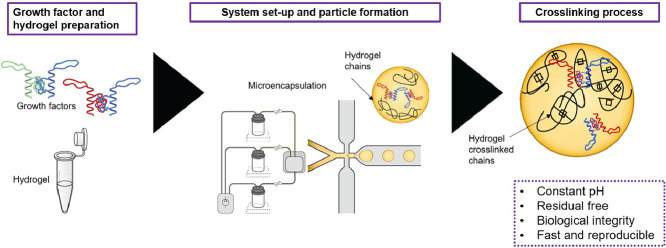
Abstract
The encapsulation of growth factors is an important component of tissue engineer- ing. Using microspheres is a convenient approach in which the dose of factors can be regulated by increasing or decreasing the number of encapsulated microspheres. Moreover, microspheres offer the possibility of delivering the growth factors directly to the target site. However, the fabrication of microspheres by traditional emulsion methods is largely variable due to the experimental procedure. We have developed a protocol using a commercially available microfluidic system that allows formation of tunable particle-size droplets loaded with growth factors. The methodology includes a guide for preparing an alginate-growth factors solution followed by the specific set-up needed for using the microfluidic system to form the microspheres. The pro- cedure also includes a unique post-crosslinking process without pH modification. These methods allow the preservation of integrity and bioactivity of the growth factors tested (BMP-6 and TGFβ -3) and their subsequent sustained delivery.
-
•
The protocol can be tuned to form particles of various sizes.
-
•
The gentle post-crosslinking process allows conformational integrity of various bioactive molecules.
Keywords: Microencapsulation, Growth Factors, Microfluidics, Microspheres
Graphical abstract
Specification Table
| Subject Area | Materials Science |
| More specific subject area | Drug delivery, encapsulation, growth factors |
| Method name | Microencapsulation by microfluidic system |
| Name and reference of original method | Utech, S., Prodanovic, R., Mao, A. S., Ostafe, R., Mooney, D. J., & Weitz, D. A. (2015). Microfluidic Generation of Monodisperse, Structurally Homogeneous Alginate Microgels for Cell Encapsulation and 3D Cell Culture. 16281633. https://doi.org/10.1002/adhm.201500021https://www.dolomite-microfluidics.com/applications/alginate-synthesis/ |
| Resource availability: | Software Dolomite Flow Control Center (Dolomite UK, part no. 3200475), Microencapsulation system (Dolomite UK) |
Introduction
The use of growth factors is crucial in directing stem cells towards specific pathways [1]. In vivo, growth factors can be found in the extracellular matrix and recruited on-demand by the cells. Furthermore, the cells themselves are able to produce certain growth factors when the right pathways have been triggered [2,3]. Unlike the natural/physiological in vivo environment, for tissue engineering approaches in vitro studies or for delivery in patients, these growth factors need to be artificially provided.
Recently, structures such as hydrogel microspheres, have been employed to encapsulate and release growth factors within tissue engineered scaffolds [4], [5], [6]. These systems allow the uniform distribution of growth factors in close proximity to the cells while avoiding the repeated manual provision of growth factors [7], [8], [9].
In this protocol we illustrate the advantages of microfluidics by describing the encapsulation of growth factors within alginate hydrogels using a method that allows high control of the particle size distribution. We have used the encapsulation of BMP-6 and TGFβ -3 as an example of growth factor encapsulation. The selected growth factors have functional roles in the development of several tissues separately. In combination, they have been proven useful during the differentiation of mesenchymal stem cells towards chondrogenic lineage.
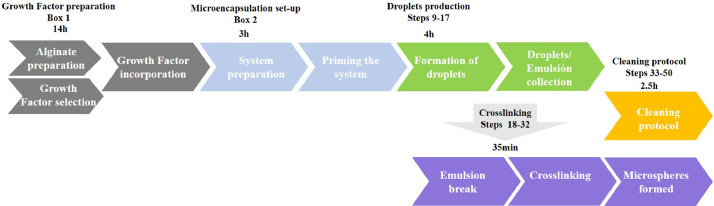
This system employs a microfluidic device that is commercially available (Dolomite, UK) and for which a detailed protocol for alginate microspheres has not been established before. This protocol addresses some of the key challenges in producing alginate microspheres using the system: establishing the flow rate parameters, growth factor loading procedure, crosslinking and cleaning of the microfluidic chip. However, the user of the present protocol is expected to have prior knowledge of microfluidics and be trained on the use of the BioDolomite (Dolomite, UK). Alternatively, the ‘µencapsulator System User Manual’ can be found in https://www.dolomite-microfluidics.com/support/downloads/. A Diagram showing a general view of the protocol described here can be seen in Fig. 1.
Fig. 1.
Scheme of the flow of the protocol. In the first part (as described in Box 1) the preparation of the alginate, the growth factors and the integration of the growth factor within the alginate is carried out. The second part (Box 2) involves the preparation of the system. In the third section we present the methodology to form the microspheres and in the fourth part a crosslink method. Finally, a specific cleaning protocol is suggested. This final step (steps 33-50) can be carried out while the crosslinking process is underway (steps 18-32) or after the microsphere formation has occurred.
Development of the protocol
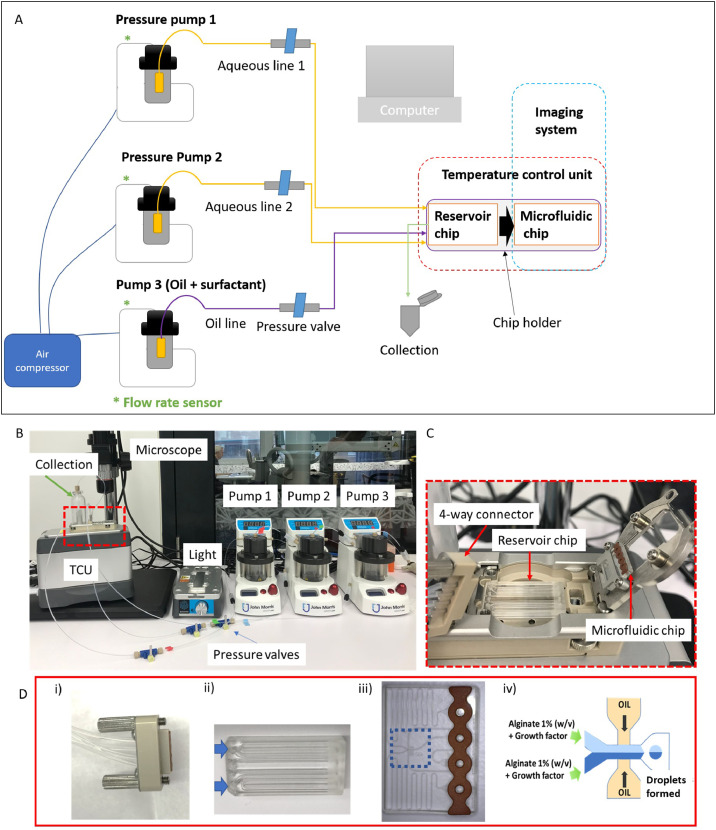
This protocol uses the multiple advantages of microfluidic systems (Fig. 2) whereby control over the pressures and flow rates, enables production of droplets with reproducible size. The microfluidic device used here is an integrated system coupled with a computer and software that allow independent and remote controlling of the pressure in each pump. This microencapsulation system is commercially available, which facilitates the translation of this protocol.
Fig. 2.
A. Diagram showing the parts of the microencapsulation system needed to produce microspheres. All the components (except for the air compressor) are connected to the computer and can be controlled or adjusted using the software. B: Picture of the microencapsulation system showing the fluidic components, the sample holder part (red box) above of the TCU (temperature-controlled unit) is shown in C. This part contains a 4-way connector. D i), a reservoir chip with two reservoir holes (indicated by blue arrows in (D ii) and a fluorophilic microfluidic chip (D iii) where the blue box shows the emulsion junction as represented in D iv.Application of the protocol
The system is composed of three pressurized pumps supplied by air from an air compressor. Driving fluids are placed in a container which is then positioned within each pump. For this protocol we suggest using Pump 1 and Pump 2 to contain the flourinated oil and Pump 3 to contain oil and surfactant (Fig. 2A-B). The pumps can be opened and closed individually by using the pressure valves. The driving fluids (oils in the pumps) will be directed towards the reservoir chip (Fig. 2C-D ii) in which the growth-factor-alginate will be located. This action will initiate the flow towards the microfluidic chip (Fig. 2C-D iii). A microemulsion will form in the microfluidic chip which will allow the formation of droplets (Fig. 2iv). The emulsion can be collected in the sample collection vial.
This encapsulation protocol for growth factors offers several advantages. For instance, the droplets size produced and the growth factor concentration can be controlled with only minimum waste [10]. This is possible due to the small volume created when using the microfluidics [11]. The total volume of emulsion produced is approximately 100 µL which also minimizes the amount of growth factors involved in the fabrication process.
Other existing methods that use the same encapsulation system have focused on the encapsulation of single or multiple cells using temperature-dependent hydrogels such as low viscosity agarose, crosslinking by pH modification, or by means of double-emulsion systems [12,13]. The encapsulation was possible in those methods by keeping the droplets entrapped in the emulsion-surfactant solution and, when the breaking-emulsion solution was added, the biological material was released. Alternatively, the use of pH variation was used to obtain alginate microspheres, however, changing the pH can modify the growth factor's conformational structure thus, damaging its functionality.
In this protocol we developed a method to form alginate microspheres using a rapid single-emulsion, because our interest was to be able to maintain the microspheres after breaking the emulsion (e.g. using PicoBreakTM), which is only possible if crosslinking is achieved before breaking the emulsion. Thus, this protocol introduces a unique crosslinking methodology which allows the manufacturing of gel-like microspheres maintaining a neutral pH.
The formation of microfluidic-formed microspheres can be adapted to a wide range of applications. There are no major limitations in testing the encapsulation of other growth factors after gaining enough confidence in managing the parameters of the alginate fluid. The formation of micro- spheres can also be coupled with other microfluidic devices, as the use of microfluidics is becoming popular in laboratories. For example, it is possible to coat the microspheres with a second polymer or couple the system with another microfluidic chip to form a double emulsion. This highlights the flexibility of the method presented here. Moreover, this protocol can be applied to encapsulate other materials such as cells.
Comparison with other fabrication techniques
In recent years the development of methods to encapsulate growth factors has been widely explored due to their potential applications. In particular the use of emulsion methods has attracted several research groups due to the simplicity of this method [14,15]. The emulsion can be formed in a number of ways, for example by using magnetic stirring to mix the water and oil phase, thus creating droplets. This simple approach has been successfully employed to form microspheres [16]. However, the stirring velocity is generally limited by the magnetic stirring plate apparatus (usually 1500 rpm) which only allows the formation of relatively large particles (200–500 µm) [17,18]. Furthermore, this type of emulsion method often needs relatively large volumes (e.g. 80 mL of emulsion) which in turn requires a large quantity of growth factors. Consequently, using a magnetic stirring-emulsion method is costly for encapsulating growth factors.
A second method to encapsulate growth factors using emulsion methods is based on homogenization. Homogenizer devices allow accurate control of the velocity, across a typical range from 1000–20,000 rpm, depending on the device used [19]. This creates a wide range of possible velocities which allows control of the emulsion and the particle size distribution. However, to obtain a relatively homogeneous particle size distribution it is often necessary to homogenize the oil and water phase for more than 2 minutes [20]. As a consequence, the fluids are submitted to high shear rates which, depending on the volume, causes an increment on the temperature that can damage the growth factors. An alternative to this is to perform a step-by-step method, in which a sequence of short emulsion pulses is applied with a resting time in between each pulse. This helps to control the temperature but, the repetition of the high velocity pulses can often be too harsh and cause growth factor damage.
In both magnetic stirring and homogenizing, a common issue is the large variability in the particle size: changing parameters such as the emulsion stabilizer type or the preparation procedure can strongly influence the particle size, which can hamper the reproducibility of the reported methods, the rate of loading and release of factors [21]. In contrast, the present method uses a commercially available system that provides confidence in producing microspheres with reproducible physical properties. Because the emulsion is always created using a size-defined microfluidic chip, the encapsulation of growth factors using this method is highly reproducible. Moreover, this computerized control serves to automate the process and, at the same time, offers the possibility of easily manipulating the alginate droplet formation. The use of microfluidic methods reduces the production costs compared to other methods, by using a relatively small amount of alginate and growth factors to produce the microspheres. This strongly reduces the workload and waste while increasing the experimental throughput.
Limitations
The use of microfluidics needs practice and blockages are a recognized issue in the field [22]. We have experienced blockage issues during the development of this protocol. Due to that recurrent issue, a cleaning procedure was developed as part of the protocol. We believe that this procedure will help the user to maintain a reliable production and provide a long working life for the microfluidic chip.
It is also suggested that the user initially practice the steps in the protocol using alginate without growth factors in order to become familiar with the procedure. Outlined in the troubleshooting section are some procedures that may be useful in case of persistent blockages of the microfluidic chip.
Experimental workflow description
The work-flow shown in Fig. 1 describes the main stages of this protocol (Boxes 1 and 2, and Procedure 1-50). These main tasks are described below.
Material preparation
Details of the growth factor preparation are given in Box 1. The sterilization of the alginate can be performed once. If ethylene oxide (ETO) sterilization is not available, it is advisable to outsource this or to perform another sterilization technique. However, the user should be aware that the use of some other sterilization techniques such as autoclaving can damage the material properties [23]. Dissolving the alginate can be performed only once and the reagent can be stored at 4 °C. Special care, such as avoiding exposure to elevated temperatures (e.g. 100 °C), needs to be taken when handling the growth factors as these biomolecules tend to degrade easily. It is recommended that the mixing of growth factors with the alginate be performed in a gentle manner to avoid growth factor damage and to restrict the introduction of air within the alginate-growth factor solution. In this example, we have used BMP-6 and TGFβ -3 with a neutral pH.
Microencapsulation system set-up
The process describing the microencapsulation system (Box 2) must be performed every time the microspheres are to be produced. This process ensures that the conditions are consistent in every cycle. In particular the priming steps are necessary in order to bring the fluids near the 4-way con- nector and prepare them to flow through the reservoir and the microfluidic chip. This procedure becomes routine once the user is familiar with the system.
Droplet production
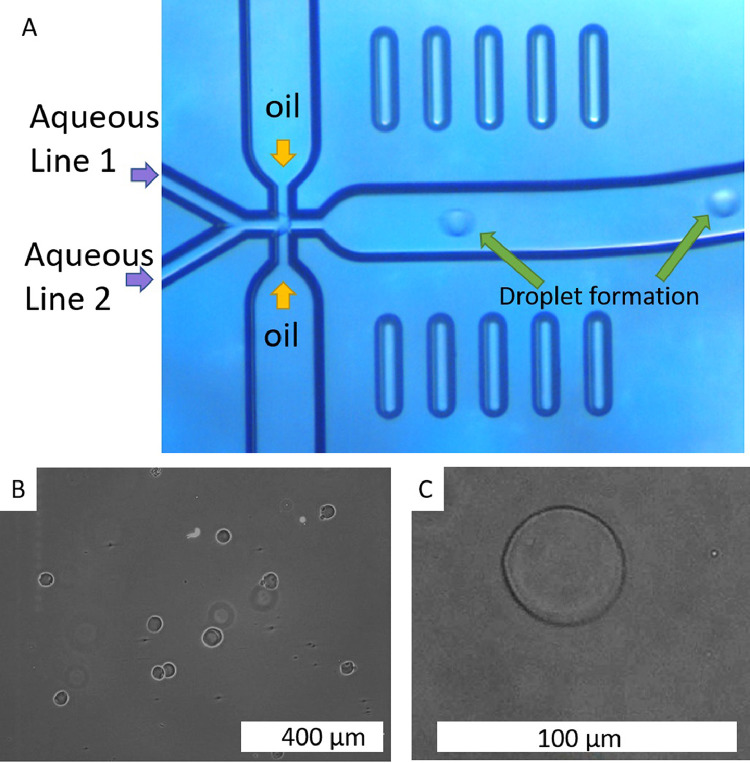
The production of microspheres (Steps 9–17) starts with the placement of all the components needed to run the microencapsulation cycle and the adjust- ments necessary for this protocol. We emphasize the importance of setting the flow rate correctly and properly closing the 4-way connector. The droplets will be produced due to a microemulsion process occurring within the microfluidic chip (Fig. 3A). The production of droplets is controlled by introducing the flow rate using the software whilst the pressure will fluctuate to maintain the flow rate constant. It is important to note any high pressures (e.g. greater than 4000 mbar) displayed in the software controlling the pressurized pumps, as this can indicate a blockage or leakage. Typical pressures and flow rates settings are shown in Table 1.
Fig. 3.
A: Microscope image of the microfluidic chip forming alginate droplets. B-C: Images of the alginate microspheres after the crosslinking process.
Table 1.
Flow rates (Q) and pressures (P) for droplet size formation.
| Pump 1 |
Pump 2 |
Pump 3 |
Particle size | |||
|---|---|---|---|---|---|---|
| P[mbar] | Q[µL/min] | P[mbar] | Q[µL/min] | P[mbar] | Q[µL/min] | [µm] |
| 2000-3000 | 3 | 2000-3000 | 3 | 100-200 | 50 | n.a (co-flow) |
| 2000-3000 | 1 | 2000-3000 | 1 | 100-200 | 50 | 50-55 |
| 2000-3000 | 0.5 | 2000-3000 | 0.5 | 100-200 | 50 | 40-45 |
Crosslinking process
This part (steps 18–32) is the most critical part of the protocol in which the user has to take care to perform the steps in a timely manner. In Steps 20–23 where a ’flicking’ is suggested, this refers to a gentle finger-tapping that will help the reagent to gently mix. This procedure is crucial to clear out the surfactant and induce the crosslinking solution to reach the droplets. After this procedure has been performed, the microtube containing the crosslinked emulsion can rest at room temperature (step 24) while the cleaning procedure is taking place. We also recommend that one person follows Step 25–32 while a second person performs the cleaning protocol. This is suggested to avoid the remaining solutions from drying inside the chips. Using a microscope to verify that the microspheres are stable is useful to confirm that the process has been successful. To do this, we suggest placing 10 µL of the washed spheres on a cell-counter glass slide and observing the spheres under the microscope at 4-40X magnification (Table 2). Examples of crosslinked spheres can be seen in Fig. 3B-C.
Table 2.
Troubleshooting table.
| Step Problem | Possible reason | Solution | |
|---|---|---|---|
| Step 3 | The software does not show a pump | Incomplete initialization | Re-start the software and pump |
| The pressure in a pump is high | 4-way connector not attached | Reconnect the 4-way connector | |
| Blockage | Unblock the system (Step 50) | ||
| Step 13 | The flow has not started | 4-way connector not attached | Reconnect connector |
| Blockage | Unblock the system (Step 50) | ||
| Incorrect particle size formed | Fluid properties have changed | Check the TCU temperature | |
| Blockage | Unblock the system (Step 50) | ||
| Step 14 | The fluids are not flowing | Fluids are too viscous | Verify the reagent dilutions |
| Blockage | Unblock the system (Step 50) | ||
| Step 32 | Microspheres not formed | Unsuccessful crosslinking | Repeat the process |
| Insufficient emulsion stabilizer | Check surfactant concentration | ||
| Leakage from the chips | Perform the process again | ||
| Step 50 | Blockage of microchip | Residuals/contamination | Replace the chip |
| Perform cleaning protocol twice | |||
| Sonicate (2 h) in SDS, then | |||
| repeat the cleaning protocol | |||
Cleaning procedure
The cleaning process (Steps 33–50) has been optimized to ensure that a minimum residue remain in the chip, in order to be able to re-use the microfluidic chip. The use of two different reagents, as suggested, facilitates thorough cleaning. However, the user is not limited to performing this procedure a single time, for instance, if blockage occurs this procedure can be repeated, as necessary. Other cleaning protocols are not recommended as introducing different reagents might damage the fluorophilic coating of the microfluidic chip. In case of persistent blockage refer to the troubleshooting section.
Level of expertise
For the selection of growth factors and the application, expertise in cell biology is recommended. The use of the microencapsulation system ideally requires training which is usually provided by the manufacturer of the ma- chine at no cost. However, to encapsulate growth factors using alginate only this protocol is available. Therefore, it is recommended to practice the pro- cedure in order to become familiar with the protocol. After proper training, a typical graduate student can master the microencapsulation process after 6 weeks of practice. A PC1 or PC2 laboratory will generally contain most of the consumables used.
| Box 1 | Growth factor and hydrogel preparation Timing 1d 3h |
| Before performing the integration of growth factors within alginate solution it is useful to revise the data sheet of the growth factors to consider the storage, half-life, and reconstitution of the growth factors. |
| Procedure |
| 1. Sterilize the alginate powder using ETO. |
| 2. Dissolve the alginate in PBS at 2% w/v at 37°C |
| CRITICAL Changing the concentration will change the rheological properties of the final solution. |
| 3. Filter the alginate solution using a 0.22 µm filter. |
| 4. Retrieve 100 µL of the filtered alginate (2% w/v). |
| □ PAUSE POINT The rest of the solution can be stored at -4°C. |
| 5. Prepare the growth factors following the reconstitution protocol suggested by the manufacturer and adjust pH to neutral. |
| 6. Add the growth factors in the desired concentration to obtain a final volume of 100 µL. PBS can be added at this step to obtain the final volume. |
| 7. Mix the growth factor solution up-and-down using a micropipette. |
| 8. Add the mixed solution of growth factors (100 µL) to 100 µL of the filtered alginate. |
| 9. Mix the alginate-growth factors solution by carefully pipetting the solution up-and-down using a micropipette. The final alginate concentration in the solution will be 1% w/v. |
| □ PAUSE POINT The mixture can be kept at 4°C up to 24h. |
| Box 2 | Microencapsulation system set-up Timing 2h |
| The user should familiarize themselves with the parts of the microencapsulation system before performing this part of the protocol. It is useful to practice this protocol part before performing the encapsulation of growth factors. All parts of the system are identified in Fig. 2 and pictures showing the steps in the software can be found in Supplementary Information. |
| Procedure |
| 1. Turn on the air compressor and wait until the pressure reaches 8 kPa. |
| CRITICAL The air compressor should reach 8 kPa before continuing to the next step, if not, the pressure may drop during the process. |
| 2. Turn on the three pressure pumps starting from the ’oil’(Pump 3), then ’aqueous line 2’ (Pump 2) and then ’aqueous line 1’ (Pump 1). |
| 3. Turn on the imaging system (light controller and microscope) and the TCU (temperature controller system) |
| 4. Turn on the PC and open the Dolomite software. |
| CRITICAL The pressure pumps must be initialized before the software is open to ensure full recognition of the devices. |
| 5. Set the TCU at 40°C using the software (Supplementary Information). |
| CRITICAL Setting the temperature at a different temperature might affect the rheological properties of the alginate-growth factor solution. |
| 6. Place the oil reagents within the pressure pumps as indicated by the pressure pump labeling (see reagents set up for the specific preparations of the oil reagents). |
| 7. Disconnect the 4-ways linear connector from the chip holder (if connected) (Fig. 2C). |
| □ PAUSE POINT The 4-way linear connector can be placed on the bench on a delicate-task wiper (e.g. KimwipesTM). |
| 8. Open the pressure valve of the pressure pump (starting with the ’oil’ pres- sure pump). |
| 9. Prime the system by setting the pressure (using the software as indicated in Supplementary Information) at 1000 mbar then press the ’enter’ key on the PC. |
| CRITICAL Confirmation by pressing the ’enter’ key is necessary to set the pressure. |
| 10. Once a droplet of the reagent starts to come out of the 4-way connector, close the valve and stop the pressure using the ’stop’ key on the software. |
| 11. Repeat the procedure (in Box 2, Procedure 8–10) for all of the pressure pumps. |
| □ PAUSE POINT Once the system is primed, it can stay on hold until the droplet formation process starts. |
Reagent set-up
Alginate sterilization and solution preparation
Use alginate of medium viscosity. We suggest weighting the desired amount of alginate to produce a large batch (e.g 10 mL) at 2% w/v, before the sterilization with ETO and to run the sterilization cycle for 12h as described in Ref. [23]. After the cycle and the ventilation has been completed it is suggested to verify the weight and the mechanical properties of the material if possi- ble to confirm that the material has not undergone any physical/chemical changes. For the solution preparation, add PBS to obtain a 2% w/v solution and place it at 37 °C until the alginate dissolves. Next, filter the alginate by first collecting it using a syringe (5 mL is recommended) and passing the fluid through a 0.22 µm PES filter (Box 1, procedure steps 1–3).
Microfluidic oil reagents
Each microfluidic pressure pump has one glass tube containing oil. Each pump is then connected to the lines which drives a reagent. The lines to be connected to the aqueous lines (aqueous line 1 and aqueous line 2) must contain at least 5 mL of oil placed inside a glass tube inside each pump, while the pump connected to the oil line must contain at least 5 mL of a solution made of oil + 1% w/v PicoSurf (in 5% oil). All of the oil reagents must be filtered with a 0.22 µm PDMS filter.
□ PAUSE POINT Once prepared these reagents can be kept at room tem- perature.
Cleaning reagents
To clean the microfluidic chip, it is necessary to use two different reagents. The first reagent is a mixture of PBS and 1% w/v SDS. The second reagent is deionized water. All reagents need to be filtered (0.22 µm) prior to their use.
Quality control of main reagents
The quality control steps we describe here are important for verifying the quality of the main reagents. They can be performed either before the actual droplets formation or for troubleshooting an unsuccessful run. The first check can verify the mechanical and physical properties of the EtO treated alginate by checking the crosslinking capacity and shear-thinning properties of the hydrogel. The second check can involve the verification of molecular weight and detection using ELISA and by western blotting (Supplementary Information).
Materials
Biological materials
-
•
Recombinant human bone morphogenetic protein 6 (R&D Sytems, cat.no. 243-B3)
-
•
Recombinant human transforming growth factor beta-3 (Preportech, cat.no. 100-36E)
Reagents
-
•
Alginic acid sodium salt (Alginate, Sigma Aldrich, cat.no. A2033) Calcium chloride (Sigma Aldrich, cat.no. C-5080)
-
•
Sodium hydroxide (Sigma Aldrich, cat.no. 221465)
-
•
Phosphate buffered saline (PBS, Sigma Aldrich, cat.no. D8537) HFE 7500 Flourinated oil (Acota Limited, cas.no.297730-93-9)
-
•
! CAUTION Avoid direct contact with this oil, as it may cause respi- ratory, skin and eye irritation. Wear appropriate laboratory clothing and equipment when handling it.
-
•
PicoSurf surfactant (Sphere Fluidics, cat.no. C023)
-
•
CRITICAL other types of surfractant can be synthesized as de- scribed in Ref. [24] or purchased from a different company.
-
•
PicoBreak emulsion breaker (Sphere Fluidics, cat.no. JMORSC- 1059132)
-
•
CRITICAL other emulsion breakers can be purchased (e.g. 3M) Sodium dodecyl sulfate (SDS, Sigma Aldrich, cas.no.151-21-3) Isopropanol (Sigma Aldrich, cat.no. 33539)
-
•
! CAUTION This reagent is highly flammable
Equipment
CRITICAL Having the equipment listed here ensures the optimal func- tioning of the microencapsulation system for this protocol. However, it is possible to buy these components from different suppliers.
-
•
Air compressor (Aircomp, model Bambi PT5 cat.no PT5) Temperature controller unit, Meros TCU-100 (TCU, Dolomite UK, part no. 3200428)
-
•
Microchip holder, (Dolomite UK, part.no. 3200444b) Reservoir Chip (Dolomite UK, part no. 3200444)
-
•
Fluorophilic microfluidic chip, microencapsulator 2 reagent droplet chip 50 µm etch (Dolomite UK, part.no. 3200445) [25]
-
•
Microscope, Meros high speed (Dolomite UK, part no. 3200531)
-
•
Flow rate sensor display (Dolomite UK, part no. 3200095)
-
•
Flow rate sensor 50 µL/min (Dolomite UK, part.no. 3200098)
-
•
Pressure pumps, Mitos P-Pump (Dolomite UK, part no. 3200016)
-
•
Four-way connector (Dolomite UK, part no. 3200454)
-
•
Adjustable 10, 200 and 1,000 µL pipettes and sterile pipette tips.
Software
-
•
Dolomite Flow Control Center (Dolomite UK, part no. 3200475).Procedure
| Procedure |
| Growth Factor preparation Timing 14h |
| CRITICAL The protocol should be carried out in a clean environment. |
| A PC1 or PC2 laboratory with temperature control is recommended. |
|
|
| Microencapsulation system set-up ° Timing 3h |
|
| CRITICAL STEP The system should recognize all of the pressure pumps, the TCU (temperature-controlled unit) component and the microscope. |
| ? TROUBLESHOOTING |
|
|
|
|
|
| CRITICAL STEP The introduction of air bubbles when retrieving and dispensing the alginate-growth factor solution should be avoided or kept to a minimum. |
| Droplet production ° Timing 4h |
|
|
| CRITICAL STEP Ensuring that the connector is correctly placed will avoid the introduction of air bubbles and prevent possible leakage between the reservoir chip and the microfluidic chip. |
|
|
|
|
| ? TROUBLESHOOTING |
|
|
|
| CRITICAL STEP Closing the valves and flow rates in this order is useful to avoid cross-flow between the lines. |
| Emulsion breaking and crosslinking process Timing 35 min. |
| CRITICAL The quantities and timing from this part must be exact to allow the calcium chloride to mix while the emulsion breaker (e.g. Picobreak) is breaking the emulsion. |
|
|
|
|
|
|
|
| □ PAUSE POINT This can be kept at room temperature. |
|
| it. |
|
|
|
|
|
|
| CRITICAL STEP Washing the microspheres prevents possible toxic effects from the reagents. |
|
| ? TROUBLESHOOTING |
| Cleaning procedure Timing 2.5 h. |
| CRITICAL This part of the protocol is crucial to ensure the re-usability of the microfluidic chip. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ? TROUBLESHOOTING |
Timing
Box 1, step 1, sterilization of material: ~ 1d Box 1, step 2, dissolving material: ~ 5h Step 1-2, preparing the materials: ~ 1d 3h
Box 1, step 3-4, filtering of material: ~ 30 min Box 1, step 5, growth factor reconstitution: ~ 1 h
Box 1, step 6-9, mixing growth factor with alginate: ~ 30min Box 2, step 1-4, turning on the system: ~ 10-30 min
Box 2, step 5-10, priming the first reagent: ~ 10-30 min
Box 2, step 11, priming the rest of the reagents: ~ 30-40 min Step 3, preparing the system: ~ 2 h
Step 4-8, placing the reagents: ~ 30 min-1 h
Step 9-13, setting the chips and flow rate: ~ 10-30 min Step 14-17, production of spheres: ~ 2-3 h
Step 18, collection of emulsion: ~ 5-10 min
Step 19-23, crosslinking and breaking the emulsion: ~ 1-2 min Step 24-32, retrieving the spheres and washing: ~ 10min- 20 min Step 33-42, cleaning tubing: ~ 15-30 min
Step 43-50, cleaning with all reagents: ~ 1-2 h.
Anticipated results
It is advisable to perform the selection of growth factors before performing this protocol. Selecting a different manufacturer can result in different encapsulation efficiency and release rates. This is most likely linked to the fact that the molecular weight of the growth factors can vary depending on the purification and extraction process. Therefore, we strongly recommend performing a trial of the growth factors to identify the detection range (e.g. using a calibration curve with known concentration) with ELISA, as well as pre-mixing the alginate with the desired concentration. It is also advisable to verify the molecular weight and functionality by western blot (Supplementary Information) and growth-factor dependent cell lines [25].
In our experience, certain reconstitution buffers can cause an electrostatic interaction of the growth factor with the alginate. Herein, we outline a process that was useful in avoiding the electrostatic interaction by maintain a neutral pH of the growth factors and alginate material and thus, reducing the possibility of blockage or growth factor damage. It is expected that the encapsulation of the growth factors as used here is around 2.5 µg/mL. Trying to encapsulate different growth factors in alginate is possible given the general approach presented here but, for the moment we have limited this protocol to the example of growth factors used. Therefore, the user will need to characterize the encapsulation performance for other growth factors. However, based on our observations similar flow rates can be set-up.
This protocol produces small volumes of microspheres containing growth factors. It is expected that following this protocol, the user can obtain a 100
µL volume that can be further reduced. To reduce the volume, the reader can simply centrifuge and remove the excess PBS while keeping the pellet formed at the bottom of the microtube. If the volume and distribution of the spheres has been changed, a variation of the delivery rate is possible and will depend on the experimental conditions such as the sink volume.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgements
Funding from the Australian Research Council Centre of Excellence Scheme (Project Number 140100012 CE) is gratefully acknowledged. Funding from St. Vincent's hospital Research Endowment Fund (Project Number 88184). The Sylvia and Charles Viertel Charitable Foundation, Clinical Investigator Award (VIERCI2118003). Studio Tulum® for graphical visualization.Author contributions statement
L.M.C.A. developed and wrote the manuscript and performed the study. S.D. developed the protocol, performed the study and designed the experiment. C.O. designed the experiment and performed the study. A.Q. developed the protocol. C.B. and S.E.M. supervised the study and helped in writing the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.mex.2021.101324.
Appendix. Supplementary materials
References
- 1.Caballero Aguilar L.M., Silva S.M., Moulton S.E. Growth factor delivery: defining the next generation platforms for tissue engineering. J. Control. Release. 2019;306:40–58. doi: 10.1016/J.JCONREL.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 2.Augustyniak E., Trzeciak T., Richter M., Kaczmarczyk J., Suchorska W. The role of growth factors in stem cell-directed chondrogenesis: a real hope for damaged cartilage regeneration. Int. Orthop. 2015;39:995–1003. doi: 10.1007/s00264-014-2619-0. [DOI] [PubMed] [Google Scholar]
- 3.Tang X. Connective tissue growth factor contributes to joint homeostasis and osteoarthritis severity by controlling the matrix sequestration and activation of latent TGFβ. Ann. Rheum. Dis. 2018;77:1372–1380. doi: 10.1136/annrheumdis-2018-212964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bian L. Enhanced MSC chondrogenesis following delivery of TGF-β 3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32:6425–6434. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sukarto A., Yu C., Flynn L.E., Amsden B.G. Co-delivery of adipose-derived stem cells and growth factor-loaded microspheres in RGD-grafted n-methacrylate glycol chitosan gels for focal chondral repair. Biomacromolecules. 2012;13:2490–2502. doi: 10.1021/bm300733n. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen A.H., McKinney J., Miller T., Bongiorno T., McDevitt T.C. Gelatin methacrylate microspheres for controlled growth factor release. Acta Biomater. 2015;13:101–110. doi: 10.1016/J.ACTBIO.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solorio L., Zwolinski C., Lund A.W., Farrell M.J., Stegemann J.P. Gelatin microspheres crosslinked with genipin for local delivery of growth factors. J. Tissue Eng. Regenerat. Med. 2010;4:514–523. doi: 10.1002/term.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Annamalai R.T. Harnessing macrophage-mediated degradation of gelatin micro- spheres for spatiotemporal control of BMP2 release. Biomaterials. 2018;161:216–227. doi: 10.1016/J.BIOMATERIALS.2018.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lan L. Implantable porous gelatin microspheres sustained release of bFGF and improved its neuroprotective effect on rats after spinal cord injury. PLoS ONE. 2017;12:1–16. doi: 10.1371/journal.pone.0173814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Streets A.M., Huang Y. Chip in a lab: Microfluidics for next generation life science research. Biomicrofluidics. 2013;7:11302. doi: 10.1063/1.4789751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mark D., Haeberle S., Roth G., von Stetten F., Zengerle R. Microfluidic lab-on-a- chip platforms: requirements, characteristics and applications. Chem. Soc. Rev. 2010;39:1153. doi: 10.1039/b820557b. [DOI] [PubMed] [Google Scholar]
- 12.Chan H.F. Rapid formation of multicellular spheroids in double-emulsion droplets with controllable microenvironment. Sci. Rep. 2013;3:3462. doi: 10.1038/srep03462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grasso M.S., Lintilhac P.M. Microbead Encapsulation of Living Plant Protoplasts: A New Tool for the Handling of Single Plant Cells. Appl. Plant Sci. 2016;4 doi: 10.3732/apps.1500140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caballero Aguilar L., Stoddart P.R., McArthur S.L., Moulton S.E. Polycaprolactone porous template facilitates modulated release of molecules from alginate hydrogels. React. Funct. Polym. 2018;133:29–36. doi: 10.1016/J.REACTFUNCTPOLYM.2018.09.016. [DOI] [Google Scholar]
- 15.Ching S.H., Bansal N., Bhandari B. Alginate gel particles–A review of production techniques and physical properties. Critical Rev. Food Sci. Nutr. 2017;57:1133–1152. doi: 10.1080/10408398.2014.965773. [DOI] [PubMed] [Google Scholar]
- 16.Lio D., Yeo D., Xu C. Control of alginate core size in alginate-poly (Lactic- Co-Glycolic) acid microparticles. Nanoscale research letters. 2016;11(9) doi: 10.1186/s11671-015-1222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corstens M.N. Emulsion-alginate beads designed to control in vitro intestinal lipolysis: towards appetite control. J. Funct. Foods. 2017;34:319–328. [Google Scholar]
- 18.Eral H.B. Biocompatible alginate microgel particles as heteronucleants and encapsulating vehicles for hydrophilic and hydrophobic drugs. Cryst. Growth Des. 2014;14:2073–2082. doi: 10.1021/cg500250e. [DOI] [Google Scholar]
- 19.Ahmed, N., an Michelin-Jamois, M., Fessi, H. & Elaissari, A. Modified double emulsion process as a new route to prepare submicron biodegradable magnetic/polycaprolactone particles for in vivo theranostics. doi: 10.1039/c2sm06872a. [DOI]
- 20.Thakur G., Mitra A., Basak A., Rousseau D., Pal K. Proceedings of the International Conference on Systems in Medicine and Biology. 2010. Characterization of oil-in-water gelatin emulsion gels: effect of homogenization time; pp. 305–308. [DOI] [Google Scholar]
- 21.Einhorn-Stoll U., Weiss M., Kunzek H. Influence of the emulsion components and preparation method on the laboratory-scale preparation of o/w emulsions containing different types of dispersed phases and/or emulsifiers. Nahrung - Food. 2002;46:294–301. doi: 10.1002/1521-3803(20020701)46:4<294::AID-FOOD294>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Hu K., Bhattacharya B.B., Chakrabarty K. Proceedings of the IEEE Transactions on Computer-Aided Design of Integrated Circuits and Systems. Vol. 35. 2016. Fault diagnosis for leakage and blockage defects in flow-based microfluidic biochips; pp. 1179–1191. [DOI] [Google Scholar]
- 23.O'Connell C.D. Evaluation of sterilisation methods for bio-ink components: gelatin, gelatin methacryloyl, hyaluronic acid and hyaluronic acid methacryloyl. Biofabrication. 2019 doi: 10.1088/1758-5090/ab0b7c. [DOI] [PubMed] [Google Scholar]
- 24.Holtze C. Biocompatible surfactants for water-in-fluorocarbon emulsions. Lab on a Chip. 2008;8:1632. doi: 10.1039/b806706f. [DOI] [PubMed] [Google Scholar]
- 25.Caballero Aguilar L.M. Formation of alginate microspheres prepared by optimized microfluidics parameters for high encapsulation of bioactive molecules. J. Colloids Interface Sci. 2021:240–251. doi: 10.1016/j.jcis.2020.12.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.