Abstract
Background:
Voriconazole is a triazole antifungal agent approved by the US Food and Drug Administration for serious fungal infections, including with Aspergillus, Fusarium, Pseudallescheria, and Scedosporium species. In initial clinical trials, approximately 2% of patients developed cutaneous reactions, including photosensitivity, cheilitis, and xerosis. Subsequent reports have implicated voriconazole as a cause of severe photosensitivity and accelerated photoaging, pseudoporphyria cutanea tarda, and aggressive squamous cell carcinoma.
Observation:
We report 5 melanoma in situ lesions in the setting of extreme photosensitivity associated with long-term voriconazole therapy.
Conclusions:
We recommend surveillance for skin cancer formation in all patients who require long-term voriconazole treatment, particularly those who manifest signs or symptoms of photosensitivity or chronic photodamage. Further study of the mechanism underlying voriconazole photosensitivity and oncogenesis is warranted.
VORICONAZOLE IS A SECOND-generation triazole anti-fungal agent that acts through inhibition of cytochrome P450–dependent synthesis, a key step in fungal cell membrane lipid formation. Voriconazole was approved by the US Food and Drug Administration (FDA) in 2002 for the treatment of serious fungal infections, including with Fusarium and Aspergillus species.1–3 It also has demonstrated efficacy in esophageal candidiasis and in endemic mycoses such as histoplasmosis and coccidioidomycosis.4 In clinical trials for invasive aspergillosis, voriconazole demonstrated superior cure rates to amphotericin B and decreased toxic effects.5 Voriconazole therapy is generally well tolerated; the most common adverse effects include visual disturbances, gastrointestinal complaints (nausea, vomiting, and diarrhea), and cutaneous eruptions.6 Although photosensitivity was initially described in 1% to 2% of patients on long-term voriconazole regimens (>12 weeks of consecutive therapy), numerous reports in the literature have described scribed pseudoporphyria,7,8 photoaging with multiple lentigines, and premature dermatoheliosis.9 Furthermore, several recent reports of aggressive squamous cell carcinoma (SCC)10–13 have been described following voriconazole administration. Herein, we describe 5 melanoma in situ lesions arising in areas of chronic photodamage in 2 patients receiving long-term voriconazole therapy.
REPORT OF CASES
CASE 1
A 39-year-old woman with Fitzpatrick type III skin was referred to the University of California, San Francisco (UCSF), Department of Dermatology in May 2007 for severe photosensitivity and extensive lentigines on the photoexposed surfaces of the face, upper trunk, and extensor extremities. Prior to the development of photosensitivity, the patient worked in the field as a California Highway Patrol officer. She reported that she enjoyed spending time in the sun and had used tanning beds episodically between ages 23 and 30 years but was not actively seeking sun exposure or using tanning beds at the time of presentation to our clinic. She denied a history of frequent sun exposure as an adolescent or teenager and reported that she only sunburned on very rare occasions (less than once per year). She had no history of photosensitivity, melanoma, or nonmelanoma skin cancer prior to voriconazole therapy. There was no family history of skin cancer or photosensitive conditions.
In August of 1992, she was diagnosed as having coccidioidomycosis meningitis while residing in the Central Valley region of California. She was otherwise healthy, was not known to be immunodeficient, and resided in an area endemic for coccidioidomycosis. She had been maintained on oral fluconazole therapy until February 2004, at which time her infection became resistant to this therapy, and she required hospitalization. The fluconazole was replaced with oral voriconazole, 300 mg, twice daily, which she continued taking for the 3 years until her dermatologic evaluation. She initially was seen by an outside dermatologist in August of 2004 with a photodistributed vesicular eruption for which clinical presentation and histopathologic findings were consistent with pseudoporphyria, which was attributed to voriconazole.
Approximately 1 year after the voriconazole treatment was started, and continuing for the following 2 years, severe erythema developed in sun-exposed areas, and eruptive new pigmented macules appeared on the face, upper trunk, and extensor extremities. In January of 2007, after 35 months of voriconazole therapy, biopsy specimens taken from a 5-mm erythematous and brown macule on the right helix revealed a compound melanocytic neoplasm with evidence of regression; histopathologic findings from a subsequent reexcision demonstrated residual melanoma in situ (Figure 1). A 6-mm tan and erythematous macule on the dorsal surface of the patient’s left wrist was also biopsied at this time, and the specimen was interpreted to indicate an atypical lentiginous compound melanocytic nevus. Because this lesion extended to the periphery of the biopsy area, it was reexcised, but no residuum was identified.
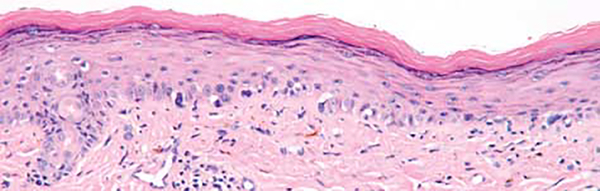
Figure 1.
Melanoma in situ, right ear (case 1). Single atypical intraepidermal melanocytes are seen in an irregular pattern along the dermoepidermal junction (hematoxylin-eosin, original magnification ×100).
At the time of presentation to the UCSF clinic, the patient had been taking hydroxychloroquine, 200 mg, by mouth twice daily for 25 months, as prescribed by her referring dermatologist, and applied mexoryl-based sunscreen daily (Anthelios; LaRoche-Posay, New York, New York), both for the management of her chronic photosensitivity. However, she acknowledged poor compliance with sun avoidance. She described ongoing severe erythema in sun-exposed areas as well as the progressive appearance of brown macules on her face, chest, and arms. Her medications on presentation included voriconazole, hydroxychloroquine, valacyclovir, levonorgestrel-ethinyl estradiol, and hydrochlorothiazide. Hydrochlorothiazide had been prescribed approximately 4 years prior for idiopathic lower extremity edema. Because of the potential for hydrochlorothiazide to cause photosensitivity, it was subsequently replaced by spironolactone and torsemide. However, her photosensitivity was unchanged following discontinuation of hydrochlorothiazide treatment.
Physical examination at the time of her initial visit revealed diffuse erythema and hyperpigmentation of the face, trunk, and extremities with a photodistributed accentuation. Innumerable tan pigmented macules were seen on the face, anterior chest, upper back, and extremities (Figure 2). Two hyperpigmented macules overlying the right mandible and the left forearm, each with an irregular border and greater pigmentation than the patient’s background lesions, were clinically suggestive of melanoma. Histopathologic analysis identified a solar lentigo with superimposed postinflammatory pigmentary alteration on the mandible and a lentiginous junctional melanocytic nevus with many associated superficial dermal melanophages on the arm.
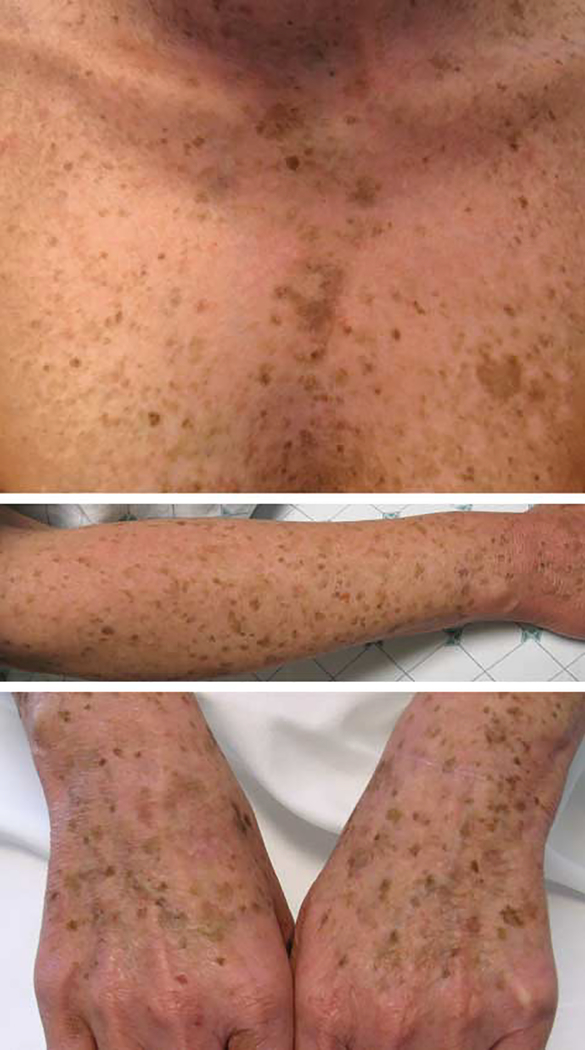
Figure 2.
Phototoxic effects and accelerated photoaging associated with chronic voriconazole therapy. Erythema, dermatoheliosis, and lentigines of the central chest (A), forearm (B), and dorsal surfaces of the hands (C) in a 39-year-old woman (case 1).
Five months later, the patient was seen at our pigmented lesion clinic with an ill-defined, 3.7 × 3.3-cm brownish-blue patch within a field of solar lentigines on severely sun-damaged skin on the mid-upper chest. A biopsy specimen demonstrated melanoma in situ, clinically consistent with lentigo maligna type. The patient underwent Mohs surgery with successful clearance. Permanent section from a debulking specimen demonstrated melanoma in situ without any evidence of an invasive component. The patient was diagnosed in September 2009 with 2 additional in situ melanomas, one on the right forearm and the other on the dorsal surface of the left hand, and underwent Mohs micrographic surgery for both lesions.
Following the second melanoma in situ diagnosis, voriconazole treatment was discontinued and therapy with fluconazole restarted. When fluconazole treatment led to alopecia, it was discontinued in October 2009, and treatment with itraconazole (200 mg, 3 times daily) was initiated. The patient plans to undergo close surveillance for additional melanomas that includes a total skin examination every 3 to 6 months in our clinic.
CASE 2
A 21-year-old man with Fitzpatrick type III skin and chronic granulomatous disease (CGD) began treatment with voriconazole, 200 mg, by mouth twice daily in March 2003 for pulmonary Aspergillus infection. The patient denied photosensitivity or a history of sunburn prior to voriconazole therapy. However, approximately 2 years after starting voriconazole treatment, he began to develop lentigines on sun-exposed areas of the body, including his face, forearms, and dorsal surfaces of his hands. He recalled an episode of blistering of both his upper and lower lips after a vacation in Florida in 2005. The patient reported that after starting voriconazole treatment, the sun seemed to bother him, and he subsequently avoided extensive sun exposure.
He did not have a family history of sun sensitivity, extensive lentigines, or melanoma, but his older sister was diagnosed as having basal cell cancer at age 19 years. He had been taking trimethoprim/sulfamethoxazole since age 5 days for bacterial prophylaxis following the diagnosis of CGD (dose at the time of presentation, 80 mg/400 mg, twice daily), but he denied photosensitivity while on this treatment regimen prior to beginning voriconazole treatment. The patient also was treated with oral isotretinoin, 30 mg/d, for approximately 6 months in 2007.
After approximately 55 months of voriconazole treatment, when the patient was 20 years old, an irregular, darkly pigmented macule was noted on his left forearm. A skin biopsy specimen revealed melanoma in situ; subsequent reexcision was performed 1 month later. Histopathologic analysis revealed cytologically atypical melanocytes arranged in nests and singly along the dermoepidermal junction, with pagetoid spread highlighted by a MART-1 stain (melanoma antigen recognized by T cells) (Figure 3).
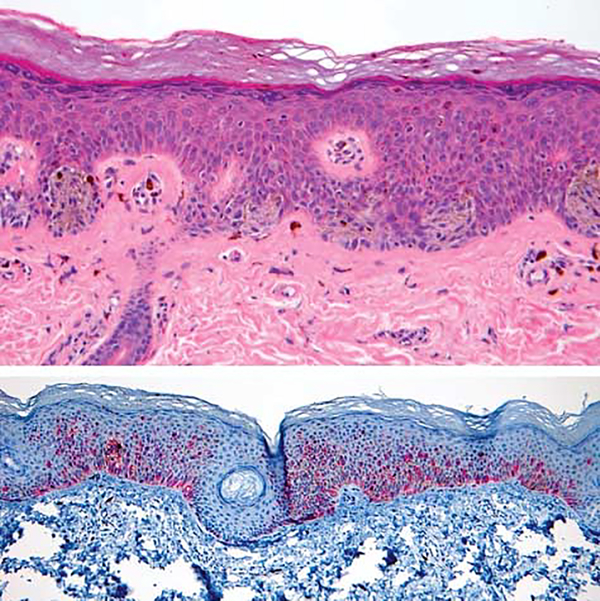
Figure 3.
Melanoma in situ, left forearm (case 2). A, Melanocytes are arranged in nests and singly along the dermoepidermal junction. The melanocytes are cytologically atypical and display pagetoid upward extension (hematoxylin-eosin, original magnification ×100). B, MART-1 stain (melanoma antigen recognized by T cells) highlighting pagetoid spread of cytologically atypical melanocytes (original magnification ×40).
On physical examination, he was found to have extensive brown, evenly pigmented lentigines varying in size between 2 and 10 mm. They involved photoexposed areas, including the face and dorsal surfaces of the hands and forearms (Figure 4). He had a well-healed scar on his left arm at the site of a previous melanoma in situ excision without residual pigmentation. Several months after his melanoma diagnosis, his antifungal therapy was changed from voriconazole to posaconazole. No subsequent lesions suggestive of malignancy were identified. The patient also described fading of the lentiginous pigmentation since he discontinued voriconazole therapy.
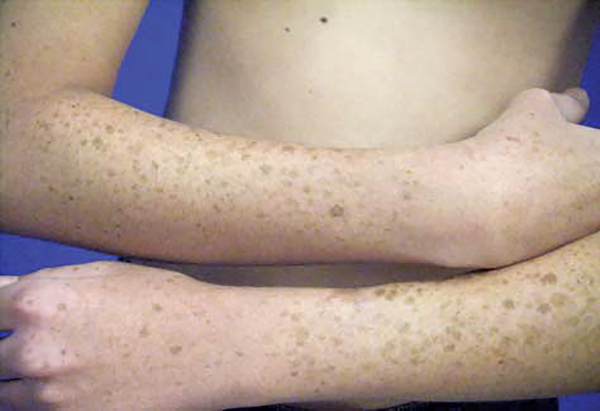
Figure 4.
Extensive lentigines of the dorsal forearms and hands in a 21-year-old man following long-term voriconazole treatment (case 2). Similar lentigines were also present on the face.
COMMENT
Following approval of voriconazole by the FDA in 2002, several case reports documented severe photosensitivity similar to that described by our patients. A constellation of erythema, blistering, dermatoheliosis, multiple lentigines, and new nevi was reported in a 15-year-old girl, with symptom onset occurring 5 weeks after initiating voriconazole therapy for a sinus infection with Curvularae species.9
Four case reports have also described a possible association between voriconazole and cutaneous SCC.10–13 In the first case,12 a patient with human immunodeficiency virus (HIV)/AIDS developed severe photosensitivity 6 months after starting voriconazole treatment, which abated within 15 days after treatment discontinuation. When the drug regimen was restarted 6 months later, the reaction reappeared and persisted throughout 19 months of additional therapy. The patient’s other medications included saquinavir, which has rarely been reported to induce photosensitivity.14 However, the photosensitive reaction abated promptly each time voriconazole treatment was stopped and reappeared on reintroduction. Multifocal SCC of the scalp was diagnosed after 3 years of voriconazole therapy.
In the second report,11 a 69-year-old man, status post–renal allograft and with photosensitivity symptoms, developed a large, poorly differentiated SCC 19 months after starting voriconazole treatment. No other photosensitizing agents were reported. The authors noted that the SCC appeared fewer than 2 years after transplant; by contrast, immunosuppression-related cutaneous malignant neoplasm in renal transplant is uncommon before 5 years posttransplant.
In the third case,10 a 29-year-old woman with CGD was treated with voriconazole for disseminated Aspergillus infection, and she subsequently developed erythema on areas of UV exposure. The patient was not reported to be using any other photosensitizing agents. Despite sun avoidance and sunscreen use, she developed SCC of the upper lip after 3 years of voriconazole therapy as well as multiple additional SCCs of the nose and cheeks the following year.
Although each of the 3 patients had some form of immune compromise that may have predisposed the individuals to cutaneous malignancy (CGD, HIV-AIDS, and iatrogenic immunosuppression post–renal transplant, respectively), in each case prominent photosensitivity and a temporal correlation between onset of therapy and the appearance of malignancy strongly implicated a contributing role of voriconazole.
In the fourth case report, our research group13 recently described 8 immunocompromised patients, including 2 children, who developed 51 cutaneous SCCs while receiving long-term voriconazole therapy. In several patients, aggressive high-risk tumors developed. Each patient also manifested additional signs of accelerated photodamage, including actinic keratosis and lentigo formation. Three patients were also taking trimethoprim/sulfamethoxazole, and 1 was taking dapsone; however, 4 patients were not using any concurrent photosensitizing agents.
The mechanism of voriconazole-induced photosensitivity is not known. The reaction pattern most closely resembles phototoxic effects, which typically manifest as sunburnlike erythema and tissue edema following sun exposure in the presence of an inciting chromophore. The action spectrum for most phototoxic reactions are UV-A wavelengths, 320 to 400 nm.15 Voriconazole does not absorb in the UV-A or UV-B ranges, although a specific metabolite, voriconazole N-oxide, may have absorption in both the UV-A and UV-B spectrum.16 Several authors have hypothesized that voriconazole may have indirect retinoid-like effects.17–20 Like voriconazole, retinoids cause photosensitivity, erythema, xerosis, and cheilitis. However, retinoid treatment does not lead to lentigo formation. Furthermore, systemic retinoid therapy is known to have a protective effect in patients at high risk for malignant neoplasms, including patients with xeroderma pigmentosum (XP) and immunosuppressed patients status post–solid organ transplant.21,22
The development of pronounced lentigines and melanoma in our patients bears clinical similarity to what happens in patients who have undergone long-term PUVA therapy and patients with XP. Stern et al23 demonstrated an increased risk of melanoma in patients receiving more than 250 treatments with psoralen plus UV-A photochemotherapy (PUVA). This risk was most pronounced more than 15 years from initial treatment (relative risk, 5.4 vs age-, sex-, and ethnicity-matched controls; 95% confidence interval, 2.2–11.1). Psoralen plus UV-A therapy involves a phototoxic reaction induced by the sensitizing agent methoxypsoralen, and patients frequently develop many small tan macules in exposed areas, so-called PUVA lentigines. These lentigines are histologically similar to the typical solar lentigo in that they demonstrate increased single melanocytes along the epidermal basal layer and clubbing of rete ridges, but they are different in that they have scattered large melanocytes with variable cytologic atypia.23
It is worth noting the similar clinical findings of pronounced lentiginosis and melanoma seen in the 2 patients described herein. The rapid onset of extensive lentigines as well as the short interval of voriconazole therapy before melanoma developed (relative to that seen due to PUVA) suggests perhaps an even more accelerated process of carcinogenesis.
The short duration between the initiation of voriconazole therapy and the onset of melanoma, the clinically severe phototoxic effects and photodamage, and the reports of SCC are all clinically reminiscent of patients with XP. As a result of genetic defects in nucleotide excision repair genes, patients with XP develop melanoma and nonmelanoma skin cancers early in life and have an approximate 1000-fold increased risk of melanoma. Recent data from a study of melanoma in patients with XP and non-XP controls identified a similar anatomic distribution and UV-induced PTEN mutations in both populations, thus implicating UV-induced mutations in tumor suppressor genes in melanomagenesis.24 Future comparisons between XP-associated melanomas and those occurring in the setting of voriconazole-associated phototoxic effects may provide valuable insight into the contribution of UV light to melanoma genesis.
Although establishing a definite causative role of voriconazole in the current melanoma cases is not possible, we believe the presence of accelerated photodamage in 2 relatively young patients, coupled with the recent reports supporting an association between voriconazole use and SCC, warrant further exploration of the role of the drug in skin cancer formation. Ideally, surveillance for photosensitivity and cutaneous malignant neoplasms should be prospectively included in future clinical trial design for the drug. Until such studies further define the skin cancer risk associated with voriconazole, we recommend surveillance for skin cancer formation in all patients who require long-term voriconazole treatment, particularly those who manifest signs or symptoms of photosensitivity or chronic photodamage.
Footnotes
Financial Disclosure: Dr McCalmont has served as a consultant for Cutera Lasers and as a medicolegal consultant for numerous law firms representing plaintiffs and defendants; no medicolegal consultancies involved voriconazole.
Contributor Information
Daniel D. Miller, Department of Dermatology, University of California, San Francisco.
Edward W. Cowen, Dermatology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Josephine C. Nguyen, Department of Dermatology, University of Pennsylvania, Philadelphia.
Timothy H. McCalmont, Department of Pathology, University of California, San Francisco; Department of Dermatology, University of California, San Francisco.
Lindy P. Fox, Department of Dermatology, University of California, San Francisco.
REFERENCES
- 1.Walsh TJ, Lutsar I, Driscoll T, et al. Voriconazole in the treatment of aspergillosis, scedosporiosis and other invasive fungal infections in children. Pediatr Infect Dis J. 2002;21(3):240–248. [DOI] [PubMed] [Google Scholar]
- 2.Johnson LB, Kauffman CA. Voriconazole: a new triazole antifungal agent. Clin Infect Dis. 2003;36(5):630–637. [DOI] [PubMed] [Google Scholar]
- 3.Hilliard T, Edwards S, Buchdahl R, et al. Voriconazole therapy in children with cystic fibrosis. J Cyst Fibros. 2005;4(4):215–220. [DOI] [PubMed] [Google Scholar]
- 4.Cortez KJ, Walsh TJ, Bennett JE. Successful treatment of coccidioidal meningitis with voriconazole. Clin Infect Dis. 2003;36(12):1619–1622. [DOI] [PubMed] [Google Scholar]
- 5.Herbrecht R, Denning DW, Patterson TF, et al. ; Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408–415. [DOI] [PubMed] [Google Scholar]
- 6.Baildon R. Pfizer global research and development: 2001. http://www.fda.gov/ohrms/dockets/ac/01/slides/3792s2_01_1-Pfizer-main/sld053.htm. Accessed August 29, 2009.
- 7.Tolland JP, McKeown PP, Corbett JR. Voriconazole-induced pseudoporphyria. Photodermatol Photoimmunol Photomed. 2007;23(1):29–31. [DOI] [PubMed] [Google Scholar]
- 8.Dolan CK, Hall MA, Blazes DL, Norwood CW. Pseudoporphyria as a result of voriconazole use: a case report. Int J Dermatol. 2004;43(10):768–771. [DOI] [PubMed] [Google Scholar]
- 9.Racette AJ, Roenigk HH Jr, Hansen R, Mendelson D, Park A. Photoaging and phototoxicity from long-term voriconazole treatment in a 15-year-old girl. J Am Acad Dermatol. 2005;52(5)(suppl 1):S81–S85. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy KL, Playford EG, Looke DF, Whitby M. Severe photosensitivity causing multifocal squamous cell carcinomas secondary to prolonged voriconazole therapy. Clin Infect Dis. 2007;44(5):e55–e56. [DOI] [PubMed] [Google Scholar]
- 11.Vanacker A, Fabre G, Van Dorpe J, Peetermans WE, Maes B. Aggressive cutaneous squamous cell carcinoma associated with prolonged voriconazole therapy in a renal transplant patient. Am J Transplant. 2008;8(4):877–880. [DOI] [PubMed] [Google Scholar]
- 12.Brunel AS, Fraisse T, Lechiche C, Pinzani V, Mauboussin JM, Sotto A. Multifocal squamous cell carcinomas in an HIV-infected patient with a long-term voriconazole therapy. AIDS. 2008;22(7):905–906. [DOI] [PubMed] [Google Scholar]
- 13.Cowen EW, Nguyen JC, Miller DD, et al. Chronic phototoxicity and aggressive squamous cell carcinoma of the skin in children and adults during treatment with voriconazole [published online November 5, 2009]. J Am Acad Dermatol. doi: 10.1016/j.jaad.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winter AJ, Pywell JM, Ilchyshyn JM, Fearn J, Natin D. Photosensitivity due to saquinavir. Genitourin Med. 1997;73(4):323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin: Clinical Dermatology. 10th ed. Philadelphia, PA: Saunders, Elsevier; 2006. [Google Scholar]
- 16.Murayama N, Imai N, Nakane T, Shimizu M, Yamazaki H. Roles of CYP3A4 and CYP2C19 in methyl hydroxylated and N-oxidized metabolite formation from voriconazole, a new anti-fungal agent, in human liver microsomes. Biochem Pharmacol. 2007;73(12):2020–2026. [DOI] [PubMed] [Google Scholar]
- 17.Auffret N, Janssen F, Chevalier P, Guillemain R, Amrein C, Le Beller C. Voriconazole photosensitivity: 7 cases. Ann Dermatol Venereol. 2006;133(4):330–332. [DOI] [PubMed] [Google Scholar]
- 18.Rubenstein M, Levy ML, Metry D. Voriconazole-induced retinoid-like photosensitivity in children. Pediatr Dermatol. 2004;21(6):675–678. [DOI] [PubMed] [Google Scholar]
- 19.Denning DW, Griffiths CE. Muco-cutaneous retinoid-effects and facial erythema related to the novel triazole antifungal agent voriconazole. Clin Exp Dermatol. 2001; 26(8):648–653. [DOI] [PubMed] [Google Scholar]
- 20.Van Wauwe JP, Coene MC, Goossens J, Cools W, Monbaliu J. Effects of cytochrome P-450 inhibitors on the in vivo metabolism of all-trans-retinoic acid in rats. J Pharmacol Exp Ther. 1990;252(1):365–369. [PubMed] [Google Scholar]
- 21.Kraemer KH, DiGiovanna JJ, Moshell AN, Tarone RE, Peck GL. Prevention of skin cancer in xeroderma pigmentosum with the use of oral isotretinoin. N Engl J Med. 1988;318(25):1633–1637. [DOI] [PubMed] [Google Scholar]
- 22.Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13(8): 1933–1938. [DOI] [PubMed] [Google Scholar]
- 23.Stern RS, Nichols KT, Vakeva LH. Malignant melanoma in patients treated for psoriasis methoxsalen (psoralen) and ultraviolet A radiation (PUVA). N Engl J Med. 1997;336(15):1041–1045. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Digiovanna JJ, Stern JB, et al. Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc Natl Acad Sci U S A. 2009;106 (15):6279–6284. [DOI] [PMC free article] [PubMed] [Google Scholar]