Abstract
Objectives
This study sought to quantify and compare the decline in volumes of cardiovascular procedures between the United States and non-U.S. institutions during the early phase of the coronavirus disease-2019 (COVID-19) pandemic.
Background
The COVID-19 pandemic has disrupted the care of many non-COVID-19 illnesses. Reductions in diagnostic cardiovascular testing around the world have led to concerns over the implications of reduced testing for cardiovascular disease (CVD) morbidity and mortality.
Methods
Data were submitted to the INCAPS-COVID (International Atomic Energy Agency Non-Invasive Cardiology Protocols Study of COVID-19), a multinational registry comprising 909 institutions in 108 countries (including 155 facilities in 40 U.S. states), assessing the impact of the COVID-19 pandemic on volumes of diagnostic cardiovascular procedures. Data were obtained for April 2020 and compared with volumes of baseline procedures from March 2019. We compared laboratory characteristics, practices, and procedure volumes between U.S. and non-U.S. facilities and between U.S. geographic regions and identified factors associated with volume reduction in the United States.
Results
Reductions in the volumes of procedures in the United States were similar to those in non-U.S. facilities (68% vs. 63%, respectively; p = 0.237), although U.S. facilities reported greater reductions in invasive coronary angiography (69% vs. 53%, respectively; p < 0.001). Significantly more U.S. facilities reported increased use of telehealth and patient screening measures than non-U.S. facilities, such as temperature checks, symptom screenings, and COVID-19 testing. Reductions in volumes of procedures differed between U.S. regions, with larger declines observed in the Northeast (76%) and Midwest (74%) than in the South (62%) and West (44%). Prevalence of COVID-19, staff redeployments, outpatient centers, and urban centers were associated with greater reductions in volume in U.S. facilities in a multivariable analysis.
Conclusions
We observed marked reductions in U.S. cardiovascular testing in the early phase of the pandemic and significant variability between U.S. regions. The association between reductions of volumes and COVID-19 prevalence in the United States highlighted the need for proactive efforts to maintain access to cardiovascular testing in areas most affected by outbreaks of COVID-19 infection.
Key Words: cardiovascular disease, cardiovascular imaging, coronavirus, COVID-19, diagnostic cardiovascular procedure
Abbreviations and Acronyms: CAC, coronary artery calcium scan; CCTA, coronary computed tomographic angiography; CMR, cardiac magnetic resonance; COVID-19, coronavirus disease-2019; CVD, cardiovascular disease; IAEA, International Atomic Energy Agency; ICA, invasive coronary angiography; PET, positron emission tomography; SPECT, single-photon emission computed tomography; TEE, transesophageal echocardiogram; TTE, transthoracic echocardiogram
Central Illustration
The coronavirus disease-2019 (COVID-19) pandemic has led to profound disruptions in the delivery of health care around the world. Clinicians have reduced in-person visits, eliminated elective procedures, and increased reliance on telehealth within a remarkably short period of time (1,2). In addition, data from several countries have confirmed declines in emergency room visits and hospitalizations for a variety of common non-COVID-19 medical and surgical conditions, leading to concerns about an emerging global health crisis from delayed or missed diagnoses during the pandemic (3, 4, 5, 6, 7).
Disruptions in medical care are especially concerning for patients with cardiovascular disease (CVD), which is the leading cause of death for men and women globally. Prior to the pandemic, CVD accounted for 17.9 million deaths worldwide annually (8). The timely performance of advanced cardiovascular diagnostic tests is essential to the accurate diagnosis, risk stratification, and management of patients with known or suspected CVD (9, 10, 11). However, diagnostic cardiovascular procedures, as with other elective or nonemergent procedures, have been reduced, delayed, or canceled entirely during the pandemic. We recently reported that worldwide volumes of cardiovascular testing declined by 64% during the early phase of the pandemic (12), whereas studies from at least 5 countries reported declines of 30% to 40% in invasive coronary angiography (ICA) procedures for acute coronary syndrome (ACS), causing growing concern over the short- and long-term implications of reductions in diagnostic cardiovascular testing on overall CVD morbidity and mortality around the world (13, 14, 15, 16, 17, 18). At the same time, imaging guidance statements amid the pandemic point to evolving indications for cardiovascular testing to now prioritize acute diagnosis, safety, and decreased downstream resource usage (19, 20, 21).
Furthermore, in addition to the acute cardiovascular complications caused by COVID-19 (22, 23, 24, 25), an increasing body of evidence is showing possible sustained cardiovascular effects related to the disease (22,26). For example, a recent study of patients who recovered from COVID-19 showed that most of those studied had signs consistent with cardiac inflammation (22), highlighting the need for cardiovascular testing to identify a large at-risk population with new, undiagnosed CVD.
The extent to which the early phase of the COVID-19 pandemic has reduced volumes of diagnostic cardiovascular procedures in the United States and the differential impact of the pandemic on U.S. and non- U.S. laboratories, has not been reported. In an effort to comprehensively quantify reductions in cardiovascular testing during the early phase of the pandemic, the International Atomic Energy Agency (IAEA, Vienna, Austria) coordinated a worldwide study, called the INCAPS-COVDI (IAEA Non-invasive Cardiology Protocols Study of COVID-19), to characterize volumes of procedures from facilities around the world that perform diagnostic cardiovascular procedures. We recently reported an analysis of the worldwide impact of the COVID-19 pandemic on cardiac diagnostic procedures (12). In this study, we compared volumes of procedure data between U.S. and non-U.S. institutions and between U.S. regions, and we identified factors associated with diagnostic procedure volume reduction in the U.S. during the early months of the COVID-19 pandemic.
Methods
Study design
The INCAPS-COVID executive committee, comprising experts in cardiac imaging from every world region, was convened to study the impact of the COVID-19 pandemic on worldwide diagnostic cardiovascular procedure volumes. A study was designed in which facilities performing cardiac diagnostic procedures were asked to report the total number and type of noninvasive and invasive procedures performed at their institution during the months of March 2019, March 2020, and April 2020. Regional and national coordinators facilitated outreach to IAEA-registered institutions and through professional organizations to invite participants to participate in the study. U.S. regional coordinators (New England, Mid Atlantic, South East, South, Midwest, South West, and West) helped recruit centers in their respective subregions to increase U.S. representation in the study. Publicizing on social media platforms (Twitter, LinkedIn, Facebook) also helped to ensure broad and diverse participation in the survey. March 2019 data were treated as baseline values when assessing reduction in procedure volume during March and April 2020 (i.e., early months of the pandemic). Data were aggregated by country and by the 8 world regions defined by the IAEA: Africa, Eastern Europe, Far East, Latin America, Middle East, South Asia, North America (i.e., Canada and the United States), South East Asia and the Pacific, and Western Europe (27). In that analysis, we compared data between U.S. and non-U.S. laboratories, as well as between U.S. regions as defined by the U.S. Census Bureau: Midwest, Northeast, South, and West (28). Notwithstanding that Puerto Rico is a U.S. territory (as for other U.S. territories), it is not considered part of the 4 statistical regions defined by the U.S. Census Bureau and was therefore included in the non-U.S. group for the purposes of this analysis. However, the inclusion of Puerto Rico in the non-U.S. group did not increase the number of non-U.S. countries reported in the results, as it is not an individual country. Participation was voluntary and no patient-level or identifiable data were collected and therefore institutional review board review was not required for this study.
Data collection
Survey data were collected by using a secure software platform hosted by the IAEA, the International Research Integration System (IRIS) (IAEA, Vienna, Austria). Using a standardized data collection form (Supplemental Appendix), each site provided data for procedure volumes for the following test types: stress electrocardiography (ECG), without subsequent imaging; stress echocardiography; stress single-photon emission computed tomography (SPECT); stress positron emission tomography (PET); stress cardiac magnetic resonance (CMR); coronary artery calcium (CAC) scanning; coronary computed tomographic angiography (CCTA); transthoracic echocardiography (TTE); transesophageal echocardiography (TEE); PET cardiac infection studies (fluorine-18 labeled fluorodeoxyglucose to assess for intracardiac infection); non-stress CMR; and ICA. All study types except for ICA were considered noninvasive testing. Facilities described as inpatient hospital only or inpatient and outpatient hospitals were defined as inpatient facilities, whereas facilities defined as outpatient hospitals only, outpatient imaging centers, or outpatient physician practices were defined as outpatient facilities. Teaching facilities were self-identified by survey respondents. Participants also responded to questions regarding the impact of the pandemic on availability of personal protective equipment, staff redeployments, staff and patient safety policies, operational capacity, increased use of telehealth, and staffing of imaging personnel at their institution. Survey questions marked as “planning” or “implemented” were considered affirmative responses compared to those marked as “no plans,” which were considered negative responses. Increased use of telehealth was defined as an affirmative response to survey questions regarding usage of telehealth for patient care (ie, direct contact with patients).
U.S. regional analysis included data compiled from external sources, including COVID-19 prevalence data (29) and U.S. demographic and socioeconomic data from the 2010 U.S. census (30). At the time of this analysis, U.S. county data were the smallest geographic unit of available COVID-19 data; therefore this was the most granular level of census data used in our analyses. County-level COVID-19 and census data were compiled based on county Federal Information Processing System (FIPS) codes (31). FIPS codes were assigned to each facility based on the county in which the facility operates.
Statistical analysis
Differences in frequency distributions were statistically compared using Pearson chi-squared and Fisher exact tests, and differences in continuous variables were compared using Wilcoxon rank sum and Kruskal-Wallis tests. A robust regression model using Huber’s M-estimator to reduce the weight of influential outliers (32) was used to determine factors associated with the percentage of reduction in procedure volume in the United States between March 2019 and April 2020. Variables with a p value ≤0.25 in univariate analyses were considered in the multivariable model, with final inclusion based on stepwise elimination of variables exceeding the significance level of 0.10. Variables considered in the multivariable model included county COVID-19 prevalence (cases per 10,000 residents) on April 30, 2020 (29); outpatient facility; redeployments; use of telehealth for patient care; urban center (defined as a facility located in a county in a metro area with population >1 million, based on U.S. Department of Agriculture 2013 Rural-Urban Continuum Codes) (33) political party affiliation of the current state governor; political party affiliation of the state electoral college vote in the 2016 presidential election; and county-level census demographics (30), including household income, and percentage of the county population that was foreign-born, black, and unemployed. A 2-tailed p value <0.05 was considered statistically significant. Statistical analysis was conducted using Stata/SE version 15.1 software (StataCorp, College Station, Texas).
Results
A total of 936 questionnaires were submitted, of which 27 duplicates were excluded from the analysis. Worldwide data were analyzed from a final sample of 909 facilities in 108 countries, including U.S. data from 155 facilities located in 107 distinct counties in 40 U.S. states. Counties included in this analysis encompassed approximately 31% of the entire U.S. population. Volumes of procedure data were submitted from 138 U.S. centers totaling 329,472 studies (170,463 in March 2019; 104,019 in March 2020; and 54,990 in April 2020) and 708 non-U.S. centers totaling 988,227 studies (508,175 in March 2019; 290,606 in March 2020; and 189,446 in April 2020) for a combined 1.3 million imaging studies.
Facility characteristics
Characteristics of U.S. and non-U.S. imaging centers are summarized in Table 1 . Compared to non-U.S. centers, a greater percentage of U.S. centers performed nearly every type of imaging test except for CCTA and CMR. PET cardiac infection was the only test used less frequently in U.S. laboratories than in non-U.S. laboratories (9% vs. 17%, respectively; p = 0.018). U.S. institutions also reported a greater number of procedures per center than non-U.S. centers (641 vs. 215, respectively; p < 0.001) and more outpatient studies (30% vs. 16%, respectively; p < 0.001), and a greater percentage of imaging staff were redeployed to nonimaging-related activities during the pandemic than non-U.S. centers (29% vs. 19%, respectively; p = 0.001). The number of hospital beds and percentage of teaching institutions were not significantly different between U.S. and non-U.S. centers. U.S. regional participation was greatest in the South (57 facilities), followed by the Northeast (43 facilities), the Midwest (28 facilities), and the West (27 facilities). The proportion of centers performing each imaging test was similar between U.S. regions, with significant differences observed only with stress ECG. Characteristics including median procedures per facility, number of hospital beds, proportion of teaching institutions, and redeployment of medical staff were similar among U.S. regions, although the proportion of inpatient and outpatient facilities was statistically different. Cardiologists submitted more surveys from U.S. facilities than non-U.S. facilities (70% vs. 31%, respectively), whereas nuclear medicine physicians submitted more surveys from non-U.S. facilities (3% vs. 42%, respectively) (Supplemental Table 1).
Table 1.
Characteristics for U.S., Non-U.S., and U.S. Regional Institutions That Perform Diagnostic Cardiovascular Testing Procedures
| U.S. Regions |
Worldwide |
|||||||
|---|---|---|---|---|---|---|---|---|
| Midwest | Northeast | South | West | p Value | U.S. | Non-U.S. | p Value | |
| Number of states/countries∗ | 10 | 7 | 16 | 7 | 40 | 107∗ | ||
| Number of centers, total | 28 | 43 | 57 | 27 | 155 | 754 | ||
| With procedure volume data | 25 | 41 | 49 | 23 | 138 | 708 | ||
| Type of test† | ||||||||
| Stress ECG | 14 (56) | 36 (88) | 35 (71) | 14 (61) | 0.023 | 99 (72) | 302 (43) | <0.001 |
| Stress echocardiography | 18 (72) | 28 (68) | 26 (53) | 14 (61) | 0.192 | 86 (62) | 202 (29) | <0.001 |
| Stress SPECT | 22 (88) | 37 (90) | 48 (98) | 20 (87) | 0.114 | 127 (92) | 513 (72) | <0.001 |
| Stress PET | 6 (24) | 9 (22) | 13 (27) | 7 (30) | 0.891 | 35 (25) | 53 (7) | <0.001 |
| Stress CMR | 11 (44) | 11 (27) | 11 (22) | 4 (17) | 0.176 | 37 (27) | 130 (18) | 0.023 |
| CT coronary calcium | 16 (64) | 20 (49) | 20 (41) | 13 (57) | 0.175 | 69 (50) | 221 (31) | <0.001 |
| CT coronary angiography | 17 (68) | 23 (56) | 27 (55) | 15 (65) | 0.527 | 82 (59) | 397 (56) | 0.468 |
| TTE | 17 (68) | 28 (68) | 30 (61) | 16 (70) | 0.801 | 91 (66) | 248 (35) | <0.001 |
| TEE | 17 (68) | 20 (49) | 21 (43) | 14 (61) | 0.111 | 72 (52) | 208 (29) | <0.001 |
| PET cardiac infection | 2 (8) | 5 (12) | 3 (6) | 2 (9) | 0.793 | 12 (9) | 118 (17) | 0.018 |
| CMR | 15 (60) | 21 (51) | 17 (35) | 14 (61) | 0.086 | 67 (49) | 292 (41) | 0.112 |
| All noninvasive testing | 25 (100) | 41 (100) | 49 (100) | 23 (100) | 1.000 | 138 (100) | 708 (100) | 1.000 |
| Invasive angiography | 13 (52) | 17 (41) | 26 (53) | 12 (52) | 0.625 | 68 (49) | 216 (31) | <0.001 |
| Baseline procedures per center‡ | 1,290 (350-2,250) | 686 (280-1,832) | 505 (240-1,052) | 470 (195-1,465) | 0.097 | 641 (242-1,709) | 215 (68-768) | <0.001 |
| Hospital beds | 500 (400-686) | 693 (350-867) | 400 (182-745) | 450 (150-600) | 0.196 | 522 (250-793) | 504 (230-900) | 0.449 |
| Type of center | ||||||||
| Inpatient | 26 (93) | 28 (65) | 37 (65) | 18 (67) | 0.023 | 108 (70) | 631 (84) | <0.001 |
| Outpatient | 2 (7) | 15 (35) | 20 (35) | 9 (33) | 47 (30) | 123 (16) | ||
| Teaching institution | 21 (78) | 28 (65) | 34 (60) | 15 (54) | 0.436 | 98 (63) | 499 (66) | 0.480 |
| Imaging staff redeployed | 4 (15) | 14 (33) | 22 (39) | 5 (18) | 0.068 | 45 (29) | 141 (19) | 0.004 |
Values are n, n (%), or median (interquartile range).
CMR = cardiac magnetic resonance; ECG = electrocardiogram; PET = positron emission tomography; SPECT = single-photon emission computed tomography; TEE = transesophageal echocardiogram; TTE = transthoracic echocardiogram.
Reflects the number of non-U.S. countries rather than U.S. states. Data from Puerto Rico are included in the non-U.S. category but do not increase the count of 107 non-U.S. countries.
Percentages displayed in parentheses refer to the percentage of centers that reported procedure volume data for each specific test (n = number of centers reporting procedure volume data).
March 2019 procedure volumes were the baseline for each facility.
Procedure volumes for U.S. versus non-U.S. Centers
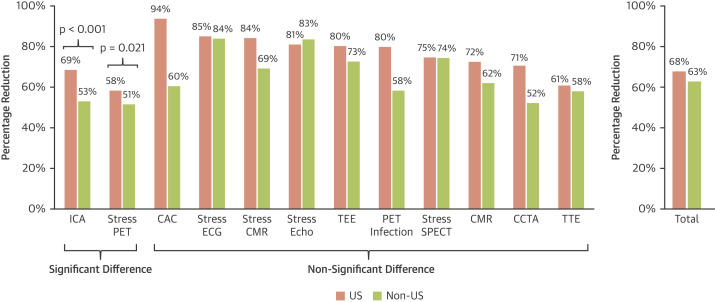
Percentage of reductions in cardiovascular procedure volumes are summarized for U.S. and non- U.S. centers from March 2019 to April 2020 (Table 2 , Figure 1 , Supplemental Table 2). Total reductions in procedure volumes during the early pandemic in U.S. facilities were similar to those in non-U.S. facilities (68% vs. 63%, respectively; p = 0.237) (Figure 1). U.S. facilities saw greater reductions in ICA (69% vs. 53%, respectively; p < 0.001) and stress PET procedures (58% vs. 51%, respectively; p = 0.020) than non-U.S. facilities. The declines in all noninvasive studies were similar between U.S. and non-U.S. facilities (68% vs. 64%, respectively; p = 0.118). Reductions were also similar between U.S. and non-U.S. facilities regardless of facility type, teaching status, redeployment of medical staff, layoffs, or increased use of technologies such as telehealth services. For both U.S. and non-U.S. facilities, declines in aerosol-generating procedures that typically require exercise-induced stress, such as stress ECG and stress echocardiography, were greater than declines in stress SPECT and stress PET, which can be performed preferentially by using pharmacological stress agents. Survey responses showed that most U.S. (71%) and non-U.S. (64%) facilities were planning or had already adopted policies to avoid exercise stress testing in favor of pharmacologic testing (Supplemental Table 3). A smaller but still significant number of U.S. (40%) and non-U.S. (43%) facilities also used modified nuclear stress protocols to prioritize shorter acquisition times and stress-first protocols when possible.
Table 2.
Reduction in Cardiac Imaging Volume by Diagnostic Test and Facility Characteristics
| U.S. Regions |
Worldwide |
|||||||
|---|---|---|---|---|---|---|---|---|
| Midwest | Northeast | South | West | p Value† | U.S. | Non-U.S. | p Value† | |
| Reduction in total procedures | ||||||||
| March 2019–March 2020 | 41 | 44 | 29 | 48 | 0.069 | 39 | 43 | 0.803 |
| March 2020–April 2020 | 56 | 58 | 47 | −7 | <0.001 | 47 | 35 | 0.470 |
| March 2019–April 2020 | 74 | 76 | 62 | 44 | <0.001 | 68 | 63 | 0.237 |
| By diagnostic test∗ | ||||||||
| Stress ECG | 85 | 91 | 81 | 61 | 0.050 | 85 | 84 | 0.426 |
| Stress echocardiography | 82 | 90 | 73 | 67 | 0.038 | 81 | 83 | 0.551 |
| Stress SPECT | 77 | 87 | 69 | 47 | <0.001 | 75 | 74 | 0.062 |
| Stress PET | 78 | 77 | 65 | 30 | 0.143 | 58 | 51 | 0.021 |
| Stress CMR | 78 | 97 | 62 | 58 | 0.642 | 84 | 69 | 0.786 |
| CT coronary calcium | 97 | 95 | 93 | 83 | 0.743 | 94 | 60 | 0.366 |
| CT coronary angiography | 60 | 82 | 72 | 48 | 0.045 | 71 | 52 | 0.753 |
| TTE | 68 | 69 | 54 | 36 | 0.037 | 61 | 58 | 0.492 |
| TEE | 86 | 83 | 69 | 71 | 0.057 | 80 | 73 | 0.114 |
| PET cardiac infection | 0 | 88 | 78 | 42 | 0.186 | 80 | 58 | 0.957 |
| CMR | 75 | 78 | 73 | 50 | 0.111 | 72 | 62 | 0.422 |
| All noninvasive testing | 74 | 76 | 62 | 44 | <0.001 | 68 | 64 | 0.118 |
| Invasive coronary angiography | 77 | 75 | 63 | 41 | 0.013 | 69 | 53 | <0.001 |
| By facility characteristic∗ | ||||||||
| Type of facility | ||||||||
| Inpatient | 74 | 76 | 63 | 45 | <0.001 | 69 | 60 | 0.176 |
| Outpatient | 54 | 87 | 54 | 40 | 0.043 | 59 | 78 | 0.674 |
| Teaching status | ||||||||
| Teaching | 74 | 75 | 61 | 46 | 0.002 | 68 | 60 | 0.187 |
| Nonteaching | 74 | 83 | 65 | 39 | 0.038 | 66 | 72 | 0.874 |
| Redeployment during pandemic | ||||||||
| Redeployed | 86 | 84 | 72 | 42 | 0.003 | 76 | 62 | 0.810 |
| Not redeployed | 71 | 74 | 54 | 44 | 0.012 | 65 | 63 | 0.045 |
| Changes in staffing | ||||||||
| Furloughed or laid off staff | 75 | 81 | 58 | 48 | 0.049 | 69 | 75 | 0.020 |
| No changes | 73 | 74 | 65 | 42 | 0.004 | 67 | 60 | 0.932 |
| Telehealth services for patient care | ||||||||
| Increased use | 75 | 76 | 64 | 44 | <0.001 | 69 | 64 | 0.135 |
| No change in use | 43 | 77 | 43 | – | 0.108 | 46 | 58 | 0.470 |
Values are %, unless otherwise indicated.
CMR = cardiac magnetic resonance imaging; ECG = electrocardiogram; PET = positron emission tomography; SPECT = single-photon emission computed tomography; TEE = transesophageal echocardiogram; TTE = transthoracic echocardiogram; U.S. = United States.
Percentage reductions were calculated as the cumulative reduction of all procedures in each category from March 2019 to April 2020.
The p values were calculated by comparing the distributions of percentage reductions of individual laboratories for each category.
Figure 1.
Percentage Reduction in Procedure Volumes from March 2019 to April 2020 for U.S. and Non-U.S. Imaging Centers
Clustered bar graphs showing the percentage reduction in procedure volumes from March 2019 to April 2020 by diagnostic test type and by U.S. (red) and non-U.S. (green) centers. ICA (p < 0.001) and stress PET (p = 0.021) were the only tests in which the difference in percent reduction between U.S. and non-U.S. centers was statistically significant. CAC = coronary artery calcium scan; CCTA = coronary computed tomographic angiography; CMR = cardiac magnetic resonance imaging; ECG = electrocardiogram; echo = echocardiogram; ICA = invasive coronary angiography; IQR = interquartile range; PET = positron emission tomography; SPECT = single-photon emission computed tomography; TEE = transesophageal echocardiogram; TTE = transthoracic echocardiogram.
Procedure volumes for U.S. regional centers
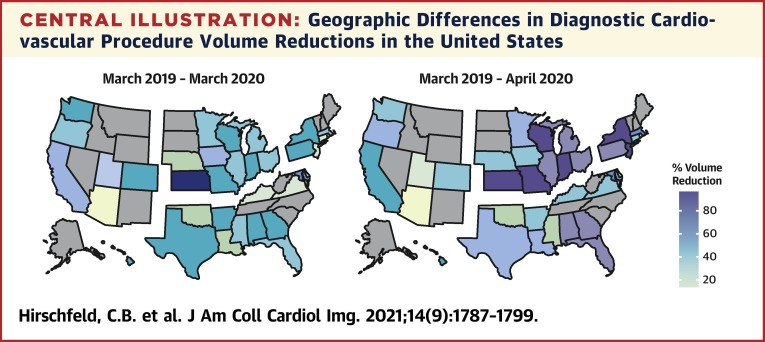
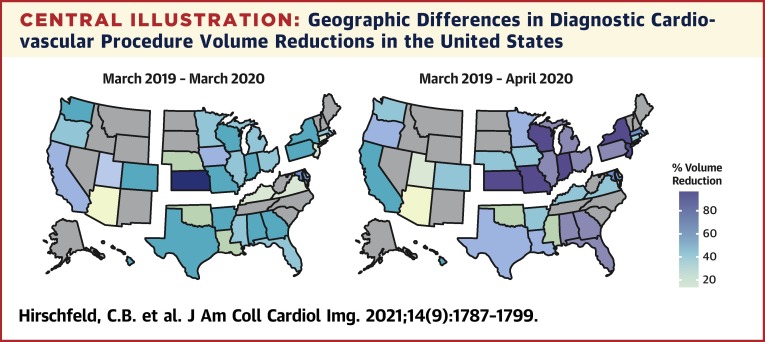
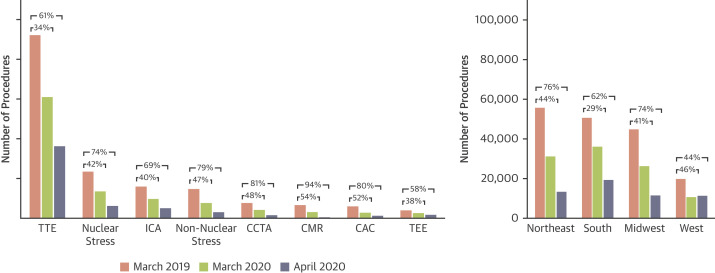
Table 2 summarizes the percentage of decline in volumes of cardiovascular procedures among U.S. regions. Total reductions in volumes among U.S. regions during the early months of the pandemic were similar from March 2019 to March 2020 (p = 0.069) but different from March 2019 to April 2020 (p < 0.001). The largest declines were observed in the Northeast (76%) and Midwest (74%) facilities, followed by facilities in the South (62%) and West (44%) (Central Illustration ). Reductions in volumes differed significantly among U.S. regions for 6 of 12 diagnostic tests, including stress ECG, stress echo, stress SPECT, CCTA, TTE, and ICA. Declines were highest in the Northeast and Midwest and lowest in the South and West for every test type except for CCTA and CMR (Figure 2 ). Reductions in volumes of procedures varied significantly among U.S. regions for every facility characteristic, except among facilities that reported no changes in telehealth usage. Reductions for each facility characteristic were greater for the Northeast and Midwest than for the South and West regions.
Central Illustration.
Geographic Differences in Diagnostic Cardiovascular Procedure Volume Reductions in the United States
U.S. maps demonstrate the percentage of reduction in diagnostic cardiovascular procedures from March 2019 to March 2020 (left map) and March 2019 to April 2020 (right map). States are grouped together by U.S. census regions: West (left), Midwest (top middle), Northeast (top right), and South (bottom right). Alaska and Hawaii are included in the West region. Differences in overall volume reductions were similar between U.S. regions in March 2020 (p = 0.069) but different in April 2020 (p < 0.001), with the greatest declines seen in the Northeast and Midwest.
Figure 2.
Reduction in U.S. Cardiovascular Procedure Volumes by Diagnostic Test and Region
Clustered bar graphs display the number of procedures for March 2019 (red), March 2020 (green), and April 2020 (blue) for each test and each U.S. region. TTE was the most represented diagnostic test in the study, followed by stress tests and ICA procedures. The greatest declines in the volume of procedures were seen in stress tests, CCTA, CMR, and CAC. Regional declines were greatest in the Northeast and Midwest, followed by the South and West. PET infection is not shown in the figure due to the small sample size. Abbreviations as in Figure 1.
Operational capacity, safety policies, and staffing
Major differences were noted in the responses for operational capacity, safety policies, and staffing between U.S. and non-U.S. facilities (Table 3 ). For example, compared to non-U.S. centers, a greater proportion of U.S. centers reported increased usage of telehealth for direct patient care (90% vs. 65%, respectively; p < 0.001) and use of patient screening measures, such as temperature checks (87% vs. 77%, respectively; p = 0.008), symptom screening (97% vs. 86%, respectively; p < 0.001), and COVID-19 testing (46% vs. 26%, respectively; p < 0.001). U.S. facilities were also more likely to require the use of face masks than non-U.S. facilities (97% vs. 81%, respectively; p < 0.001). Furloughs of nonphysician imaging staff were reported in more U.S. centers than in non-U.S. centers (35% vs. 22%, respectively; p = 0.001), whereas furloughs of physicians were reported in fewer U.S. centers than in non-U.S. centers (13% vs. 21%, respectively; p = 0.025). Survey responses were mostly similar among U.S. regions, with slight differences in reports of increased time to clean and disinfect equipment, and nonphysician layoffs, which were notably higher in the South than in other regions.
Table 3.
Changes in Institutional Capacity, Practices, and Staffing That Were Implemented in March 2020 and April 2020 During the COVID-19 Pandemic
| U.S. Regions |
Worldwide |
|||||||
|---|---|---|---|---|---|---|---|---|
| Midwest (n = 28) | Northeast (n = 43) | South (n = 57) | West (n = 27) | p Value | U.S. (n = 155) | Non-U.S. (n = 754) | p Value | |
| Change in capacity | ||||||||
| Some outpatient activities cancelled | 27 (96) | 42 (98) | 50 (88) | 26 (96) | 0.217 | 145 (94) | 678 (91) | 0.246 |
| All outpatient activities cancelled | 15 (54) | 27 (66) | 31 (54) | 16 (59) | 0.662 | 89 (58) | 432 (58) | 0.991 |
| Phased re-opening after peak pandemic | 26 (93) | 42 (98) | 53 (93) | 26 (96) | 0.759 | 147 (95) | 663 (89) | 0.027∗ |
| Extended hours | 13 (46) | 20 (47) | 25 (44) | 9 (33) | 0.707 | 67 (43) | 321 (43) | 0.943 |
| New weekend hours | 8 (29) | 17 (40) | 15 (27) | 8 (30) | 0.568 | 48 (31) | 227 (31) | 0.872 |
| Increased use of telehealth for patient care | 26 (93) | 38 (88) | 51 (89) | 24 (92) | 0.945 | 139 (90) | 481 (65) | <0.001∗ |
| Increased time per study for cleaning/disinfection | 21 (75) | 42 (98) | 48 (84) | 21 (78) | 0.014∗ | 132 (85) | 643 (86) | 0.824 |
| Eliminate protocols requiring close contact | 23 (82) | 36 (84) | 42 (75) | 24 (89) | 0.498 | 125 (81) | 570 (76) | 0.191 |
| Change in practice | ||||||||
| Physical distancing | 28 (100) | 43 (100) | 55 (98) | 25 (93) | 0.150 | 151 (98) | 720 (96) | 0.294 |
| Separate spaces for patients with COVID-19 | 25 (93) | 31 (74) | 49 (89) | 23 (92) | 0.098 | 128 (86) | 685 (92) | 0.016∗ |
| Reduced waiting room time | 26 (93) | 39 (93) | 50 (89) | 26 (96) | 0.849 | 141 (92) | 687 (92) | 0.897 |
| Limit visitors | 28 (100) | 43 (100) | 54 (96) | 27 (100) | 0.663 | 152 (99) | 731 (98) | 0.469 |
| Temperature checks | 24 (89) | 37 (86) | 50 (89) | 22 (81) | 0.770 | 133 (87) | 579 (77) | 0.008∗ |
| Symptom screening | 28 (100) | 42 (98) | 51 (94) | 27 (100) | 0.523 | 148 (97) | 640 (86) | <0.001∗ |
| COVID-19 testing | 14 (50) | 21 (49) | 23 (40) | 13 (48) | 0.777 | 71 (46) | 194 (26) | <0.001∗ |
| Require masks | 27 (96) | 43 (100) | 54 (95) | 26 (96) | 0.478 | 150 (97) | 606 (81) | <0.001∗ |
| Change in staffing | ||||||||
| Furlough non-physician imaging staff | 13 (50) | 10 (26) | 19 (34) | 10 (36) | 0.176 | 52 (35) | 162 (22) | 0.001∗ |
| Furlough imaging physicians | 1 (4) | 5 (13) | 8 (14) | 5 (18) | 0.404 | 19 (13) | 152 (21) | 0.025∗ |
| Reduce salaries of non-physician imaging staff | 9 (35) | 7 (17) | 15 (26) | 8 (29) | 0.330 | 39 (26) | 185 (25) | 0.810 |
| Reduce salaries of imaging physicians | 12 (48) | 10 (24) | 17 (30) | 9 (32) | 0.278 | 48 (32) | 188 (25) | 0.101 |
| Laid off non-physician imaging staff | 3 (11) | 2 (5) | 12 (21) | 1 (4) | 0.049∗ | 18 (12) | 65 (9) | 0.229 |
| Laid off imaging physicians | 0 (0) | 3 (7) | 2 (4) | 2 (7) | 0.487 | 7 (5) | 39 (5) | 0.739 |
Values are n (%). Figures reflect the proportion of laboratories with planned or implemented changes.
U.S. = United States.
Indicates significant p values.
Factors associated with procedure volume reduction in the U.S
Results of a linear regression analysis are presented in Table 4 . In a multivariable analysis, the mean reduction in volumes of procedures during the early phase of the COVID-19 pandemic was 11.5% greater for facilities reporting staff redeployments than those reporting no redeployments (95% confidence interval [CI]: 5.3% to 17.7%; p < 0.001), 12.5% greater for outpatient facilities than for inpatient facilities (95% CI: 6.3% to 18.7%; p < 0.001), 9.7% greater for urban centers than for nonurban centers (95% CI: 3.3% to 16.1%; p = 0.003), and 0.6% greater for every 1 case increase per 10,000 residents in the county COVID-19 prevalence (95% CI: 0.1% to 1.1%; p = 0.011). The remaining variables described in the methods, including increased usage of telehealth, political factors, and U.S. census demographic characteristics, were not found to be associated with volume reduction in a multivariable analysis.
Table 4.
Factors Associated with Reduction of Diagnostic Cardiovascular Procedure Volumes during the Early Phase of the COVID-19 Pandemic in a Multivariable Analysis
| Mean Volume Change∗ | 95% CI |
p Value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| COVID-19 prevalence | 0.6 | 0.1 | 1.1 | 0.011 |
| Staff redeployments | 11.5 | 5.3 | 17.7 | <0.001 |
| Outpatient center | 12.5 | 6.3 | 18.7 | <0.001 |
| Urban center | 9.7 | 3.3 | 16.1 | 0.003 |
Values are %.
Percentage change that can be expected in the mean volume reduction for each variable. For example, mean volume reduction is 11.5% greater in facilities that reported staff redeployments and 12.5% higher in outpatient centers. For the COVID-19 prevalence (continuous variable), every increase in 1 case per 10,000 county residents is expected to increase the mean volume reduction of a facility by an additional 0.6%.
Discussion
This report examined worldwide data from 909 institutions in 108 countries to investigate how the COVID-19 pandemic has impacted the volume of diagnostic cardiovascular procedures performed in the U.S. and non-U.S. facilities and to determine factors associated with volume reduction in U.S. facilities. We found that volume reductions were generally similar between U.S. and non-U.S. facilities for all diagnostic procedures apart from ICA, in which the U.S. experienced greater declines (69% vs. 53%, respectively; p < 0.001). Conversely, we observed significant differences between U.S. regions, with the greatest declines seen in the Northeast and Midwest for nearly every type of cardiovascular test. Factors statistically correlated with greater reduction in volumes in a multivariable analysis included COVID-19 prevalence, staff redeployments, outpatient centers, and urban centers.
The impact of the pandemic on worldwide CVD morbidity and mortality is an area of growing concern. Already, multiple reports have described worrisome declines in the rates of percutaneous revascularization procedures for ACS. Garcia et al. (18) evaluated 9 high-volume cardiac catheterization facilities in the United States and found that laboratory activations for ST-segment elevation myocardial infarctions (STEMI) declined from baseline values by 38% at the end of March 2020. This decrease was similar to reductions in STEMI activations reported in separate studies from Spain (40%) (16) and Italy (33%) (15). One possible explanation could be increased usage of noninvasive management pathways for ACS. However, studies have shown that, in fact, overall hospitalizations for ACS have also declined by a similar percentage (13,14). Mafham et al. (13) evaluated hospital admission data in England and found that admissions for ACS in March 2020 had declined by 40% (13). Our data also showed reductions in ICA volumes of 40% in U.S. facilities and 43% in non-U.S. facilities at the end of March 2020, similar to those in previous studies. However, in April 2020, we observed even greater worldwide declines in ICA procedures, with a significantly greater reduction in U.S. centers than in non-U.S. centers (69% vs. 53%, respectively; p < 0.001).
The greater reduction of ICA procedures in U.S. facilities could relate to several factors, including the rapid rise in COVID-19 cases in the U.S. during March and April of 2020. New York City was widely considered to be one of the epicenters of the COVID-19 pandemic in April (34). Thus, it is not surprising that our data also revealed significant differences in volume reductions between U.S. regions, with a nearly 2-fold greater decline in ICA procedures in the Northeast than in the West (77% vs. 41%, respectively; p <0.001). These declines are unlikely due to a true decrease in the incidence of ACS. In fact, Kwong et al. (35) showed that the risk of acute myocardial infarction was approximately 3 to 6 times higher in the first 7 days of viral respiratory infection. A more alarming, and more likely, alternative is the decline in emergency room visits for chest pain due to the reluctance of patients to seek medical attention during the pandemic. A recent report from the Centers for Disease Control and Prevention showed that, during the early phase of the pandemic, emergency room visits for chest pain decreased by 24,258 visits per week across the United States compared to the same period in 2019, whereas visits for acute myocardial infarction declined by 1,156 per week, suggesting that delayed care in these cases might have resulted in “additional mortality” (36). Similar declines in emergency room presentations have been described for acute stroke (37,38), acute surgical complaints (4,39), and even emergency mental health services (40), which are largely believed to be the result of decreased usage of health care services generally during the pandemic, rather than the decreased incidence of non-COVID-19 illnesses. Although the reported declines in hospital presentations and procedures for ACS are a major cause for concern, additional data are urgently needed to better establish the direct impact of these findings on the morbidity and mortality of CVD around the world.
In addition to reductions in ICA procedures, we found that rates of noninvasive cardiovascular procedures also fell sharply during the early pandemic. It is possible that declines in worldwide cardiovascular testing might have curtailed transmission of COVID-19 while permitting an increase in hospital capacity and a decrease in inappropriate testing (41). However, it may also signify a potential looming global health crisis from the millions of CVD diagnoses that could be missed during the pandemic. Overall, declines in noninvasive cardiovascular procedure volumes were similar for U.S. and non-U.S. laboratories (68% vs. 64% reported declines; p = 0.118), which is more likely explained by offsetting procedure volume reductions in non-U.S. regions than a true resemblance between U.S. and non-U.S. centers. For example, our international report showed that regions beyond the peak of transmission in April 2020 (e.g., Far East and South East Asia) reported the lowest reductions in volume of procedures, whereas regions at the peak or in the early stages of community transmission during the same time period (e.g., Europe and South America, respectively) reported greater reductions in volumes (12).
Variable rates of reductions in procedure volumes for each modality also suggests that factors other than restricted access during the pandemic likely impacted the relative reductions observed. For example, declines in TEE volumes were generally greater than other modalities, likely due to fears of aerosolization with endotracheal intubation. We also found that most U.S. and non-U.S. facilities implemented policies to avoid aerosol-generating exercise stress tests in favor of pharmacologic stress tests while optimizing protocols to shorten patient-staff contact time (e.g., reduced acquisition times and use of stress-first protocols). Consequently, both U.S. and non-U.S. facilities reported greater reductions in stress ECG and stress echocardiography than nuclear stress tests, where image acquisition can be performed at a distance by using pharmacologic stress agents. Reductions in CCTA were also lower than exercise stress tests, raising the possibility that facilities could have used alternative nonstress modalities to diagnose CAD.
In contrast, there were significant differences in reductions of procedure volumes reported among U.S. regions, with greater declines generally observed in the Northeast and Midwest. This difference did not emerge until April 2020 when declines in procedure volumes in the Northeast and Midwest outpaced declines in the South and West. We found that facilities operating in counties with a greater prevalence of COVID-19 in April of 2020 reported greater reductions in cardiovascular testing (p = 0.011). This was likely due to the mounting effects of the pandemic in these areas, which led to the abrupt cessation of elective procedures and the suspension of many outpatient medical practices (42,43). Consequently, our analysis revealed that classification as an outpatient practice was also associated with a 12.5% greater reduction in volumes of diagnostic procedures (p < 0.001). A common practice in the most affected areas of the pandemic has been to redeploy medical staff to accommodate surges in the number of hospitalized patients (44). In our study, redeployment of imaging staff was associated with an 11.5% greater overall procedure volume reduction, independent of the COVID-19 prevalence in the surrounding area (p < 0.001). However, whether redeployments were the direct cause of procedure volume reductions or a consequence of reductions during the early pandemic (e.g., redeployments to reduce overhead) is unknown. Although health care systems must prioritize provision of resources to maintain flexibility and scalability during the pandemic, further examination of the potential adverse consequences of such strategies (i.e., decreased availability of essential health care services) and the development of approaches to mitigate them in the future are warranted. Additionally, despite the divisive politicization surrounding the U.S. response to the COVID-19 pandemic (45) factors associated with U.S. political alignment (i.e., the political party affiliation of the current governor and whether the state electoral college voted for the Republican or Democratic nominee in the 2016 presidential election) were also not significantly associated with procedure volume reduction in a multivariable analysis.
Finally, population density has been shown in some studies to be an important factor in both the incidence and death rates resulting from COVID-19 infection (46, 47, 48). We found that facilities located in urban counties with a metropolitan population of >1 million had 9.7% fewer procedures than facilities in more rural counties (p = 0.003). Although the reason for this is not entirely apparent, it could signify the existence of disparities in access to cardiovascular testing during the pandemic. Major health inequities related to COVID-19 have already been described, with black populations experiencing greater rates of infection, hospitalizations, and even deaths in some studies (49, 50, 51). At baseline, black patients in the United States have disproportionately higher morbidity and mortality from CVD and are less likely to receive the same standards of cardiovascular care as nonminority patients (52). Minority populations are also overrepresented in urban communities (53), raising the possibility that minority groups could be more affected by the greater declines in diagnostic cardiac procedures seen in more densely populated counties. Variables accounting for racial and economic differences were not significant in our analysis; however, this study was not designed to detect discrepancies in these characteristics (e.g., county-level data do not fully reflect the demographic characteristics of the neighborhood served by an individual institution, and study participants did not provide patient demographic data associated with their procedure volumes). Nonetheless, communities and individual laboratories should be aware of possible disparities in access to cardiovascular testing that affect the communities most in need of these essential services during the COVID-19 pandemic.
This study has several limitations. First, U.S. regional participation in the INCAPS-COVID study was variable, and data collection is prone to potential biases (e.g., volunteer bias or sampling bias). Thus, the extent to which regional data are representative of the true regional changes in cardiovascular testing is unknown. Additionally, facilities that participated in the survey may not represent the exact distribution of facilities that perform diagnostic cardiac imaging in the community (e.g., only 35% of non-U.S. facilities reported procedure volume data for TTE, a commonly used imaging modality), and the specialty of the survey respondent may have affected the mixture of procedures reported (e.g., nuclear medicine physicians may only report nuclear procedures rather than procedures for the entire department or practice). Nevertheless, the INCAPS-COVID registry constitutes a diverse group of diagnostic facilities representing a broad range of clinical practice settings in each world region. Furthermore, our regression analysis was limited by the granularity of U.S. COVID-19 data, which at the time of this writing, were available only at the county level in most U.S. states. Ideally, a smaller geographical unit of measurement (e.g., census tract) would better reflect the demographic characteristics of the community served by each individual U.S. imaging center. Still, county-level data were sufficient to account for a great degree of variability in our model and enabled us to identify variables significantly associated with U.S. procedure volume reduction. Finally, our results reflect only the early phase of the COVID-19 pandemic. Since the collection of INCAPS-COVID data, institutional and governmental strategies related to the delivery of health care have likely changed, and shifts in diagnostic cardiac testing during the second and third waves of the pandemic remains unknown. In view of this, the INCAPS-COVID Investigators Group is planning to reconvene for additional data collection in early 2021, which is expected to provide additional insights into ongoing changes in worldwide diagnostic cardiovascular testing throughout the COVID-19 pandemic.
Conclusions
In this study, we observed marked reductions in worldwide cardiovascular testing during the early phase of the COVID-19 pandemic that were generally similar between U.S. and non-U.S. facilities. The major exception was a greater decline in ICA procedures in the U.S. that could be linked to the outbreak of COVID-19 in the United States during this time. Conversely, we observed variations between U.S. regions, with the greatest reductions in procedure volumes seen in the Northeast and Midwest. We found that COVID-19 prevalence, staff redeployments, outpatient centers, and urban centers were all associated with greater declines in total cardiovascular procedure volumes in the United States. The substantial reduction in cardiovascular testing during the early phase of the pandemic highlights the need for strategies to maintain access to this essential resource in areas most affected by COVID-19 outbreaks and to mitigate the predicted burden of CVD morbidity and mortality in the wake of the pandemic.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: INCAPS-COVID is the first study to quantify the marked declines in cardiovascular testing around the world during the early phase of the COVID-19 pandemic. Reduction in volumes of procedures in the United States were similar to those in non-U.S. institutions but differed significantly among U.S. regions, with the greatest declines associated with COVID-19 prevalence, staff redeployments, urban centers, and outpatient centers.
TRANSLATIONAL OUTLOOK: The substantial reduction in diagnostic cardiovascular procedures during the early phase of the COVID-19 pandemic highlights the need to identify strategies to maintain access to cardiac testing in areas most affected by COVID-19 outbreaks in order to mitigate the predicted burden of CVD morbidity and mortality in the wake of the pandemic. Further studies are needed to correlate reductions in cardiovascular testing to clinical outcomes.
Funding Support and Author Disclosures
This work was supported by the International Atomic Energy Agency. Dr. Blankstein has previously received research support from Amgen and Astellas Inc. Dr. Ferencik has been previously supported by U.S. National Institutes of Health (NIH) and American Heart Association; and is a consultant for Biograph, Inc. Dr. Nørgaard has previously received unrestricted institutional research grants from Siemens and HeartFlow. Dr. Maurovich-Horvat has been a shareholder of Neumann Medical Ltd. Dr. Einstein has previously received grants from NIH, International Atomic Energy Agency, Canon Medical Systems, Roche Medical Systems, WL Gore, and GE Health care; consultant for WL Gore; on the Speakers Bureau for Ionetix; has received travel/accommodations/meeting expenses from HeartFlow; and is a stockholder in Emergent BioSolutions Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment(s)
The INCAPS-COVID Investigators Group, listed by name in the Supplemental Appendix, thank cardiology and imaging professional societies worldwide for their assistance in disseminating the survey to their memberships. This group includes, alphabetically, but not limited to, American Society of Nuclear Cardiology, Arab Society of Nuclear Medicine, Australasian Association of Nuclear Medicine Specialists, Australia-New Zealand Society of Nuclear Medicine, Belgian Society of Nuclear Medicine, Brazilian Nuclear Medicine Society, British Society of Cardiovascular Imaging, Conjoint Committee for the Recognition of Training in CT Coronary Angiography Australia and New Zealand, Consortium of Universities and Institutions in Japan, Danish Society of Cardiology, Gruppo Italiano Cardiologia Nucleare, Indonesian Society of Nuclear Medicine, Japanese Society of Nuclear Cardiology, Moscow Regional Department of Russian Nuclear Medicine Society, Philippine Society of Nuclear Medicine, Russian Society of Radiology, Sociedad Española de Medicina Nuclear e Imagen Molecular, Society of Cardiovascular Computed Tomography, and Thailand Society of Nuclear Medicine. The authors also thank Olga Morozova for assistance with graphics.
Footnotes
James Udelson, MD, served as Guest Editor for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a list of the INCAPS COVID investigators group and country participation as well as supplemental tables, please see the online version of this paper.
Contributor Information
INCAPS-COVID Investigators Group:
Andrew J. Einstein, Diana Paez, Maurizio Dondi, Nathan Better, Rodrigo Cerci, Sharmila Dorbala, Thomas N.B. Pascual, Paolo Raggi, Leslee J. Shaw, Todd C. Villines, Joao V. Vitola, Michelle C. Williams, Yaroslav Pynda, Gerd Hinterleitner, Yao Lu, Olga Morozova, Zhuoran Xu, Cole B. Hirschfeld, Yosef Cohen, Benjamin Goebel, Eli Malkovskiy, Michael Randazzo, Andrew Choi, Juan Lopez-Mattei, Purvi Parwani, Mohammad Nawaz Nasery, Artan Goda, Ervina Shirka, Rabie Benlabgaa, Salah Bouyoucef, Abdelkader Medjahedi, Qais Nailli, Mariela Agolti, Roberto Nicolas Aguero, Maria del Carmen Alak, Lucia Graciela Alberguina, Guillermo Arroñada, Andrea Astesiano, Alfredo Astesiano, Carolina Bas Norton, Pablo Benteo, Juan Blanco, Juan Manuel Bonelli, Jose Javier Bustos, Raul Cabrejas, Jorge Cachero, Roxana Campisi, Alejandro Canderoli, Silvia Carames, Patrícia Carrascosa, Ricardo Castro, Oscar Cendoya, Luciano Martin Cognigni, Carlos Collaud, Carlos Collaud, Claudia Cortes, Javier Courtis, Daniel Cragnolino, Mariana Daicz, Alejandro De La Vega, Silvia Teresa De Maria, Horacio Del Riego, Fernando Dettori, Alejandro Deviggiano, Laura Dragonetti, Mario Embon, Ruben Emilio Enriquez, Jorge Ensinas, Fernando Faccio, Adolfo Facello, Diego Garofalo, Ricardo Geronazzo, Natalia Gonza, Lucas Gutierrez, Miguel Angel Guzzo, Miguel Angel Guzzo, Victor Hasbani, Melina Huerin, Victor Jäger, Julio Manuel Lewkowicz, Maria Nieves A López De Munaín, Jose Maria Lotti, Alejandra Marquez, Osvaldo Masoli, Osvaldo Horacio Masoli, Edgardo Mastrovito, Matias Mayoraz, Graciela Eva Melado, Anibal Mele, Maria Fernanda Merani, Alejandro Horacio Meretta, Susana Molteni, Marcos Montecinos, Eduardo Noguera, Carlos Novoa, Claudio Pereyra Sueldo, Sebastian Perez Ascani, Pablo Pollono, Maria Paula Pujol, Alejandro Radzinschi, Gustavo Raimondi, Marcela Redruello, Marina Rodríguez, Matías Rodríguez, Romina Lorena Romero, Arturo Romero Acuña, Federico Rovaletti, Lucas San Miguel, Lucrecia Solari, Bruno Strada, Sonia Traverso, Sonia Simona Traverzo, Maria del Huerto Velazquez Espeche, Juan Sebastian Weihmuller, Juan Wolcan, Susana Zeffiro, Mari Sakanyan, Scott Beuzeville, Raef Boktor, Patrick Butler, Jennifer Calcott, Loretta Carr, Virgil Chan, Charles Chao, Woon Chong, Mark Dobson, D'Arne Downie, Girish Dwivedi, Barry Elison, Jean Engela, Roslyn Francis, Anand Gaikwad, Ashok Gangasandra Basavaraj, Bruce Goodwin, Robert Greenough, Christian Hamilton-Craig, Victar Hsieh, Subodh Joshi, Karin Lederer, Kenneth Lee, Joseph Lee, John Magnussen, Nghi Mai, Gordon Mander, Fiona Murton, Dee Nandurkar, Johanne Neill, Edward O'Rourke, Patricia O'Sullivan, George Pandos, Kunthi Pathmaraj, Alexander Pitman, Rohan Poulter, Manuja Premaratne, David Prior, Lloyd Ridley, Natalie Rutherford, Hamid Salehi, Connor Saunders, Luke Scarlett, Sujith Seneviratne, Deepa Shetty, Ganesh Shrestha, Jonathan Shulman, Vijay Solanki, Tony Stanton, Murch Stuart, Michael Stubbs, Ian Swainson, Kim Taubman, Andrew Taylor, Paul Thomas, Steven Unger, Anthony Upton, Shankar Vamadevan, William Van Gaal, Johan Verjans, Demetrius Voutnis, Victor Wayne, Peter Wilson, David Wong, Kirby Wong, John Younger, Gudrun Feuchtner, Siroos Mirzaei, Konrad Weiss, Natallia Maroz-Vadalazhskaya, Olivier Gheysens, Filip Homans, Rodrigo Moreno-Reyes, Agnès Pasquet, Veronique Roelants, Caroline M. Van De Heyning, Raúl Araujo Ríos, Valentina Soldat-Stankovic, Sinisa Stankovic, Maria Helena Albernaz Siqueira, Augusto Almeida, Paulo Henrique Alves Togni, Jose Henrique Andrade, Luciana Andrade, Carlos Anselmi, Roberta Araújo, Guilherme Azevedo, Sabbrina Bezerra, Rodrigo Biancardi, Gabriel Blacher Grossman, Simone Brandão, Diego Bromfman Pianta, Lara Carreira, Bruno Castro, Tien Chang, Fernando Cunali, Jr., Roberto Cury, Roberto Dantas, Fernando de Amorim Fernandes, Andrea De Lorenzo, Robson De Macedo Filho, Fernanda Erthal, Fabio Fernandes, Juliano Fernandes, Fabio Fernandes, Thiago Ferreira De Souza, Wilson Furlan Alves, Bruno Ghini, Luiz Goncalves, Ilan Gottlieb, Marcelo Hadlich, Vinícius Kameoka, Ronaldo Lima, Adna Lima, Rafael Willain Lopes, Ricardo Machado e Silva, Tiago Magalhães, Fábio Martins Silva, Luiz Eduardo Mastrocola, Fábio Medeiros, José Claudio Meneghetti, Vania Naue, Danilo Naves, Roberto Nolasco, Cesar Nomura, Joao Bruno Oliveira, Eduardo Paixao, Filipe Penna De Carvalho, Ibraim Pinto, Priscila Possetti, Mayra Quinta, Rodrigo Rizzo Nogueira Ramos, Ricardo Rocha, Alfredo Rodrigues, Carlos Rodrigues, Leila Romantini, Adelina Sanches, Sara Santana, Leonardo Sara da Silva, Paulo Schvartzman, Cristina Sebastião Matushita, Tiago Senra, Afonso Shiozaki, Maria Eduarda Menezes de Siqueira, Cristiano Siqueira, Paola Smanio, Carlos Eduardo Soares, José Soares Junior, Marcio Sommer Bittencourt, Bernardo Spiro, Cláudio Tinoco Mesquita, Jorge Torreao, Rafael Torres, Marly Uellendahl, Guilherme Urpia Monte, Otávia Veríssimo, Estevan Vieira Cabeda, Felipe Villela Pedras, Roberto Waltrick, Marcello Zapparoli, Hamid Naseer, Marina Garcheva-Tsacheva, Irena Kostadinova, Youdaline Theng, Gad Abikhzer, Rene Barette, Benjamin Chow, Dominique Dabreo, Matthias Friedrich, Ria Garg, Mohammed Nassoh Hafez, Chris Johnson, Marla Kiess, Jonathon Leipsic, Eugene Leung, Robert Miller, Anastasia Oikonomou, Stephan Probst, Idan Roifman, Gary Small, Vikas Tandon, Adwait Trivedi, James White, Katherine Zukotynski, Jose Canessa, Gabriel Castro Muñoz, Carmen Concha, Pablo Hidalgo, Cesar Lovera, Teresa Massardo, Luis Salazar Vargas, Pedro Abad, Harold Arturo, Sandra Ayala, Luis Benitez, Alberto Cadena, Carlos Caicedo, Antonio Calderón Moncayo, Antonio Calderón Moncayo, Sharon Gomez, Claudia T. Gutierrez Villamil, Claudia Jaimes, Juan Londoño, Juan Luis Londoño Blair, Luz Pabon, Mauricio Pineda, Juan Carlos Rojas, Diego Ruiz, Manuel Valencia Escobar, Andres Vasquez, Damiana Vergel, Alejandro Zuluaga, Isabel Berrocal Gamboa, Gabriel Castro, Ulises González, Ana Baric, Tonci Batinic, Maja Franceschi, Maja Hrabak Paar, Mladen Jukic, Petar Medakovic, Viktor Persic, Marina Prpic, Ante Punda, Juan Felipe Batista, Juan Manuel Gómez Lauchy, Yamile Marcos Gutierrez, Yamile Marcos Gutierrez, Rayner Menéndez, Amalia Peix, Luis Rochela, Christoforos Panagidis, Ioannis Petrou, Vaclav Engelmann, Milan Kaminek, Vladimír Kincl, Otto Lang, Milan Simanek, Jawdat Abdulla, Morten Bøttcher, Mette Christensen, Lars Christian Gormsen, Philip Hasbak, Søren Hess, Paw Holdgaard, Allan Johansen, Kasper Kyhl, Bjarne Linde Norgaard, Kristian Altern Øvrehus, Niels Peter Rønnow Sand, Rolf Steffensen, Anders Thomassen, Bo Zerahn, Alfredo Perez, Giovanni Alejandro Escorza Velez, Mayra Sanchez Velez, Islam Shawky Abdel Aziz, Mahasen Abougabal, Taghreed Ahmed, Adel Allam, Ahmed Asfour, Mona Hassan, Alia Hassan, Ahmed Ibrahim, Sameh Kaffas, Ahmed Kandeel, Mohamed Mandour Ali, Ahmad Mansy, Hany Maurice, Sherif Nabil, Mahmoud Shaaban, Ana Camila Flores, Anne Poksi, Juhani Knuuti, Velipekka Kokkonen, Martti Larikka, Valtteri Uusitalo, Matthieu Bailly, Samuel Burg, Jean-François Deux, Vincent Habouzit, Fabien Hyafil, Olivier Lairez, Franck Proffit, Hamza Regaieg, Laure Sarda-Mantel, Vania Tacher, Roman P. Schneider, Harold Ayetey, George Angelidis, Aikaterini Archontaki, Sofia Chatziioannou, Ioannis Datseris, Christina Fragkaki, Panagiotis Georgoulias, Sophia Koukouraki, Maria Koutelou, Eleni Kyrozi, Evangelos Repasos, Petros Stavrou, Pipitsa Valsamaki, Carla Gonzalez, Goleat Gutierrez, Alejandro Maldonado, Klara Buga, Ildiko Garai, Pál Maurovich-Horvat, Erzsébet Schmidt, Balint Szilveszter, Edit Várady, Nilesh Banthia, Jinendra Kumar Bhagat, Rishi Bhargava, Vivek Bhat, Mona Bhatia, Partha Choudhury, Vijay Sai Chowdekar, Aparna Irodi, Shashank Jain, Elizabeth Joseph, Sukriti Kumar, Prof Dr Girijanandan Mahapatra, Deepanjan Mitra, Bhagwant Rai Mittal, Ahmad Ozair, Chetan Patel, Tapan Patel, Ravi Patel, Shivani Patel, Sudhir Saxena, Shantanu Sengupta, Santosh Singh, Bhanupriya Singh, Ashwani Sood, Atul Verma, Erwin Affandi, Padma Savenadia Alam, Edison, Gani Gunawan, Habusari Hapkido, Basuki Hidayat, Aulia Huda, Anggoro Praja Mukti, Djoko Prawiro, Erwin Affandi Soeriadi, Hilman Syawaluddin, Amjed Albadr, Majid Assadi, Farshad Emami, Golnaz Houshmand, Majid Maleki, Maryam Tajik Rostami, Seyed Rasoul Zakavi, Eed Abu Zaid, Svetlana Agranovich, Yoav Arnson, Rachel Bar-Shalom, Alex Frenkel, Galit Knafo, Rachel Lugassi, Israel Shlomo Maor Moalem, Maya Mor, Noam Muskal, Sara Ranser, Aryeh Shalev, Domenico Albano, Pierpaolo Alongi, Gaspare Arnone, Elisa Bagatin, Sergio Baldari, Matteo Bauckneht, Paolo Bertelli, Francesco Bianco, Rachele Bonfiglioli, Roberto Boni, Andrea Bruno, Isabella Bruno, Elena Busnardo, Elena Califaretti, Luca Camoni, Aldo Carnevale, Roberta Casoni, Armando Ugo Cavallo, Giorgio Cavenaghi, Franca Chierichetti, Marcello Chiocchi, Corrado Cittanti, Mauro Colletta, Umberto Conti, Alberto Cossu, Alberto Cuocolo, Marco Cuzzocrea, Maria Luisa De Rimini, Giuseppe De Vincentis, Eleonora Del Giudice, Alberico Del Torto, Veronica Della Tommasina, Rexhep Durmo, Paola Anna Erba, Laura Evangelista, Riccardo Faletti, Evelina Faragasso, Mohsen Farsad, Paola Ferro, Luigia Florimonte, Viviana Frantellizzi, Fabio Massimo Fringuelli, Marco Gatti, Angela Gaudiano, Alessia Gimelli, Raffaele Giubbini, Francesca Giuffrida, Salvatore Ialuna, Riccardo Laudicella, Lucia Leccisotti, Lucia Leva, Riccardo Liga, Carlo Liguori, Giampiero Longo, Margherita Maffione, Maria Elisabetta Mancini, Claudio Marcassa, Elisa Milan, Barbara Nardi, Sara Pacella, Giovanna Pepe, Gianluca Pontone, Sabina Pulizzi, Natale Quartuccio, Lucia Rampin, Fabrizio Ricci, Pierluigi Rossini, Giuseppe Rubini, Vincenzo Russo, Gian Mauro Sacchetti, Gianmario Sambuceti, Massimo Scarano, Roberto Sciagrà, Massimiliano Sperandio, Antonella Stefanelli, Guido Ventroni, Stefania Zoboli, Dainia Baugh, Duane Chambers, Ernest Madu, Felix Nunura, Hiroshi Asano, Chimura Misato Chimura, Shinichiro Fujimoto, Koichiro Fujisue, Tomohisa Fukunaga, Yoshimitsu Fukushima, Kae Fukuyama, Jun Hashimoto, Yasutaka Ichikawa, Nobuo Iguchi, Masamichi Imai, Anri Inaki, Hayato Ishimura, Satoshi Isobe, Toshiaki Kadokami, Takao Kato, Takashi Kudo, Shinichiro Kumita, Hirotaka Maruno, Hiroyuki Mataki, Masao Miyagawa, Ryota Morimoto, Masao Moroi, Shigeki Nagamachi, Kenichi Nakajima, Tomoaki Nakata, Ryo Nakazato, Mamoru Nanasato, Masanao Naya, Takashi Norikane, Yasutoshi Ohta, Satoshi Okayama, Atsutaka Okizaki, Yoichi Otomi, Hideki Otsuka, Masaki Saito, Sakata Yasushi Sakata, Masayoshi Sarai, Daisuke Sato, Shinya Shiraishi, Yoshinobu Suwa, Kentaro Takanami, Kazuya Takehana, Junichi Taki, Nagara Tamaki, Yasuyo Taniguchi, Hiroki Teragawa, Nobuo Tomizawa, Kenichi Tsujita, Kyoko Umeji, Yasushi Wakabayashi, Shinichiro Yamada, Shinya Yamazaki, Tatsuya Yoneyama, Mohammad Rawashdeh, Daultai Batyrkhanov, Tairkhan Dautov, Khalid Makhdomi, Kevin Ombati, Faridah Alkandari, Masoud Garashi, Tchoyoson Lim Coie, Sonexay Rajvong, Artem Kalinin, Marika Kalnina, Mohamad Haidar, Renata Komiagiene, Giedre Kviecinskiene, Mindaugas Mataciunas, Donatas Vajauskas, Christian Picard, Noor Khairiah A. Karim, Luise Reichmuth, Anthony Samuel, Mohammad Aaftaab Allarakha, Ambedhkar Shantaram Naojee, Erick Alexanderson-Rosas, Erika Barragan, Alejandro Becerril González-Montecinos, Manuel Cabada, Daniel Calderon Rodriguez, Isabel Carvajal-Juarez, Violeta Cortés, Filiberto Cortés, Erasmo De La Peña, Manlio Gama-Moreno, Luis González, Nelsy Gonzalez Ramírez, Moisés Jiménez-Santos, Luis Matos, Edgar Monroy, Martha Morelos, Mario Ornelas, Jose Alberto Ortga Ramirez, Andrés Preciado-Anaya, Óscar Ulises Preciado-Gutiérrez, Adriana Puente Barragan, Sandra Graciela Rosales Uvera, Sigelinda Sandoval, Miguel Santaularia Tomas, Lilia M. Sierra-Galan, Lilia M. Sierra-Galan, Silvia Siu, Enrique Vallejo, Mario Valles, Marc Faraggi, Erdenechimeg Sereegotov, Srdja Ilic, Nozha Ben-Rais, Nadia Ismaili Alaoui, Sara Taleb, Khin Pa Myo, Phyo Si Thu, Ram Kumar Ghimire, Bijoy Rajbanshi, Peter Barneveld, Andor Glaudemans, Jesse Habets, Klaas Pieter Koopmans, Jeroen Manders, Stefan Pool, Arthur Scholte, Asbjørn Scholtens, Riemer Slart, Paul Thimister, Erik-Jan Van Asperen, Niels Veltman, Derk Verschure, Nils Wagenaar, John Edmond, Chris Ellis, Kerryanne Johnson, Ross Keenan, Shaw Hua, Christopher Occleshaw, Alexander Sasse, Andrew To, Niels Van Pelt, Calum Young, Teresa Cuadra, Hector Bladimir Roque Vanegas, Idrissa Adamou Soli, Djibrillou Moussa Issoufou, Tolulope Ayodele, Chibuzo Madu, Yetunde Onimode, Elen Efros-Monsen, Signe Helene Forsdahl, Jenni-Mari Hildre Dimmen, Arve Jørgensen, Isabel Krohn, Pål Løvhaugen, Anders Tjellaug Bråten, Humoud Al Dhuhli, Faiza Al Kindi, Naeema Al-Bulushi, Zabah Jawa, Naima Tag, Muhammad Shehzad Afzal, Shazia Fatima, Muhammad Numair Younis, Musab Riaz, Mohammad Saadullah, Yariela Herrera, Dora Lenturut-Katal, Manuel Castillo Vázquez, José Ortellado, Afroza Akhter, Dianbo Cao, Stephen Cheung, Xu Dai, Lianggeng Gong, Dan Han, Yang Hou, Caiying Li, Tao Li, Dong Li, Sijin Li, Jinkang Liu, Hui Liu, Bin Lu, Ming Yen Ng, Kai Sun, Gongshun Tang, Jian Wang, Ximing Wang, Zhao-Qian Wang, Yining Wang, Yifan Wang, Jiang Wu, Zhifang Wu, Liming Xia, Jiangxi Xiao, Lei Xu, Youyou Yang, Wu Yin, Jianqun Yu, Li Yuan, Tong Zhang, Longjiang Zhang, Yong-Gao Zhang, Xiaoli Zhang, Li Zhu, Ana Alfaro, Paz Abrihan, Asela Barroso, Eric Cruz, Marie Rhiamar Gomez, Vincent Peter Magboo, John Michael Medina, Jerry Obaldo, Davidson Pastrana, Christian Michael Pawhay, Alvin Quinon, Jeanelle Margareth Tang, Bettina Tecson, Kristine Joy Uson, Mila Uy, Magdalena Kostkiewicz, Jolanta Kunikowska, Nuno Bettencourt, Guilhermina Cantinho, Antonio Ferreira, Ghulam Syed, Samer Arnous, Said Atyani, Angela Byrne, Tadhg Gleeson, David Kerins, Conor Meehan, David Murphy, Mark Murphy, John Murray, Julie O'Brien, Ji-In Bang, Henry Bom, Sang-Geon Cho, Chae Moon Hong, Su Jin Jang, Yong Hyu Jeong, Won Jun Kang, Ji-Young Kim, Jaetae Lee, Chang Kyeong Namgung, Young So, Kyoung Sook Won, Venjamin Majstorov, Marija Vavlukis, Barbara Gužic Salobir, Monika Štalc, Theodora Benedek, Imre Benedek, Raluca Mititelu, Claudiu Adrian Stan, Alexey Ansheles, Olga Dariy, Olga Drozdova, Nina Gagarina, Vsevolod Milyevich Gulyaev, Irina Itskovich, Anatoly Karalkin, Alexander Kokov, Ekaterina Migunova, Viktor Pospelov, Daria Ryzhkova, Guzaliya Saifullina, Svetlana Sazonova, Vladimir Sergienko, Irina Shurupova, Tatjana Trifonova, Wladimir Yurievich Ussov, Margarita Vakhromeeva, Nailya Valiullina, Konstantin Zavadovsky, Kirill Zhuravlev, Mirvat Alasnag, Subhani Okarvi, Dragana Sobic Saranovic, Felix Keng, Jia Hao Jason See, Ramkumar Sekar, Min Sen Yew, Andrej Vondrak, Shereen Bejai, George Bennie, Ria Bester, Gerrit Engelbrecht, Osayande Evbuomwan, Harlem Gongxeka, Magritha Jv Vuuren, Mitchell Kaplan, Purbhoo Khushica, Hoosen Lakhi, Lizette Louw, Nico Malan, Katarina Milos, Moshe Modiselle, Stuart More, Mathava Naidoo, Leonie Scholtz, Mboyo Vangu, Santiago Aguadé-Bruix, Isabel Blanco, Antonio Cabrera, Alicia Camarero, Irene Casáns-Tormo, Hug Cuellar-Calabria, Albert Flotats, Maria Eugenia Fuentes Cañamero, María Elia García, Amelia Jimenez-Heffernan, Rubén Leta, Javier Lopez Diaz, Luis Lumbreras, Juan Javier Marquez-Cabeza, Francisco Martin, Anxo Martinez de Alegria, Francisco Medina, Maria Pedrera Canal, Virginia Peiro, Virginia Pubul-Nuñez, Juan Ignacio Rayo Madrid, Cristina Rodríguez Rey, Ricardo Ruano Perez, Joaquín Ruiz, Gertrudis Sabatel Hernández, Ana Sevilla, Nahla Zeidán, Damayanthi Nanayakkara, Chandraguptha Udugama, Magnus Simonsson, Hatem Alkadhi, Ronny Ralf Buechel, Peter Burger, Luca Ceriani, Bart De Boeck, Christoph Gräni, Alix Juillet de Saint Lager Lucas, Christel H. Kamani, Nadine Kawel-Boehm, Robert Manka, John O. Prior, Axel Rominger, Jean-Paul Vallée, Benjapa Khiewvan, Teerapon Premprabha, Tanyaluck Thientunyakit, Ali Sellem, Kemal Metin Kir, Haluk Sayman, Mugisha Julius Sebikali, Zerida Muyinda, Yaroslav Kmetyuk, Pavlo Korol, Olena Mykhalchenko, Volodymyr Pliatsek, Maryna Satyr, Batool Albalooshi, Mohamed Ismail Ahmed Hassan, Jill Anderson, Punit Bedi, Thomas Biggans, Anda Bularga, Russell Bull, Rajesh Burgul, John-Paul Carpenter, Duncan Coles, David Cusack, Aparna Deshpande, John Dougan, Timothy Fairbairn, Alexia Farrugia, Deepa Gopalan, Alistair Gummow, Prasad Guntur Ramkumar, Mark Hamilton, Mark Harbinson, Thomas Hartley, Benjamin Hudson, Nikhil Joshi, Michael Kay, Andrew Kelion, Azhar Khokhar, Jamie Kitt, Ken Lee, Chen Low, Sze Mun Mak, Ntouskou Marousa, Jon Martin, Elisa Mcalindon, Leon Menezes, Gareth Morgan-Hughes, Alastair Moss, Anthony Murray, Edward Nicol, Dilip Patel, Charles Peebles, Francesca Pugliese, Jonathan Carl Luis Rodrigues, Christopher Rofe, Nikant Sabharwal, Rebecca Schofield, Thomas Semple, Naveen Sharma, Peter Strouhal, Deepak Subedi, William Topping, Katharine Tweed, Jonathan Weir-Mccall, Suhny Abbara, Taimur Abbasi, Brian Abbott, Shady Abohashem, Sandra Abramson, Tarek Al-Abboud, Mouaz Al-Mallah, Omar Almousalli, Karthikeyan Ananthasubramaniam, Mohan Ashok Kumar, Jeffrey Askew, Lea Attanasio, Mallory Balmer-Swain, Richard R. Bayer, Adam Bernheim, Sabha Bhatti, Erik Bieging, Ron Blankstein, Stephen Bloom, Sean Blue, David Bluemke, Andressa Borges, Kelley Branch, Paco Bravo, Jessica Brothers, Matthew Budoff, Renée Bullock-Palmer, Angela Burandt, Floyd W. Burke, Kelvin Bush, Candace Candela, Elizabeth Capasso, Joao Cavalcante, Donald Chang, Saurav Chatterjee, Yiannis Chatzizisis, Michael Cheezum, Tiffany Chen, Jennifer Chen, Marcus Chen, Andrew Choi, James Clarcq, Ayreen Cordero, Matthew Crim, Sorin Danciu, Bruce Decter, Nimish Dhruva, Neil Doherty, Rami Doukky, Anjori Dunbar, William Duvall, Rachael Edwards, Kerry Esquitin, Husam Farah, Emilio Fentanes, Maros Ferencik, Daniel Fisher, Daniel Fitzpatrick, Cameron Foster, Tony Fuisz, Michael Gannon, Lori Gastner, Myron Gerson, Brian Ghoshhajra, Alan Goldberg, Brian Goldner, Jorge Gonzalez, Rosco Gore, Sandra Gracia-López, Fadi Hage, Agha Haider, Sofia Haider, Yasmin Hamirani, Karen Hassen, Mallory Hatfield, Carolyn Hawkins, Katie Hawthorne, Nicholas Heath, Robert Hendel, Phillip Hernandez, Gregory Hill, Stephen Horgan, Jeff Huffman, Lynne Hurwitz, Ami Iskandrian, Rajesh Janardhanan, Christine Jellis, Scott Jerome, Dinesh Kalra, Summanther Kaviratne, Fernando Kay, Faith Kelly, Omar Khalique, Mona Kinkhabwala, George Kinzfogl Iii, Jacqueline Kircher, Rachael Kirkbride, Michael Kontos, Anupama Kottam, Joseph Krepp, Jay Layer, Steven H. Lee, Jeffrey Leppo, John Lesser, Steve Leung, Howard Lewin, Diana Litmanovich, Yiyan Liu, Juan Lopez-Mattei, Kathleen Magurany, Jeremy Markowitz, Amanda Marn, Stephen E. Matis, Michael Mckenna, Tony Mcrae, Fernando Mendoza, Michael Merhige, David Min, Chanan Moffitt, Karen Moncher, Warren Moore, Shamil Morayati, Michael Morris, Mahmud Mossa-Basha, Zorana Mrsic, Venkatesh Murthy, Prashant Nagpal, Kyle Napier, Jagat Narula, Katarina Nelson, Prabhjot Nijjar, Medhat Osman, Purvi Parwani, Edward Passen, Amit Patel, Pravin Patil, Ryan Paul, Lawrence Phillips, Venkateshwar Polsani, Rajaram Poludasu, Brian Pomerantz, Thomas Porter, Ryan Prentice, Amit Pursnani, Mark Rabbat, Suresh Ramamurti, Florence Rich, Hiram Rivera Luna, Austin Robinson, Kim Robles, Cesar Rodríguez, Mark Rorie, John Rumberger, Raymond Russell, Philip Sabra, Diego Sadler, Mary Schemmer, U. Joseph Schoepf, Samir Shah, Nishant Shah, Sujata Shanbhag, Gaurav Sharma, Steven Shayani, Jamshid Shirani, Pushpa Shivaram, Steven Sigman, Mitch Simon, Ahmad Slim, David Smith, Alexandra Smith, Prem Soman, Aditya Sood, Monvadi Barbara Srichai-Parsia, James Streeter, Albert T, Ahmed Tawakol, Dustin Thomas, Randall Thompson, Tara Torbet, Desiree Trinidad, Shawn Ullery, Samuel Unzek, Seth Uretsky, Srikanth Vallurupalli, Vikas Verma, Alfonso Waller, Ellen Wang, Parker Ward, Gaby Weissman, George Wesbey, Kelly White, David Winchester, David Wolinsky, Sandra Yost, Michael Zgaljardic, Omar Alonso, Mario Beretta, Rodolfo Ferrando, Miguel Kapitan, Fernando Mut, Omoa Djuraev, Gulnora Rozikhodjaeva, Ha Le Ngoc, Son Hong Mai, and Xuan Canh Nguyen
Appendix
References
- 1.Mann D.M., Chen J., Chunara R., Testa P.A., Nov O. COVID-19 transforms health care through telemedicine: Evidence from the field. J Am Med Inform Assoc. 2020;27:1132–1135. doi: 10.1093/jamia/ocaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keesara S., Jonas A., Schulman K. Covid-19 and health care's digital revolution. N Engl J Med. 2020;382:e82. doi: 10.1056/NEJMp2005835. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton W. Cancer diagnostic delay in the COVID-19 era: what happens next? Lancet Oncol. 2020;21:1000–1002. doi: 10.1016/S1470-2045(20)30391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anteby R., Zager Y., Barash Y., et al. The impact of the coronavirus disease 2019 outbreak on the attendance of patients with surgical complaints at a tertiary hospital emergency department. J Laparoendosc Adv Surg Tech A. 2020;30(9):1001–1007. doi: 10.1089/lap.2020.0465. [DOI] [PubMed] [Google Scholar]
- 5.Eshraghian A., Taghavi A., Nikeghbalian S., Malek-Hosseini S.A. Reduced rate of hospital admissions for liver-related morbidities during the initial COVID-19 outbreak. Lancet Gastroenterol Hepatol. 2020;5:803–804. doi: 10.1016/S2468-1253(20)30207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Hamamsy I., Brinster D.R., DeRose J.J., et al. The COVID-19 pandemic and acute aortic dissections in New York: a matter of public health. J Am Coll Cardiol. 2020;76:227–229. doi: 10.1016/j.jacc.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Range G., Hakim R., Motreff P. Where have the ST-segment elevation myocardial infarctions gone during COVID-19 lockdown? Eur Heart J Qual Care Clin Outcomes. 2020;6:223–224. doi: 10.1093/ehjqcco/qcaa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000-2016. World Health Organization; Geneva, Switzerland: 2020. http://www.who.int/healthinfo/global_burden_disease/estimates/en/ Available at: [Google Scholar]
- 9.Roifman I., Han L., Koh M., et al. Clinical effectiveness of cardiac noninvasive diagnostic testing in patients discharged from the emergency department for chest pain. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Carli M.F., Geva T., Davidoff R. The future of cardiovascular imaging. Circulation. 2016;133:2640–2661. doi: 10.1161/CIRCULATIONAHA.116.023511. [DOI] [PubMed] [Google Scholar]
- 11.Roifman I., Austin P.C., Qiu F., Wijeysundera H.C. Impact of the publication of appropriate use criteria on usage rates of myocardial perfusion imaging studies in Ontario, Canada: a population-based study. J Am Heart Assoc. 2017;6:e005961. doi: 10.1161/JAHA.117.005961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Einstein A.J., Shaw L.J., Hirschfeld C., et al. International impact of COVID-19 on the diagnosis of heart disease. J Am Coll Cardiol. 2021;77:173–185. doi: 10.1016/j.jacc.2020.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mafham M.M., Spata E., Goldacre R., et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396:381–389. doi: 10.1016/S0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lantelme P., Couray Targe S., Metral P., et al. Worrying decrease in hospital admissions for myocardial infarction during the COVID-19 pandemic. Arch Cardiovasc Dis. 2020;113:443–447. doi: 10.1016/j.acvd.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piccolo R., Bruzzese D., Mauro C., et al. Population trends in rates of percutaneous coronary revascularization for acute coronary syndromes associated with the COVID-19 outbreak. Circulation. 2020;141:2035–2037. doi: 10.1161/CIRCULATIONAHA.120.047457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez-Leor O., Alvarez-Álvarez B., Ojeda S., et al. Impact of the COVID-19 pandemic on interventional cardiology activity in Spain. REC: Intervent Cardiol. 2020;2:82–89. [Google Scholar]
- 17.Negreira Caamano M., Piqueras Flores J., Mateo Gomez C. Impact of COVID-19 pandemic in cardiology admissions. Med Clin (Barc) 2020;155:179–180. doi: 10.1016/j.medcle.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia S., Albaghdadi M.S., Meraj P.M., et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States During COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skali H., Murthy V.L., Al-Mallah M.H., et al. Guidance and best practices for nuclear cardiology laboratories during the coronavirus disease 2019 (COVID-19) pandemic: an information statement from ASNC and SNMMI. J Nucl Med. 2020;27:1022–1029. doi: 10.1007/s12350-020-02123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi A.D., Abbara S., Branch K.R., et al. Society of Cardiovascular Computed Tomography guidance for use of cardiac computed tomography amidst the COVID-19 pandemic endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr. 2020;14:101–104. doi: 10.1016/j.jcct.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zoghbi W.A., DiCarli M.F., Blankstein R., et al. Multimodality cardiovascular imaging in the midst of the covid-19 pandemic: ramping up safely to a new normal. J Am Coll Cardiol Img. 2020;13:1615–1626. doi: 10.1016/j.jcmg.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tahir F., Bin Arif T., Ahmed J., Malik F., Khalid M. Cardiac manifestations of coronavirus disease 2019 (COVID-19): a comprehensive review. Cureus. 2020;12 doi: 10.7759/cureus.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guzik T.J., Mohiddin S.A., Dimarco A., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boukhris M., Hillani A., Moroni F., et al. Cardiovascular implications of the COVID-19 pandemic: a global perspective. Can J Cardiol. 2020;36:1068–1080. doi: 10.1016/j.cjca.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang L., Zhao P., Tang D., et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. J Am Coll Cardiol Img. 2020;13:2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Statute of the IAEA. https://www.iaea.org/about/statute Available at:
- 28.U.S. Census Bureau (2010) Census Regions and Divisions of the United States. https://www.census.gov/geographies/reference-maps/2010/geo/2010-census-regions-and-divisions-of-the-united-states.html Available at: Accessed August 10, 2020.
- 29.US Coronavirus Cases and Deaths USAFacts.org. https://usafacts.org/visualizations/coronavirus-covid-19-spread-map/ Available at:
- 30.U.S. Census Bureau Decennial Census Tables (2010) https://www.census.gov/programs-surveys/decennial-census/data/tables Available at:
- 31.U.S. Census Bureau (2020). 2019 State, County, Minor Civil Division, and Incorporated Place FIPS Codes. Available at: https://www.census.gov/geographies/reference-files/2019/demo/popest/2019-fips.html. Accessed August 10, 2020.
- 32.Huber P.J. Robust estimation of a location parameter. Ann Math Statist. 1964;35:73–101. [Google Scholar]
- 33.US Department of Agriculture Economic Research Service Rural-Urban Continuum Codes. https://www.ers.usda.gov/data-products/rural-urban-continuum-codes/documentation/ Available at:
- 34.Goyal P., Choi J.J., Pinheiro L.C., et al. Clinical characteristics of covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwong J.C., Schwartz K.L., Campitelli M.A. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378:2540–2541. doi: 10.1056/NEJMc1805679. [DOI] [PubMed] [Google Scholar]
- 36.Hartnett K.P., Kite-Powell A., DeVies J., et al. Impact of the COVID-19 pandemic on emergency department visits--United States, January 1, 2019--May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:699–704. doi: 10.15585/mmwr.mm6923e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siegler J.E., Heslin M.E., Thau L., Smith A., Jovin T.G. Falling stroke rates during COVID-19 pandemic at a comprehensive stroke center. J Stroke Cerebrovasc Dis. 2020;29:104953. doi: 10.1016/j.jstrokecerebrovasdis.2020.104953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uchino K., Kolikonda M.K., Brown D., et al. Decline in stroke presentations during COVID-19 surge. Stroke. 2020;51:2544–2547. doi: 10.1161/STROKEAHA.120.030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solis E., Hameed A., Brown K., Pleass H., Johnston E. Delayed emergency surgical presentation: impact of corona virus disease (COVID-19) on non-COVID patients. ANZ J Surg. 2020;90:1482–1483. doi: 10.1111/ans.16048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoyer C., Ebert A., Szabo K., Platten M., Meyer-Lindenberg A., Kranaster L. Decreased usage of mental health emergency service during the COVID-19 pandemic. Eur Arch Psychiatry Clin Neurosci. 2020;271:377–379. doi: 10.1007/s00406-020-01151-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward R.P., Lee L., Ward T.J., Lang R.M. Utilization and appropriateness of transthoracic echocardiography in response to the COVID-19 pandemic. J Am Soc Echocardiogr. 2020;33:690–691. doi: 10.1016/j.echo.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Provenzano D.A., Sitzman B.T., Florentino S.A., Buterbaugh G.A. Clinical and economic strategies in outpatient medical care during the COVID-19 pandemic. Reg Anesth Pain Med. 2020;45:579–585. doi: 10.1136/rapm-2020-101640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diaz A., Sarac B.A., Schoenbrunner A.R., Janis J.E., Pawlik T.M. Elective surgery in the time of COVID-19. Am J Surg. 2020;219:900–902. doi: 10.1016/j.amjsurg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim M.K., Rabinowitz L.G., Nagula S., et al. A primer for clinician deployment to the medicine floors from an epicenter of covid-19. N Engl J Med Catal Innov Care Deliv. 2020 doi: 10.1056/CAT.20.0180. [DOI] [Google Scholar]
- 45.Dorn A.V., Cooney R.E., Sabin M.L. COVID-19 exacerbating inequalities in the US. Lancet. 2020;395:1243–1244. doi: 10.1016/S0140-6736(20)30893-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Team C.C.-R. Geographic differences in COVID-19 cases, deaths, and incidence--United States, February 12--April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:465–471. doi: 10.15585/mmwr.mm6915e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rocklov J., Sjodin H. High population densities catalyse the spread of COVID-19. J Travel Med. 2020;27:taaa038. doi: 10.1093/jtm/taaa038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang C.H., Schwartz G.G. Spatial disparities in coronavirus incidence and mortality in the United States: an ecological analysis as of May 2020. J Rural Health. 2020;36:433–445. doi: 10.1111/jrh.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laurencin C.T., McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7:398–402. doi: 10.1007/s40615-020-00756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wadhera R.K., Wadhera P., Gaba P., et al. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA. 2020;323:2192–2195. doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382:2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graham G. Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev. 2015;11:238–245. doi: 10.2174/1573403X11666141122220003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vlahov D., Galea S. Urbanization, urbanicity, and health. J Urban Health. 2002;79:S1–S12. doi: 10.1093/jurban/79.suppl_1.S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.