Abstract
Calpains are Ca2+-dependent cysteine proteases; their aberrant activation is associated with several neurodegenerative diseases. The μ-calpain catalytic subunit, calpain-1, is located in the cytoplasm as well as in the mitochondria. Mitochondrial calpain-1 cleaves apoptosis-inducing factor (AIF), leading to apoptotic cell death. We have previously reported that short peptides of calpain-1 C2-like domain conjugated with cell penetrating peptide HIV-Tat (Tat-μCL) selectively inhibit mitochondrial calpain-1 and effectively prevent neurodegenerative diseases of the eye. In this study, we determined whether mitochondrial calpain-1 mediates oxytosis (oxidative glutamate toxicity) in hippocampal HT22 cells using Tat-μCL and newly generated polyhistidine-conjugated μCL peptide and compared their efficacies in preventing oxytosis. TUNEL assay and single strand DNA staining revealed that both μCL peptides inhibited glutamate-induced oxytosis. Additionally, both the peptides suppressed the mitochondrial AIF translocation into the nucleus. All polyhistidine-μCL peptides (containing 4–16 histidine residues) showed higher cell permeability than Tat-μCL. Notably, tetrahistidine (H4)-μCL exerted the highest cytoprotective activity. Thus, H4-μCL may be a potential peptide drug for calpain-1-mediated neurodegenerative diseases such as Alzheimer's disease.
Keywords: Mitochondrial calpain-1, Oxytosis, Cell penetrating peptide, Hippocampal HT22 cells, Neurodegeneration
Abbreviations: AIF, apoptosis-inducing factor; BBB, blood–brain barrier; CPP, cell penetrating peptide; DAPI, 4′,6-diamidino-2-phenylindole; DMEM, Dulbecco's Modified Eagle Medium; Hn-μCL, polyhistidine-conjugated μCL; PBS, phosphate-buffered saline; ssDNA, single stranded DNA; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
Highlights
-
•
Mitochondrial calpain-1 mediates glutamate-induced oxytosis in HT22 cells.
-
•
CPP-μCL inhibits AIF translocation and DNA fragmentation in oxytosis.
-
•
Polyhistidine conjugation enhances intracellular μCL peptide uptake efficiency.
1. Introduction
Calpains are Ca2+-dependent cysteine proteases; their hyperactivation is associated with the pathologies of neurodegenerative diseases, muscular dystrophy, type II diabetes, and eye diseases [[1], [2], [3], [4], [5]]. The calpain family is comprised of 15 genes. Of these, calpain-1 (μ-calpain catalytic subunit) is a member of the classical calpain subfamily that is expressed ubiquitously [6]. Most calpains localize in the cytoplasm and mediate the processing of a number of substrates in response to elevation in intracellular Ca2+ levels [7,8]. Additionally, mitochondrially-localized calpains such as calpain-1, calpain-2 (m-calpain catalytic subunit), calpain-5, and calpain-10 have been reported to regulate mitochondrial homeostasis and pathological neuronal cell death [[9], [10], [11], [12], [13], [14]]. Apoptosis-inducing factor (AIF), a mitochondrial oxidoreductase that regulates the respiratory chain as well as apoptotic cell death, is a substrate of mitochondrially-localized calpain-1 [9]. Truncated AIF released from the mitochondria translocates into the nucleus to induce DNA fragmentation in apoptotic cells [10,15,16].
Previously, we have reported that a peptide derived from calpain-1 C2-like (C2L) domain specifically inhibits the mitochondrial calpain-1 by preventing its interaction with the chaperone protein ERp57 (Fig. 1a) [17]. The inhibitory peptide conjugated with a cell penetrating peptide (CPP) derived from HIV-Tat, Tat-μCL, was capable of preventing photoreceptor cell death in retinitis pigmentosa model Royal College of Surgeons rats [17] and retinal ganglion cell death in ischemia/reperfusion-induced glaucoma model rats [18]. Ischemia causes glutamate-mediated excitotoxicity via the NMDA receptor [19]. Glutamate also induces oxidative stress-induced regulated cell death called oxytosis, which is distinct from classical apoptosis [20]; it depends on AIF and not caspase activation for cell death. In oxytosis, extracellular glutamate leads to glutathione depletion by antagonizing cystine/glutamate antiporter and resulting oxidative stress induces cell death [21]. Since oxytosis is considered to be involved in the pathology of neurodegenerative diseases such as Alzheimer's disease, mouse hippocampus-derived HT22 cells have been utilized as a model cell system to elucidate the oxytosis mechanism. Oxytosis usually proceeds via several complex and consequent processes involving glutathione depletion, reactive oxygen species production, and lipoxygenase and AIF activation [22]. Although calpain-1 is thought to be involved in the late execution of cell death in oxytosis, any direct evidence of mitochondrial calpain-1 in oxytosis is lacking.
Fig. 1.
Intracellular uptake and apoptosis inhibitory effect of Tat-μCL.
(a) Model of mitochondrial calpain-1 inhibition by CPP-μCL. Mitochondrial μ-calpain composed of calpain-1 (L) and calpain small subunit (S) associates with chaperone protein ERp57 in the intermembrane space (IMS). Increase in mitochondrial Ca2+ level leads to mitochondrial calpain-1 activation, leading to the cleavage of mitochondrial inner membrane (IM) protein AIF into truncated form (tAIF). CPP-μCL inhibits mitochondrial calpain-1 by competitive binding to ERp57. (b) Intracellular uptake of Tat-μCL peptide in HT22 cells. HT22 cells were incubated for 1 h in a culture medium containing 50 μM of Tat-μCL peptide. Fixed cells were stained with DAPI (blue) and Tat antibody (red). Scale bars: 20 μm. (c) TUNEL-positive cells induced by glutamate. Following incubation of HT22 cells with or without 50 μM Tat-μCL or Tat-μCL scramble and 2 mM glutamate for 18 h, apoptotic cells were detected using TUNEL assay. Fixed cells were stained with DAPI (blue) and TUNEL (green). Representative data are shown. Scale bars: 100 μm. (d) Tat-μCL suppresses glutamate-induced apoptosis. The number of TUNEL-positive cells was normalized by nuclei count and expressed as percentages. The experiments were repeated at least thrice. Data represent mean ± SD (n = 3). *; p < 0.05 vs Glu, ***; p < 0.001 vs Glu (One-way ANOVA, Tukey–Kramer). . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
CPPs such as HIV-Tat peptide (GRKKRRQRRRPPQ), penetratin (RQIKIWFQNRRMKWKK), and polyarginine (RRRRRRRRR) are often used to deliver bioactive substances into cells [[23], [24], [25]]; however, there are limitations regarding their delivery and/or retention in certain tissues. Polyhistidine peptide is a CPP that does not have a positive charge in neutral pH but has a high cell-penetrating efficiency with minimal interference by serum components [26]. The previous study has also shown that cell-penetrating efficiency depends on the length of polyhistidine [27]. In this study, we evaluated the role of mitochondrial calpain-1 against glutamate-induced oxytosis and examined the intracellular delivery efficacies of Tat-μCL and polyhistidine-conjugated μCL (Hn-μCL) peptides in HT22 cells.
2. Materials and methods
2.1. Peptide synthesis
All peptides used were synthesized using an automated peptide synthesizer (PSSM-8; Shimadzu, Kyoto, Japan). The sequences of peptides are presented in Table 1. The synthesized peptides were purified on a C18 column (Jupiter, 250 mm × 10 mm; Phenomenex, Torrance, CA, USA) using reverse-phase high-pressure liquid chromatography. The purified peptides were dissolved in phosphate-buffered saline (PBS) at a concentration of 10 mM, and aliquots were stored at −20 °C.
Table 1.
List of cell penetrating peptide conjugated μCL sequences.
| Peptides | Sequence |
|---|---|
| Tat-μCL | GRKKRRQRRRPPQ-PDALKSRTIR |
| Tat-μCL scramble | GRKKRRQRRRPPQ-ASLRIDRPTK |
| H4-μCL | HHHH-PDALKSRTIR |
| H8-μCL | HHHHHHHH-PDALKSRTIR |
| H12-μCL | HHHHHHHHHHHH-PDALKSRTIR |
| H16-μCL | HHHHHHHHHHHHHHHH-PDALKSRTIR |
2.2. Cell culture
Mouse hippocampus-derived neuronal HT22 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM, 05919, NISSUI PHARMACEUTICAL CO., LTD., Tokyo, Japan) containing NaHCO3 (S8761, Sigma-Aldrich, St. Louis, MO, USA), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM l-glutamine (PSG, 10378-016, Thermo Fisher Scientific, Tokyo, Japan), and 10% heat-inactivated fetal bovine serum (10270-106, Thermo Fisher Scientific, Tokyo, Japan).
The cells were seeded onto a 24-well plate at a concentration of 5 × 104 cells/well and incubated for 24 h. To induce glutamate-induced oxytosis, the cells were treated with 2 mM l-glutamate (194–02032, Wako Pure chemical industries, Ltd., Osaka, Japan) in fresh culture medium and incubated for certain time periods as described. To evaluate protective effect of peptide, the cells were pretreated with peptide by replacing the culture media containing 50 μM peptides for 1 h and then cotreated with the peptide and 2 mM l-glutamate for 18 h.
2.3. Immunocytochemistry
HT22 cells were seeded onto a 13-mm round cover glass placed in a 24-well plate at a concentration of 5 × 104 cells/well. After 24 h, cells were treated with each peptide at 50 μM for 1 h, followed by washing with PBS containing 0.05% Tween 20 (Tw-PBS) and fixation with 4% paraformaldehyde in 0.2 M phosphate buffer (pH 7.4) at 4 °C for 30 min. Cells were permeabilized with acetone at 25 °C for 10 min, blocked with 5% goat serum at 37 °C for 30 min, and subsequently incubated with rabbit polyclonal anti-HIV1-Tat antibody (1:200, ab43014, Abcam, Cambridge, UK), chicken anti-μCL antibody (1:200, T. K. Craft, Gunma, Japan), anti-single stranded DNA (ssDNA) rabbit antibody (1:200, 18731, Immuno-Biological Laboratories, Minneapolis, MN, USA), rabbit polyclonal anti-AIF antibody (1:200, ab1998, Abcam), or rabbit polyclonal anti-COX IV antibody (1:200, ab16056, Abcam) in Tw-PBS at 4 °C overnight. The primary antibody was stained with either a goat polyclonal anti-rabbit IgG conjugated to Alexa Fluor® 594 (1:500, A11037, Invitrogen, Carlsbad, CA, USA) or goat polyclonal anti-chicken IgY conjugated to Alexa Fluor® 488 (1:500, ab150169, Abcam) at 37 °C for 40 min in Tw-PBS. Nuclei were stained with 1 μg/mL of 4′,6-diamidino-2-phenylindole (DAPI) concomitantly with the secondary antibody. Fluorescent images were acquired using a confocal laser scanning microscope (C2+, Nikon Institute, Tokyo, Japan). The numbers of ssDNA-positive cells and total nuclei were counted as apoptotic cell population.
2.4. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
HT22 cells grown on a cover glass were pretreated with or without 50 μM peptide for 1 h followed by incubation with culture medium containing 2 mM l-glutamate and each peptide for 18 h. The assay was performed using the in situ apoptosis detection kit (Takara Bio, Shiga, Japan) according to the manufacturer's instructions. Fluorescent images were acquired using a confocal laser scanning microscope (C2+). TUNEL-positive cells and total nuclei were counted and used for statistical analysis.
2.5. MTS assay
HT22 cells were seeded onto a 96-well plate at a concentration of 1 × 103 cells/well and cultured for 24 h. Culture media were replaced with fresh DMEM containing 50 μM of each peptide or 2 mM glutamate as a positive control and incubated for 6–24 h. Following incubation, 20 μL MTS reagent (Promega, Wisconsin, USA) was added to each well containing 100 μL culture media. After incubation for 1 h, absorbance was measured at 492 nm.
2.6. Statistical analysis
All experiments were repeated at least thrice, and data were expressed as mean ± standard deviation (SD). Statistical analysis was performed by one-way or two-way ANOVA and post hoc Tukey-Kramer test using R, and p < 0.05 was considered as statistically significant.
3. Results
3.1. Tat-μCL protects HT22 cells against glutamate-induced oxytosis
The permeability of Tat-μCL in HT22 cells was analyzed using immunofluorescent anti-Tat antibody staining. Since Tat-μCL treatment showed cytotoxicity at the concentrations above 100 μM, cells were treated with 50 μM peptide. The intracellular translocation of Tat-μCL was observed 1 h after peptide treatment (Fig. 1b); it was found to be distributed in various cellular compartments including mitochondria, showing colocalization with mitochondrial marker COX IV (Fig. S1). Next we analyzed whether mitochondrial calpain-1 is involved in glutamate-induced oxytosis, HT22 cells were incubated in culture medium containing 2 mM glutamate for 18 h, which induces oxidative glutamate toxicity but not necrosis in this cell line [28].
TUNEL assay revealed that treatment of HT22 cells with glutamate significantly increased TUNEL-positive cells (up to 73%) (Fig. 1c and d), whereas preincubation with 50 μM Tat-μCL peptide for 1 h and following cotreatment with the peptide and glutamate significantly decreased TUNEL-positive cell population. Conversely, Tat-conjugated scrambled μCL failed to attenuate glutamate toxicity (Fig. 1c and d).
3.2. Intracellular uptake of polyhistidine-μCL
To compare Tat-μCL and Hn-μCL cell permeabilities, we synthesized μCL peptides with 4–16 histidine residues fused to the N-termini (H4- to H16-μCL, Table 1). HT22 cells were incubated with 50 μM of each peptide for 1 h, and the intracellular incorporation of either peptide was detected using immunofluorescent anti-μCL antibody staining. Similar to Tat-μCL, the intracellular uptake of all Hn-μCL peptides was observed in various cellular compartments (Fig. S1). Moreover, Hn-μCL displayed increased cell permeability compared to Tat-μCL (Fig. S1).
3.3. Cytotoxicity of Tat-μCL and Hn-μCL
The cytotoxicity of each peptide was determined microscopically as well as by using MTS assay. Although glutamate used as a positive control induced cell detachment and shrinkage, no abnormalities were observed in cell morphology on treatment with any of the peptides (Fig. 2b). Consistent with this observation, none of the peptides affected cell viability, as determined using MTS assay, although glutamate decreased the viability in a time-dependent manner (Fig. 2b).
Fig. 2.
Cytotoxicity of Tat-μCL and Hn-μCL in HT22 cells.
(a and b) HT22 cells were cultured for 6–24 h in a culture medium containing 50 μM of peptide or 2 mM glutamate. (a) Phase contrast images of HT22 cells treated with Tat-μCL or Hn-μCL for 24 h. Scale bars: 100 μm. (b) MTS assay. After each incubation period, the cells were treated with MTS reagent for 1 h, and absorbance was measured at 492 nm. The experiments were repeated at least thrice. Data represent mean ± SD. *; p < 0.05 (Two-way ANOVA, Tukey–Kramer).
3.4. Cytoprotective effect of Hn-μCL on oxytosis
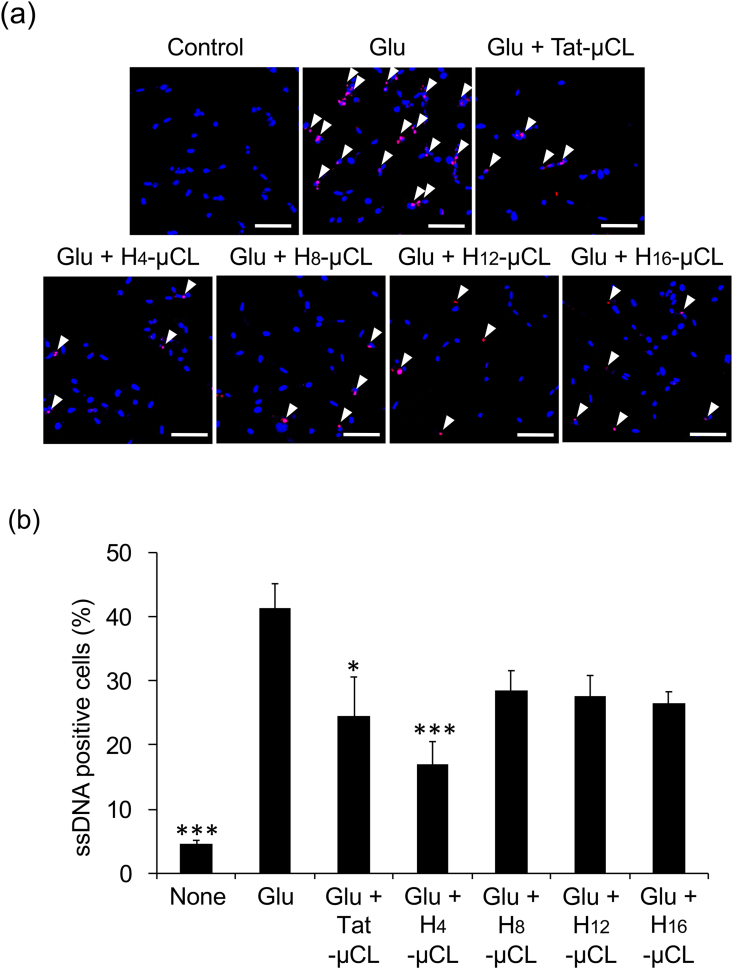
The protective effect of Hn-μCL against glutamate-induced oxytosis was examined using immunofluorescent staining of ssDNA. ssDNA-positive cells were not detected in the control; however, 40% cells were found to be ssDNA-positive following glutamate treatment. Conversely, treatment with Tat-μCL or H4-μCL significantly reduced ssDNA-positive cells induced by glutamate toxicity (Fig. 3a and b).
Fig. 3.
Apoptosis inhibitory effect of Tat-μCL and Hn-μCL.
(a) s
sDNA-positive cells induced by glutamate and protective effect of Hn-μCL. Apoptotic cells were detected using immunocytochemistry following pretreatment of HT22 cells with 50 μM peptide for 1 h and cotreatment with 2 mM glutamate for 18 h. Cells were fixed and stained with DAPI (blue) and ssDNA (red). Arrows indicate apoptotic cells. Representative data is shown. Scale bars: 50 μm. (b) Quantification of ssDNA positive cells. Experiments were repeated at least thrice. Data represent mean ± SD (n = 6 fields). *; p < 0.05 vs Glu, **; p < 0.01 vs Glu, ***; p < 0.001 vs Glu (One-way ANOVA, Tukey–Kramer). . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.5. Tat-μCL and Hn-μCL prevent AIF nuclear translocation
Since AIF proteolytic processing by calpain-1 induces AIF translocation from the mitochondria into the nucleus, we analyzed AIF nuclear translocation in cells treated with glutamate and/or Hn-μCL peptides. AIF was detected as a fiber-like network in the cytoplasm (Fig. 4a and c) as has been reported previously [29]. The treatment of cells with glutamate induced diffuse localization of AIF in both cytoplasm and nucleus (Fig. 4a and b). In contrast, treatment with Tat-μCL or Hn-μCL significantly reduced the number of cells with AIF nuclear translocation (Fig. 4c and d).
Fig. 4.
Prevention of AIF translocation by Tat-μCL and Hn-μCL.
(a and b) AIF nuclear translocation induced by glutamate. After incubation of HT22 cells with 2 mM glutamate for up to 18 h, apoptotic cells were detected using immunocytochemistry. Cells were stained with DAPI (blue) and AIF (red). (a) Arrows indicate cells with translocated AIF. Representative data is shown. Scale bars: 50 μm. (b) The number of cells with nuclear AIF translocation was counted using ImageJ and expressed as a percentage of total nuclei. Data represent mean ± SD (n = 6 fields). ***; p < 0.001 (One-way ANOVA, Tukey–Kramer). (c and d) Hn-μCL peptide inhibits AIF nuclear translocation. Following pretreatment of HT22 cells with or without 50 μM of peptide for 1 h and cotreatment with 2 mM glutamate for another 18 h, AIF translocation was detected using immunocytochemistry. (c) Images of staining with DAPI (blue) and AIF (red) are shown. Arrows indicate cells with translocated AIF. Scale bars: 100 μm. (d) The number of cells with nuclear AIF translocation was counted as described in (b). Data represent mean ± SD (n = 3 fields). *; p < 0.05 vs Glu, **; p < 0.01 vs Glu, ***; p < 0.001 vs Glu (One-way ANOVA, Tukey–Kramer). . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
In the present study, we evaluated the role of mitochondrial calpain-1 in glutamate-induced oxytosis and compared the cellular permeabilities of Tat-μCL and Hn-μCL in HT22 cells as well as analyzed their protective effects against glutamate-induced oxytosis. The intracellular uptake of all Hn-μCL peptides was higher than that of Tat-μCL (Fig. S1). The increased uptake may be conferred by the non-ionic polyhistidine property and/or improved intracellular stability by higher protease resistance than that of Tat-μCL. Most CPPs are known to bind to negatively-charged molecules on the membrane surface during intracellular uptake, whereas polyhistidine is incorporated into cells by a charge-independent pathway [26]. Despite the comparable uptake efficiency of Hn-μCL, H4-μCL treatment lowered glutamate-induced DNA fragmentation the most compared to other polyhistidine peptides (Fig. 3a and b). Although difference in the protective effect was statistically insignificant in the present experimental condition, tetrahistidine may be more suitable for the inhibition of mitochondrial calpain-1 activity or longer polyhistidine peptides may weakly interrupt the competition of mitochondrial calpain-1/ERp57 complex.
It is suggested that the regulatory mechanisms of oxytosis and ferroptosis are highly similar in neurological diseases [20]; features including glutamate neurotoxicity, iron dysregulation, and glutathione depletion are involved in eye diseases including retinitis pigmentosa and glaucoma [30] that are alleviated by Tat-μCL administration in rat models [17,18]. Glutamate-induced oxytosis prevention by mitochondrial calpain-1 inhibition may be applied to neurodegenerative diseases. Alzheimer's disease is a kind of neurodegenerative disease characterized by the extracellular deposition of amyloid β plaques and intracellular accumulation of hyperphosphorylated tau [[31], [32], [33], [34]]. Calpain-1 is involved in the cleavage of tau protein at multiple sites that contribute to both clearance and accumulation of fibril formation [35]. Intriguingly, Alzheimer's disease is often associated with age-related macular degeneration and glaucoma, and common features including amyloid β plaque deposition, chronic inflammation, iron accumulation, and oxidative stress have been reported [36].
The blood–brain barrier (BBB) restricts substance exchange between blood and tissue fluid of the brain; thus, an efficient and safe delivery system is required for treatment of the central nervous system with a macromolecule [37]. In recent years, intranasal administration has attracted attention as a drug administration route capable of delivering a drug into the brain bypassing the BBB [[38], [39], [40]]. We attempted an intranasal administration of Tat-μCL to mice as a preliminary experiment. Tat-μCL was delivered to the olfactory epithelium; however, it was undetectable in the hippocampus (data not shown). In the present study, Hn-μCL showed improved uptake efficiency without adverse effect in HT22 cells. The delivery efficiency of Hn-μCL in brain by intranasal administration and its protective effect in neurodegenerative disease model mice should be elucidated in future studies. In contrast to mitochondrial calpain-1-mediated neurodegeneration, cytoplasmic calpain-1 activity is required for long term potentiation by cleaving RhoA, PHLPP1β, and STEP [1]. Since μ-CL peptides selectively inhibit mitochondrial calpain-1, Hn-μCL may be a more useful tool than conventional calpain inhibitors to analyze calpain-1 function in the mitochondria without affecting its cytoplasmic counterpart.
In conclusion, mitochondrial calpain-1-specific inhibitor peptide prevents glutamate-induced oxytosis in HT22 cells. In addition, new inhibitory peptides Hn-μCL is incorporated into HT22 cells more efficiently than Tat-μCL. H4-μCL most effectively inhibits AIF nuclear translocation and DNA fragmentation. The present study added valuable information regarding the mechanisms of oxytosis and provided new insight in developing treatment strategies for neurodegenerative diseases such as Alzheimer's disease.
Funding
The present study was supported in part by the Uehara Memorial Foundation, Japan for Taku Ozaki.
Role of the sponsor
The funding organizations had no role in the study design, conducting the study, data collection, management, analysis, interpretation, preparation, or review. The funding organization had no role in the approval of the manuscript or the decision to submit the manuscript for publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Prof. Tetsuro Yamashita for helpful discussions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2021.101101.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Intracellular uptake of Tat-μCL and Hn-μCL in HT22 cells.
After HT22 cells were incubated with or without 50 μM Tat-μCL or Hn-μCL for 1 h, cells were fixed and stained with DAPI (blue), μCL antibody (green), and mitochondrial marker COX IV (red). Scale bars: 50 μm.
References
- 1.Baudry M., Bi X. Calpain-1 and calpain-2: the yin and yang of synaptic plasticity and neurodegeneration. Trends Neurosci. 2016;39:235–245. doi: 10.1016/j.tins.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lasa-Elgarresta J., Mosqueira-Martín L., Naldaiz-Gastesi N., Sáenz A., de Munain A.L., Vallejo-Illarramendi A. Calcium mechanisms in limb-girdle muscular dystrophy with CAPN3 mutations. Int. J. Mol. Sci. 2019;20:4548. doi: 10.3390/ijms20184548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pánico P., Salazar A.M., Burns A.L., Ostrosky-Wegman P. Role of calpain-10 in the development of diabetes mellitus and its complications. Arch. Med. Res. 2014;45:103–115. doi: 10.1016/j.arcmed.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Mahajan V.B., Skeie J.M., Bassuk A.G., Fingert J.H., Braun T.A., Daggett H.T., Folk J.C., Sheffield V.C., Stone E.M. Calpain-5 mutations cause autoimmune uveitis, retinal neovascularization, and photoreceptor degeneration. PLoS Genet. 2012;8:e1003001. doi: 10.1371/journal.pgen.1003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y., Wang K.K.W. The calpain family and human disease. Trends Mol. Med. 2001;7:355–362. doi: 10.1016/S1471-4914(01)02049-4. [DOI] [PubMed] [Google Scholar]
- 6.Ono Y., Sorimachi H. Calpains - an elaborate proteolytic system. Biochim. Biophys. Acta Protein Proteonomics. 2012;1824:224–236. doi: 10.1016/j.bbapap.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Goll D.E., Thompson V.F., Li H., Wei W.E.I., Cong J. The calpain system. Physiol. Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 8.Raynaud F., Carnac G., Marcilhac A., Benyamin Y. m-Calpain implication in cell cycle during muscle precursor cell activation. Exp. Cell Res. 2004;298:48–57. doi: 10.1016/j.yexcr.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 9.Ozaki T., Tomita H., Tamai M., Ishiguro S.I. Characteristics of mitochondrial calpains. J. Biochem. 2007;142:365–376. doi: 10.1093/jb/mvm143. [DOI] [PubMed] [Google Scholar]
- 10.Ozaki T., Yamashita T., ichi Ishiguro S. Mitochondrial m-calpain plays a role in the release of truncated apoptosis-inducing factor from the mitochondria. Biochim. Biophys. Acta Mol. Cell Res. 2009;1793:1848–1859. doi: 10.1016/j.bbamcr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Ozaki T., Yamashita T., ichi Ishiguro S. ERp57-associated mitochondrial μ-calpain truncates apoptosis-inducing factor. Biochim. Biophys. Acta Mol. Cell Res. 2008;1783:1955–1963. doi: 10.1016/j.bbamcr.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Iwamoto T., Ishiyama E., Ishida K., Yamashita T., Tomita H., Ozaki T. Presence of calpain-5 in mitochondria. Biochem. Biophys. Res. Commun. 2018;504:454–459. doi: 10.1016/j.bbrc.2018.08.144. [DOI] [PubMed] [Google Scholar]
- 13.Chukai Y., Iwamoto T., Itoh K., Tomita H., Ozaki T. Characterization of Mitochondrial Calpain-5. Biochim. Biophys. Acta Mol. Cell Res. 2021;1868:118989. doi: 10.1016/j.bbamcr.2021.118989. [DOI] [PubMed] [Google Scholar]
- 14.Arrington D.D., Van Vleet T.R., Schnellmann R.G. Calpain 10: a mitochondrial calpain and its role in calcium-induced mitochondrial dysfunction. Am. J. Physiol. Cell Physiol. 2006;291:1159–1171. doi: 10.1152/ajpcell.00207.2006. [DOI] [PubMed] [Google Scholar]
- 15.Han X., Liu C., Zhang K., Guo M., Shen Z., Liu Y. Calpain and JNK pathways participate in isoflurane – induced nucleus translocation of apoptosis-inducing factor in the brain of neonatal rats. Toxicol. Lett. 2018;285:60–73. doi: 10.1016/j.toxlet.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Polster B.M., Basańez G., Etxebarria A., Hardwick J.M., Nicholls D.G. Calpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. J. Biol. Chem. 2005;280:6447–6454. doi: 10.1074/jbc.M413269200. [DOI] [PubMed] [Google Scholar]
- 17.Ozaki T., Nakazawa M., Yamashita T., Sorimachi H., Hata S. Intravitreal injection or topical eye-drop application of a μ-calpain C2L domain peptide protects against photoreceptor cell death in Royal College of Surgeons' rats, a model of retinitis pigmentosa. BBA - Mol. Basis Dis. 2012;1822:1783–1795. doi: 10.1016/j.bbadis.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Ozaki T., Yamashita T., Tomita H., Sugano E. The protection of rat retinal ganglion cells from ischemia/reperfusion injury by the inhibitory peptide of mitochondrial μ-calpain. Biochem. Biophys. Res. Commun. 2016;478:1700–1705. doi: 10.1016/j.bbrc.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Belov Kirdajova D., Kriska J., Tureckova J., Anderova M. Ischemia-triggered glutamate excitotoxicity from the perspective of glial cells. Front. Cell. Neurosci. 2020;14:51. doi: 10.3389/fncel.2020.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewerenz J., Ates G., Methner A., Conrad M., Maher P. Oxytosis/ferroptosis-(Re-) emerging roles for oxidative stress-dependent non-apoptotic cell death in diseases of the central nervous system. Front. Neurosci. 2018;12:214. doi: 10.3389/fnins.2018.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fricker M., Tolkovsky A.M., Borutaite V., Coleman M., Brown G.C. Neuronal cell death. Physiol. Rev. 2018;98:813–880. doi: 10.1152/physrev.00011.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maher P., van Leyen K., Dey P.N., Honrath B., Dolga A., Methner A. The role of Ca2+ in cell death caused by oxidative glutamate toxicity and ferroptosis. Cell Calcium. 2018;70:47–55. doi: 10.1016/j.ceca.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou L., Peng Q., Wang P., Zhou B. Progress in Research and application of HIV-1 TAT-derived cell-penetrating peptide. J. Membr. Biol. 2017;250:115–122. doi: 10.1007/s00232-016-9940-z. [DOI] [PubMed] [Google Scholar]
- 24.Tünnemann G., Ter-Avetisyan G., Martin R.M., Stöckl M., Herrmann A., Cardoso M.C. Live-cell analysis of cell penetration ability and toxicity of oligo-arginines. J. Pept. Sci. 2008;14:469–476. doi: 10.1002/psc.968. [DOI] [PubMed] [Google Scholar]
- 25.Kamei N. Nose-to-Brain delivery of peptide drugs enhanced by coadministration of cell-penetrating peptides: therapeutic potential for dementia. Pharm. Soc. Japan. 2017;137:1247–1253. doi: 10.1248/yakushi.17-00138. [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki T., Tokuda Y., Kotake A., Okada H., Takeda S., Kawano T., Nakayama Y. Cellular uptake and in vivo distribution of polyhistidine peptides. J. Contr. Release. 2015;210:115–124. doi: 10.1016/j.jconrel.2015.05.268. [DOI] [PubMed] [Google Scholar]
- 27.Kimura S., Kawano T., Iwasaki T. Short polyhistidine peptides penetrate effectively into Nicotiana tabacum-cultured cells and Saccharomyces cerevisiae cells. Biosci. Biotechnol. Biochem. 2017;81:112–118. doi: 10.1080/09168451.2016.1234925. [DOI] [PubMed] [Google Scholar]
- 28.Fukui M., Song J.H., Choi J., Choi H.J., Zhu B.T. Mechanism of glutamate-induced neurotoxicity in HT22 mouse hippocampal cells. Eur. J. Pharmacol. 2009;617:1–11. doi: 10.1016/j.ejphar.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 29.Sevrioukova I.F. Apoptosis-inducing factor: structure, function, and redox regulation. Antioxidants Redox Signal. 2011;14:2545–2579. doi: 10.1089/ars.2010.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng J.J., Song W.T., Yao F., Zhang X., Peng J., Luo X.J., Xia X.B. Involvement of regulated necrosis in blinding diseases: focus on necroptosis and ferroptosis. Exp. Eye Res. 2020;191:107922. doi: 10.1016/j.exer.2020.107922. [DOI] [PubMed] [Google Scholar]
- 31.Chévez-Gutiérrez L., Bammens L., Benilova I., Vandersteen A., Benurwar M., Borgers M., Lismont S., Zhou L., Van Cleynenbreugel S., Esselmann H., Wiltfang J., Serneels L., Karran E., Gijsen H., Schymkowitz J., Rousseau F., Broersen K., De Strooper B. The mechanism of γ-Secretase dysfunction in familial Alzheimer disease. EMBO J. 2012;31:2261–2274. doi: 10.1038/emboj.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haass C., Kaether C., Thinakaran G., Sisodia S. Trafficking and proteolytic processing of APP. Cold Spring Harb. Perspect. Med. 2012;2:a006270. doi: 10.1101/cshperspect.a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bateman R.J., Xiong C., Benzinger T.L.S., Fagan A.M., Goate A., Fox N.C., Marcus D.S., Cairns N.J., Xie X., Blazey T.M., Holtzman D.M., Santacruz A., Buckles V., Oliver A., Moulder K., Aisen P.S., Ghetti B., Klunk W.E., McDade E., Martins R.N., Masters C.L., Mayeux R., Ringman J.M., Rossor M.N., Schofield P.R., Sperling R.A., Salloway S., Morris J.C. Clinical and biomarker changes in dominantly inherited alzheimer's disease. N. Engl. J. Med. 2012;367:795–804. doi: 10.1056/nejmoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberson E.D., Scearce-Levie K., Palop J.J., Yan F., Cheng I.H., Wu T., Gerstein H., Yu G.-Q., Mucke L. Reducing endogenous tau ameliorates amyloid β–induced deficits in an alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 35.Chen H.H., Liu P., Auger P., Lee S.H., Adolfsson O., Rey-Bellet L., Lafrance-Vanasse J., Friedman B.A., Pihlgren M., Muhs A., Pfeifer A., Ernst J., Ayalon G., Wildsmith K.R., Beach T.G., van der Brug M.P. Calpain-mediated tau fragmentation is altered in Alzheimer’s disease progression. Sci. Rep. 2018;8:16725. doi: 10.1038/s41598-018-35130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashok A., Singh N., Chaudhary S., Bellamkonda V., Kritikos A.E., Wise A.S., Rana N., McDonald D., Ayyagari R. Retinal degeneration and alzheimer’s disease: an evolving link. Int. J. Mol. Sci. 2020;21:7290. doi: 10.3390/ijms21197290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamei N., Shingaki T., Kanayama Y., Tanaka M., Zochi R., Hasegawa K., Watanabe Y., Takeda-morishita M. Visualization and quantitative assessment of the brain distribution of insulin through nose-to-brain delivery based on the cell- penetrating peptide noncovalent strategy. Mol. Pharm. 2016;13:1004–1011. doi: 10.1021/acs.molpharmaceut.5b00854. [DOI] [PubMed] [Google Scholar]
- 38.Ohtsuki S., Terasaki T. Contribution of carrier-mediated transport systems to the blood–brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm. Res. (N. Y.) 2007;24:1745–1758. doi: 10.1007/s11095-007-9374-5. [DOI] [PubMed] [Google Scholar]
- 39.Tajes M., Ramos-fernández E., Weng-jiang X., Bosch-morató M., Guivernau B., Eraso-pichot A., Salvador B., Fernàndez X., Roquer J., Muñoz F.J., Tajes M., Ramos-fernández E., Weng-jiang X., Bosch M., Guivernau B., Eraso-pichot A., Salvador B., Fernàndez-busquets X., Guivernau B., Tajes M., Ramos-ferna E., Ferna X., Roquer J., Mun F.J., Eraso-pichot A. The blood-brain barrier: structure, function and therapeutic approaches to cross it. Mol. Membr. Biol. 2014;31:152–167. doi: 10.3109/09687688.2014.937468. [DOI] [PubMed] [Google Scholar]
- 40.Keaney J., Campbell M. The dynamic blood–brain barrier. FEBS J. 2015;282:4067–4079. doi: 10.1111/febs.13412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intracellular uptake of Tat-μCL and Hn-μCL in HT22 cells.
After HT22 cells were incubated with or without 50 μM Tat-μCL or Hn-μCL for 1 h, cells were fixed and stained with DAPI (blue), μCL antibody (green), and mitochondrial marker COX IV (red). Scale bars: 50 μm.