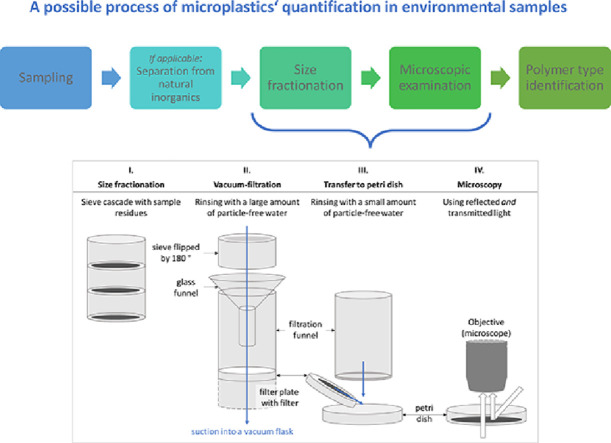
Abstract
In the field of microplastics’ quantification, efficient and reproducible methodology is still needed. Procedures of sample fractionation and transfer are often insufficiently reported, although fractionating a sample in similarly sized particles is a crucial prerequisite for the subsequent detection and identification process. At the same time, fractionation is error-prone as particles can be lost during transfer between different vessels. This article presents a four-step technique of sample preparation and microscopic examination, suited for different kind of environmental samples (e.g., water, sediment, soil): The sample is size-fractionated in a sieve cascade (I), rinsed from the sieve and vacuum-filtrated onto a filter (II), rinsed from the filter into a glass petri dish with a low amount of water (III), and examined under the microscope in wet or dry condition (IV). The technique manages on standard laboratory equipment and is reliable for fragments > 300 µm: In a validation experiment with polypropylene, the average recovery was 94 ± 13.5% (arithmetic mean ± standard deviation) and 100% (median), respectively.
-
•
Reliable sample transfer after wet-sieving.
-
•
Concentration of the pretreated sample in a very small amount of water.
-
•
Usage of transmitted light in microscopy.
Keywords: Sample preparation, Wet sieving, Vacuum filtration, Microscopic detection, Method validation
Graphical abstract
Specifications table
| Subject Area: | Environmental Science |
| More specific subject area: | Microplastics’ research |
| Method name: | Sample transfer from sieve to microscope |
| Name and reference of original method: | Not applicable |
| Resource availability: |
|
*Method details
Description of the method
In the process of microplastics’ quantification, the presented technique (Fig. 1) is suited for different environmental matrices, such as sediments, soils, and waters, containing larger, hardly decomposable natural organic matter, which complicates microscopic examination. In case of sediments or soils, the inorganic fraction must be removed before the first step.
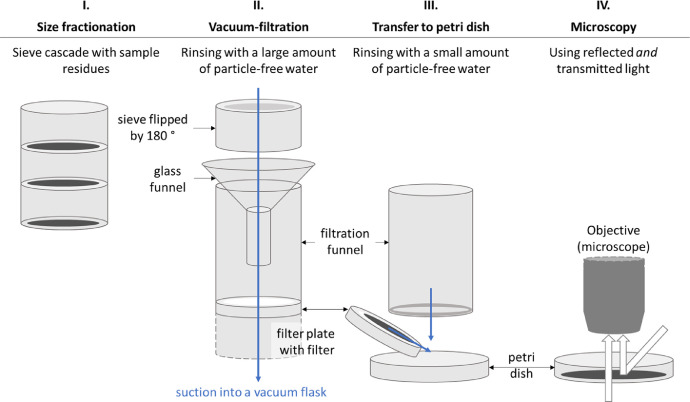
Fig. 1.
Sketch of the four-step method for sample transfer from sieve to microscope. Note that the vacuum flask of the filtration setup and the microscope are only rudimentarily shown. In the validation experiment presented here, the sieves had a diameter of 75 mm and mesh sizes of 1000 µm, 500 µm and 300 µm (decreasing from the top to the bottom).
Step I – Size fractionation: To allow for microscopy of similarly sized particles, the natural organic phase is fractionated in different size classes by wet-sieving in a sieve cascade (Fig. 2a). We used sieve mesh sizes of 300, 500, and 1000 µm, and a sieve diameter of 75 mm. As particles can be trapped in the sieves’ edges (Fig. 2b), it is highly advisable to use sieves with additional soldered seams. During wet-sieving it is important to rinse gently to avoid fragmentation of particles and spilling of water, and thoroughly to ensure proper separation of particles. It is advisable to internally standardize the extent of rinsing.
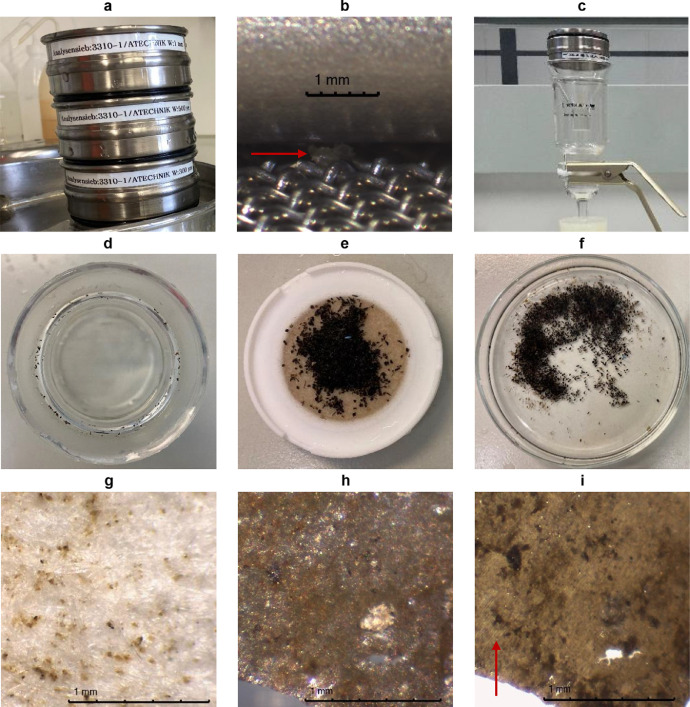
Fig. 2.
Cell phone photos (without scale) and microscope photos (with scale) illustrating different aspects of size fractionation, sieve residue transfer, and microscopy. (a) Sieve cascade (sieve diameter: 75 mm) with top-down decreasing mesh size. (b) A particle trapped in the edge of a 300 µm-sieve without additional soldered seam. (c) Filtration setup for 47 mm filters consisting of (top-down): flipped sieve, glass funnel, filtration funnel, filter plate, and vacuum flask (not shown). (d) Top view on the filtration funnel's undersurface where particles can stick to after a vacuum filtration. (e) Cellulosic filter (diameter: 47 mm) on its filter plate loaded with a fractionated post-density-separation riverbed sample (size fraction: 500–1000 µm). (f) Petri dish containing the sample from e (diameter of the petri dish: 90 mm). (g) Sample residues < 300 µm sticking to a cellulosic filter after the larger particles were rinsed off. (h) A suspicious particle from riverbed samples of local river Lahn illuminated by reflected light. (i) The same particle shown in h illuminated by reflected and transmitted light revealing cellular structures.
Step II – Vacuum filtration: Having finished the top sieve residue, the sieve is set on a glass funnel mounted above a vacuum filtration unit and thereby flipped by 180° (Fig. 2c). The funnel (upper diameter here 80 mm) serves as an adapter between sieve and filtration unit. The remaining sieve cascade must be protected from airborne contamination by a plastic-free cover. While rinsing the sieve residue into the funnel, special attention must be paid to the edge of the sieve where particles can be trapped if no soldered seams were added (compare with step I). Having rinsed all particles from the sieve into the filtration funnel, all inner walls contacted by the sample must be rinsed carefully as well. After this, steps I and II are repeated with the next sieve.
The advantage of transferring a sieve residue onto a filter is that we can use as much filtered water as necessary to remove all particles from the sieve mesh as the rinsing water is sucked off. Simultaneously, the sample is concentrated without water on a filter. If a sieve residue was rinsed into a beaker, the amount of water would be limited by the beaker's volume (further vessels are usually unwanted). Further, the sample would be suspended in a larger amount of water, making a microscopic examination in wet condition elaborate as only small volumes can be processed. Clearly, the presented technique works especially well if the natural organic matter content of a sample is not too high to overload and clog sieves and filters. In case of samples with very high organic matter content, a preceeding step of matrix reduction, for instance by sample homogenization and splitting, and/or several repeated runs of size fractionation and sample transfer are necessary.
Step III – Transfer to petri dish: Rinsing the sample off the filter requires only low water volumes allowing for a sample transfer into a low-volume vessel like a petri dish. During vacuum filtration, particles can move to the underside of the filtration funnel (Fig. 2d). To not loose these particles, they are rinsed with a few drops of water into the petri dish. The filter plate is taken from the vacuum flask (Fig. 2e), and the freshly concentrated filter residue is rinsed with low amounts of water into the petri dish (Fig. 2f). A petri dish diameter of about 90 mm is recommended; smaller dishes may be chosen for smaller matrices whereas larger dishes complicate systematic stereomicroscopy. The concentration technique (step I to III) proved to be reliable for particles > 300 µm (compare section Validation of the method) whereas significantly smaller components can stick to the cellulosic filter used here (example from an environmental sample in Fig. 2g). Cellulosic filters are easily available and low-cost but other filter types, such as glass fiber filters or stainless-steel filters, which are reusable, are probably suitable as well – the crucial point is that the sample can be rinsed off the filter with a low amount of water.
Step IV – Microscopy: Finally, the petri dish is covered and – if a dry sample is wished for microscopy – heated for a few days at no more than 65 °C to evaporate the water. The temperature is necessary to speed up the evaporation process. It is recommended to homogenize the particles after placing them in the oven, for instance by means of forceps, to avoid particle accumulation as in (Fig. 2f) and to achieve an even distribution of particles within the petri dish. Investigating the particles under the microscope in a glass petri dish is advantageous compared to a filter for several reasons: Warping of cellulosic filter papers requiring a frequent refocusing is prevented, compared to a stainless-steel background the contrast is higher, and particles can be observed with both reflected and transmitted light. During microscopy, it is advisable to determine an internal standard to scan the petri dish systematically, e.g., in circles inwards. Good practices of selecting suspicious microplastic particles have been summarized elsewhere [1], [2], [3]. After having sorted out all potential microplastic particles, the remaining natural organic matter can be transferred to a repository for storage to meet the requirements of good scientific practice. The operator can scan the sample at the same time again to find overlooked particles; if this post-processing is performed by a second operator, according to the four-eyes principle, the risk of overlooking microplastics can be reduced even further. It is highly recommended and meanwhile a standard in microplastics’ analysis to subject the selected putative microplastics to a chemical identification, e.g., by a spectroscopic technique, to both verify the particles’ synthetic nature and determine their polymer type [4].
Possible variations of the method
The following modifications of the presented technique were not tested here but are conceivable:
-
(1)
Modifications of the setup itself concern sieve diameter, mesh size, filter type, and petri dish diameter. Instead of a petri dish, a Bogorov counting chamber may be used to examine the wet sample under the microscope in a very systematic way. In this case, it is recommended to rinse the filter residue into a small beaker which facilitates pouring into the counting chamber.
-
(2)
Individual size fractions may be transferred from the filter into beakers for storage until further processing. In this case, it is advisable to constantly add filtered water to avoid sticking of particles to the inner wall which easily happens when water evaporates from a non-airtightly covered beaker. Alternatively, the samples may be frozen (consider to use cold-resistant glass and to fill the beaker only two-thirds).
-
(3)
Individual size fractions may be subjected to a chemical treatment before microscopy, either to stain the sample with a fluorescent dye like Nile Red, or to decompose the particles’ biofilm, for instance by means of Fenton's reagent, to enhance spectroscopic identification. Instead of adding the chemical to the petri dish, the operator could also submerge the sieve into a reaction vessel as suggested by Nakajima et al. [5]. This approach appears especially useful if several different chemicals and enzymes [6] are applied.
-
(4)
When stereomicroscopic detection is not required, size fractions smaller than 500 µm may be directly transferred from the sieve via a glass funnel onto an analytic filter, e.g., an aluminum oxide filter for µFTIR-Imaging.
Validation of the method
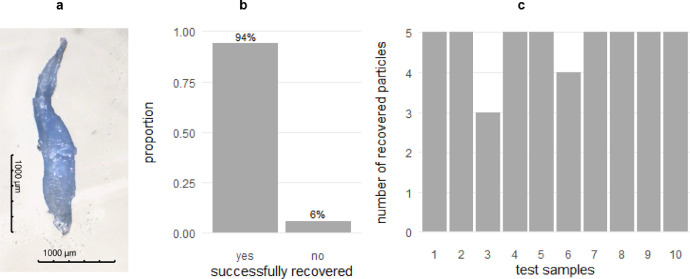
To characterize the reliability of the presented technique, a recovery experiment with ten test samples was performed. Each test sample contained 150 mg of dried natural organic matter obtained from previous investigations of the local river Lahn. The amount of natural organic matter approximately corresponded to the usual amount in our local riverbed sediment samples with a total sample mass of about 2 kg dry weight. Each test sample was weighed in a crystallizing dish and manually spiked with five test particles: blue polypropylene (PP) fragments obtained as waste from automotive industry (General Industries Deutschland GmbH). Before spiking, test particles had been picked from a 300 µm-sieve and photographed (Motic Images 3.0) to determine their exact size dimensions (example for a long, thin test particle in Fig. 3a).
Fig. 3.
Recovery experiment with test particles. (a) Example for an especially long and thin test particle. (b) Proportion of successfully recovered test particles (47 out of 50 test particles). (b) Absolute number of recovered test particles per test sample (number of test particles per sample: 5).
All ten samples were processed as described in section Description of the method. Contamination-free water was produced by passing tap water through a stainless-steel sieve (mesh size: 200 µm; Atechnik GmbH) mounted below the tap. After having transferred a sample into a petri dish, recovered test particles were hand sorted and counted immediately without microscope – they could be easily spotted due to their blue color. The relative recovery was calculated as the quotient of recovered and total particle number per sample (= five). Data analysis was performed in RStudio (version 1.1.463).
In total, 94% of the test particles were recovered (47 out of 50) (Fig. 3b). The average recovery was 94 ± 13.5% (arithmetic mean ± standard deviation) and the median was 100%: In eight of ten samples, a full recovery was obtained; in sample 3 and 6 two and one particle got lost, respectively (Fig. 3c). The loss of two particles in sample 3 might have occurred during wet-sieving where the particle hit the 300 µm sieve in a passing configuration although being larger than 300 µm in the largest dimension. The particle loss in sample 6 is attributed to accidental flooding during rinsing of a sieve.
Background information and reasoning
In microplastics’ quantification, the technique of sieving divides an environmental sample into subsamples with similar particle sizes each. This can serve as sample volume reduction, but is especially important for subsequent detection and identification of microplastics: Microscopy is more efficient when particles have similar sizes and do not overlap each other. FTIR spectroscopy, a common identification method, offers different measurement systems for different size classes (e.g., particles > 500 µm: ATR FTIR spectroscopy; particles < 500 µm: µFTIR Imaging). The following variations of sieving exist: A sample can be sieved
-
•
in wet or dry condition.
-
•
with a configuration of mesh sizes which corresponds to the geoscientific standard or not.
-
•
before and/or after removing the inorganic fraction.
Sieving of sediment or soil samples is commonly performed before inorganic matter removal [3]. However, sieving after removing the mineral fraction or sieving of water samples can be reasonable in large and/or organic-rich samples. Usually, for microscopic examination, samples are transferred to filters [3]. As filters are opaque, they prevent exploiting a microscope's full illumination: Transmitted light cannot be used although this is important to reveal cellular structures. For instance, Song et al. (2015) reported that in a validation experiment all sheets counted as microplastics under the microscope turned out to be of biological origin when identified with FTIR spectroscopy [7]. An example for a sheet-type particle which is suspicious using reflected light but obviously biogenic using transmitted light is shown in Fig. 2(h),(i). In light of high misinterpretation in microscopic detection [7], filters appear suboptimal as microscopic underlay. A glass underlay is more suitable, but a manual particle-by-particle transfer to a conventional specimen slide can target only a manageable number of large particles which is often not given in practice.
Against this background, we aimed to develop a technique to transfer a sieve residue into a glass petri dish. Such a procedure has not yet been described in the scientific literature; in contrast, the step of sieve residue transfer is often not or only vaguely described. The presented technique is based on our observation that a sample (particle sizes between 300 µm and 1000 µm) freshly concentrated on a cellulosic filter can easily be rinsed off the filter into a petri dish using only a small amount of water. After a few days of heating at 65 °C, the water is evaporated, and the dried sample can be examined microscopically in the petri dish. The detected particles can be manually sorted out and chemically identified, e.g., via ATR FTIR spectroscopy.
Rinsing a sample off a filter had already been described in the literature in the context of the chemical treatment of microplastics [5]. In Enders et al. [8], a similar technique is applied: A sample is fractionated in a vacuum filtration setup with stainless-steel filters (diameter: 47 mm); subsequently, the filter residue is rinsed from the steel filter into a petri dish. While this might be suitable for samples with a low organic matter content, our technique based on sieves with diameters of 75 mm is suitable for samples with a higher organic matter content. Moreover, using vacuum filtration setups for size fractionation has the disadvantage of rinsing the relatively large inner surface of a vacuum flask to not loose particles.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the German Federal Ministry for Education and Research (BMBF) for funding through the project 01BF1705. We acknowledge the support of the German Research Foundation (DFG) - reference number 391977956 - SFB 1357.
References
- 1.Norén F. KIMO; 2007. Small Plastic Particles in Coastal Swedish Waters. [Google Scholar]
- 2.Hidalgo-Ruz V., Gutow L., Thompson R.C., Thiel M. Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ. Sci. Technol. 2012;46:3060–3075. doi: 10.1021/es2031505. [DOI] [PubMed] [Google Scholar]
- 3.Yang L., Zhang Y., Kang S., Wang Z., Wu C. Microplastics in freshwater sediment: a review on methods, occurrence, and sources. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.141948. [DOI] [PubMed] [Google Scholar]
- 4.Löder M.G.J., Gerdts G. Marine Anthropogenic Litter. Springer; Berlin: 2015. Methodology used for the detection and identification of microplastics – a critical appraisal; pp. 201–227. [Google Scholar]
- 5.Nakajima R., Lindsay D.J., Tsuchiya M., Matsui R., Kitahashi T., Fujikura K., Fukushima T. A small, stainless-steel sieve optimized for laboratory beaker-based extraction of microplastics from environmental samples. MethodsX. 2019;6:1677–1682. doi: 10.1016/j.mex.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Löder M.G.J., Imhof H.K., Ladehoff M., Löschel L.A., Lorenz C., Mintenig S., Piehl S., Primpke S., Schrank I., Laforsch C. Enzymatic purification of microplastics in environmental samples. Environ. Sci. Technol. 2017;51:14283–14292. doi: 10.1021/acs.est.7b03055. [DOI] [PubMed] [Google Scholar]
- 7.Song Y.K., Hong S.H., Jang M., Han G.M., Rani M., Lee J., Shim W.J. A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Mar. Pollut. Bull. 2015;93:202–209. doi: 10.1016/j.marpolbul.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Enders K., Lenz R., do Sul J.A.I., Tagg A.S., Labrenz M. When every particle matters: a QuEChERS approach to extract microplastics from environmental samples. MethodsX. 2020;7 doi: 10.1016/j.mex.2020.100784. [DOI] [PMC free article] [PubMed] [Google Scholar]