Abstract
A relationship between Bifidobacterium and defecation has previously been reported. Our hypothesis on the effectiveness of alkaline electrolyzed water (AEW) proposes that ingestion of AEW, considered possessing antioxidative properties, increases the number of Bifidobacteria and improves stool hardness and gastrointestinal symptoms. A double-blind, randomized study was conducted to evaluate the connection between stool consistency and change in gut microbiota composition induced by drinking hydrogen-dissolved AEW. The participants drank 500 mL of purified tap water or AEW every day for 2 weeks. In this study, drinking AEW did not drastically change gut microbiota, but it appeared to act on a specific bacterial species. Drinking AEW was confirmed to cause an increase in Bifidobacterium. The AEW group also saw stool consistency significantly converge to Bristol stool scale Type 4 (“normal”). Therefore, it is highly likely that the gut microbiota will be changed by drinking AEW. This study was retrospectively registered in University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN ID: UMIN000039507) on February 18, 2020, and was approved by the Ethics Committee of University of Yamanashi (approval No. H30-25) on January 9, 2018.
Keywords: 16S rRNA, alkaline electrolyzed water, antioxidative properties, bifidobacterium, double-blind randomized trial, gastrointestinal symptoms, gut microbiota, hydrogen, next-generation sequencing, stool consistency
INTRODUCTION
In 2007, it was reported that water in which hydrogen has been dissolved (hydrogen water) possesses an antioxidant effect that efficiently and directly removes hydroxyl radicals.1 Water with alkaline properties including hydrogen generated by electrolysis has been known as alkaline electrolyzed water (AEW) in Japan.2 Naito et al.3 reported that AEW possesses antioxidant activity. Tashiro et al.4 at around the same time performed a large-scale clinical evaluation of the effectiveness of AEW in improving gastrointestinal symptoms. Taking as their participants showing abdominal complaints such as heartburn, stomach discomfort, abdominal distension, diarrhea, and constipation, they investigated the general improvement in abdominal issues in participants drinking 500 mL of either AEW or non-electrolyzed purified tap water (PW) daily over a period of 4 weeks. They report that the AEW-drinking group saw better results, while symptoms such as diarrhea and constipation were also substantially ameliorated in that group compared to the PW-drinking control group.4 We have previously investigated the effects of drinking AEW in healthy participants who do not experience abdominal complaints, and have reported that AEW ingestion normalizes stool consistency.5 Recently, the effects of the alkaline-reduced drinking water on irritable bowel syndrome with diarrhea have also been reported by Shin et al.6
Takagi et al.7 have been investigating and researching the stool consistency of Japanese participants using the Bristol stool scale (BSS),8 an evaluation method for categorizing the consistency of stool into seven types: Type 1 (separate hard lumps, like nuts); Type 2 (sausage-shaped, but lumpy); Type 3 (like a sausage but with cracks on its surface); Type 4 (like a sausage or snake, smooth and soft); Type 5 (soft blobs with clear cut edges); Type 6 (fluffy pieces with ragged edges, a mushy stool); and Type 7 (watery, no solids, entirely liquid). They reported that 60% of healthy males generally tended toward loose stool (BSS Type 5 or 6), and discussed the possibility that the balance of the gut microbiota composition had an effect on stool consistency trends. Other studies9,10 also reported numerous microbes affecting stool consistency.
We have previously published a paper regarding a mouse experiment, in which the participants ingested hydrogen-dissolved AEW in advance of the experiment. We found not only did the antioxidant mechanisms act directly and indirectly on oxidative stress in mice, but also confirmed that the balance of the gut microbiota composition changed, with active compounds such as short-chain fatty acids increasing, even under non-stress conditions during which no oxidative stress was applied.11 In an experiment on gut microbiota in humans who were ingesting hydrogen-rich water, Sha et al.12 investigated the diversity and abundance of gut microbiota based on 4-week and 8-week ingestion of hydrogen-rich water by soccer players undergoing long-term, high-intensity sports training. They found that an oxidative stress marker, malondialdehyde, decreased and that total antioxidant capacity increased.
There have hitherto been no published studies on the connection between stool consistency and changes in gut microbiota composition caused by the ingestion of hydrogen-dissolved AEW by normal healthy individuals who do are not subject to intense oxidative stress due to factors such as excessive exercise. The present study investigates the link between stool consistency and changes in gut microbiota in the stool of healthy individuals who have drunk hydrogen-dissolved AEW.
PARTICIPANTS AND METHODS
Participants
We recruited healthy volunteers as test participants, but excluding smokers, people who regularly take antioxidants, and people who already regularly ingest AEW. The participants were instructed not to engage in strenuous exercise for the duration of this study. Written informed consent was obtained from all the participants. The randomization was carried out by the researcher who had no clinical involvement in this trial. Participants and researchers were all blinded to the test water allocation. Using block randomization, he divided a group of 20 male test participants aged 30–59 years and living in Kofu City, Yamanashi Prefecture, into two test groups: AEW group, consisting of 10 participants (C, F, G, K, L, N, O, P, Q, S) who drank AEW, and PW group, consisting of 10 participants (A, B, D, E, H, I, J, M, R, T) who drank PW. Participant characteristics are presented in Table 1. No significant differences existed between the groups with respect to age, height and weight. Each test participant was handed two hermetically sealed aluminum pouches (each containing 250 mL of AEW or PW) per day. We investigated the influence of drinking AEW on gut microbiota and stool condition by means of a double-blind randomized trial in which each participant was asked to ingest two pouches (500 mL) daily over a period of 2 weeks (January 2018). This study was retrospectively registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN ID: UMIN000039507; Date of Registration: February 18, 2020). All experimental protocols were approved by the Ethics Committee of the University of Yamanashi (approval No. H30-25) on January 9, 2018, and all techniques were performed in accordance with the relevant guidelines and regulations. The writing and editing of the article were performed in accordance with the CONsolidated Standards Of Reporting Trials (CONSORT) Statement (Additional file) and the trial flow chart is shown in Figure 1.
Table 1.
Age, weight and height distribution of the participants
| Group | Age (yr) | Weight (kg) | Height (cm) |
|---|---|---|---|
| PW | 43.7±8.4 | 75.8±11.6 | 173.2±3.5 |
| AEW | 44.3±7.8 | 71.2±8.2 | 172.0±5.7 |
Note: Data are expressed as mean ± SD (n = 10). AEW: Alkaline electrolyzed water; PW: purified tap water.
CONSORT 2010 checklist of information to include when reporting a randomised trial*
| Section/Topic | Item No | Checklist item | Reported on page No |
|---|---|---|---|
| Title and abstract | |||
|
|
|||
| 1a | Identification as a randomised trial in the title | 1 | |
|
|
|||
| 1b | Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts) | 1 | |
|
|
|||
| Introduction | |||
|
|
|||
| Background and | 2a | Scientific background and explanation of rationale | 2-3 |
|
|
|||
| objectives | 2b | Specific objectives or hypotheses | 3 |
|
|
|||
| Methods | |||
|
|
|||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | 4 |
|
|
|||
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | 4 | |
|
|
|||
| Participants | 4a | Eligibility criteria for participants | 4 |
|
|
|||
| 4b | Settings and locations where the data were collected | 4 | |
|
|
|||
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | 4 |
|
|
|||
| Outcomes | 6a | Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed | 6-8 |
|
|
|||
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | N/A | |
|
|
|||
| Sample size | 7a | How sample size was determined | 4 |
|
|
|||
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | N/A | |
|
|
|||
| Randomisation: | |||
|
|
|||
| Sequence generation | 8a | Method used to generate the random allocation sequence | 4 |
|
|
|||
| 8b | Type of randomisation; details of any restriction (such as blocking and block size) | 4 | |
|
|
|||
| Allocation concealment mechanism | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | 4 |
|
|
|||
| Implementation | 10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions | 4 |
|
|
|||
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how | 4 |
|
|
|||
| 11b | If relevant, description of the similarity of interventions | N/A | |
|
|
|||
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | 8 |
|
|
|||
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | N/A | |
|
|
|||
| Results | |||
|
|
|||
| Participant flow (a diagram is strongly recommended) | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome | 5 |
|
|
|||
| 13b | For each group, losses and exclusions after randomisation, together with reasons | N/A | |
|
|
|||
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | 2 |
|
|
|||
| 14b | Why the trial ended or was stopped | N/A | |
|
|
|||
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | 5 |
|
|
|||
| Numbers analysed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | 11-13 |
|
|
|||
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) | 11-14 |
|
|
|||
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | N/A | |
|
|
|||
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory | NA |
|
|
|||
| Harms | 19 | All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) | NA |
|
|
|||
| Discussion | |||
|
|
|||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | 15-16 |
|
|
|||
| Generalisability | 21 | Generalisability (external validity, applicability) of the trial findings | 15-16 |
|
|
|||
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | 15-16 |
|
|
|||
| Other information | |||
|
|
|||
| Registration | 23 | Registration number and name of trial registry | 16 |
|
|
|||
| Protocol | 24 | Where the full trial protocol can be accessed, if available | 16 |
|
|
|||
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders | 16 |
*We strongly recommend reading this statement in conjunction with the CONSORT 2010 Explanation and Elaboration for important clarifications on all the items. If relevant, we also recommend reading CONSORT extensions for cluster randomised trials, non-inferiority and equivalence trials, non-pharmacological treatments, herbal interventions, and pragmatic trials. Additional extensions are forthcoming: for those and for up to date references relevant to this checklist, see www.consort-statement.org
Figure 1.
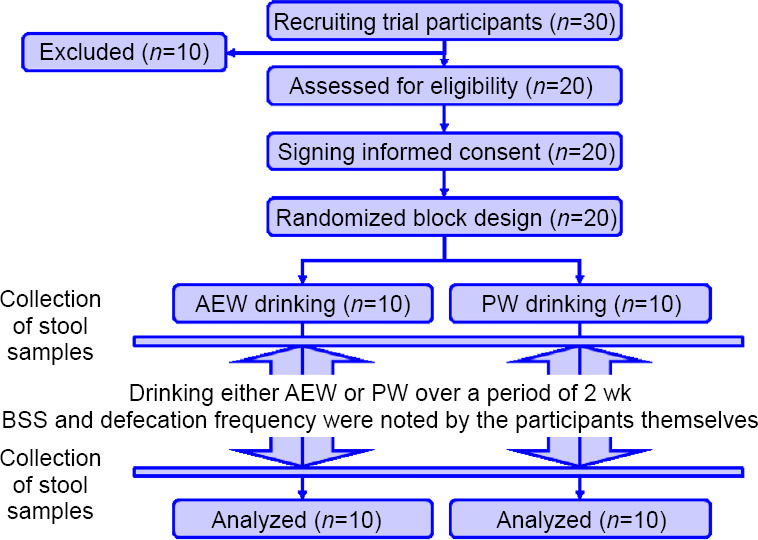
A trial flow chart.
Note: AEW: Alkaline electrolyzed water; PW: purified tap water.
Preparation of test water
One pouch-worth of each of the different types of test water was generated using the methods outlined below. Aluminum pouches were used so as not to allow hydrogen molecules to pass through easily. We confirmed that there was no change in water quality – such as loss of hydrogen concentration in the test water – during the storage time between collecting the water in the pouch and drinking the water.
PW: PW was made from ordinary University of Yamanashi tap water, purified using the activated carbon filter of an alkaline ionized water apparatus (TK-HS92, modified for this study, Panasonic, Kusatsu, Japan),13 and the water being passed through at a flow rate of 2.0 L/min without electrolysis. About 250 mL of the PW was collected in each aluminum pouch before it was hermetically sealed.
AEW: After purifying tap water from the University of Yamanashi in the same manner as the PW, the diaphragm-type Pt electrode electrolytic cell inside the alkaline ionized water apparatus was used to electrolyze the water at a constant current. As a result of this electrolysis, a certain quantity of hydrogen molecules and hydroxide ions were generated on the cathode side and dissolved by running the water at a flow rate of 2.0 L/min, so that the pH and hydrogen dissolution amount generated stable AEW. About 250 mL of AEW was collected in each aluminum pouch, which was then hermetically sealed.
The test water characteristics were: (for PW) hydrogen concentration 0.0 mg/L, pH 7.6; (for AEW) hydrogen concentration 0.3 mg/L, pH 9.5. In this test, the water ingested by all the participants came from the same tap water source, with the water ingested by one test group being purified only, while that of the other test group was purified and then electrolyzed. The difference in ingested ingredients from the AEW and the PW was therefore limited to only the alkalinity resulting from the hydrogen molecules and hydroxide ions (OH–) generated by the electrolysis of the water. The pH of the test water was measured using a pH meter (HM-20P, DKK-TOA, Tokyo, Japan), and the hydrogen concentration was measured using a portable dissolved hydrogen meter (DH-35A, DKK-TOA, Tokyo, Japan).
Collection of stool samples
To measure gut microbiota, samples were taken from the participants’ stool. Sampling was performed twice, the day before initiating the ingestion test and the day the 2-week test ended, with the participants themselves taking the samples immediately after getting up in the morning using the stool-sampling kit (FS-0006, Techno Suruga Lab, Shizuoka, Japan).
Classification of stool consistency
Stool consistency and defecation frequency were noted by the participants themselves in a daily survey, which was collected each week. The participants categorized their stool into types 1–7 using the BSS.
The stool consistency types 1 to 7 as determined by the participants were converted into points (1 to 7 points) and set as BSS values. Because the number of defecations can be 0 or multiple times per day, the Modified BSS value was collected based on the mean score for each individual over 2 days. This was arrived at by adding up the BSS values for all defecations over 2 days and dividing by the number of defecations. The following Equation (1) shows this process.

To compare the results from before and after drinking the test water, we calculated both the Modified BSS value before drinking from the day before the test started and the day of test start, and the Modified BSS value after drinking from the test end date and the day before the test end date.
Microbiota analysis by 16S rRNA gene sequencing
Stool samples were suspended in guanidine thiocyanate solution in the Stool collection kit (FS-0006, Techno Suruga Lab, Shizuoka, Japan).14 Bacterial genomic DNA was extracted using a NucleoSpin Microbial DNA kit (Macherey-Nagel, Düren, Germany). Approximately 500 μL of the stored stool sample was placed in a microcentrifuge tube containing 100 μL of elution buffer. The mixture was then placed into a NucleoSpin Beads Tube (Macherey-Nagel) with proteinase K and was then subjected to beating with mechanical beads for 12 minutes at 30 Hz in a TissueLyzer LT (Qiagen, Hilden, Germany). The subsequent extraction procedure was performed according to the manufacturer’s instructions.15 Extracted DNA samples were purified using an Agencourt AMPure XP (Beckman Coulter, Brea, CA, USA). Microbiota analyses by 16S rRNA gene sequencing were conducted by Takara Bio Inc. The V3–V4 region was amplified using forward primer 341F (5′-TCG TCG GCA GCG TCA GAT GTG TAT AAG AGA CAG CCT ACG GGN GGC WGC AG-3′) and reverse primer 806R (5′-GTC TCG TGG GCT CGG AGA TGT GTA TAA GAG ACA GGG ACT ACH VGG GTW TCT AAT-3′). The italicized sequences in the primers are the Illumina overhang adapter. PCR amplification was done using the following program: initial denaturation at 94˚C for 1 minute; 28 cycles of 98˚C for 10 seconds, 50˚C for 15 seconds, and 68˚C for 15 seconds; and a hold at 4˚C. The amplicons were adapted with Illumina sequencing adapters and dual-index barcode sequences using a kit (Nextera XT Index Kit v2 SetA/B/C/D; Illumina Inc., San Diego, CA, USA), after which they were purified (AMPure XP Beads; Beckman Coulter Inc., Brea, CA, USA) according to the manufacturer’s instructions. The prepared libraries were subjected to sequencing of 250 paired-end bases using the MiSeq Reagent v3 kit and the MiSeq (Illumina) at the Biomedical Center at Takara Bio.11
Sequence data analysis
Post-processing sequencing data were analyzed using Quantitative insights into Microbial Ecology (QIIME pipeline, ver. 1.8.0., http://qiime.org/).16 First, sequenced paired-end reads were joined to construct contigs. In the representative sequence preparation step, CD-HIT-OTU (ver. 0.0.1., http://weizhongli-lab.org/cd-hit-otu/) was conducted for clustering operational taxonomic units (OTUs). Next, chimeric contigs were removed as far as possible by applying the CD-HIT-OTU algorithm. After removing the chimeric contigs, the remaining contigs were clustered into OTUs with 97% sequence similarity.
Taxonomic identification was performed at the phylum and genus levels. To acquire taxonomic information for each OTU, representative sequences were aligned to the Greengenes 16S rRNA database (g_13.8)17 by PyNAST (Version 1.2.2, https://pypi.org/project/pynast/) and assigned to its database for classification by RDP classifier (Version 2.2, https://rdp.cme.msu.edu/classifier/). Likewise, a homology search was conducted for representative sequences and assigned to the DNA Data Bank of Japan 16S ribosomal RNA database by BLASTN (Version 2.2.20, https://blast.ncbi.nlm.nih.gov/). Alpha diversity indices were computed at a sequence depth of 87,474 sequences for the sample with the lowest sequence. To construct a phylogenetic tree based on the aligned sequences to the Greengenes 16S rRNA database, and the unweighted-pair group method using arithmetic means (UPGMA), clustering was applied to unweighted UniFrac distance matrices to build an UPGMA tree using FastTree (Version 2.1.3, http://www.microbesonline.org/fasttree/).
Statistical analysis
With regard to microbiota analysis, we compared the before- and after-drinking data from each test group using the Wilcoxon signed-rank test. The observed species, Chao 1 and Shannon indices were calculated using the R “phyloseq” package and were statistically analyzed using a two-sample t-test. Statistical analyses were applied using GraphPad Prism Ver. 8.2.0 (GraphPad Software Inc., San Diego, CA, USA). An F-test was performed to investigate the population variance ratio and estimate whether the variance of the Modified BSS values before drinking was equal to the variance of the Modified BSS values after drinking. The test was performed using the Microsoft Excel add-in Statcel4 (OMS Publishing, Saitama, Japan). Differences were regarded as statistically significant for P values of < 0.05.
RESULTS
Microbial diversity and cluster analysis
Alpha-diversity between before and after the tests of the two groups was compared using three different indices [the observed species, the Chao 1 index (OTU richness estimation), and the Shannon index (OTU evenness estimation)] Figure 2A–C). However, there was no statistically significant difference in alpha-diversity between before and after the test.
Figure 2.
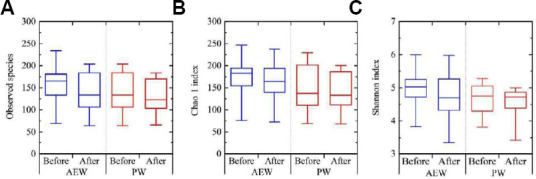
Alpha-diversity of gut microbiota in the healthy participants before and after of 2-week drinking either hydrogen-dissolved alkaline electrolyzed water (AEW) or purified tap water (PW).
Note: (A) Observed species; (B) Chao 1 index; (C) Shannon index. Data are expressed as mean ± SD, and were analyzed by two-sample t-test.
Hierarchical clustering analysis was performed on the gut microbiota of the test participants both before and after drinking each type of water. For all participants in the AEW group except for K and O, changes in microbiota before and after drinking were all within the same cluster, and did not cross the boundaries of individuals (Figure 3). For the PW group, on the other hand, changes in the microbiota of all participants before and after drinking were within the same cluster. Drinking AEW therefore did not drastically change gut microbiota.
Figure 3.
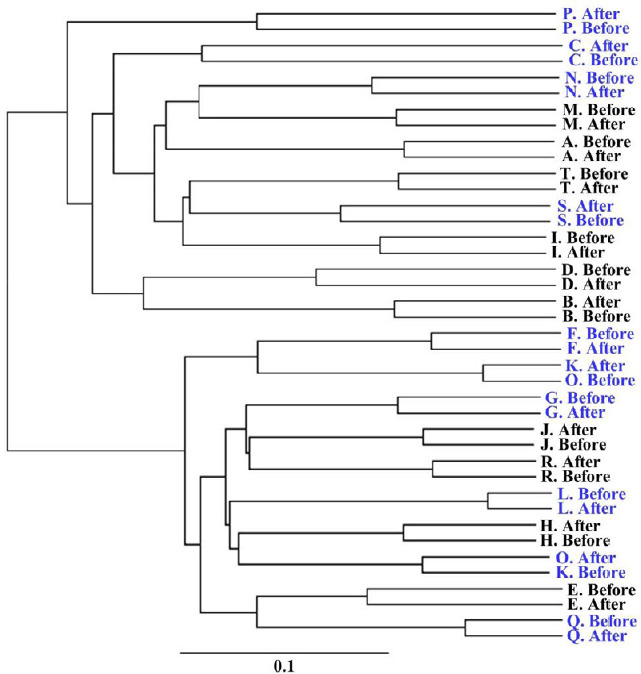
Cluster analysis of gut microbiota in the healthy participants before and after 2-week drinking either hydrogen-dissolved alkaline electrolyzed water (AEW) or purified tap water (PW).
Note: Blue participant (consisting of participants C, F, G, K, L, N, O, P, Q, S): AEW, Black participants (consisting of participants A, B, D, E, H, I, J, M, R, T): PW. Abscissa axis indicates the evolutionary distance.
Comparison of changes in gut microbiota
We compared, by means of a double-blind comparison test, the changes in gut microbiota and stool consistency induced by drinking either AEW or PW over a period of 2 weeks. Participants were randomly divided into two groups, consisting of an AEW group (n = 10) and a PW group (n = 10), who ingested 500 mL of AEW or PW per day.
First, the relative abundance, at the phylum level, of gut microbiota in each participant was compared before and after the test (Figure 4A and B). At the phylum level, the gut microbiota of the participants were, as often seen in Japanese participants, made up largely of Actinobacteria, Bacteroides, and Firmicutes.18,19 The AEW group’s relative abundance of Actinobacteria increased in nine participants (all but K) as shown in Figure 5A, with the mean value increasing significantly, while the PW group’s relative abundance of Actinobacteria, as shown in Figure 5B, decreased in five while increasing in the other five, but with no statistically significant change. There was no significant difference in the relative abundance of Bacteroides or Firmicutes.
Figure 4.
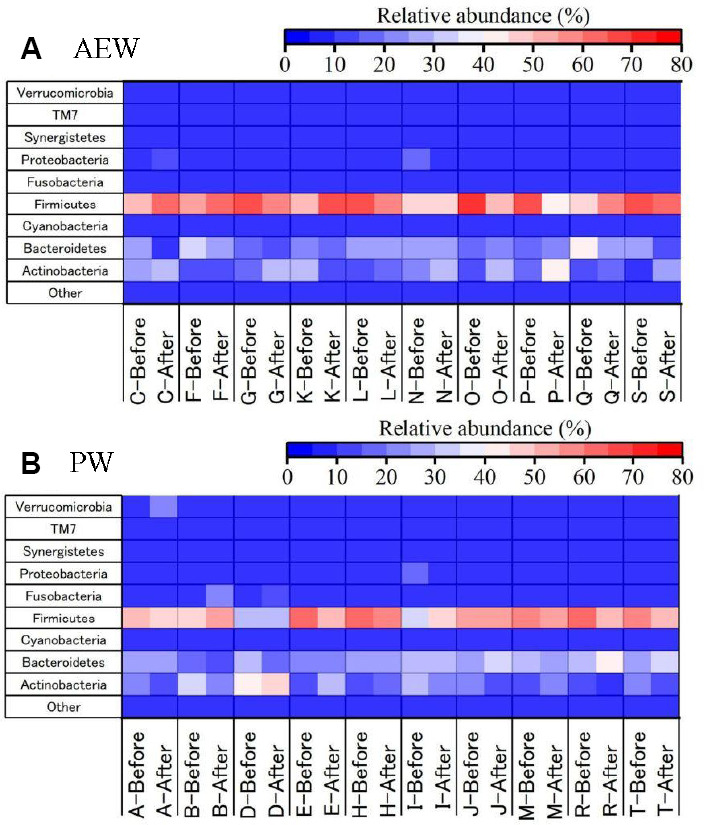
Heatmap of microbial communities (phylum-level) in the stool samples in healthy participants between before and after of 2-week drinking either hydrogen-dissolved alkaline electrolyzed water (AEW; A) or purified tap water (PW; B), as obtained by next-generation sequencing.
Figure 5.
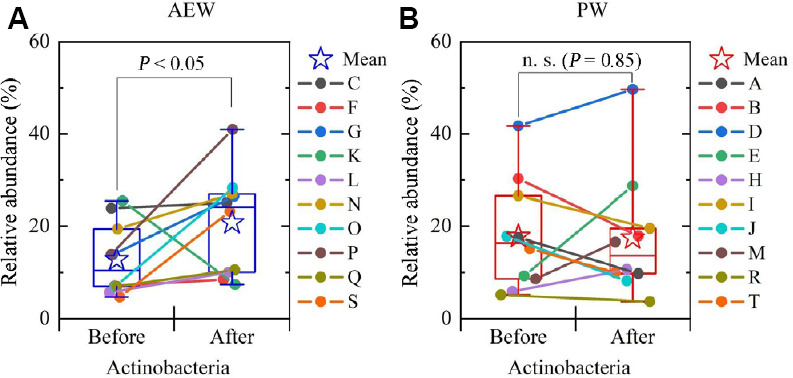
The change of the relative abundance of Actinobacteria before and after the experiment (boxplot) and the change in each test participant (indicated by lines connecting “before” and “after” points on the graph) for the AEW group (A) and the PW group (B).
Note: Data are expressed as mean ± SD, and were analyzed by Wilcoxon signed-rank test. AEW: Alkaline electrolyzed water; n.s.: not significant; PW: purified tap water.
Next, the relative abundance at the genus level of gut microbiota in all of the participants was compared before and after the test (Figure 6A and B). There was no significant change (increase or decrease) in the various genera that make up the Bacteroides and Firmicutes phyla in either the AEW group or the PW group between before and after the experiment. However, in the AEW group, the relative abundance of Bifidobacterium increased in nine participants (all but K), similar to the analysis of the Actinobacteria phylum, even among the genera comprising the Actinobacteria that significantly increased in number, with the mean value also increasing significantly (Figure 7A). The relative abundance of Bacteroides and Clostridium revealed a decreasing trend in the AEW group, though the difference was not statistically significant. Just as on the phylum level, the relative abundance of Bifidobacterium in the PW group showed an increase in five participants and a decrease in the other five (Figure 7B), with no significant increase or decrease.
Figure 6.
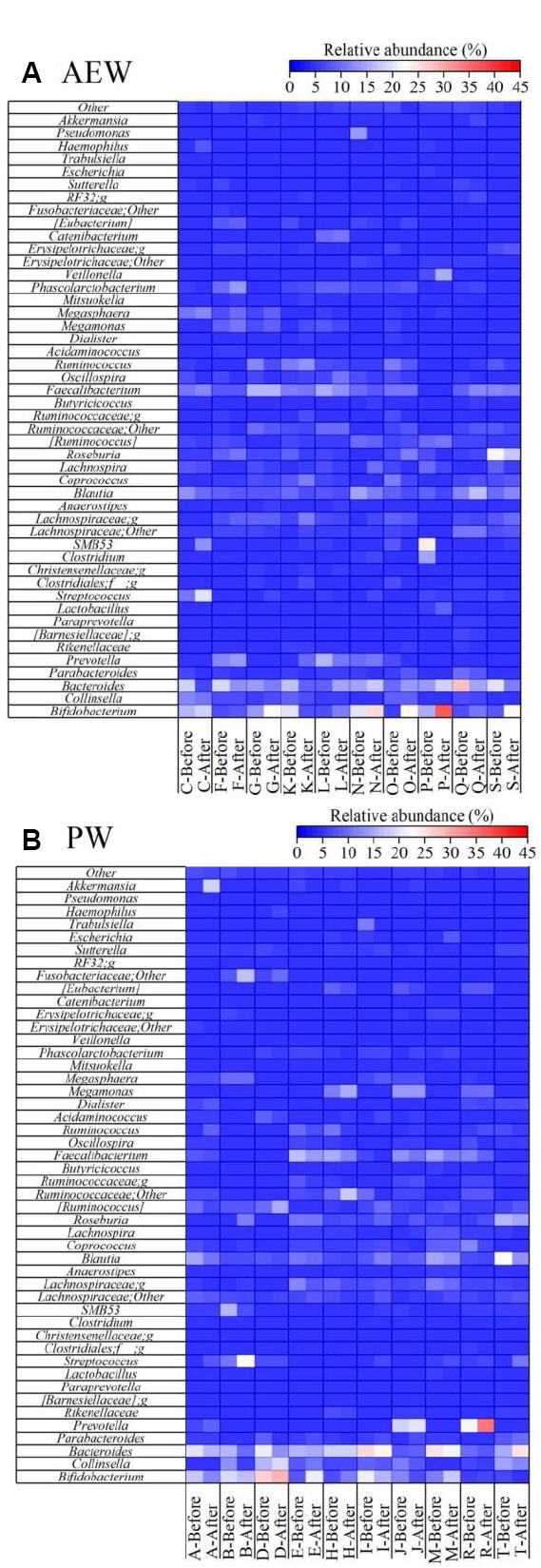
Heatmap of microbial communities (genus-level) in the stool samples, as obtained by next-generation sequencing in healthy participants before and after 2-week drinking either hydrogen-dissolved alkaline electrolyzed water (AEW, A) or purified tap water (PW, B).
Figure 7.
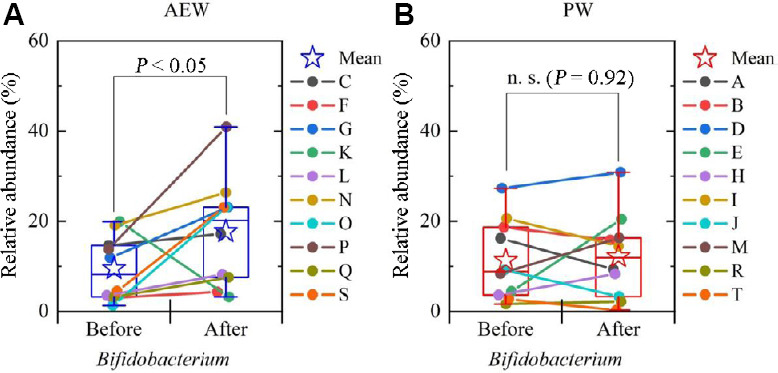
The change in mean value of the relative abundance of Bifidobacterium before and after the experiment (boxplot) and the change in each test participant (indicated by lines connecting “before” and “after” points on the graph) for the AEW group (A) and the PW group (B).
Note: Data are expressed as mean ± SD, and were analyzed by Wilcoxon signed-rank test. AEW: Alkaline electrolyzed water; n.s.: not significant; PW: purified tap water.
The closest relatives of OTU Cluster 0 and OTU Cluster 2, which were detected as the predominant OTUs among Bifidobacterium (which were significantly increased by AEW intake), were Bifidobacterium longum and Bifidobacterium adolescentis, respectively. As shown in Figure 8, both showed a significant increase (P < 0.05).
Figure 8.
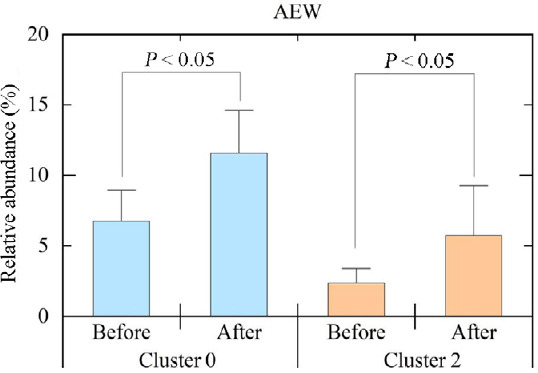
Comparison of OTU Cluster 0 and OTU Cluster 2, which were detected as predominant OTUs among Bifidobacterium (which were increased significantly by AEW intake) before and after ingesting the AEW.
Note: Data are expressed as mean ± SD, and were analyzed by Wilcoxon signed-rank test. AEW: Alkaline electrolyzed water; OTU: operational taxonomic unit.
Drinking AEW does not change gut microbiota drastically, but it is considered to act on specific bacteria, and this study was able to confirm that it caused an increase in Bifidobacterium.
Test of distribution of modified BSS values before and after ingestion
By performing an F-test on the modified BSS value population variance ratio before and after the experiment, we were able to confirm that the population variance of the Modified BSS value after the test was significantly smaller than that for the modified BSS value before the test; and that even in healthy participants, intake of AEW showed a significant tendency to cause stool consistency to converge to a “normal” state (Type 4). These results are shown in Figure 9A. With regard to the PW group, by performing an F-test on the modified BSS value population variance ratio before and after the test, we were able to confirm that there was no significant difference in population variance in Figure 9B.
Figure 9.
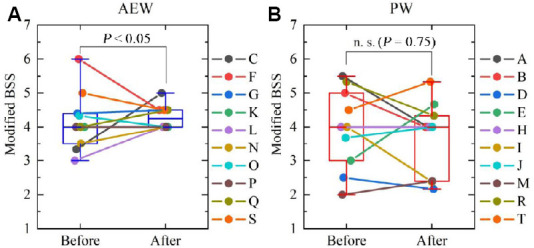
Comparison of modified Bristol stool scale (BSS) values for the 2-day period immediately after beginning the drinking test and the 2-day period immediately before finishing the drinking test for the AEW group (A) and the PW group (B).
Note: Data are expressed as mean ± SD, and were analyzed by F-test.
DISCUSSION
We compared, by means of a double-blind comparison test, the changes in gut microbiota and stool consistency induced by drinking either hydrogen-dissolved AEW or PW over a period of 2 weeks. The relative abundance of the Bifidobacterium genus increased in the AEW group, and showed a significant increase after the test compared to beforehand. Among Bifidobacterium, the closest relatives of OTU were Bifidobacterium longum and Bifidobacterium adolescentis, with both showing a significant increase. In addition, the AEW group saw their stool consistency significantly converge to BSS Type 4 (“normal”). Kohno et al.20 reported on the relationship between gut microbiota and stool consistency, and reported that by having humans ingest Bifidobacterium in the form of tablets, the gut microbiota composition was transformed and stool consistency was normalized.
Sha et al.12 published a report on changes in the relative abundance of Bifidobacterium resulting from intake of hydrogen water. It did not provide numerical data, but they reported that, prior to drinking AEW, the relative abundance of Bifidobacterium in their hydrogen water group was less than that of their control water group, but that the gap was largely eliminated after drinking AEW. In our study, we performed a comparative evaluation of two groups with largely equal (i.e., no significant difference) relative abundances of Bifidobacterium who drank either AEW containing hydrogen or PW, and were thereby able to confirm an increase in the Bifidobacterium population in the intestine as a result of drinking AEW. Consequently, we were able to support their findings. Although Sha et al.12 reported that the diversity of the gut microbiota changed significantly, our results indicated no recognizable or significant difference regarding alpha-diversity, and demonstrated the possibility of intake of AEW acting not on a wide range of bacteria but rather on specific types (Bifidobacterium in our present study).
For example, Blautia is a well-known intestinal bacterium that generates short-chain fatty acids via hydrogen metabolism.21 We assumed that Bifidobacterium might also metabolize hydrogen, but as Wolf et al.22 noted, “hydrogenases were entirely absent from the Bifidobacteria”, the probability of this occurring may be quite low. On the other hand, as reported by Shimamura et al.23,24 intestinal bacteria such as anaerobic Bifidobacteria are harmed by even small amounts of oxygen. Based on their behavior under microaerobic conditions, Rodríguez et al.25 classified Bifidobacteria into three classes: unable to grow (Class 1), able to grow (Class 2), and only able to grow in the presence of catalase (Class 3).
The Bifidobacterium longum seen to increase in our present study has high sensitivity to oxygen, is in Class 3, and can grow in the presence of catalase, which breaks down H2O2. Furthermore, Bifidobacterium adolescentis has exceptionally high (Class 1) sensitivity, and cannot grow even in the presence of traces of oxygen. With regard to the growth potential of anaerobic bacteria in low-oxygen environments, it is surmised that there is a relationship between the enzyme activity involved in the generation and elimination of substances such as reactive oxygen and H2O2. It can be hypothesized that hydrogen molecules, which exhibit antioxidant action, are involved in this oxidation/antioxidation pathway, and that this acts as an indirect growth factor for Bifidobacteria, which are highly sensitive to oxygen.
Before our study began, we did not anticipate any increase in specific bacterial species, so statistically relevant sample sizes could therefore not be determined in advance. We hope that future studies will be carried out on hydrogen as an antioxidant and that the reliability of the results obtained on gut microbiota can thereby be further secured.26,27,28,29,30,31 In this trial, male participants were selected. But the effects of drinking AEW on irritable bowel syndrome or the stool consistency have been reported using male and female mixed participants in previous researches.5,6 Therefore, we think that it is highly likely that the gut microbiota will be changed by drinking AEW, even in female.
Additional file
Additional file 1: CONSORT checklist.
Footnotes
Conflicts of interest
YT is a salaried employee of the Panasonic Corporation. This study does not alter our adherence to Medical Gas Research policies on sharing data and materials. Other authors report no conflict of interest related to this manuscript.
Financial support
This study was funded by Panasonic Co., Ltd.
Institutional review board statement
This study was retrospectively registered in University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN ID: UMIN000039507) on February 18, 2020, and was approved by the Ethics Committee of University of Yamanashi (approval No. H30-25) on January 9, 2018.
Declaration of participant consent
The authors certify that they have obtained all appropriate participant consent forms. In the form the participants have given their consent for the images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published.
Reporting statement
The writing and editing of the article were performed in accordance with the CONsolidated Standards Of Reporting Trials (CONSORT) Statement.
Biostatistics statement
The statistical methods of this study were reviewed by the biochemistry researcher of Yamanashi University, Japan.
Copyright transfer agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices). Study protocol and informed consent form will be available immediately following publication, without end date.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Funding: This study was funded by Panasonic Co., Ltd.
REFERENCES
- 1.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 2.Japanese Standards Association. Water electrolyzer for home use. JIS-T2004. 2018 [Google Scholar]
- 3.Naito Y, Takagi T, Uchiyama K, et al. Chronic administration with electrolyzed alkaline water inhibits aspirin-induced gastric mucosal injury in rats through the inhibition of tumor necrosis factor-α expression. J Clin Biochem Nutr. 2002;32:69–81. [Google Scholar]
- 4.Tashiro H, Kitahora T, Fujiyama Y, Bammba T. Clinical evaluation of alkaline-ionized water for chronic diarrhea: placebo-controlled double-blind study. Dig Absorpt. 2000;23:52–56. [Google Scholar]
- 5.Tanaka Y, Saihara Y, Izumotani K, Nakamura H. Daily ingestion of alkaline electrolyzed water containing hydrogen influences human health, including gastrointestinal symptoms. Med Gas Res. 2018;8:160–166. doi: 10.4103/2045-9912.248267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin DW, Yoon H, Kim HS, et al. Effects of alkaline-reduced drinking water on irritable bowel syndrome with diarrhea: a randomized double-blind, placebo-controlled pilot study. Evid Based Complement Alternat Med 2018. 2018 doi: 10.1155/2018/9147914. 9147914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takagi T, Naito Y, Inoue R, et al. Differences in gut microbiota associated with age, sex, and stool consistency in healthy Japanese subjects. J Gastroenterol. 2019;54:53–63. doi: 10.1007/s00535-018-1488-5. [DOI] [PubMed] [Google Scholar]
- 8.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 9.Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 2016;65:57–62. doi: 10.1136/gutjnl-2015-309618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins SM. A role for the gut microbiota in IBS. Nat Rev Gastroenterol Hepatol. 2014;11:497–505. doi: 10.1038/nrgastro.2014.40. [DOI] [PubMed] [Google Scholar]
- 11.Higashimura Y, Baba Y, Inoue R, et al. Effects of molecular hydrogen-dissolved alkaline electrolyzed water on intestinal environment in mice. Med Gas Res. 2018;8:6–11. doi: 10.4103/2045-9912.229597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sha JB, Zhang SS, Lu YM, et al. Effects of the long-term consumption of hydrogen-rich water on the antioxidant activity and the gut flora in female juvenile soccer players from Suzhou, China. Med Gas Res. 2018;8:135–143. doi: 10.4103/2045-9912.248263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka Y. Structure and function of alkaline ionized water apparatus. J Functional Water. 2017;12:29–34. [Google Scholar]
- 14.Hosomi K, Ohno H, Murakami H, et al. Method for preparing DNA from feces in guanidine thiocyanate solution affects 16S rRNA-based profiling of human microbiota diversity. Sci Rep. 2017;7:4339. doi: 10.1038/s41598-017-04511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naito Y, Takagi T, Inoue R, et al. Gut microbiota differences in elderly subjects between rural city Kyotango and urban city Kyoto: an age-gender-matched study. J Clin Biochem Nutr. 2019;65:125–131. doi: 10.3164/jcbn.19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugimoto T, Shima T, Amamoto R, et al. Impacts of habitual diets intake on gut microbial counts in healthy japanese adults. Nutrients. 2020;12:2414. doi: 10.3390/nu12082414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odamaki T, Kato K, Sugahara H, et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:90. doi: 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohno M, Yoshino T, Matsuura Y, Asada M, Kawahara Y. Effects of enteric capsules containing bifidobacteria and lactic acid bacteria on the fecal properties in healthy volunteers. J Intest Microbiol. 2004;18:87–92. [Google Scholar]
- 21.Rey FE, Faith JJ, Bain J, et al. Dissecting the in vivo metabolic potential of two human gut acetogens. J Biol Chem. 2010;285:22082–22090. doi: 10.1074/jbc.M110.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf PG, Biswas A, Morales SE, Greening C, Gaskins HR. H2 metabolism is widespread and diverse among human colonic microbes. Gut Microbes. 2016;7:235–245. doi: 10.1080/19490976.2016.1182288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimamura S, Abe F, Ishibashi N, et al. Relationship between oxygen sensitivity and oxygen metabolism of Bifidobacterium species. J Dairy Sci. 1992;75:3296–3306. doi: 10.3168/jds.S0022-0302(92)78105-3. [DOI] [PubMed] [Google Scholar]
- 24.Kawasaki S, Watanabe M, Fukiya S, Yokota A. Chapter 7 - Stress responses of bifidobacteria: oxygen and bile acid as the stressors. In: Mattarelli P, Biavati B, Holzapfel WH, Wood BJB, editors. The bifidobacteria and related organisms. Cambridge: Academic Press; 2018. pp. 131–143. [Google Scholar]
- 25.Rodríguez E, Peirotén Á, Landete JM, Medina M, Arqués JL. Gut catalase-positive bacteria cross-protect adjacent bifidobacteria from oxidative stress. Microbes Environ. 2015;30:270–272. doi: 10.1264/jsme2.ME15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostojic SM. Hydrogen-rich water as a modulator of gut microbiota? J Funct Foods. 2021;78:104360. [Google Scholar]
- 27.Shortt C, McGovern NM, Lafferty E, Bromley SA, Falck P, Pasricha PJ. S0480 portable hydrogen breath testing and microbiome analysis identifies prebiotic-induced increases in colonic fermentation and bifidobacterium. Am J Gastroenterol. 2020;115:S240. [Google Scholar]
- 28.Bordoni L, Gabbianelli R, Fedeli D, et al. Positive effect of an electrolyzed reduced water on gut permeability, fecal microbiota and liver in an animal model of Parkinson’s disease. PLoS One. 2019;14:e0223238. doi: 10.1371/journal.pone.0223238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lian N, Shen M, Zhang K, et al. Drinking hydrogen-rich water alleviates chemotherapy-induced neuropathic pain through the regulation of gut microbiota. J Pain Res. 2021;14:681–691. doi: 10.2147/JPR.S288289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao HW, Li Y, Luo D, et al. Hydrogen-water ameliorates radiation-induced gastrointestinal toxicity via MyD88’s effects on the gut microbiota. Exp Mol Med. 2018;50:e433. doi: 10.1038/emm.2017.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikeda M, Shimizu K, Ogura H, et al. Hydrogen-rich saline regulates intestinal barrier dysfunction, dysbiosis, and bacterial translocation in a murine model of sepsis. Shock. 2018;50:640–647. doi: 10.1097/SHK.0000000000001098. [DOI] [PubMed] [Google Scholar]


