ng/mL spiked in the matrix (matrix matched standards).
Fig. 1.
Reconstructed ion chromatograms showing the SRM 1st transition obtained in the spiked samples at 5 ng/mL and in the procedural blanks using positive ionization (ESI+).
Fig. 2.
Reconstructed ion chromatograms showing the SRM 1st transition obtained in the spiked samples at 5 ng/mL and in the procedural blanks using negative ionization (ESI-).
Method validation
A number of the received samples was pooled to obtain a representative mixture of the umbilical cord serum, that was needed to validate the proposed method. Ten aliquots of 500 µL of the pool samples were collected to elaborate the validation samples. These 10 samples were spiked at two concentrations (50 and 400 ng/mL) with the mix of the target compounds. The developed method was evaluated under optimized conditions in terms of linearity range, sensitivity, accuracy, precision, and matrix effects.
The method limits of detection (MLODs) and quantification (MLOQs), and the coefficient of determination (r2) are listed in Table 5. MLODs and MLOQs were calculated as the concentration of each compound giving a signal-to noise ratio of 3 and 10, respectively. A wide linearity interval 1–700 ng/mL was obtained for all the compounds, with r2 > 0.9969. The method was highly sensitive, with MLODs in the range 0.01–0.42 ng/mL blood.
Table 5.
Limits of detection (MLODs) and quantification (MLOQs) of the method (expressed in ng/ml blood sample) and determination coefficient (r2) for each compound.
| MLOD (ng/ml) | MLOQ (ng/mL) | r2 | |
|---|---|---|---|
| BP3 | 0.3 | 1.01 | 0.9997 |
| BP1 | 0.08 | 0.28 | 0.9984 |
| 4HB | 0.42 | 1.39 | 0.9982 |
| DHB | 0.05 | 0.18 | 0.9982 |
| DHMB | 0.14 | 0.48 | 0.9995 |
| BP2 | 0.16 | 0.53 | 0.9974 |
| BP4 (-) | 0.26 | 0.85 | 0.9988 |
| AVO | 0.35 | 1.17 | 0.9992 |
| MePB (-) | 0.41 | 1.38 | 0.9993 |
| PrPB (-) | 0.23 | 0.75 | 0.9991 |
| BuPB (-) | 0.18 | 0.61 | 0.9969 |
| BePB (-) | 0.01 | 0.04 | 0.9986 |
MLOD: Limit of detection of the method; MLOQ: Limit of quantification of the method.
Considering the high complexity of the sample composition, matrix effects were expected, and consequently, evaluated. Two representative examples of the calibration curves in the matrix extract (matrix-matched standards) and in the organic solvent are shown in Figs. 3 and 4. The large differences in the slope of the curves showed that, in these cases, BP3 signal suffers from signal enhancement in the presence of the matrix, and MePB experienced signal suppression, as indicated by the different slope obtained in the two media. Therefore, the matrix effects observed were significant, and demanded consideration. Thus, matrix matched calibration curves were used for all the analytes studied and were prepared using the pool of the samples created for the validation of the method.
Fig. 3.
Calibration curves for BP3 showing the enhancement of the signal in the serum matrix in comparisson with MeOH.
Fig. 4.
Calibration curves for MePB showing the suppression of the signal in the serum matrix in comparisson with MeOH in comp.
Table 6 lists the recovery rates obtained at the two spiked concentration levels. Despite generally good recoveries were obtained, between 80 and 120%, BP2 was scarcely recovered (c.a. 20%) at the higher spike level (400 ng/mL); however, at low concentration the recovery was quite good (≈ 91.3%). Considering the complexity of the samples analyzed, occurrence levels are not expected to reach this high concentration, and thus BP2 was also included in the method. AVO, on the other hand, presented medium-to-low recoveries (15.5–31.4%) at both concentrations, so it was also included in the method, but the obtained concentration values were considered semi-quantitative. Finally, BP4 had the opposite behavior than BP2. It showed a good recovery at high concentration (c.a. 71.9%), but small recovery at the low concentration (c.a.19%), and, therefore we decided to proceed as for AVO.
Table 6.
Recovery rates (%) obtained from the spiked samples at the two concentration levels tested.
| Validation sample | BP3 | BP1 | 4HB | DHB | DHMB | BP2 | BP4 (-) | AVO | BePB (-) | BuPB (-) | PrPB (-) | MEPB (-) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 ng/mL (1) | 89.8 | 134.4 | 109.4 | 94.2 | 107.4 | 94.4 | 17.08 | 31.2 | 86.4 | 100.2 | 111 | 121.6 |
| 50 ng/mL (2) | 83.4 | 101.2 | 110.4 | 87 | 114.4 | 72.6 | 28.4 | 31.4 | 87.8 | 97.4 | 108 | 110.6 |
| 50 ng/mL (3) | 83.8 | 142.8 | 123.6 | 128.8 | 82.6 | 82.8 | 14.28 | 24.8 | 81.6 | 93.8 | 105.4 | 114.2 |
| 50 ng/mL (4) | 85.6 | 131.2 | 136.4 | 101.2 | 110.8 | 100.4 | 19.3 | 23.6 | 81.4 | 93.2 | 103.4 | 111.6 |
| 50 ng/mL (5) | 90.6 | 146.4 | 120.4 | 121.2 | 121.4 | 106.4 | 16.58 | 21.6 | 80.4 | 92.4 | 104.8 | 118.6 |
| 400 ng/mL (1) | 91.5 | 95 | 104 | 98.75 | 74.5 | 21.6 | 68.25 | 15.52 | 85.75 | 101.2 | 91 | 77 |
| 400 ng/mL (2) | 106 | 96.25 | 97.5 | 99.75 | 65 | 27.25 | 66.25 | 21.77 | 84.5 | 103.5 | 91.75 | 74.5 |
| 400 ng/mL (3) | 110 | 92 | 104.2 | 103.2 | 57.75 | 20.5 | 68 | 25.25 | 88.75 | 100 | 95.25 | 75.75 |
| 400 ng/mL (4) | 106.5 | 93 | 94.5 | 84.75 | 63.5 | 14.75 | 82.75 | 15.8 | 90.5 | 97.75 | 97.75 | 72.25 |
| 400 ng/mL (5) | 113.7 | 96 | 103.7 | 105.2 | 74.5 | 18.57 | 74.25 | 19.17 | 86.75 | 101.5 | 92.75 | 74.75 |
| Average 50 ng/mL | 86.6 | 131.2 | 120.0 | 106.5 | 107.3 | 91.3 | 19.1 | 26.5 | 83.5 | 95.4 | 106.5 | 115.3 |
| Average 400 ng/mL | 105.5 | 94.45 | 100.8 | 98.35 | 67.05 | 20.53 | 71.9 | 19.50 | 87.25 | 100.8 | 93.7 | 74.85 |
(1), (2), (3), (4), (5): Number of replica; (-); Analyzed in negative mode.
Repeatability and reproducibility were evaluated (Table 7). Intra-day RSD values (1.5–32%) and inter-day RSD values (0.5–18%) indicated quite good precision for the complex matrix.
Table 7.
Relative standard deviation (RSD%) for inter- and intra-day precision.
| RSD% Intra C1 | RSD% Intra C2 | RSD% Inter C1 | RSD% Inter C2 | |
|---|---|---|---|---|
| BP3 | 1.68 | 33.8 | 8.45 | 10.41 |
| BP1 | 8.93 | 7.5 | 2.56 | 15.07 |
| 4HB | 5.51 | 18.05 | 0.72 | 12.09 |
| DHB | 8.91 | 32.17 | 2.7 | 2.8 |
| DHMB | 7.38 | 29.28 | 0.46 | 15.47 |
| BP2 | 6.81 | 18.3 | 7.04 | 18.49 |
| BP4 (-) | 1.66 | 9.56 | 4.45 | 15.77 |
| AVO | 2.25 | 16.47 | 1.59 | 0.82 |
| MePB (-) | 1.64 | 8.47 | 3.1 | 7.68 |
| PrPB (-) | 1.5 | 11.1 | 7.21 | 14.21 |
| BuPB (-) | 2.34 | 7.02 | 5.99 | 11.61 |
| BePB (-) | 2.74 | 27.11 | 9.25 | 17.84 |
C1: Spiked concentration 1; C2: Spiked concentration 2; RSD%: Relative standard deviation; Intra: Intra-day; Inter: Inter-day.
As an applicability example of the developed method, Fig. 5 shows the reconstructed ion chromatograms corresponding to the UV filters and parabens detected in the serum of a cord blood sample. All the target compounds were detected and quantifiable, at concentrations from 0.20 to 53.3 ng/mL.
Fig. 5.
Reconstructed ion chromatograms showing the SMR first selected transition, in ascendant chromatographic retention time (tR) order, corresponding to a cord serum sample.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge the Generalitat de Catalunya (Water and Soil Quality Unit 2017-SGR-1404) and the Spanish Ministry of Science and Innovation (Project CEX2018-000794-S) for its financial support. We also want to thank the voluntary mothers for their contribution to science through this study.
References
- 1.Boberg J., Taxvig C., Christiansen S., Hass U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod. Toxicol. 2010;30(2):301–312. doi: 10.1016/j.reprotox.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Darbre P.D., Harvey P.W. Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J. Appl. Toxicol. 2008;28(5):561–578. doi: 10.1002/jat.1358. [DOI] [PubMed] [Google Scholar]
- 3.Fent K., Zenker A., Rapp M. Widespread occurrence of estrogenic UV-filters in aquatic ecosystems in Switzerland. Environ. Pollut. 2010;158(5):1817–1824. doi: 10.1016/j.envpol.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Fleming T.P., Watkins A.J., Velazquez M.A., Mathers J.C., Prentice A.M., Stephenson J., Barker M., Saffery R., Yajnik C.S., Eckert J.J., Hanson M.A., Forrester T., Gluckman P.D., Godfrey K.M. Origins of lifetime health around the time of conception: causes and consequences. Lancet. 2018;391(10132):1842–1852. doi: 10.1016/S0140-6736(18)30312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gago-Ferrero P., Díaz-Cruz M.S., Barceló D. An overview of UV-absorbing compounds (organic UV filters) in aquatic biota. Anal. Bioanal. Chem. 2012;404(9):2597–2610. doi: 10.1007/s00216-012-6067-7. [DOI] [PubMed] [Google Scholar]
- 6.Gago-Ferrero P., Díaz-Cruz M.S., Barceló D. UV filters bioaccumulation in fish from Iberian river basins. Sci. Total Environ. 2015;518–519:518–525. doi: 10.1016/j.scitotenv.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Geer L.A., Pycke B.F.G., Waxenbaum J., Sherer D.M., Abulafia O., Halden R.U. Association of birth outcomes with fetal exposure to parabens, triclosan and triclocarban in an immigrant population in Brooklyn, New York. J. Hazard. Mater. 2017;323:177–183. doi: 10.1016/j.jhazmat.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hines E.P., Mendola P., von Ehrenstein O.S., Ye X., Calafat A.M., Fenton S.E. Concentrations of environmental phenols and parabens in milk, urine and serum of lactating North Carolina women. Reprod. Toxicol. 2015;54:120–128. doi: 10.1016/j.reprotox.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kameda Y., Kimura K., Miyazaki M. Occurrence and profiles of organic sun-blocking agents in surface waters and sediments in Japanese rivers and lakes. Environ. Pollut. 2011;159(6):1570–1576. doi: 10.1016/j.envpol.2011.02.055. [DOI] [PubMed] [Google Scholar]
- 10.Kinnberg K.L., Petersen G.I., Albrektsen M., Minghlani M., Awad S.M., Holbech B.F., Green J.W., Bjerregaard P., Holbech H. Endocrine-disrupting effect of the ultraviolet filter benzophenone-3 in zebrafish, Danio rerio. Environ. Toxicol. Chem. 2015;34(12):2833–2840. doi: 10.1002/etc.3129. [DOI] [PubMed] [Google Scholar]
- 11.Kolatorova L., Vitku J., Hampl R., Adamcova K., Skodova T., Simkova M., Parizek A., Starka L., Duskova M. Exposure to bisphenols and parabens during pregnancy and relations to steroid changes. Environ. Res. 2018;163:115–122. doi: 10.1016/j.envres.2018.01.031. Februaryhttps://doi.org/ [DOI] [PubMed] [Google Scholar]
- 12.Krause M., Frederiksen H., Sundberg K., Jørgensen F.S., Jensen L.N., Nørgaard P., Jørgensen C., Ertberg P., Juul A., Drzewiecki K.T., Skakkebaek N.E., Andersson A.M. Presence of benzophenones commonly used as UV filters and absorbers in paired maternal and fetal samples. Environ. Int. 2018;110:51–60. doi: 10.1016/j.envint.2017.10.005. April 2017https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 13.Krause M., Klit A., Blomberg Jensen M., Søeborg T., Frederiksen H., Schlumpf M., Lichtensteiger W., Skakkebaek N.E., Drzewiecki K.T. Sunscreens: are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. Int. J. Androl. 2012;35(3):424–436. doi: 10.1111/j.1365-2605.2012.01280.x. [DOI] [PubMed] [Google Scholar]
- 14.Kunz P.Y., Galicia H.F., Fent K. Comparison of in vitro and in vivo estrogenic activity of UV filters in fish. Toxicol. Sci. 2006;90(2):349–361. doi: 10.1093/toxsci/kfj082. [DOI] [PubMed] [Google Scholar]
- 15.Molins-Delgado D., Olmo-Campos M.d.M., Valeta-Juan G., Pleguezuelos-Hernández V., Barceló D., Díaz-Cruz M.S. Determination of UV filters in human breast milk using turbulent flow chromatography and babies’ daily intake estimation. Environ. Res. 2018;161:532–539. doi: 10.1016/j.envres.2017.11.033. September 2017https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 16.Pycke B.F.G., Geer L.A., Dalloul M., Abulafia O., Halden R.U. Maternal and fetal exposure to parabens in a multiethnic urban U.S. population. Environ. Int. 2015;84:193–200. doi: 10.1016/j.envint.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song S., He Y., Huang Y., Huang X., Guo Y., Zhu H., Kannan K., Zhang T. Occurrence and transfer of benzophenone-type ultraviolet filters from the pregnant women to fetuses. Sci. Total Environ. 2020;726 doi: 10.1016/j.scitotenv.2020.138503. [DOI] [PubMed] [Google Scholar]
- 18.Towers C.V., Terry P.D., Lewis D., Howard B., Chambers W., Armistead C., Weitz B., Porter S., Borman C.J., Kennedy R.C.M., Chen J. Transplacental passage of antimicrobial paraben preservatives. J. Expo. Sci. Environ. Epidemiol. 2015;25(6):604–607. doi: 10.1038/jes.2015.27. [DOI] [PubMed] [Google Scholar]
- 19.Valle-Sistac J., Molins-Delgado D., Díaz M., Ibáñez L., Barceló D., Diaz-Cruz M.S. Determination of parabens and benzophenone-type UV filters in human placenta: first description of the existence of benzyl paraben and benzophenone-4. Environ. Int. 2016;88:243–249. doi: 10.1016/j.envint.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 20.Zhang T., Sun H., Qin X., Wu Q., Zhang Y., Ma J., Kannan K. Benzophenone-type UV filters in urine and blood from children, adults, and pregnant women in China: partitioning between blood and urine as well as maternal and fetal cord blood. Sci. Total Environ. 2013;461–462:49–55. doi: 10.1016/j.scitotenv.2013.04.074. [DOI] [PubMed] [Google Scholar]