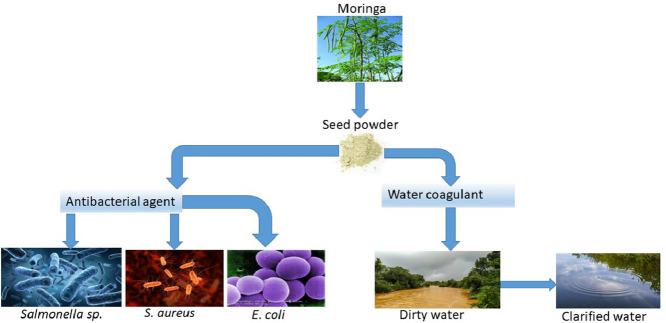
Abstract
This research evaluated Moringa oleifera seed powder (MOSP) as an antibacterial agent, and a coagulant. In the former, clinical isolates of Salmonella sp., Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) were used, and in the latter, river and stream water were used. Both the isolates and water samples were treated with MOSP at varying final concentrations of 0.001, 0.002, 0.004 and 0.017 g/ml.
For the antibacterial assay, a dose of 0.017 g/ml of MOSP was effective on all three isolates with CFU/ml reduction of 99.4, 78.8 and 57.3% on Salmonella sp., E. coli and S. aureus respectively. An ANOVA confirmed this finding at P<0.05; 0.0014 between the treated and control samples. The water treatment assay also showed a reduction of total hardness, fluoride, phosphate, nitrate, total iron and manganese levels below the water quality standards.
The MOSP could serve as a cost-effective product for process integration in raw water treatment systems in rural and urban settings. The study shows bioactivity of the seed powder of Moringa, and provides grounds to isolate the active component for commercialization and usage by the wider population with limited or no access to potable water.
Keywords: Moringa oleifera seed powder, Antibacterial, Water coagulant, Potable water
Graphical abstract
Specifications Table
| Subject Area |
|
| More specific subject area | Microbiology |
| Protocol name | Moringa oleifera – an antibacterial agent and water clarifier |
| Reagents/tools |
|
| Experimental design | Empirical data collection and analysis |
| Trial registration | Not applicable |
| Ethics |
|
| Value of the Protocol |
|
Rationale of the study
Africa is among other continents that are challenged with inadequate water supply coupled with alterations in the quality of final treated water delivered for public use. In Ghana, many households within cities, towns, remote villages and slums, lack adequate and safe water for their daily needs. This has contributed to poor sanitation through the disposal of human excreta in some communities. Also, the surge of Galamsey (illegal gold mining) has rendered many raw water sources murky, hence increased the recycling of both chemical and biological hazards in abstracted water destined for household potable water production. This has increased the expenditure of the government for safe household water production. Aluminium sulfate popularly known as Alum and other chlorine-coated tablets have therefore been sold in local markets for household water treatment where potable water is lacking. Latest research reports indicate that protracted use of these chemicals poses health problems. There is therefore the urgent need for alternative water treatment solutions which are safe, inexpensive, reproducible, accessible, user-friendly, and sustainable.
Background of the study
Moringa oleifera (MO) is a multipurpose tree with considerable potential hence, many developing countries promote its cultivation. It belongs to the family Moringaceae and is one of the 14 known species. In particular, Moringaceae family is rich in a unique group of glycoside compounds i.e. glucosinolates and isothiocyanates [1]. The Latin word “oleifera” means 'oil bearing'. More common names for the tree are 'drumstick', 'never die', 'Ben tree' or 'horseradish' [2]. The plant is an outstanding indigenous source of highly digestible protein, calcium, iron, vitamin C and carotenoids. It is found in medicinal uses, cosmetics, food supplements, and water treatment. Amazingly, MO propagates where clean water is needed the most i.e. Africa, Asia, and Latin America [3]. Seeds of MO are organic polymers that contain water-soluble, positively charged dimeric proteins that are stable, and act as an effective coagulant for water and wastewater treatment [4]. The proteins within the seeds also contain 1% active polyelectrolytes that neutralize negatively charged colloids in dirty water, and can therefore be a nontoxic natural polypeptide for sedimentation of mineral particles and organic compounds in the purification of drinking water [5]. In addition, MO seeds contain active antimicrobial agent namely 4 alpha rhamnosyloxy-benzyl isothiocyanate [6] which according to Sapana [5], can flocculate Gram-positive and Gram-negative bacterial cells or act directly on them resulting in growth reduction or inhibition. The selection of materials and chemicals used in water supply systems for treatment is an important consideration since they may have a potentially adverse effect on drinking water quality. Chemicals added to water include disinfectants, oxidants, coagulants, flocculants, algaecides, antioxidants and chemicals for softening, pH adjustment and scale prevention [7]. Earlier studies found that MO seeds are non-toxic, and recommended their use as a coagulant in water treatment in developing countries [8]. The use of MO for coagulation, co-coagulation, or coagulant aid has been a subject of investigation in many parts of the world [9]. Several studies conducted, comparing MO to traditional coagulants, like aluminium sulfate with synthetic polyelectrolytes, indicated that the former may have many more advantages and non-complex processes with no requisite maintenance [10] and has a broader dose response, resulting in a more robust treatment process. Also, a reduction in the use of synthetic chemicals in the treatment of potable water is of benefit to water companies pursuing environmentally sensitive policies [2]. Developing countries are faced with problems of potable water supply due to inadequate financial resources [8]. The conventional method of water purification in these countries using aluminium sulfate popularly called alum can result in extra financial constraints. This may render treated water very expensive and beyond the reach of most rural inhabitants hence, they resort to other water sources such as dams, streams, rivers and lakes [11]. Natural coagulants however are of much interest to many researchers because of their abundant sources, low price, environmental friendliness, multifunction and biodegradable nature in water purification [12]. Currently, they are widely researched due to their effectiveness and unlimited user advantages over synthetic products [13]. This study sought to explore protocols for assessing the potency of MO in water treatment by evaluating the ability of its seed powder to inhibit growth of known bacterial isolates as well as exhibit water coagulant properties in comparison with a chemical coagulant.
Experimental design, reagents/tools, data measurement and analysis
Source of materials
Sixteen litres each of stream and river water samples were collected into sterile plastic containers and stored in a cool dry place until required. Eight litres of each sample was used for preliminary work on the antibacterial and water coagulant properties of MOSP. Out of the remaining 8 L, 6 L was reserved for physicochemical analysis and 2 L for microbiological work.
River water
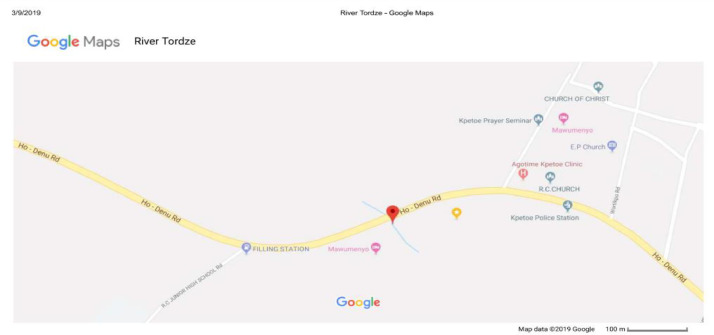
Water was drawn from river Tordze, located in Agortime-Kpetoe, which serves as raw water source for domestic use. The river as shown in Fig. 1, is situated at 410 m from the Agortime-Kpetoe Police Station, along Ho-Denu road (Google, n.d.).
Fig. 1.
Location of river Tordze.
Stream water
The stream is located 400 m North from the University of Health and Allied Sciences in Ho, along Ho-Denu road and was chosen based on its high usage by the public.
Moringa oleifera seeds
One kilogram of freshly harvested Moringa oleifera seeds were ordered from Africa Moringa Hub, a local farm in Sunyani, Ghana for the research.
Aluminium sulfate (Alum)
Five hundred grams of alum pellets were obtained from Ghana Water Company Limited, Ho branch in the Volta Region.
Microbiological media
These were Mannitol salt agar (Research-lab Fine Chem Industries, Mumbai 400 002. India), Nutrient agar (Biomark laboratories, Pune 411 041. India) and Salmonella-Shigella agar (Biomark laboratories, Pune 411 041. India).
Preparation of MOSP
De-hulled MO seeds weighing 250 g were left to dry at room temperature for seven days, after which they were ground into powder using a domestic grinder (Akai BD018IDA-309) and passed through a plastic sieve to obtain smooth texture. The sample was then sealed in an air-tight container until needed.
Preliminary determination of MOSP dosage
Experiment 1
River and stream water were used here including two coagulants, MOSP and alum. For stream water, ten disposable cups labelled 1 to 10 containing 150 ml of the sample were used for each coagulant type. The dosage ranged from 0.1 g to 1.0 g and were assigned in the same order as the labelled cups. Each river and stream water sample were stirred vigorously for 1 min after the coagulant was added. This procedure was repeated for river water. The samples were left for 12 h and visually observed for color change. The optimum dose based on observation was found to be 1.0 g/150 ml.
Experiment 2
Observation made in Experiment 1 informed the decision to lower the dosage of the coagulant to a range of 0.01 to 0.09 g. The same coagulants were used, with the addition of a third coagulant variation, consisting of a combination of MOSP and alum in different proportions. Experiment 1 was then repeated, this time with only nine cups per coagulant.
Experiment 3
Observations made in Experiment 2 suggested 0.01 g/150 ml for both MOSP and alum and ratio of 0.09 g/150 ml MOSP to 0.01 g/150 ml alum as the optimum dosages. These doses were then added to varied volumes of water (150, 200, 300, 400, and 500 ml). This was to determine in which volume of water selected doses worked best. The samples were left for 12 h as before.
Coagulation assay
Experiment 3 showed that, all selected doses were most efficient in 150 ml of water sample. Samples were then coded and analyzed for physico-chemical parameters. Raw water samples from the river and stream were also used as controls.
Determination of physico-chemical parameters
Physicochemical parameters were analyzed according to standard methods by Baird and Bridgewater [14]. The parameters evaluated were: pH, Conductivity (mS/m), salinity (ppt), temperature (°C), turbidity (NTU), alkalinity (as CaCO3), as well as total dissolved solids, total hardness, content of chloride, sodium, fluoride, phosphates, nitrates, nitrites, total iron and manganese, all in mg/L. The various methods and respective instruments used for the analysis are shown in Table 4.
Table 4.
Summary of physico-chemical methods.
| Parameters | Test Method | Instrument/Process |
|---|---|---|
| pH | SM4500H+B | HQ30d HACH pH reader |
| Conductivity | SM2520 B | HACH Conductivity/TDS meter Model 44,600 |
| Salinity | SM2520 A | TDS/1000 |
| Temperature | SM2520 B | HACH Conductivity/TDS meter Model 44,600 |
| Turbidity | SM2130 B | HACH 2100Q |
| Alkalinity (as CaCO3) | SM2320 B | Titration |
| Total Dissolved Solids | SM2540 C | HACH Conductivity/TDS meter Model 44,600 |
| Total Hardness | SM2340 C | Titration |
| Chloride | SM4500Cl¯ B | Titration |
| Sodium (Na+) | SM3500-Na B | Jenway PFP7 Flame photometer |
| Fluoride | PHOT 14 | Palintest Photometer 7500 Bluetooth smart |
| Phosphate | PHOT 28 | Palintest Photometer 7500 Bluetooth smart |
| Nitrate | PHOT 23 | Palintest Photometer 7500 Bluetooth smart |
| Nitrite | PHOT 24 | Palintest Photometer 7500 Bluetooth smart |
| Total Iron | PHOT 39 | Palintest Photometer 7500 Bluetooth smart |
| Manganese | PHOT 20 | Palintest Photometer 7500 Bluetooth smart |
Antibacterial assay
Preparation of bacterial cultures
Under aseptic conditions, nutrient broth was prepared according to manufacturer's instructions and autoclaved at 121 °C for 15 min. Three test tubes were then filled each with 9 ml of the sample. For the first test tube, an inoculating loop was heated over a flame, cooled by dipping into sterile nutrient agar, and then used to pick a colony of Staphylococcus aureus. The content was shaken off into the test tube containing nutrient broth. The same process was repeated for second and third test tubes, using Escherichia coli and Salmonella spp., respectively. The three test tubes containing these bacteria were then incubated at 37 °C for 24 h.
Preparation of serial dilutions
Results from Experiment 2 suggested that 0.01 g of MOSP was effective in 150 ml of water. It was therefore necessary to verify if the said dose was equally effective in preventing or reducing growth of the selected bacteria. In order to establish a sequence, the dose was multiplied by a factor of 2 to obtain five doses, 0.01 g, 0.02 g, 0.04 g, 0.08 g and 0.16 g.
For each cultured microorganism, 6 sets of serial dilutions up to the sixth dilution were made. Five sets were assigned each a MOSP dose and the sixth set was used as control. After appropriate doses were added, dilution bottles were closed firmly, and contents shaken vigorously for 1 min to activate antibacterial property of MO seeds. They were then left at room temperature to settle for 12 h.
Evaluation of MOSP effectiveness
Mannitol salt agar, Nutrient agar and Salmonella-Shigella agar were prepared for Staphylococcus aureus, Escherichia coli and Salmonella spp., respectively, according to manufacturer's instructions. Using Staphylococcus aureus, 1 ml of each MOSP-treated serial dilution as well as blank, were plated on petri dishes. Designated media was then added by pour-plate method. The samples were gently shaken for 20 s to ensure a uniform mixture. This procedure was repeated for Escherichia coli and Salmonella spp. Samples were then incubated at 37 °C for 24 h.
Physico-chemical parameters of river Tordze before and after treatment with coagulants
Table 5 below shows the results obtained from coagulation assay, before and after treatment of river Tordze water with the various coagulants.
Table 5.
Physico-chemical analyses of water obtained from river Tordze.
| Parameters | R | RM | RA | RMA | GS |
|---|---|---|---|---|---|
| Physical | |||||
| pH | 7.65 | 6.97 | 7.45 | 6.51 | 6.50–8.50 |
| Color | 225 | 7.5 | 5 | 9 | |
| Conductivity (mS/m) | 21.00 | 21.00 | 23.00 | 26.00 | 100.00 |
| Salinity (ppt) | 0.01 | 0.01 | 0.01 | 0.01 | |
| Temperature (⁰C) | 27.40 | 26.60 | 26.40 | 28.00 | |
| Turbidity (NTU) | 72.50 | 4.10 | 1.65 | 9.03 | 5.00 |
| Anions (mg/L) | |||||
| Alkalinity (as CaCO3) | 20.00 | 20.00 | 10.00 | 20.00 | |
| Total Dissolved Solids (TDS) | 10.50 | 10.50 | 11.50 | 13.00 | 1000.00 |
| Total Hardness | 90.00 | 80.00 | 100.00 | 100.00 | 500.00 |
| Chloride | 10.00 | 15.00 | 15.00 | 15.00 | 250.00 |
| Fluoride | 0.79 | 0.46 | 0.10 | 0.10 | 1.50 |
| Phosphate | 0.20 | 0.05 | 1.05 | 0.14 | 30.00 |
| Nitrate | 3.15 | 2.05 | 1.04 | 1.64 | 50.00 |
| Nitrite | 0.03 | 0.18 | 0.15 | 1.35 | 3.00 |
| Cations (mg/L) | |||||
| Sodium (Na+) | 6.49 | 9.74 | 9.74 | 9.74 | 200.00 |
| Total Iron | 0.03 | 2.80 | 4.62 | 0.17 | 0.30 |
| Manganese | 0.20 | 1.18 | 0.57 | 0.03 | 0.40 |
Where, R: untreated River water; RM: River water with MOSP; RA: River water with alum; RMA: River water with MOSP and alum, GS: Ghana standards
River Tordze water (Fig. 1) with MOSP was effective in reducing pH, color and turbidity. However, treatment with alum (RA) showed greater reduction than RM. Salinity remained the same for all samples. The combination of the two gave a significant reduction in pH, however, lower than the individual coagulants on other parameters.
Anion content of river Tordze before and after treatment
All values of the parameters analysed were within the Ghana standards for water quality. River water with MOSP was effective in reducing total hardness, fluoride, phosphate and nitrate content of water. However, it had no significant effect on alkalinity and TDS. When compared with RA, it had a better effect in reducing total hardness and phosphate levels. River water with alum on the other hand reduced the alkaline and fluoride contents. It also increased the levels of TDS, total hardness, chloride, phosphate and nitrite. Overall, the efficacy of RMA on anion content reduction of the river water was lower than the individual coagulants.
Cation content of river Tordze water before and after treatment
In reference to Table 5, the total hardness for RM was slightly lower than the other treatment coagulants. As related to the standard value of 200 mg/L for sodium, treatment using RM, RA, and RMA did not cause any significant increase in the mineral which recorded 4.9% (9.74/200) in the samples compared to 3.2% (6.49/200) in the raw untreated water. Thus, the ratio between the raw untreated and treated river Tordze samples was 1:1.5. Also, RMA was better in the reduction of total iron and Manganese compared to the rest of the treatment coagulants. These results were achieved using a total of 6 litres of river Tordze water over triplicate measurements out of which the average readings are presented.
Physico-chemical parameters of stream water before and after treatment with coagulants
Table 6 below shows the results obtained from coagulation assay, before and after treatment of stream water with various coagulants.
Table 6.
Physico-chemical water analysis results of stream water.
| Parameters | S | SM | SA | SMA | GS |
|---|---|---|---|---|---|
| Physical | |||||
| pH | 7.47 | 7.48 | 7.66 | 7.52 | 6.50–8.50 |
| Color | 10 | 7.5 | 7.5 | 6 | |
| Conductivity (mS/m) | 71.00 | 73.00 | 75.00 | 79.00 | 100.00 |
| Salinity (ppt) | 0.04 | 0.04 | 0.04 | 0.04 | |
| Temperature (⁰C) | 29.90 | 29.80 | 29.50 | 27.20 | |
| Turbidity (NTU) | 1.80 | 6.00 | 2.42 | 5.38 | 5.00 |
| Anions (mg/L) | |||||
| Alkalinity (as CaCO3) | 20.00 | 20.00 | 20.00 | 20.00 | |
| Total Dissolved Solids | 35.50 | 36.50 | 37.50 | 39.50 | 1000.00 |
| Total Hardness | 60.00 | 190.00 | 130.00 | 200.00 | 500.00 |
| Chloride | 65.00 | 65.00 | 65.00 | 40.00 | 250.00 |
| Fluoride | 0.46 | 1.15 | 0.79 | 0.06 | 1.50 |
| Phosphate | 1.65 | 1.10 | 0.19 | 1.25 | 30.00 |
| Nitrate | 15.66 | 20.55 | 18.88 | 0.79 | 50.00 |
| Nitrite | 0.28 | 11.88 | 7.92 | 5.78 | 3.00 |
| Cations (mg/L) | |||||
| Sodium (Na+) | 42.19 | 42.19 | 42.19 | 25.96 | 200.00 |
| Total Iron | 0.13 | 0.04 | 0.04 | 0.00 | 0.30 |
| Manganese | 0.14 | 0.11 | 0.46 | 0.23 | 0.40 |
Where S: Untreated Stream water; SM: Stream water with MOSP; SA: Stream water with alum; SMA: Stream water with MOSP and alum; GS: Ghana standards.
Physical parameters of stream water before and after treatment
In Table 6, the stream water samples also recorded varied levels of treatment parameters. Total dissolved solids were reduced by SM compared to SA and SMA. However, in comparison with the standard values for cation reduction or removal, the SMA was effective for Sodium reduction, and total iron removal followed by both SM and SA. For Manganese however, SM performed better. Chloride, Phosphate, Nitrate and Nitrite were reduced better with SMA compared with SM and SA. The results obtained from the stream water analysis also utilised a total of 6 litres over triplicate measurements and averages.
Anion content of stream water before and after treatment
Alkalinity remained unchanged with all coagulants. Both SA and SM were only effective in reducing phosphate, as other parameters were either increased or remained unchanged. On the other hand, SMA caused a significant reduction in chloride, fluoride, phosphate and nitrate levels. This indicated that, the synergy of the coagulants produced a much better effect.
Cation content of stream water before and after treatment
Stream water with MOSP caused a reduction in total iron and manganese but had no effect on sodium. The SA increased the content of manganese with no effect on sodium, however, it reduced the total iron levels. Amazingly, SMA not only reduced all cations significantly, but also the removal of total iron content.
Antibacterial assay
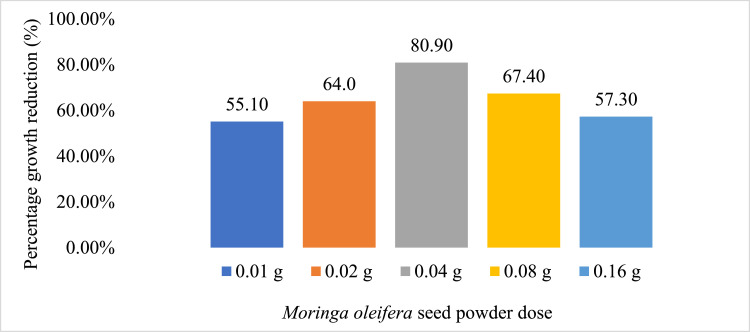
Effect of MOSP on S. aureus
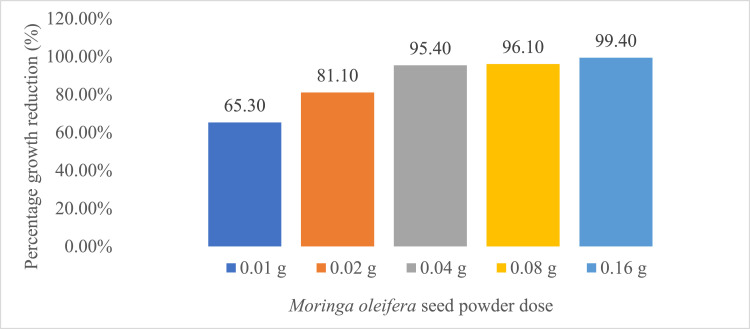
The figure above shows that 0.04 g of MOSP was most effective in inhibiting microbial growth. This is evident by its percentage CFU/ml reduction of 80.90%. It was also observed that the ability of doses 0.08 g and 0.16 g to inhibit bacterial growth was reduced. This could be that the dose of MOSP beyond 0.04 g partly served as a substrate upon which the bacteria fed, thereby increasing their growth. However, considering the fact that the highest reduction came from 0.04 g could imply that a specific dose of MOSP was effective against the bacteria.
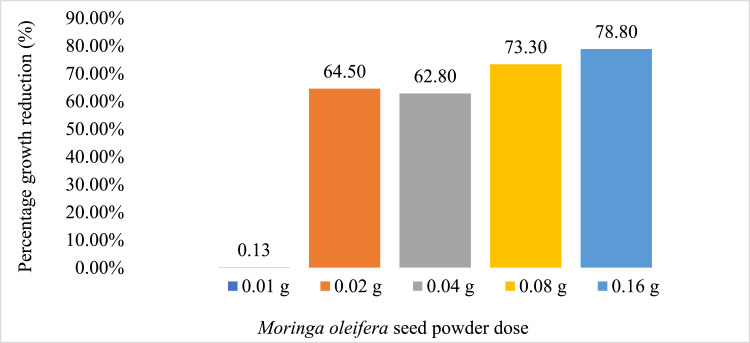
Effect of MOSP on E. coli
The figure gives a trend that suggests that a higher concentration of MOSP was required to inhibit growth of E. coli. This was evident by the highest MOSP dose of 0.16 g.
Effect of MOSP on Salmonella sp
It could be seen in Fig. 4 that MOSP had a significant effect on Salmonella. The trend showed that the higher the MOSP dose, the greater the effect on the bacteria.
Fig. 4.
Effect of MOSP on Salmonella sp.
Bactericidal effect of MOSP on bacterial isolates
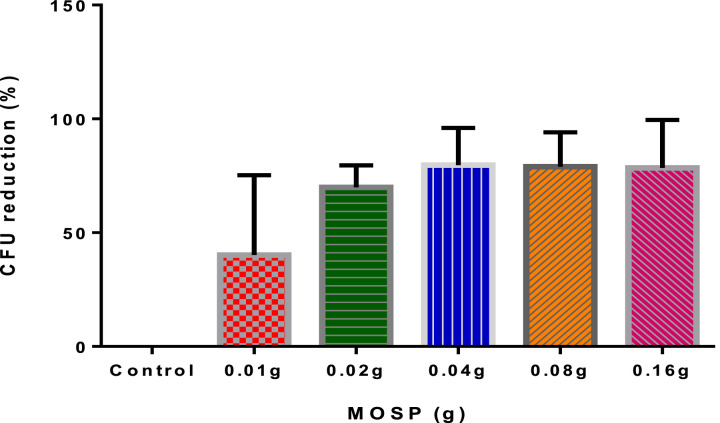
It could be seen in Fig. 5 that, generally, a higher dose of MOSP resulted in a greater reduction of the bacteria. The control sample, however, had no CFU reduction because, it was not treated with MOSP. Additionally, the p-value of 0.0014 obtained from the One-way ANOVA further confirmed the significant difference between the treated samples and the control. This finding thus prove that MO seeds possess an antibacterial property.
Fig. 5.
Bactericidal effect of MOSP on isolates.
At the onset of the study, concentrations of the coagulants and their requisite water volumes were determined (Table 1, Table 2, Table 3). The pH of river Tordze water was reduced by both RM and RMA, while that of stream water was increased by these coagulants as shown in Tables 5 and 6. Based on findings from Table 5, pH values obtained were similar, with highly reduced color by all the coagulants from the raw river water whilst maintaining slight alterations in conductivity by the raw river water sample. Salinity remained unchanged, however, turbidity reduction was also observed for RM RA and RMA which were great reductions from the raw water sample of 72.50 NTU. For stream water treatment (Table 6), the pH remained fairly unchanged using all the coagulants. The values recorded were within the standard values. In addition, the conductivity readings were not highly affected by the coagulants, with values below the standard. Salinity was also the same in the river water treatment, at 0.04 across the coagulants. Sutherland [15] argued that performance of MO is maintained irrespective of raw water pH, and application has no effect on pH of treated water. However, since river water had a higher turbidity than stream water, it could be that turbidity influenced the pH levels. The changes in pH were also non-conforming with findings from Delelegn et al. [16]. Sutherland [15] observed that the effectiveness of MO seeds in coagulation is dependent on turbidity levels. This may account for the fact that, the overall effect of MO on river water which had 72.5 NTU was much better than that of the stream water with 1.80 NTU. Additionally, a study by Dorea [17] as cited in Lea [3] suggested that MO is unsuitable for low-turbidity waters below 50 NTU. The reason is that, low turbidity waters generally contain low levels of suspended particulate matter. Under such conditions, the opportunity for inter-particle interactions is greatly reduced thereby resulting in reduced floc formation and an adverse effect on treatment performance [15]. Individual MO coagulant was effective in reducing total hardness of river water, which conforms to similar research by Mangale [6]. The authors explained that, in the presence of MO coagulant, light, slow-settling solids or flocks formed, causing a precipitation reaction that led to the conversion of soluble hardness-causing ions to insoluble compounds. They also suggested that the required dosage of MOSP increases as the value of water hardness increases. From the results, MO was effective in reducing the levels of fluoride, phosphate and nitrate within the required turbidity levels. However, even in stream water which had low turbidity, its effect was improved when combined with alum. The advantage of the synergy was also observed within the cations, where, even though individual coagulants were ineffective, the combination resulted in great reduction of manganese, total iron and sodium. Removal of iron and manganese may have resulted from the formation of protein-iron and protein-manganese complexes with MO and subsequent floc formation. The settled matter resulted in the concomitant removal of iron and manganese. This indicates the propensity for MO to form organo-metallic complexes. The potential therefore exists for MO to be employed in the removal of specific metal ions from solution which may include the removal of heavy metals from wastewater discharges [15,18]. Decreased levels of metals may also occur because Moringa amphoteric protein binds to the oppositely charged metal ions and causes them to precipitate [19]. The antibacterial assay revealed that MOSP was effective on all three isolates (Fig. 2, Fig. 3, Fig. 4, Fig. 5). However, the effect varied with each organism; Salmonella sp. and E. coli were significantly reduced. This partly conforms with the study by Ferreira [20] on the ability of MO to cause significant reduction of E. coli. Several studies undertaken to evaluate the effectiveness of MO in clarifying raw natural waters also determined bacterial removal. In most cases, 90–99% reductions in total bacterial counts within two hours of treatment were recorded. However, in all cases it was also reported that regrowth of bacteria occurred at or above original levels within a 12-h period following treatment [15]. It has been postulated, therefore, that the addition of whole seed material to effect clarification has the disadvantage of supplying a suitable substrate that promotes bacterial regrowth [21], [22], [23]. Bacterial removal does occur to a significant level using MO, but only due to coagulation and filtration. Therefore, in order to achieve microbiological safety of potable water quality, a disinfection stage would need to be included in the treatment process [2]. This research confirms MOSP as an effective antibacterial agent and coagulant, hence, a potential alternative to synthetic coagulants. The major drawback is its ineffectiveness in water bodies of low turbidity. Therefore, MOSP may be useful during the wet seasons where turbidity levels increase and could potentially provide significant savings in chemical coagulant cost.
Table 1.
Determination of optimum dose of two coagulants in g/150 ml of water sample.
| Coagulant | Coagulant and dose per 150 ml of water sample (g/150 ml) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| MOSP | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 |
| Alum | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 |
Table 2.
Determination of optimum dose of three coagulant variations in 150 ml of water.
| Coagulant | Coagulant dose in cups per 150 ml of water (g/150 ml) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| MOSP | 0.01 | 0.02 | 0.03 | 0.04 | 0.05 | 0.06 | 0.07 | 0.08 | 0.09 |
| Alum | 0.01 | 0.02 | 0.03 | 0.04 | 0.05 | 0.06 | 0.07 | 0.08 | 0.09 |
| MOSP: Alum | 0.01: 0.09 | 0.02: 0.08 | 0.03: 0.07 | 0.04: 0.06 | 0.05: 0.05 | 0.06: 0.04 | 0.07: 0.03 | 0.08: 0.02 | 0.09: 0.01 |
Table 3.
Determination of optimum volume of water for selected coagulant doses.
| Coagulant dose (g) | Volumes of Water (ml) |
||||
|---|---|---|---|---|---|
| 150 ml | 200 ml | 300 ml | 400 ml | 500 ml | |
| 0.01 g of MOSP | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 0.01 g of Alum | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 0.09 g of MOSP: 0.01 g of Alum | 0.09: 0.01 | 0.09: 0.01 | 0.09: 0.01 | 0.09: 0.01 | 0.09: 0.01 |
Fig. 2.
Effect of MOSP on S. aureus.
Fig. 3.
Effect of MOSP on E. coli.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Direct submission or co-submission
Co-submissions are papers that have been submitted alongside an original research paper accepted for publication by another Elsevier journal Direct Submission.
References
- 1.Yongabi K.A. Biocoagulants for water and waste water purification : a review. Int. Rev. Chem. Eng. 2010;2(3):444–458. doi: 10.1006/cyto.1995.0069. [DOI] [Google Scholar]
- 2.Al-Khalili, R.S. (1999). Contact flocculation filtration using natural coagulants for developing countries; https://queens.ezp1.qub.ac.uk/login?url=https://search.proquest.com/docview/301610368?accountid=13374%0Ahttp://resolver.ebscohost.com/openurl?ctx_ver=Z39.882004&ctx_enc=info:ofi/enc:UTF8&rfr_id=info:sid/ProQuest+Dissertations+%26+Theses%3A+UK+%26+Irelan.
- 3.Lea M. Bioremediation of turbid surface water using seed extract from the Moringa oleifera Lam. (Drumstick) tree. Curr. Protoc. Microbiol. 2014;(SUPPL.33):1–14. doi: 10.1002/9780471729259.mc01g02s33. [DOI] [PubMed] [Google Scholar]
- 4.Shan T.C., Matar M.Al, Makky E.A., Ali E.N. The use of Moringa oleifera seed as a natural coagulant for wastewater treatment and heavy metals removal. Appl. Water Sci. 2017;7(3):1369–1376. doi: 10.1007/s13201-016-0499-8. [DOI] [Google Scholar]
- 5.Sapana M.M., Sonal G.C., Raut P. Use of Moringa oleifera (Drumstick) seed as natural absorbent and an antimicrobial agent for ground water treatment. Res. J. Recent Sci. 2012;1(3):31–40. https://doi.org/2231-3184. [Google Scholar]
- 6.Mangale S.M., Chonde S.G., Jadhav S., Raut P.D. Study of Moringa oleifera (Drumstick) seed as natural absorbent and antimicrobial agent for river water treatment. J. Nat. Prod. Pl. Resour. 2012;2(1):89–100. https://doi.org/2231-3184. [Google Scholar]
- 7.Ghana M.W.R.W.H. National drinking water quality management framework for ministry of water resources, works and housing government of Ghana. Res. J. 2015 June. [Google Scholar]
- 8.Ali E.N., Muyibi S.A., Salleh H.M., Alam M.Z., Salleh M.R.M. Production of natural coagulant from Moringa oleifera seed for application in treatment of low turbidity water. J. Water Resour. Protoc. 2010;02(03):259–266. doi: 10.4236/jwarp.2010.23030. [DOI] [Google Scholar]
- 9.Pastawski P.F., Yao N., Jiang L., Lukin M., Cirac J.I. Quantum memory based encryption : tolerance and security proofs. Civ. Environ. Res. 2011;3(8):1–5. [Google Scholar]
- 10.Sánchez-Martín J., Beltrán-Heredia J., Peres J.A. Improvement of the flocculation process in water treatment by using Moringa oleifera seeds extract. Braz. J. Chem. Eng. 2012;29(3):495–501. doi: 10.1590/S0104-66322012000300006. [DOI] [Google Scholar]
- 11.Amagloh F.K., Benang A. Effectiveness of Moringa oleifera seed as coagulant for water purification. Afr. J. Agric. Res. 2009;4(2):119–123. doi: 10.1164/RCCM.201707-1543ED. [DOI] [Google Scholar]
- 12.Asrafuzzaman M., Fakhruddin A.N.M., Hossain M.A. Reduction of turbidity of water using locally available natural coagulants. ISRN Microbiol. 2011;2011:1–6. doi: 10.5402/2011/632189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feria Diaz J.J., Ballut Dajud G., Rodriguez Miranda J.P. Influence of storage time of Moringa oleifera seed on the coagulant activity efficiency for raw water treatment. Indian J. Sci. Technol. 2018;11(9):1–4. doi: 10.17485/ijst/2018/v11i9/121221. [DOI] [Google Scholar]
- 14.Baird R., Bridgewater L. 23rd ed. American Public Health Association; Washington, D.C.: 2017. Standard Methods For the Examination of Water and Wastewater. [Google Scholar]
- 15.Sutherland, J.P. (2000). The application of Moringa oleifera as a coagulant for water treatment in developing countries. PQDT - UK & Ireland, (June), 170. Retrieved from https://queens.ezp1.qub.ac.uk/login?url=https://search.proquest.com/docview/301583298?accountid=13374%0Ahttp://resolver.ebscohost.com/openurl?ctx_ver=Z39.88-2004&ctx_enc=info:ofi/enc:UTF-8&rfr_id=info:sid/ ProQuest±Dissertations±%26±Theses%3A±UK±%26±Irelan
- 16.Delelegn A., Sahile S., Husen A. Water purification and antibacterial efficacy of Moringa oleifera Lam. Agric. Food Secur. 2018;7(1):25. doi: 10.1186/s40066-018-0177-1. [DOI] [Google Scholar]
- 17.Dorea C.C. Use of Moringa spp. seeds for coagulation: a review of a sustainable option. Water Sci. Technol. Water Supply. 2006;6:219–227. [Google Scholar]
- 18.Alam M.W., Pandey P., Khan F., Souayeh B., Farhan M. Study to investigate the potential of combined extract of leaves and seeds of Moringa oleifera in groundwater purification. Int. J. Environ. Res. Public Health. 2020;17(20):7468. doi: 10.3390/ijerph17207468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendrawati H., Yuliastri I.R., Nurhasni, Rohaeti E., Effendi H., Darusman L.K. The use of Moringa oleifera seed powder as coagulant to improve the quality of wastewater and ground water. IOP Conf. Ser. Earth Environ. Sci. 2016;31(1):0–10. doi: 10.1088/1755-1315/31/1/012033. [DOI] [Google Scholar]
- 20.Ferreira R.S., Napoleão T.H., Santos A.F.S., Sá R.A., Carneiro-da-Cunha M.G., Morais M.M.C., Paiva P.M.G. Coagulant and antibacterial activities of the water-soluble seed lectin from Moringa oleifera. Lett. Appl. Microbiol. 2011;53(2):186–192. doi: 10.1111/j.1472-765X.2011.03089.x. [DOI] [PubMed] [Google Scholar]
- 21.Jahn S.A.A., Dirar J.H. Studies on natural water coagulants in the Sudan with special reference to Moringa oleifera seeds. Water SA. 1979;5(2):pp90–pp97. [Google Scholar]
- 22.Jahn S.A.A. Effectiveness of traditional flocculants as primary coagulants and coagulant aids for the treatment of tropical raw water with more than a thousand-fold fluctuation in turbidity. Water Supply, 1984;2(3/4):pp8–p10. Special Subject. [Google Scholar]
- 23.Jahn S.A.A. GTZ Manual; 1986. Proper Use of African Natural Coagulants for Rural Water Supplies Research in the Sudan and a Guide to New Projects. [Google Scholar]