Abstract
Among medical gases, including gases used therapeutically, this review discusses the comparative physiological activity of three gases – ozone (O3), xenon (Xe) and molecular hydrogen (H2), which together form representatives of three types of substances – typical oxidizing, inert, and typical reducing agents. Upon analysis of published and proprietary data, we concluded that these three medical gases can manipulate the neuroendocrine system, by modulating the production or release of hormones via the hypothalamic-pituitary-adrenal, hypothalamic-pituitary-thyroid, hypothalamic-pituitary-gonadal axes, or the gastrointestinal pathway. With repeated administration of the gases over time, these modulations become a predictable consequence of conditioned homeostatic reflexes, resulting in regulation of physiological activity. For example, the regular activation of the unconditioned defense reflex in response to repeated intoxication by ozone leads to the formation of an anticipatory stable conditioned response, which counteracts the toxic action of O3. The concept of a Pavlovian conditioned reflex (or hormoligosis) is a brief metaphor for the understanding the therapeutic effect of systemic ozone therapy.
Keywords: homeostasis, hormones, medical gases, molecular hydrogen, ozone, Pavlovian conditioned reflex, xenon
INTRODUCTION
The term “medical gases” (MGs) includes all gases used for therapy – oxygen (O2), nitric oxide, carbon monoxide, ozone (O3)-O2 mixture, noble gases (xenon (Xe), krypton, argon, helium), hydrogen sulfide, and molecular hydrogen (H2). This short review focuses on the use of the MGs,1,2,3,4 especially O3, H2, and Xe.
O3 is made up of three oxygen atoms. The Earth’s upper atmosphere contains an ozone layer, which protects the Earth from ultraviolet radiation from the Sun. However, O3 is a harmful air pollutant on the ground level. Inhalation of ozone leads to lung and throat irritation. High exposure can cause lung damage and can even be fatal. Nevertheless, O3 is also reported to have therapeutic effects in medical contexts. For example, it has been suggested that ozone therapy is useful in the treatment of arthritis, fighting viral diseases, such as human immunodeficiency virus, severe acute respiratory syndrome, and coronavirus disease 2019 (COVID-19). It also showed beneficial effects on disinfecting wounds, activating the immune system, treating ischemic heart disease, treating macular degeneration, or treating cancer. However, there are currently no human randomized controlled trials, and research remains to be done on its safety and effectiveness in humans.5
H2 was sensationally reported in 2007 as an antioxidant medical gas. In cellular studies it showed an inhibitory effect on hydroxy radicals (the most toxic reactive oxygen species (ROS)), and demonstrably attenuated infarct volume in the rat stroke model.4 Since then, hundreds of reports have been published on how H2 would be beneficial as an anti-oxidative, anti-inflammatory, and neuroprotective gas for any kind of ROS-related diseases or symptoms. This is concluded on the basis of hydrogen’s high accessibility to cells and tissues due to its small molecular size.
Xe is an inert, mono-atomic gas first identified in 1898 by the British chemists William Ramsay (1852–1916) and Morris Travers (1872–1961). Xe mainly acts as a radioapharmaceuticl diagnostic agent in clinical imaging, and as an inhaled anesthetic in general anesthesia. Other applications include organ protection, ophthalmology, and dermatology.6 Xe is also capable of providing neuroprotection. The lack of side effects, safe cardiovascular and organoprotective profile and effective neuroprotective role after hypoxic-ischemic injury make it desirable in clinical practice, despite its high cost.
Some MGs have already been used in medical practice (carbon dioxide, O2, O3, nitric oxide, and Xe). The rest, such as argon and H2, are the subjects of scientific research in the field of physiotherapy, balneology, biochemistry, and emergency medical care among many others.7,8 From a chemical perspective, MGs include oxidizing agents (O3 and nitric oxide), reducing agents (H2 and hydrogen sulfide) or inert substances (noble gases including Xe). At high dosages: O3 (more than 200 μg/kg), H2 (with rectal insufflation of 10 mL/kg), or Xe (when inhaling an O2-Xe mixture with a percentage of Xe of more than 30–50%) signs of oxidative-reductive stress and soporific intoxication are observed. Considering the same gases in low doses: O3 (parenteral administration at a dosage of 10–20 μg/kg), H2 (up to 8 ppm in drinking water or inhalation of 2–4% in air), or Xe-inhalation (5–15% in air), have a remarkable therapeutic effect.9 The pharmacological profiles of these three gases are partially listed in Table 1. Thus, at high dosages, O3, Xe, and H2 exhibit different physiological activities, directly or indirectly associated with their chemical nature. What is the reason for the paradoxical convergence of the pharmacological spectrum of these gases at low dosages? At present, there is no answer to this question. However, the practice of using O3 and Xe in medicine has been continued for more than a dozen years, even leading to the emergence of the terms “ozone therapy” – especially in recent treatments for the COVID-19,10,11,12 “xenon therapy,”9,12,13,14,15,16,17,18,19,20,21,22 and more recently, “hydrogen therapy.”23,24
Table 1.
Spectrum of physiological activity of xenon (Xe), ozone (O3) and molecular hydrogen (H2)
| Properties | Xe | O3 | H2 |
|---|---|---|---|
| Analgesic | √ | √ | √ |
| Antihypoxic | √ | √ | √ |
| Immunostimulator | √ | √ | √ |
| Anti-inflammatory | √ | √ | √ |
| Anabolic | √ | ? | ? |
| Neurohumoral | √ | √ | √ |
| Vasoplegic | √ | √ | √ |
| Antispasmodic | √ | √ | ? |
| Cardiotonic | √ | ? | √ |
| Neuroprotective | √ | √ | √ |
| Anti-stress | √ | √ | √ |
| Antioxidative | √ | √ | √ |
| Detoxication | √ | √ | √ |
| Adjuvant or a direct antitumor | √ | √ | √ |
Note: The symbol “?” means that this property of the corresponding gas has not yet been studied or has not yet been described in the scientific literature.
This situation is in sharp contradiction with the basic principles of pharmacology, which are based on the existence of causal relationships between the chemical structure and chemical properties of substances on one hand and their physiological activity, on the other. To overcome this contradiction, an assumption was made about the antioxidant properties of MGs as the basis of their therapeutic effect.3 However, can all the features of the physiological action of MGs, for example H2, be explained by antioxidant activity? Doubts about this arise because the following properties of MGs cannot be explained by only the antioxidant effects: 1) long-term remission of diseases after a course of treatment, 2) the unprecedented breadth of the spectrum of pharmacological activity, 3) normotropic nature of the pharmacological effects.9,20,23,25 The first feature indicates that MGs are capable of activating systems which are able to ensure the continuity of their effects. The second feature indicates that the operation of such a system does not depend on the pathogenesis of the disease. The third feature, confirming the conclusion about the systemic action of MGs, focuses our attention on the search for a mechanism capable of realizing this normotropic effect. The identification of such a system is obvious from the fact that the “disease-health” dichotomy essentially reduces to the “norm and deviation from the norm” dichotomy. Obviously, the homeostatic system plays a role, the main component of which is the neuroendocrine system.
A search of the PubMed database with the key words “xenon and hormones” showed only 67 papers published from 1986 to 2020 (0–7 articles per year). The maximum number of articles (7 items) was achieved in 2003. A few references describe Xe’s neurohumoral effects.26,27,28 Searching the key words “ozone therapy and hormones,” 130 publications were published on average (about 4 publications per year). Although about 650 scientific articles have been published to date on studying the physiological or therapeutic effects of H2 (see, for example, the constantly updated literature database29), only a few of them are devoted to highlighting the role of the endocrine system in the body’s response to hydrogen. Thus, we can state that although the data on the interaction of Xe, H2 and O3 with hormones are well known, the role of the hormonal system in implementing the therapeutic effect of MGs remains poorly understood. Therefore, the need to analyze the role of the neuroendocrine system in the mechanism of action of MGs is relevant. The most important role of the neuroendocrine system is its self-regulation of the underlying nonspecific adaptive system of the body. This is carried out by the hypothalamic-pituitary-adrenal (HPA)-, hypothalamic-pituitary-thyroid (HPT)-, and hypothalamic-pituitary-gonadal (HPG)-axis.30,31,32
THE ROLE OF THE NEUROENDOCRINE SYSTEM IN THE THERAPEUTIC EFFECT OF OZONE, XENON, AND MOLECULAR Hydrogen
Medical gases and HPA-axis
The HPA-axis is a highly adaptive neuroendocrine system, strongly implicated in stress resilience and vulnerability.33 As a representative example, inhalation of 0.8 ppm O3 for 4 hours in whole body chambers (1.61 mg/m3) activates the HPA-axis of male Fischer-344 rats and doubles the plasma level of glucocorticoid corticosterone, whose role in rodents is similar to that of cortisol in humans.34 Prior administration of the corticosteroid synthesis blocker metyrapone, can prevent the specific effect of O3. Glucocorticoids mediate the immunosuppressive and metabolic effects of O3 on the lungs, heart, liver, kidneys and spleen, since they are blocked by metyrapone and reproduced by administering corticosterone (10 mg/kg body weight). The effects of O3 on glucose tolerance and metabolic (triglyceride), endocrine/energy regulation (such as insulin, glucagon, galanin-like peptide 1, leptin, ghrelin, and corticosterone) and inflammatory/endothelial response, such as tumor necrosis factor, interleukin-6, vascular endothelial growth factor, and plasminogen activator inhibitor-1 were evaluated.35,36 O3 reduces the responses of glucagon, galanin-like peptide 1 and ghrelin to glucose, but does not have a significant effect on inflammatory/endothelial parameters. This emphasizes the primary effects of O3 on the endocrine system with respect to other manifestations of toxicity. In previous studies, it was shown that the administration of corticosterone reproduced the profile of the effects of O3.36
This was an implicit adaptation approach to explain the effect of O3.36 Based on the hypothesis that the body’s response to O3 is triggered by hormones of the adrenal medulla, the authors studied the effect of 1 ppm O3 inhalation on blood biochemical parameters of three groups of rats. One group underwent complete bilateral adrenalectomy (ADREX group); another underwent bilateral adrenal demodulation where the cerebral layer of the adrenal glands was destroyed (DEMED group). These were compared with animals on which the surgical intervention was simulated (SHAM group). The circulating adrenaline level in both DEMED and ADREX rats decreased to zero relative to SHAM. Corticosterone tended to be lower in DEMED rats and decreased to zero in ADREX rats. Adrenalectomy in rats exposed to air caused modest changes in metabolites and parameters of pulmonary toxicity. O3-induced hyperglycemia and glucose intolerance were markedly attenuated in DEMED rats and completely reversed in ADREX rats, whereas O3 increased circulating adrenaline and corticosterone in the SHAM group, but not in DEMED or ADREX rats. Free fatty acids and branched chain amino acids increased after exposure to O3 in SHAM, but not in DEMED or ADREX rats. Surgery or O3 did not affect the minute lung volume, but O3-induced shortness of breath was less pronounced in ADREX rats. It was concluded that O3-induced peripheral metabolic effects and lung damage/inflammation were mediated by adrenal hormones, rather than by the direct pro-inflammatory and oxidative effects of O3. The above data relate to conditions when the dosage of O3 is many times higher than the levels used in ozone therapy. In this regard, the question arises as to what extent it is possible to transfer the conclusion about the relationship between the toxic effect of O3 and the selective effect of O3 on the HPA-axis in overdose conditions to understand the mechanism of therapeutic doses. From the pathogenetic viewpoint, the toxic effect of a drug is a consequence of 1) side effects associated with the pharmacological properties of the drug, 2) toxic complications caused by relative and absolute overdose of the drug, 3) side effects caused by increased tissue sensitivity to the drug (idiosyncrasy, allergic reactions), 4) side effects of the drug caused by features of the functional state of the body.1 The first type of side effect is characteristic of a drug with the same type of receptor located in various organs and tissues of the body, or as the second type of side effect, on other types of receptors and specialized sites of receptive tissues of target organs (for example, the nonselective blocker of β-adrenergic receptors, propranolol). The third type of side effect is characteristic only of potential allergens (complete or incomplete antigens), as well as drugs capable of forming complexes with proteins.
Obviously, the first, third, and even the fourth type of toxic side effect are impossible for substances like O3, given its low molecular weight and its lack of specific receptors. It remains to be assumed that the above-described effect of O3 on the sympathoadrenal system is a characteristic feature of the systemic effect of O3, which manifests itself in the therapeutic effect at low doses and the toxic effect in an overdose. We obtained confirmation of this assumption in experiments on the dynamics of the level of cortisol in the blood plasma of patients receiving procedures of autohemotherapy major with O3.37 As shown in Figure 1, the level of cortisol, normalized to an individual normal level, began to vary sharply after the start of a course of ozone therapy. After completing the course of ozone therapy, the level of cortisol remains virtually unchanged for several weeks.
Figure 1.
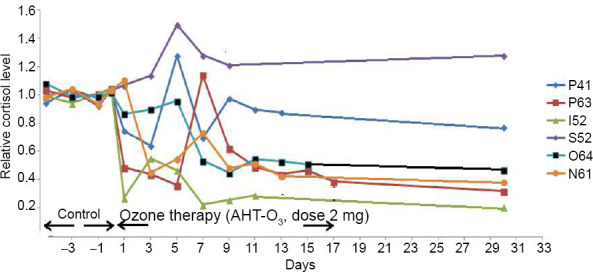
Dynamics normalized level of cortisol, in the blood of six patients (41–64 years old) in control, during AHT-O3 procedures (27 μg/kg O3, every other day) and within 1 month after completion of the course.
Note: AHT-O3: Autohemotherapy major with O3.
The dynamics of cortisol levels is strictly individual and seems unpredictable. However, as described above, this seemingly unpredictable behavior of the adrenal system has an internal logic aimed at achieving hormonal balance, namely of cortisol, aldosterone, and thyroid-stimulating hormone (TSH).38 The pioneer of the study of the body’s adaptive system, Hans Selye, noted the oscillatory mode of operation of the adrenal system described above.1 According to his “pendulum hypothesis,” antagonistic oscillations of mineralocorticoids and glucocorticoids are aimed at maintaining homeostasis and accompany the transition from eustress to distress. He believed that “an excess of mineralocorticoids predisposes the body to inflammation, and an excess of glucocorticoids increases the risk of infection.” Selye’s ideas were consistently developed in the theory of an organism’s nonspecific adaptive reactions.39 According to the main provisions of this theory, the oscillatory nature of the level of corticosteroids is reflected in hormonal changes in the body. Based on these hormonal changes, the cortisol dynamics that occur upon commencing treatment is considered to be a reflection of the adaptive response to intravenous administration of O3, and this process stops immediately after the cessation of therapy. This fact is critical, since interrupting the course until hormonal balance is restructured leaves the neuroendocrine system in an unbalanced state. An unbalanced neuroendocrine system will inevitably provoke an imbalance of the immune system, which is closely associated with the endocrine system.40 To avoid complications caused by premature termination of the ozone therapy course, certain tactics for working with primary patients were developed and conveyed to patients, which can be found on the O3 navigator (www.ozoneprotocols.org).
Xe also has a pronounced effect on the endocrine system in general and the adrenal system in particular.41 The study was conducted on a group of healthy volunteers aged 18 to 40 years. Blood was taken for analysis 60 minutes after the end of the short-term inhalation (several minutes) of the xenon-oxygen mixture (1:1). In the cited work, it was found that the level of cortisol is reduced by more than 30% after 60 minutes after Xe inhalation (Figure 2). The causes of the described hormonal changes are, a) the direct effect of Xe atoms on the molecular mechanisms of hormone synthesis in the pituitary gland, and the effect of statins and stimulators on the hypothalamus (for example, transport of pro-hormone molecules are complicated by Xe modification of the lipid membranes of the endoplasmic reticulum or ribosomes), b) the effect on the receptors of statins or releasing hormones of the hypothalamus in the pituitary gland, or adrenocorticotropic hormone receptors in the adrenal cortex. Besides, a blockade of nicotinic-acetylcholine receptors and/or a potentiation of inhibitory glycinergic system caused by Xe control the switching adaptation mechanisms.26 The influence of Xe and krypton on hormonal status is also observed in animals.42 In particular, it was shown that the long-term exposure of rats to the air-krypton or air-Xe atmosphere causes a noticeable increase in the concentration of cortisol in the rats’ blood.
Figure 2.
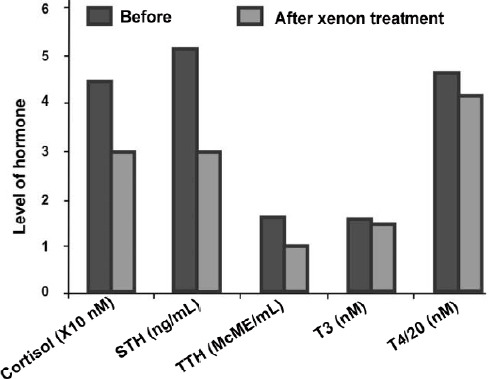
Effect of xenon inhalation on the hormonal system of healthy volunteers.
Note: Data are expressed as mean. McME/mL: Micro-international unit per milliliter; STH: serotonin; T3: triiodothyronine; T4: thyroxine; TSH: thyroid-stimulating hormone. The data are adapted from Naumov and Khlusov.41
As for the interaction between H2 and HPA-axis, H2 inhibits the HPA-axis and inflammatory responses to stress. Repeated inhalation of mixed H2-O2 gas [67%:33% (V/V)] significantly decreased both the acute and chronic stress-induced depressive and anxiety-like behaviors of mice, by blocking the stress-induced increase in the serum levels of corticosterone, adrenocorticotropic hormone, interleukin-6, and tumor necrosis factor-α.43 Furthermore, the inhalation of mixed H2-O2 gas in adolescence significantly increased the resilience to acute stress in early adulthood, illustrating the long-lasting effects of H2 on stress resilience in mice. Clinical studies regarding the use of hydrogen therapy against chronic stress will be warranted.
Medical gases and HPT-axis
The regulation of thyroid hormones is critically important for the processes of growth and differentiation, as well as for the energy regulation of all human tissues. It is not surprising that even slight deviations of thyroid hormones from the norm entail the development of depression,44 metabolic syndrome,45 dementia,46 atrial fibrillation47 and increased mortality from cardiovascular diseases,48 decreased bone density,49 and psoriasis.50 The correction of the HPT-axis with antithyroid agents or replacement therapy is effective for treating these diseases.51,52 The use of ozone therapy in the form of a course of 15 procedures for intravenous administration of 100 mL of ozonized autologous blood showed that O3 as a pharmacological agent has an excellent normotropic effect on the pituitary-thyroid system: initially reduced level (1.25 ± 0.15 pM) of triiodothyronine (T3) increased to normal (2.1 ± 0.17 pM; P < 0.01) and the content of TSH, thyroxine (T4), TSH and the relationships of T4 and T3, T3, and TSH, T4 and TSH are normalized.52 The return of the HPT to normal is accompanied by complete cleansing of the skin of psoriatic plaques within 30 days after the completion of the course of ozone therapy procedures. Ozone therapy in the form of intravenous infusion of ozonized saline (IVOS) is used for treating thyrotoxic goiter as a means of normalizing the activity of the thyroid gland.53 It is shown in the work that ozone therapy reduces the level of thyroid hormones by 22.8–44.5%, the thyroid index by 2.3 times, and increases the duration of drug remission by 2.6 times. It is possible that the therapeutic effect of O3 for treating the above diseases9,14 is also associated with the correction of the HPT-axis. Another medical gas, H2, is also effective in treating psoriasis.54 It is possible that the cause of this effect is also related to the effect on H2 on the HPT. Xe also has a thyrotropic effect.41 A sharp decrease in the level of TSH and a decrease in the average body temperature of patients undergoing xenon therapy noted in this work imply a restructuring of the adaptation system from a resistance strategy to a tolerance strategy.
Vice versa, the patients receiving Xe:O2 (70:30) showed an anabolic pattern and better preserved defense mechanisms compared with nitrous oxide + phentanyl anesthesia.28 The activities of thyroid hormones (TSH, T3, T4) fluctuated within the normal range.
Medical gases and HPG-axis
It is known that the endocrine system and its HPG-axis play a central role in the processes of conception, the progression of pregnancy and the correct course of childbirth. The entire endocrine system after the onset of pregnancy undergoes a dramatic change, which is reflected in the activation of the production of placental hormones estriol, estrone and estradiol. Pregnancy also affects the hypothalamic-pituitary complex, as evidenced by a 2–3-fold increase in the volume of the anterior pituitary gland.55 It is obvious that such a large-scale rearrangement of the endocrine system should be especially sensitive to agents modulating its activity. Moreover, we can say that the effectiveness of some pharmacological agents for treating diseases associated with pregnancy is the most important sign of its effect on the endocrine system. Treatment within pregnancy is especially evident in relation to O3, which is widely used in Russia as a means of correcting the hormone-producing function of the fetoplacental complex and the homeostasis system of various etiologies. As reported previously, data are presented on the treatment of 200 patients with a compensated form of chronic renal failure by intravenous infusion of O3 (with 200 mL of an ozonated saline with concentration of 400 μg/L for 5–7 days), alternating with the introduction of Actovegin at a dose of 160 mg also for 5–7 days.56 As a control, a group of 100 patients was used, for treating which they used traditional methods; administration of Rheopolyglucin solution 200–400 mL with trental 5 mL intravenously along with Actovegin of 160 mg each. The average level of free estriol significantly increased after ozone therapy by 59.9% – from 78.2 ± 2.5 to 92.3 ± 3.2 nM (P < 0.05). Evidence of the strong stimulating effect of O3 on estriol secretion was the average weekly increase in estriol in patients in the O3 group in the third trimester of pregnancy, which was 6.6 times higher (P < 0.05) compared to the control patient group (33.02 ± 5.2 nM). Conventional treatment was less effective – a lag behind normal levels of the hormone was observed in 70% of women. A study on the efficiency of ozone in the normalization of human placental lactogen in patients with compensated chronic renal failure showed that the achieved hormone concentration was adequate for the gestational age in 77% of patients receiving ozone therapy, and the increase in concentration was significant (from 173.8 ± 6.7 to 208.6 ± 8.1 nM (P < 0.05), i.e., 34.8 nM, or 20.2%). In patients treated by conventional methods, the average of weekly increase in the content of placental lactogen was 3.9 times less than after ozone therapy, and normalization was achieved only in 50% of women in the control group. Similar findings were made in another study57 based on the data from a study of ozone therapy in obstetrics. For example, a group of 135 women with a threat of miscarriage in the first trimester received ozone therapy. In the control group, a group of 89 women with the same diagnosis was studied. Ozone therapy was conducted in the form of an IVOS with an O3 concentration of 0.4 mg/L. Simultaneously, the incidence of sub-compensated forms of placental insufficiency decreased by 2.4 times and 5.5 times, respectively. The frequency of intrauterine growth retardation and the frequency of anemia in pregnant women decreased to 4.7 and 7.3 times, respectively. A comprehensive review of the use of ozone therapy in obstetrics can be found in Menéndez’s study.58 The phenomenal results of using ozone therapy in obstetrics described above indicate the undoubtable effect of O3 on the HPG axis.
The effects of noble gases on the HPG axis are also reported. It is interesting to note the selective action of Xe on the level of male and female sex hormones. The level of estradiol in the blood of healthy women increased 2.2 times, and the level of progesterone increased 51.9% (P < 0.05). Simultaneously, a decrease in the content of testosterone by 20.8%, prolactin by 17.8% and cortisol by 43.4% was recorded.1 Simultaneously, in studies addressing healthy young athletes, men did not show marked fluctuations of testosterone during inhalation of the Xe/O2 mixture.59 The effect of Xe on the prolactin level in the plasma of patients after Xe anesthesia was noted.28 The effect of Xe and krypton on hormonal status was also studied in animals.42 In particular, it was shown that the long-term residence of rats in the krypton-air or Xe-air atmosphere increases the concentration of progesterone, as well as a decrease in the concentration of total testosterone in the blood.
Regarding H2 on the HPG axis of the endocrine system, H2 in drinking water reversed the fall in brain estrogen level and estrogen receptor-β in female mice within a transgenic Alzheimer’s disease mouse model.60 Alternatively, there is a hypothetical assumption that H2 can influence testosterone production, based on experimental data suggesting testosterone’s dependence on the ROS level. This hypothesis agrees with the usual arguments about H2 as an anti-oxidant and does not yet have experimental evidence.61 There is no report on the use of H2 in obstetrics, except using animal models62,63,64 due to the particular conservatism of this field of medicine. However, O3 has been used in innovative ways, with its reputation as a dangerous oxidizing agent, and O3 therapy has been introduced in obstetrics. Therefore, the beneficial effects of H2 can be expected as well.
H2 and brain-stomach interaction
In addition to the abovementioned hormones, drinking water saturated with H2 significantly increases the concentration of ghrelin in blood plasma.65,66,67,68 Drinking water saturated with H2 for 4 days induces the release of ghrelin from the walls of the stomach in mice (Figure 3A, left). An intraperitoneal injection of a competitive inhibitor of the β1-adrenergic receptor, atenolol (10 mg/kg) blocks the increase in ghrelin levels caused by H2 (Figure 3A, right). Ghrelin is a peptide hormone with metabolic and endocrine functions, synthesized by cells in the gastrointestinal tract.69 Ghrelin activates the cells of the anterior pituitary gland and the arcuate nucleus of the hypothalamus, increasing the level of neuropeptide Y, which stimulates appetite. Ghrelin stimulates growth hormone secretagogue receptor-1a, as one of the growth hormones. Ghrelin is also involved in the regulation of reward, learning and memory processes, the sleep and wake cycle, taste sensations, and glucose metabolism. Growth hormone secretagogue receptor-1a is robustly expressed in dopaminergic substantia nigra and the striatum, and ghrelin increases tyrosine hydroxylase mRNA levels and dopamine concentration in the dorsal striatum. The administration of exogenous ghrelin prevents the loss of dopamine in the substantia nigra and striatum. Ghrelin-induced neuroprotection depends on the redox state of mitochondria through uncoupling of protein 2-dependent changes in mitochondrial respiration.70 However, H2-induced neuroprotection was not dependent on the uncoupling of protein 2,71 nor on the intracellular cyclic adenosine monophosphate.65 It was also suggested that the neuroprotective effect of H2-induced ghrelin was not mediated by vagus nerve stimulation.71 The solution to the questions on how the neuroprotective effect of ghrelin is induced requires additional studies (Figure 3B). In addition, the question whether or not H2 upregulates sex hormones such as testosterone and estrogen59,60 in gender-dependent manner remains unsolved. Nevertheless, the participation of the neuroendocrine system in the implementation of H2’s therapeutic effect can be considered firmly established.
Figure 3.
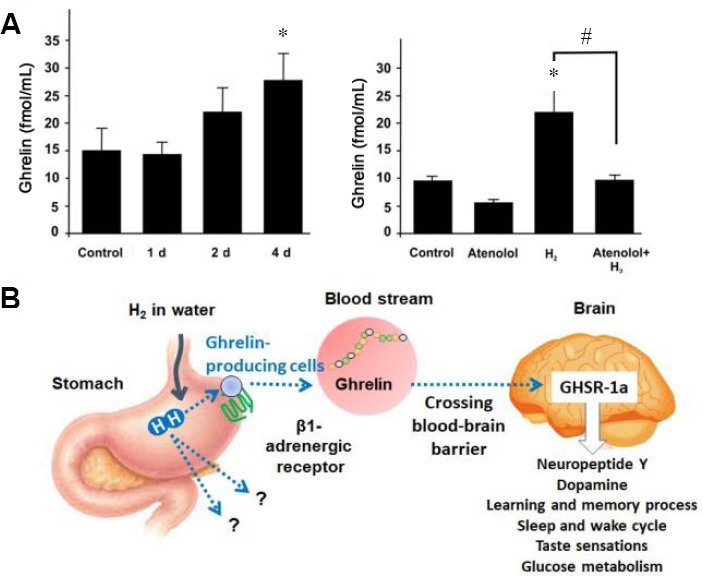
H2 induces the release of Ghrelin from the walls of the stomach, depending on the β1-adrenergic receptor.
Note: (A) Drinking water saturated with, H2 for 4 days, increases the release of ghrelin to plasma in mice. An intraperitoneal injection of a competitive inhibitor of the β1-adrenergic receptor, Atenolol (10 mg/kg) blocks the increase in ghrelin levels caused by hydrogen water. Data are expressed as mean ± SD. *P < 0.05, vs. control group; #P < 0.05, vs. H2 group. (B) A diagram of the release of ghrelin when drinking water saturated with H2. The data are adapted from Noda et al.65 GHSR-1a: Growth hormone secretagogue receptor-1a; H2: molecular hydrogen.
PAVLOVIAN CONDITIONALITY OF HOMEOSTATIC REACTION TO DRUGS
As mentioned above, the physiological activity of these gases in various fields of biology and medicine is repeatedly encountered through hormonal system reactions. Often it is considered that these reactions occur due to the influence of MGs on lipid peroxidation in the case of O32,9 and H272,73 or on modifying the lipid matrix of membranes in the case of Xe.20
The effect of MGs on the body has a corrective effect on the neuro-immuno-endocrine system,25,41 which determines the functioning of the body’s homeostatic system.74 The adjustment of homeostasis using MGs explains their pharmacological breadth, but not the continuity of their therapeutic effect over time. What is the mechanism for maintaining a new state of homeostasis after the end of the course of MG therapy? The answer to this question is sought in the epigenetic potential of the conditioned reflex (CR) to drugs.
About a century ago, students of I.P. Pavlov showed that the reflex of maintaining individual biological constants of an organism within qualitatively-quantitative boundaries specified by evolution can be used as unconditioned stimuli for the formation of Pavlovian CR (Pavlovian-CR). In other words, the reflex of maintaining homeostasis belongs to the same class of vital reflexes as food, temperature, orienting or sexual behaviour. For example, the experiments described the development of a Pavlovian-CR on the administration of adrenaline to dogs.75 Adrenaline was administered to animals in a 1:1000 dilution in Ringer’s solution of 5 mL each day. The result was an increase in blood pressure and heart rate, followed by a “vagus effect” – a decrease in pressure and heart rate. After multiple combinations of these factors, the introduction of Ringer’s solution alone had a “vagus effect” as a preventive measure against the expected increase in blood pressure.
A second example of an adaptive homeostatic reaction was also described.76 The essence of the experiment consisted of the development of Pavlovian-CR regulation of the animal’s water-salt balance. For this purpose, a series of experiments was conducted on dogs with excreted ureters and established fistulas of the stomach in an amount of 70 mL/kg of water. The infusion of a large volume of water decreases the osmotic pressure of the blood, leading to a reflex inhibition of the production of antidiuretic hormone (vasopressin) by the hypothalamus. A series of such experiments, where the introduction of water into the stomach is a conditioned irritant, and the release of vasopressin is an unconditional reaction, leads to the formation of a Pavlovian-CR of increased urine excretion. Subsequent imitation of the introduction of water into the stomach causes a Pavlovian-CR increasing in urine excretion. It would be concluded that the defense reaction against hyperhydration occurs before it arises. It was shown that if a conditioned signal (bell) was turned on half an hour after injection of an unconditioned insulin stimulus (20 units) during a period of severe hypoglycemia, then after 10 combinations, ringing without an insulin injection increased the glucose level.77 Thus, it was concluded that the conditioned signal “connects the processes of excitation in the cerebral cortex, which determines the formation of compensatory mechanisms, leading to the equalization of blood sugar levels.”
The homeostatic Pavlovian-CR is also observed at the level of the immune system. Homeostasis in the immune system was shown in a study to develop a Pavlovian-CR, in which scratching or heating the skin of the animal was used as a neutral conditioned stimulus, and the well-known reaction of increasing the level of polymorphonuclear leukocytes in the abdominal exudate after intraperitoneal administration of neutral material was used as a Pavlovian unconditioned reflex. After a certain number of combinations of these stimuli, scratching or heating the skin of the animal caused a Pavlovian-CR reaction of leukocytes in the abdominal exudate.78 French scientists also published similar results in the 1950s.79 Many contemporary works that explicitly or implicitly confirm this possibility have been done. For example, it was shown that the Pavlovian-CR reaction of the body to imitate insulin administration consists of a paradoxical hyperglycemic response versus the normal hypoglycemic response that was observed during the formation of the Pavlovian-CR.79,80 Conversely, if intragastric injection of glucose repeatedly induces hyperglycemia, the imitation of glucose administration leads to a hypoglycemic response.81,82 The Pavlovian-CR developed to simulate the administration of chlorpromazine manifests itself as an increase in the spontaneous locomotor activity of laboratory rats, while this tranquilizer reduced it when the reflex was developed.83 These and many other studies of Pavlovian-CR reactions of animals to medications were continued in clinical practice. The formation of a Pavlovian-CR to the pharmacotherapeutic effect is so obvious that it can be successfully used to reduce the dosage of drugs or the frequency of drug use and to replace the drug with a placebo.84,85,86,87
The above examples indicate the most important feature of the Pavlovian-CR-adaptive reaction, manifesting an anticipatory response to a conditioned stimulus, for example, a hyperglycemic reaction as a preparation for the possible administration of insulin, or a hypoglycemic reaction as a preparation for the possible administration of glucose. Thus, any adaptation process in the body includes elements of the formation of a Pavlovian-CR, and any factor causing an adaptive reaction is a conditional stimulus for this reflex. Registration of an adaptive reaction to such a stimulus in a Pavlovian-CR can be successful or not. It depends on the frequency, constancy and duration of its presentation.
How can these observations be used for medical purposes? To begin with, taking into account that the homeostatic systems of laboratory animals are normal before the experiments, and the described experiments consist of the intentional violation of sympathoadrenal,75 glucose81 and osmotic homeostasis.76 In the case of working with patients having certain diseases, there is a situation of deviation in the work of various homeostatic systems in patients. Therefore, the therapeutic effect in this situation should consist of the activation of the homeostatic system. It will be proposed that there are two modes of activation of the homeostasis system within the framework of the Pavlovian paradigm: 1) The activation mode arising in conditions if the influence of the higher parts of the nervous system limit the action of the activating factor on the hypothalamic-pituitary complex, 2) The activation mode arising in conditions of t a neurosomatic origin of the activating factor, causing a complete response of the neuroendocrine system.
The first type of activation begins in the hypothalamic-pituitary complex, because of the influence of cortical processes upon expectation of improvement. The second type of activation occurs in the structures of the central and peripheral endocrine glands, because of direct exposure to a physiologically active substance. The first mode (placebo mode) is transient and ends shortly after the completion of the exposure (Figure 4, left). The reason that the placebo effects are not of a long-term nature is because the expression of the genes responsible for the formation of a stable Pavlovian-CR is insufficient to consolidate the long-term memory of the event (Figure 4, lower left). In contrast, the simultaneous inclusion of the central and peripheral glands of the endocrine system in response to a material rather than imaginary adaptogenic factor (Figure 4, top right) creates metabolic prerequisites for the expression of target genes of Fos/Jun transcriptional proteins at a level sufficient to consolidate long-term memory.88,89,90 Schematically, such an option is shown in (Figure 4, bottom right). Pavlovian conditioning of the first type, in an explicit form, appears with any drug exposure.86 However, this effect is transient and too dependent on the patient’s expectation of cure, the individual treating physician, and other uncontrollable factors. It is obvious that the second type of inclusion that can ensure the continuity of the therapeutic action in the timeframe of Pavlovian conditioning and the implementation of the therapeutic activity of the substances is more attractive. This type occurs in systemic ozone therapy.
Figure 4.
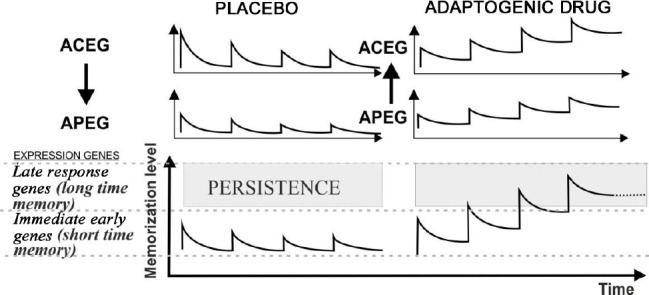
A diagram explaining the differences in the manifestation of Pavlovian conditioning during a course of procedures using a neutral substance against the expectations of the patient (placebo effect, on the left) and the substance that affects the patient’s homeostasis (adaptogen substance, on the right).
Note: ACEG: Hormonal activity of the central endocrine glands (hypothalamus, pituitary, pineal gland); APEG: hormonal activity of the peripheral glands (thyroid, parathyroid, pancreas, adrenal glands, genital. The arrows indicate the direction of the causal relationship of the activity of the endocrine glands. ACEG > APEG from central to peripheral: the arrow pointing down, and APEG > ACEG from peripheral to the central: the arrow pointing up.
THE ROLE OF PAVLOVIAN CONDITIONALITY IN THE PHYSIOLOGICAL ACTIVITY OF MEDICAL GASES
The role of Pavlovian-CR reflex in O3 therapy
As indicated above, the most important features of the conditioned homeostatic reflex are anticipatory and antagonistic in relation to the conditioned stimulus. Thus, the formation of a Pavlovian-CR to ozone therapy procedures should be accompanied by the development of reactions inverse to the physiological effect of O3. For example, it was shown that blood treatment with O3 increases the viscosity of whole blood and plasma viscosity, the aggregation of erythrocytes, increased hematocrit, erythrocyte sedimentation rate, osmotic resistance, and reduced erythrocyte deformability.91 Inflammation and vasoconstriction are the toxic effects of O3.92 The therapeutic effects of ozone therapy are diametrically opposed to the listed toxic effects of O3,93 which is in full accordance with the provision on the anticipatory and antagonistic response of the body to a conditioned stimulus, being the characteristic of O3 molecules in the blood. The approach explaining the mechanism of long-term improvement of the homeostatic system leaves an unresolved question about what ensures the stability of the conditioned homeostatic reflex after completing the course of ozone therapy procedures. The classic version of maintaining the developed Pavlovian-CR requires periodic reinforcement, otherwise, the developed Pavlovian-CR quickly fades. How does the course of ozone therapy support the increased activity of the homeostatic system for a long time, and why does this activity sharply decrease after a few months? The course of ozone therapy creates a second-order reflex by temporarily changing the mode of operation of the mechanism of homeostasis. The sequence of reflex formation is as following: 1) A course of ozone therapy procedures, which is a sequence of dosed administration of the O3 toxicant, which causes a systemic reaction of antagonistic (detoxification) orientation. 2) The formation of a new norm of qualitative-quantitative parameters of homeostasis at the peak of development of the aforementioned systemic reaction. 3) The formation of an arc of a first-order Pavlovian-CR between the dominant center of the unconditional homeostatic reaction and the center of excitation of the indifferent stimulus formed by the receptor fields - the visual, auditory, pain, kinesthetic and other sensations that arise during the procedures. 4) The occurrence of a second-order reflex caused by an unconditional homeostatic reaction associated with the new norm of homeostasis parameters. 5) A self-sustaining Pavlovian-CR of the second order, and hence the resulting improvement in the quality of life of the patient, continues as long as the reflex arc remains relevant. After its violation, the homeostatic reactions of the first and second order die out and the patient returns to the original age norms of homeostatic reactions.
As it was indicated earlier25 the normalization of hormonal homeostasis, meaning that the completion of the formation of a homeostatic reflex is perceived as rejuvenation, and its fading and return to the age norm is perceived as aging. If rejuvenation (the establishment and maintenance of a new norm of homeostasis) occurred against the background of symptoms of a disease, then adaptive rejuvenation is equivalent to remission of the disease, since the new mode of operation of the neuro-endocrine-immune system of homeostasis has a universal therapeutic effect on the body, regardless of the pathogenesis of the disease. Figuratively speaking, a 60-year-old patient suffering from hypertension and experiencing 15-year adaptive rejuvenation with the successful formation of this reflex experiences this suffering in an intensity corresponding to 45 years of age. Evenly, the attenuation of the homeostatic reflex and the return of the patient’s adaptive age to the biological one are perceived by him as a relapse of the disease. The experience shows that timely repetition of the course of procedures prevents the increase in adaptive age, and therefore prolongs the remission of diseases, regardless of their nature. It means that as unique way of healing using systemic ozone therapy is based on the formation and maintenance of the second-order homeostatic reflex described above.
The role of Pavlovian-CR Xe and H2 therapy
As indicated above, the therapeutic effects of O3 are an anticipatory compensatory reaction to anticipate the toxic effects of this oxidizing agent. Are there signs of such an anticipatory reaction to the action of Xe and H2? The physiological effects of Xe, which are used to achieve anesthesia, are inhibition of the central nervous system leading to loss of consciousness, relaxation of skeletal muscles, and the reduction or shutdown of certain reflexes. As well knowing, poisoning is a violation of the vital functions of the body resulting from the ingestion of a toxicant. In this sense, Xe can be considered a toxicant, with a focus on the nervous system.3 Accordingly, for Xe, we can expect the development of the same five stages in the formation of a conditioned therapeutic reflex as for O3 (see the previous paragraph). However, unlike O3, the homeostatic reaction of the body aimed at overcoming the described effects of Xe should consist of the excitation of the central nervous system, increased muscle tone, and activation of congenital reflexes,41,94,95 which can be considered a sign of an anticipatory compensatory reaction to Xe. However, unlike O3, Xe is used in relatively high concentrations, and its therapeutic effect is mixed from the named reflex reaction and the typical pharmacological action of Xe (analgesic, sedative, anti-inflammatory, neuroprotective, etc.). As for the role of unconditional reflex in hydrogen therapy, practically little is known so far, apart from the fact that the delayed anti-inflammatory effect of H2 can be associated with the early local pro-inflammatory effect.96
FROM HORMESIS TO PAVLOVIAN CONDITIONAL HORMOLIGOSIS AS METAPHOR OF THERAPEUTIC ACTIVITY OF MEDICAL GASES
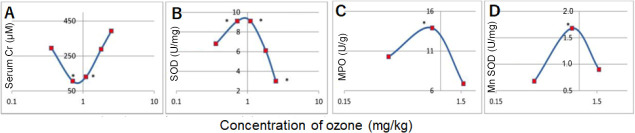
One of the most unusual qualities of O3 as a pharmacological agent is an extremely wide range of active concentrations. For example, the O3 dosage for IVOS procedures ranges from 1 to 10 μg/kg, and for autohemotherapy with O3 in the range of 25–125 μg/kg. The effectiveness of these procedures is approximately the same, which is in sharp contradiction with the typical monotonic S-shaped dose-response curve characteristic of conventional medicines. To illustrate, the dependence curves (Figure 5) of serum creatinine and superoxide dismutase, myeloperoxidase, and manganese-dependent superoxide dismutase in homogenates of internal organs of laboratory animals were presented.97 It was explained that this character of dependence occurs by the hormesis effect (ancient Greek hormáein “to set in motion, impel, urge on”), advanced by S. Sontman and D. Erlich in 1943.98 In accordance with this concept, stress factors in small doses have a beneficial effect by activating the body’s defense systems (for example, radiation in small doses activates membrane receptors, causes proliferation of splenocytes, and stimulates the immune system), but with increasing dosages, the stimulating effect is replaced by oppression and damaging effects (for example radiation damage). According to Re et al.’s study,97 the fact that O3 acts as a pharmaco-modulator causing “an adaptive response following its oxidative reactions” explains various dosages of O3 in general, and the two-phase nature of the dose-dependence. The two-phase dose-dependence corresponds to the concept we are developing, but does not disclose the adaptive reaction mechanism, which is responsible, for example, for the stimulating effect of O3 shown previously. As can be seen from the legend to Figure 5, the extreme of the dose-response curves refers to the interval 0.7–1.1 mg/L, regardless of the type of animals – rats or rabbits, and regardless of the type of organ – blood, lungs, liver or kidneys (not a local tissue-specific nature). However, in contrast, a certain integral system of the body determines the dose-dependence, being common to the animals of the rodent order. Such a system would be a nonspecific adaptation system of the body. Within the framework of the proposed concept, the homeostatic reflex in the ascending phase (Figure 5B–D) or the descending phase (Figure 5A) determines the biphasic curves, the activity of which increases as the dosage increases. The next section after the extremum reflects the inability of this reflex to cope with the toxic effect of the increasing dose of the oxidant. Such interpretation of the above graphs not only satisfactorily explains the huge width of the “therapeutic window” of O3 but also justifies the preference for using lower concentrations of O3, all other factors being equal. In fact, lower doses of O3 (for example, in the form of IVOS) are preferable, because the toxic effect of the oxidizing agent does not burden a homeostatic reflex.
Figure 5.
Hormetic shape of serum creatinine (Cr, A), kidney superoxide dismutase (SOD, B), lung myeloperoxidase (MPO, C), and- liver manganese superoxide dismutase (MnSOD, D) in rats and rabbits.
Note: (A, B) The experiments were performed on rats, dose 0.36, 0.72, 1.1, 1.8 and 2.5 mg/kg. (C, D) The experiments were performed on rabbits, dose 0.36, 0.85 and 1.57 mg/kg. The abscissa of the graphs is the ozone dose in logarithmic coordinates. Graphic materials are adapted from Re et al.97
A good characteristic of the physiological activity of O3 and other MGs is the term Hormoligosis. Luckey99 proposed this term in 1968 to refer to the phenomenon in which subharmful quantities of many stress agents may be helpful when presented to organisms in suboptimal environments. The term hormoligosis is compound words, the first part of which comes from the Greek: homo, hormao = excite, hormone, and the second from oligo = small quantities. This term was chosen because it contains two principles that are fundamentally important for understanding the mechanism of action of MGs – their hormone-modulating effect, and the low concentrations at which these substances exert their effects. Such an interpretation is fully consistent with the meaning that the author put in the term in relation to pharmacology.100 What is the proposed mechanism for implementing hormoligosis? In accordance with the concept of Pavlovian conditionality and considering the well-proven connection between the process of formation of the Pavlovian-CR and the endocrine system,101 a specific mechanism for the implementation of the leading reflex reaction to MGs is a change in hormonal balance. In fact, the endocrine system has a complete set of hormonal tools at its disposal that can mimic any known pharmacological effects of the MGs under consideration in Figure 6. For example, to develop an anticipatory homeostatic response to O3 administration for stopping disorders of blood rheology, the adaptive system can activate the synthesis of kidney erythropoietin, which is a major “viscoregulatory” factor, akin to norepinephrine in the adrenal gland and natriuretic peptide in the heart.102 Accordingly, adrenal cortisol and aldosterone or vasopressin, respectively, can be used to stop the pro-inflammatory and vasoconstrictive effects of O3.
Figure 6.

Scheme of the implementation of hormoligosis action of medical gases.
Note: The systemic therapeutic effects of medical gases and the effects of hormones that are activated by the action of medical gases are shown. H2: Molecular hydrogen; NRF2: nuclear factor E2-related factor 2; O3: ozone; TNF: tumor necrosis factor: Xe: xenon.
A generalized scheme for the development of a homeostatic reflex to the MGs under consideration is shown in Figure 6. It is assumed that MGs act first on the endocrine organs (namely the HPA axis) leading to an advanced protective reaction; this reaction occurs partially (for H2 and Xe) and completely (for O3). For O3, the diagram shows a lipid mediator, which is formed during systemic ozone therapy procedures. The presence of O3 in the blood serves as a signal to the homeostatic system for developing a protective reaction. As stable substances, H2 and Xe can reach the corresponding endocrine organs unchanged. In the diagram, a question mark near the arrow pointing to the names of the main endocrine glands question whether it is only the walls of the stomach that release the hormone ghrelin. In addition, it is obvious that the hormoligosis action of H2 is not limited to an increase in the level of ghrelin, but also testosterone and estrogen.59,60
The idea that ghrelin is not the only hormone induced by H2 also follows from the fact that mice, that are genetically incapable of producing ghrelin still exhibit H2-induced neuroprotection in a Parkinson’s disease model.71 If H2 can cause the expression of the gene responsible for the synthesis of one of the hormones – ghrelin, then it is possible that over time, other hormones ensuing from the action of H2 will be found. Figure 7, showing the scheme for the synthesis of eicosanoids from arachidonic acid, also shows an incomplete list of substances (hydroperoxides, malonicdialdehyde) which are traditionally considered important indicators of the body’s response to ozone therapy. In the diagram, they are indicated as side effects of the adaptation system. In the concept of hormoligosis, these substances must include byproducts of eicosanoids (prostaglandins, thromboxane, prostacyclin, etc.).1 Other substances are malonicdialdehyde and hydroperoxides, or other signaling factors that are involved in the expression of the corresponding genes and the consolidation of long-term memory of the effects of O3 as Nrf2. It is clear that the adaptation reaction requires both eicosanoids and signaling factors. However, their role is considered secondary, compared with the role of the endocrine system, which produces the primary reaction to MGs in the form of a homeostatic reflex.
Figure 7.
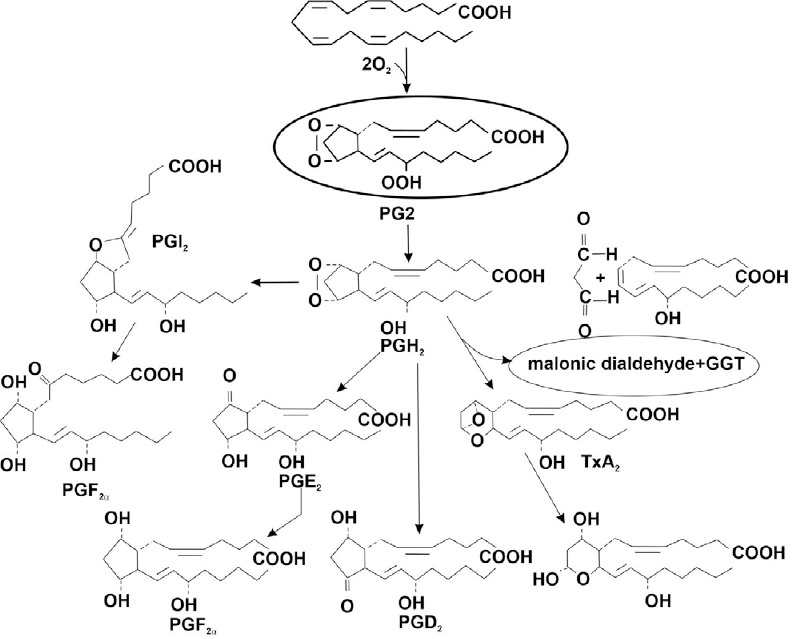
Scheme for the synthesis of eicosanoids from arachidonic acid.
Note: GGT: γ-Glutamyl transferase; PGD2: prostaglandin D2; PGE2: prostaglandin E2; PGF2α: prostaglandin F2α; TxA2: thromboxane A2.
PERSPECTIVES FOR THE FUTURE AND CONCLUSION
The studies on MGs have a close pharmacological profile, although their chemical activities have nothing in common. Based on the accumulation of data, in this article we tried approaching the problem of studying the mechanism of the action of O3, Xe, and H2. We took this approach from the viewpoint of searching for an integrating system of the body for which differences in the chemical properties of these gases are not fundamentally important. Such a system is the neuro-endocrine-immune system of homeostasis. In fact, the concept of homeostasis in the body includes the homeostasis of gases dissolved in biological fluids of the body. The obvious fact is that all biochemical reactions occur in an environment saturated with O2, nitrogen, and carbon dioxide, which remains outside the attention of researchers. However, lately an increasing amount of data have been accumulating that dissolved gases in the form of micro- and nanobubbles actively participate in numerous biochemical reactions.103 Based on these data, the idea that cells, subcellular particles, and individual molecular ensembles located far from the phase boundary are immersed in a homogeneous and monophasic medium consisting of a mixture of water molecules and low molecular weight compounds, including dissolved gases, is incorrect. Instead, the actual picture of the biochemical reaction conditions partially corresponds to the conditions of heterophase reactions at the water-gas interface. Degassing of blood plasma decreases glucose concentration, leads to an abnormal activation of blood coagulation, increases the rate of aggregation of blood cells, decreases the effectiveness of aspirin as an inhibitor of platelet aggregation, and slows down the action of indirect anticoagulants. Therefore, degassing of blood plasma radically changes its physiological and chemical properties, which leads to a dramatic acceleration of spontaneous and induced platelet aggregation. The effect of degassing is largely reversible by mixing with an equivalent amount of the original plasma.25,104,105 Therefore, we have the right to assume that the rate of biochemical reactions, and hence the homeostasis of many vital parameters is disturbed during the treatment of blood with an O3-O2 mixture, drinking water saturated with H2, and inhaling gas mixtures containing Xe or H2. The adaptation system responds to these effects by activating the innate unconditioned homeostatic reflex, based on the reaction of the neuroendocrine system and aimed at eliminating the consequences of any disturbed homeostasis of dissolved gases. The repeated challenge of this reflex during therapy leads to the consolidation of the Pavlovian conditioning mechanism and, due to its anticipatory nature, creates a long normotropic effect, which is equivalent to the long-term remission of various diseases. Such is the mechanism of action of the gases under consideration, relatively independent of the chemical properties of MGs and the broadest in therapeutic spectrum.
Based on the literature and data presented in this review, we propose to consider Pavlovian conditionalism. Hence, a brief metaphor for the mechanism of physiological activity of MGs and the adaptation approach (devised in Naumov and Khlusov41 for Xe and in Nazarov et al.38 for O3) is a promising direction for research into the mechanism of action of MGs and their clinical use.
The proposed concept sheds light on homeopathic treatment, which also mainly consists of the course of prescribing poisons in safe dosages. The selection of poisons is conducted in accordance with the expression “similia similibus curantur” (like that can be cured by like), which served as an epigraph to S. Hahnemann’s monograph, “The Organon of the Medical Art.” It will be useful to conduct experiments to verify the Pavlovian-CR concept described above. It develops a tactic for treating cardiac arrhythmias in laboratory animals. To induce a homeostatic conditional reaction, an appropriate toxicant that causes rat arrhythmia shall be chosen. A well-known arrhythmic of plant origin – aconitine is chosen. A subtoxic dose will be chosen and applied daily for a week. The rat adaptation system comprises trial-and-error alteration of hormonal balance to lower the excitability of the heart tissue. After fixing this Pavlovian-CR, rats should become less sensitive to any arrhythmogenic factors. Of course, this phenomenon is only an assumption, but a real practice confirms it – Aconitine is used in the homeopathic treatment of cardiac arrhythmias.106 To conclude, three typical MGs, O3, Xe, and H2, show beneficial effects in various diseases and symptoms in complex manners. Though these MGs exhibit different physiological activities, a common mechanism of these MGs are manipulating the neuroendocrine system, modulating the production or release of hormones, such as corticosteroids, testosterone, estrogen, and ghrelin. These modulations were caused by the conditioned homeostatic reflex, as a part of its general regulation of physiological activity. From the example of O3’s reactions in the body, the concept of Pavlovian-CR or hormoligosis is proposed. As for the mechanism at the molecular level, it remains an open question. In the case of H2-induced upregulation of the hormones, the modulation of gene expression is involved, though how H2 (as the smallest molecule) works at the genetic level is still completely unknown. H2 is also speculated to work in mitochondria, reducing the mitochondrial membrane potential.107 Indeed, taking H2 in drinking water for a long period (for a couple of months) increased the production of adenosine trisphosphate at Complex I and Complex II in rat cardiac mitochondria.108 As for the Xe-induced reaction, much less evidence has been shown. Therefore, further experimental and clinical studies are needed to elucidate the mechanism of each MG.
Acknowledgements
We thank that the experimental calculations for Xe were carried out at Tomsk Polytechnic University within the framework of Tomsk Polytechnic University Competitiveness Enhancement Program.
Footnotes
Conflicts of interest
The authors declare that they have no competing interests.
Financial support
This work was partly supported by Econika Medical Engineering and a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science to MN (No. 20K07133).
Copyright transfer agreement
The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Funding: This work was partly supported by Econika Medical Engineering and a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science to MN (No. 20K07133).
REFERENCES
- 1.Huang JL, Zhao BL, Manaenko A, Liu F, Sun XJ, Hu Q. Medical gases for stroke therapy: summary of progress 2015-2016. Med Gas Res. 2017;7:107–112. doi: 10.4103/2045-9912.208516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bocci V, Zanardi I, Travagli V. Oxygen/ozone as a medical gas mixture. A critical evaluation of the various methods clarifies positive and negative aspects. Med Gas Res. 2011;1:6. doi: 10.1186/2045-9912-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakao A, Sugimoto R, Billiar TR, McCurry KR. Therapeutic antioxidant medical gas. J Clin Biochem Nutr. 2009;44:1–13. doi: 10.3164/jcbn.08-193R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 5.Biggers A. What is ozone therapy? Benefits and risks. [Accessed by October 6, 2020]. https://www.medicalnewstoday.com/articles/320759#overview .
- 6.Bajaj T, Cascella M. Xenon. [Assessed by October 6, 2020]. https://www.statpearls.com/ArticleLibrary/viewarticle/43071 .
- 7.Nowrangi DS, Tang J, Zhang JH. Argon gas: a potential neuroprotectant and promising medical therapy. Med Gas Res. 2014;4:3. doi: 10.1186/2045-9912-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhai X, Chen X, Ohta S, Sun X. Review and prospect of the biomedical effects of hydrogen. Med Gas Res. 2014;4:19. doi: 10.1186/s13618-014-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bocci V. Ozone as a bioregulator. Pharmacology and toxicology of ozonetherapy today. J Biol Regul Homeost Agents. 1996;10:31–53. [PubMed] [Google Scholar]
- 10.Fernández-Cuadros ME, Albaladejo-Florín MJ, Álava-Rabasa S, et al. Effect of rectal ozone (O(3)) in severe COVID-19 pneumonia: preliminary results. SN Compr Clin Med. 2020 doi: 10.1007/s42399-020-00374-1. doi: 10.1007/s42399-020-00374-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma A, Shah M, Lakshmi S, et al. A pilot study for treatment of COVID-19 patients in moderate stage using intravenous administration of ozonized saline as an adjuvant treatment-registered clinical trial. Int Immunopharmacol. 2021;96:107743. doi: 10.1016/j.intimp.2021.107743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Z, Dong M, Hu K. A preliminary evaluation on the efficacy of ozone therapy in the treatment of COVID-19. J Med Virol. 2020;92:2348–2350. doi: 10.1002/jmv.26040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burov NE, Molchanov IV, Nikolaev LL. Xenon in medicine: the past, the present and the future. J Clin Pract. 2011;2:3–11. [Google Scholar]
- 14.Re L, Mawsouf MN, Menéndez S, León OS, Sánchez GM, Hernández F. Ozone therapy: clinical and basic evidence of its therapeutic potential. Arch Med Res. 2008;39:17–26. doi: 10.1016/j.arcmed.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Amer AR, Oorschot DE. Xenon combined with hypothermia in perinatal hypoxic-ischemic encephalopathy: a noble gas, a noble mission. Pediatr Neurol. 2018;84:5–10. doi: 10.1016/j.pediatrneurol.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Roostan M, Frishman WH. Xenon: an emerging neuroprotectant with potential application for cardiac arrest care. Cardiol Rev. 2018;26:207–212. doi: 10.1097/CRD.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 17.Maze M. Preclinical neuroprotective actions of xenon and possible implications for human therapeutics: a narrative review. Can J Anaesth. 2016;63:212–226. doi: 10.1007/s12630-015-0507-8. [DOI] [PubMed] [Google Scholar]
- 18.Azzopardi D, Robertson NJ, Kapetanakis A, et al. Anticonvulsant effect of xenon on neonatal asphyxial seizures. Arch Dis Child Fetal Neonatal Ed. 2013;98:F437–439. doi: 10.1136/archdischild-2013-303786. [DOI] [PubMed] [Google Scholar]
- 19.Yagi M, Mashimo T, Kawaguchi T, Yoshiya I. Analgesic and hypnotic effects of subanaesthetic concentrations of xenon in human volunteers: comparison with nitrous oxide. Br J Anaesth. 1995;74:670–673. doi: 10.1093/bja/74.6.670. [DOI] [PubMed] [Google Scholar]
- 20.Burov NE, Makeev GN, Potapov VN. Applying xenon technologies in Russia. Appl Cardiopulm Pathophysiol. 2000;9:132–133. [Google Scholar]
- 21.Rüegger CM, Davis PG, Cheong JL. Xenon as an adjuvant to therapeutic hypothermia in near-term and term newborns with hypoxic-ischaemic encephalopathy. Cochrane Database Syst Rev. 2018;8:CD012753. doi: 10.1002/14651858.CD012753.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobrovolsky A, Ichim TE, Ma D, Kesari S, Bogin V. Xenon in the treatment of panic disorder: an open label study. J Transl Med. 2017;15:137. doi: 10.1186/s12967-017-1237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohta S. Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. Pharmacol Ther. 2014;144:1–11. doi: 10.1016/j.pharmthera.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Guan WJ, Wei CH, Chen AL, et al. Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with Coronavirus disease 2019 in a recent multicenter, open-label clinical trial. J Thorac Dis. 2020;12:3448–3452. doi: 10.21037/jtd-2020-057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nazarov E. Adaptive hypothesis of system ozone therapy or why the ozone shifts always physiological and biochemical parameters of the organism towards the standard level? J Ozone Ther. 2019;3:47. [Google Scholar]
- 26.Khlusov IA, Naumov SA, Vovk SM, et al. Xenon effects on cells and receptors. Bull Russ Acad Med Sci. 2003:32–37. [PubMed] [Google Scholar]
- 27.Uchida T, Suzuki S, Hirano Y, Ito D, Nagayama M, Gohara K. Xenon-induced inhibition of synchronized bursts in a rat cortical neuronal network. Neuroscience. 2012;214:149–158. doi: 10.1016/j.neuroscience.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 28.Dingley J, Ivanova-Stoilova TM, Grundler S, Wall T. Xenon: recent developments. Anaesthesia. 1999;54:335–346. doi: 10.1046/j.1365-2044.1999.00807.x. [DOI] [PubMed] [Google Scholar]
- 29.Molecular Hydrogen Institute. Scientific studies. [Assessed by October 6, 2020]. http://www.molecularhydrogeninstitute.com/studies .
- 30.Malenka RC, Nestler EJ, Hyman SE. Sydor A, Brown RY. Molecular neuropharmacology: a foundation for clinical neuroscience. 2nd ed. New York: McGraw-Hill Medical; 2009. Chapter 10: Neural and neuroendocrine control of the internal milieu; pp. 248–259. [Google Scholar]
- 31.Papathanasiou AE, Nolen-Doerr E, Farr OM, Mantzoros CS. Geoffrey harris prize lecture 2018: novel pathways regulating neuroendocrine function, energy homeostasis and metabolism in humans. Eur J Endocrinol. 2019;180:R59–71. doi: 10.1530/EJE-18-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cameron JL. Stress and behaviorally induced reproductive dysfunction in primates. Semin Reprod Endocrinol. 1997;15:37–45. doi: 10.1055/s-2008-1067966. [DOI] [PubMed] [Google Scholar]
- 33.Cramer T, Kisliouk T, Yeshurun S, Meiri N. The balance between stress resilience and vulnerability is regulated by corticotropin-releasing hormone during the critical postnatal period for sensory development. Dev Neurobiol. 2015;75:842–853. doi: 10.1002/dneu.22252. [DOI] [PubMed] [Google Scholar]
- 34.Thomson EM, Pilon S, Guénette J, Williams A, Holloway AC. Ozone modifies the metabolic and endocrine response to glucose: Reproduction of effects with the stress hormone corticosterone. Toxicol Appl Pharmacol. 2018;342:31–38. doi: 10.1016/j.taap.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 35.Thomson EM, Pal S, Guénette J, et al. Ozone inhalation provokes glucocorticoid-dependent and -independent effects on inflammatory and metabolic pathways. Toxicol Sci. 2016;152:17–28. doi: 10.1093/toxsci/kfw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller DB, Snow SJ, Schladweiler MC, et al. Acute ozone-induced pulmonary and systemic metabolic effects are diminished in adrenalectomized rats. Toxicol Sci. 2016;150:312–322. doi: 10.1093/toxsci/kfv331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kryzhanovsky SA, Nikiforova TD, Durnev AD. Epac proteins and their role in the physiological and pathological processes in the cardiovascular system. Part 1: The role of epac proteins in the physiological and pathological processes of the vasculature. Hum Physiol. 2020;46:200–215. [Google Scholar]
- 38.Nazarov EI, Vongai VG, Glukhenkaya TA. Ozone-xenon correction of stress. Med Almanac. 2013;3:27. [Google Scholar]
- 39.Garkavi L, Kvakina EB, Mulatova AK, Sheĭko EA, Shikhliarova AI. Morphofunctional features of lymph nodes, thyroid gland and testis of rats during adaptation reactions to stress and activation. Biull Eksp Biol Med. 1989;108:634–637. [PubMed] [Google Scholar]
- 40.Downing JE, Miyan JA. Neural immunoregulation: emerging roles for nerves in immune homeostasis and disease. Immunol Today. 2000;21:281–289. doi: 10.1016/s0167-5699(00)01635-2. [DOI] [PubMed] [Google Scholar]
- 41.Naumov SA, Khlusov IA. Adaptive effects of xenon. Intensive Ther. 2007;1:10–16. [Google Scholar]
- 42.Kussmaul AR, Bogacheva MA, Shkurat TP, Pavlov BN. Effects of xenon and krypton-containing breathing mixtures on clinical and biochemical blood indices in animals. Aviakosm Ekolog Med. 2007;41:60–64. [PubMed] [Google Scholar]
- 43.Gao Q, Song H, Wang XT, et al. Molecular hydrogen increases resilience to stress in mice. Sci Rep. 2017;7:9625. doi: 10.1038/s41598-017-10362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medici M, Direk N, Visser WE, et al. Thyroid function within the normal range and the risk of depression: a population-based cohort study. J Clin Endocrinol Metab. 2014;99:1213–1219. doi: 10.1210/jc.2013-3589. [DOI] [PubMed] [Google Scholar]
- 45.Ruhla S, Weickert MO, Arafat AM, et al. A high normal TSH is associated with the metabolic syndrome. Clin Endocrinol (Oxf) 2010;72:696–701. doi: 10.1111/j.1365-2265.2009.03698.x. [DOI] [PubMed] [Google Scholar]
- 46.Cappola AR, Arnold AM, Wulczyn K, Carlson M, Robbins J, Psaty BM. Thyroid function in the euthyroid range and adverse outcomes in older adults. J Clin Endocrinol Metab. 2015;100:1088–1096. doi: 10.1210/jc.2014-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heeringa J, Hoogendoorn EH, van der Deure WM, et al. High-normal thyroid function and risk of atrial fibrillation: the Rotterdam study. Arch Intern Med. 2008;168:2219–2224. doi: 10.1001/archinte.168.20.2219. [DOI] [PubMed] [Google Scholar]
- 48.Asvold BO, Bjøro T, Nilsen TI, Gunnell D, Vatten LJ. Thyrotropin levels and risk of fatal coronary heart disease: the HUNT study. Arch Intern Med. 2008;168:855–860. doi: 10.1001/archinte.168.8.855. [DOI] [PubMed] [Google Scholar]
- 49.Murphy E, Glüer CC, Reid DM, et al. Thyroid function within the upper normal range is associated with reduced bone mineral density and an increased risk of nonvertebral fractures in healthy euthyroid postmenopausal women. J Clin Endocrinol Metab. 2010;95:3173–3181. doi: 10.1210/jc.2009-2630. [DOI] [PubMed] [Google Scholar]
- 50.Vani AC, Ingleshwar DG, Patil V, Patil V, S SA. A study of thyroid profile in patients with psoriasis. Natl J Lab Med. 2017;6:BO01–04. [Google Scholar]
- 51.Ferrari C, Reschini E, Paracchi A. Treatment of the autonomous thyroid nodule: a review. Eur J Endocrinol. 1996;135:383–390. doi: 10.1530/eje.0.1350383. [DOI] [PubMed] [Google Scholar]
- 52.Elias AR, Barr RJ. Low-dose oral propylthiouracil in the treatment of plaque psoriasis. Int J Dermatol. 1995;34:519–520. doi: 10.1111/j.1365-4362.1995.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 53.Romanov MD, Vodyakova AV, Kireeva EM, Lewina TM. The rationale for the use of infusion ozone therapy in patients with toxic goiter. Mod Probl Sci Educ. 2015;5:125. [Google Scholar]
- 54.Zhu Q, Wu Y, Li Y, et al. Positive effects of hydrogen-water bathing in patients of psoriasis and parapsoriasis en plaques. Sci Rep. 2018;8:8051. doi: 10.1038/s41598-018-26388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gabbe S, Niebyl J, Simpson J, et al. Obstetrics: normal and problem pregnancies. 7th ed. Elsevier; 2016. [Google Scholar]
- 56.Apumaita K, Murashko AV, Pak SV, Grechkanev GO, Dvoryansky SA, Iutinsky EM. The effect of ozone therapy and hyperbarotherapy on the hormonal function of the placental complex, the state of the blood coagulation system and placental morphology in patients with chronic placental insufficiency. Russ Bull Obstet Gynaecol. 2010:35–38. [Google Scholar]
- 57.Buranova FB, Fedorova TA. Plasmapheresis and medical ozone in the treatment of pregnant women with placental insufficiency after in vitro fertilization. Russ Bull Obstet Gynaecol. 2012:43–47. [Google Scholar]
- 58.Menendez-Cepero S. Ozone therapy: teratogenic study of ozone. Possible indications in obstetrics and gynecology. J Ozone Ther. 2018;2:20. [Google Scholar]
- 59.Bukhtiyarov IV, Кalmanov АS, Кislyakov UU, et al. Study of the possibility of using xenon in the training process to correct the functional state of athletes. Physiother Sports Med. 2010;6:22–29. [Google Scholar]
- 60.Hou C, Peng Y, Qin C, Fan F, Liu J, Long J. Hydrogen-rich water improves cognitive impairment gender-dependently in APP/PS1 mice without affecting Aβ clearance. Free Radic Res. 2018;52:1311–1322. doi: 10.1080/10715762.2018.1460749. [DOI] [PubMed] [Google Scholar]
- 61.Begum R, Bajgai J, Fadriquela A, Kim CS, Kim SK, Lee KJ. Molecular hydrogen may enhance the production of testosterone hormone in male infertility through hormone signal modulation and redox balance. Med Hypotheses. 2018;121:6–9. doi: 10.1016/j.mehy.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 62.Mano Y, Kotani T, Ito M, et al. Maternal molecular hydrogen administration ameliorates rat fetal hippocampal damage caused by in utero ischemia-reperfusion. Free Radic Biol Med. 2014;69:324–330. doi: 10.1016/j.freeradbiomed.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 63.Ushida T, Kotani T, Tsuda H, et al. Molecular hydrogen ameliorates several characteristics of preeclampsia in the Reduced Uterine Perfusion Pressure (RUPP) rat model. Free Radic Biol Med. 2016;101:524–533. doi: 10.1016/j.freeradbiomed.2016.10.491. [DOI] [PubMed] [Google Scholar]
- 64.Imai K, Kotani T, Tsuda H, et al. Neuroprotective potential of molecular hydrogen against perinatal brain injury via suppression of activated microglia. Free Radic Biol Med. 2016;91:154–163. doi: 10.1016/j.freeradbiomed.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 65.Noda M, Uemura Y, Yoshii Y, et al. Circulating messenger for neuroprotection induced by molecular hydrogen. Can J Physiol Pharmacol. 2019;97:909–915. doi: 10.1139/cjpp-2019-0098. [DOI] [PubMed] [Google Scholar]
- 66.Noda M, Ito M, Ohsawa I, Ohno K. Beneficial effects of hydrogen in the CNS and a new brain-stomach interaction. Eur J Neurodegener Dis. 2014;3:25–34. [Google Scholar]
- 67.Matsumoto A, Yamafuji M, Tachibana T, Nakabeppu Y, Noda M, Nakaya H. Oral ‘hydrogen water’ induces neuroprotective ghrelin secretion in mice. Sci Rep. 2013;3:3273. doi: 10.1038/srep03273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Noda M, Liu J, Long J. Neuroprotective and preventative effects of molecular hydrogen. Curr Pharm Des. 2021;27:585–591. doi: 10.2174/1381612826666201019103020. [DOI] [PubMed] [Google Scholar]
- 69.Inui A. Ghrelin: an orexigenic and somatotrophic signal from the stomach. Nat Rev Neurosci. 2001;2:551–560. doi: 10.1038/35086018. [DOI] [PubMed] [Google Scholar]
- 70.Andrews ZB, Erion D, Beiler R, et al. Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J Neurosci. 2009;29:14057–14065. doi: 10.1523/JNEUROSCI.3890-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshii Y, Inoue T, Uemura Y, et al. Complexity of Stomach-Brain Interaction Induced by Molecular Hydrogen in Parkinson’s Disease Model Mice. Neurochem Res. 2017;42:2658–2665. doi: 10.1007/s11064-017-2281-1. [DOI] [PubMed] [Google Scholar]
- 72.Iuchi K, Imoto A, Kamimura N, et al. Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Sci Rep. 2016;6:18971. doi: 10.1038/srep18971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iuchi K, Nishimaki K, Kamimura N, Ohta S. Molecular hydrogen suppresses free-radical-induced cell death by mitigating fatty acid peroxidation and mitochondrial dysfunction. Can J Physiol Pharmacol. 2019;97:999–1005. doi: 10.1139/cjpp-2018-0741. [DOI] [PubMed] [Google Scholar]
- 74.Draca SR. Endocrine-immunological homeostasis: the interrelationship between the immune system and sex steroids involves the hypothalamo-pituitary-gonadal axis. Panminerva Med. 1995;37:71–76. [PubMed] [Google Scholar]
- 75.Subkov AA, Zilov GN. The role of conditioned reflex adaptation in the origin of hyperergic reactions. Bulletin de Biologie et de Médicine Expérimentale. 1937;4:294–296. [Google Scholar]
- 76.Androsova ZG, Ginetsinskii AG, Gnedina TN, Kurduban LL, Natochin IuV, Tolkunov BF. Conditioned reactions developing under the action of humoral factors. Zh Vyssh Nerv Deiat Im I P Pavlova. 1959;9:388–397. [PubMed] [Google Scholar]
- 77.Leites MS, Pavlov GT. Conditional reaction to the sugar-lowering effect of insulin in experimental diabetes. J High Nerv Act. 1954;2:242. [PubMed] [Google Scholar]
- 78.Ader R. A historical account of conditioned immunobiologic responses. In: Ader R, editor. Psychoneuroimmunology. New York: Academic Press; 1981. pp. 321–354. [Google Scholar]
- 79.Siegel S. Conditioning of insulin-induced glycemia. J Comp Physiol Psychol. 1972;78:233–241. doi: 10.1037/h0032180. [DOI] [PubMed] [Google Scholar]
- 80.Woods SC, Shogren RE., Jr Glycemic responses following conditioning with different doses of insulin in rats. J Comp Physiol Psychol. 1972;81:220–225. doi: 10.1037/h0033529. [DOI] [PubMed] [Google Scholar]
- 81.Deutsch R. Conditioned hypoglycemia: a mechanism for saccharin-induced sensitivity to insulin in the rat. J Comp Physiol Psychol. 1974;86:350–358. doi: 10.1037/h0035948. [DOI] [PubMed] [Google Scholar]
- 82.Matysiak J, Green L. On the directionality of classically-conditioned glycemic responses. Physiol Behav. 1984;32:5–9. doi: 10.1016/0031-9384(84)90061-1. [DOI] [PubMed] [Google Scholar]
- 83.Pihl RO, Altman J. An experimental analysis of the placebo effect. J Clin Pharmacol New Drugs. 1971;11:91–95. doi: 10.1177/009127007101100203. [DOI] [PubMed] [Google Scholar]
- 84.Baptista MA, Siegel S, MacQueen G, Young LT. Pre-drug cues modulate morphine tolerance, striatal c-Fos, and AP-1 DNA binding. Neuroreport. 1998;9:3387–3390. doi: 10.1097/00001756-199810260-00010. [DOI] [PubMed] [Google Scholar]
- 85.Siegel S. Jarvik ME. Psychopharmacology in the practice of medicine. New York: Appleton-Century-Crofts; 1977. Learning and psychopharmacology. [Google Scholar]
- 86.Siegel S, Baptista MA, Kim JA, McDonald RV, Weise-Kelly L. Pavlovian psychopharmacology: the associative basis of tolerance. Exp Clin Psychopharmacol. 2000;8:276–293. doi: 10.1037//1064-1297.8.3.276. [DOI] [PubMed] [Google Scholar]
- 87.Ader R, Mercurio MG, Walton J, et al. Conditioned pharmacotherapeutic effects: a preliminary study. Psychosom Med. 2010;72:192–197. doi: 10.1097/PSY.0b013e3181cbd38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schafe GE, Nader K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 2001;24:540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- 89.Stanciu M, Radulovic J, Spiess J. Phosphorylated cAMP response element binding protein in the mouse brain after fear conditioning: relationship to Fos production. Brain Res Mol Brain Res. 2001;94:15–24. doi: 10.1016/s0169-328x(01)00174-7. [DOI] [PubMed] [Google Scholar]
- 90.Monsey MS, Boyle LM, Zhang ML, et al. Chronic corticosterone exposure persistently elevates the expression of memory-related genes in the lateral amygdala and enhances the consolidation of a Pavlovian fear memory. PLoS One. 2014;9:e91530. doi: 10.1371/journal.pone.0091530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pavlova OE. The effect of ozone on the rheological properties of blood. Moscow: Moscow State Pedagogical University; 1998. [Google Scholar]
- 92.Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–1536. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- 93.Bocci VA. Scientific and medical aspects of ozone therapy. State of the artArch Med Res. 2006;37:425–435. doi: 10.1016/j.arcmed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 94.Burov NE. Concept of mechanisms of anesthetic and therapeutic properties of xenon. Anesteziol Reanimatol. 2011:58–62. [PubMed] [Google Scholar]
- 95.Hughes RL, Yonas H, Gur D, Latchaw R. Cerebral blood flow determination within the first 8 hours of cerebral infarction using stable xenon-enhanced computed tomography. Stroke. 1989;20:754–760. doi: 10.1161/01.str.20.6.754. [DOI] [PubMed] [Google Scholar]
- 96.Hirayama M, Ito M, Minato T, Yoritaka A, LeBaron TW, Ohno K. Inhalation of hydrogen gas elevates urinary 8-hydroxy-2’-deoxyguanine in Parkinson’s disease. Med Gas Res. 2018;8:144–149. doi: 10.4103/2045-9912.248264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Re L, Malcangi G, Martinez-Sanchez G. Medical ozone is now ready for a scientific challenge: current status and future perspectives. J Exp Integr Med. 2012;3:193–196. [Google Scholar]
- 98.Southam CM, Erlich J. Effects of extracts of western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathology. 1943;33:517–524. [Google Scholar]
- 99.Luckey TD. Insecticide hormoligosis. J Econ Entomol. 1968;61:7–12. doi: 10.1093/jee/61.1.7. [DOI] [PubMed] [Google Scholar]
- 100.Townsend JF, Luckey TD. Hormoligosis in pharmacology. J Am Med Assoc. 1960;173:44–48. doi: 10.1001/jama.1960.73020190007010. [DOI] [PubMed] [Google Scholar]
- 101.Stanton ME, Levine S. Pavlovian conditioning of endocrine responses. In: Ader R, Weiner H, Baum A, editors. Experimental foundations of behavioral medicine: Conditioning approaches. Psychology Press; 1988. pp. 25–46. [Google Scholar]
- 102.Brun JF. Hormones, metabolism and body composition as major determinants of blood rheology: potential pathophysiological meaning. Clin Hemorheol Microcirc. 2002;26:63–79. [PubMed] [Google Scholar]
- 103.Graves DJ, Idicula J, Lambertsen CJ, Quinn JA. Bubble formation in physical and biological systems: a manifestation of counterdiffusion in composite media. Science. 1973;179:582–584. doi: 10.1126/science.179.4073.582. [DOI] [PubMed] [Google Scholar]
- 104.Shatalov VM. Mechanism of the biological impact of weak electromagnetic fields and in vitro effects of degassing of blood. Biofizika. 2012;57:1034–1040. [PubMed] [Google Scholar]
- 105.Shatalov VM, Zinchenko AA. Dissolved air effects on blood clotting dynamics in vitro. Phys Alive. 2010;1:43–50. [Google Scholar]
- 106.Reichenberg J, Ullman R. Healing hearts with homeopathy. Townsend Lett Doctors Patients. 2004;253-254:48–50. [Google Scholar]
- 107.Ishihara G, Kawamoto K, Komori N, Ishibashi T. Molecular hydrogen suppresses superoxide generation in the mitochondrial complex I and reduced mitochondrial membrane potential. Biochem Biophys Res Commun. 2020;522:965–970. doi: 10.1016/j.bbrc.2019.11.135. [DOI] [PubMed] [Google Scholar]
- 108.Gvozdjáková A, Kucharská J, Kura B, et al. A new insight into the molecular hydrogen effect on coenzyme Q and mitochondrial function of rats. Can J Physiol Pharmacol. 2020;98:29–34. doi: 10.1139/cjpp-2019-0281. [DOI] [PubMed] [Google Scholar]