Abstract
Purpose:
Given the limitations of prostate specific antigen and standard biopsies for detecting prostate cancer, we evaluated the cancer detection rate and external validity of a magnetic resonance imaging/transrectal ultrasound fusion guided prostate biopsy system used at the National Institutes of Health.
Materials and Methods:
We performed a phase III trial of a magnetic resonance imaging/transrectal ultrasound fusion guided prostate biopsy system with participants enrolled between 2012 and 2013. A total of 153 men consented to the study and underwent 3 Tesla multiparametric magnetic resonance imaging with an endorectal coil for clinical suspicion of prostate cancer. Lesions were classified as low or moderate/high risk for prostate cancer. Magnetic resonance imaging/transrectal ultrasound fusion guided biopsy and standard 12-core prostate biopsy were performed and 105 men were eligible for analysis.
Results:
Mean patient age was 65.8 years and mean prostate specific antigen was 9.5 ng/ml. The overall cancer detection rate was 62.9% (66 of 105 patients). The cancer detection rate in those with moderate/high risk on imaging was 72.3% (47 of 65) vs 47.5% (19 of 40) in those classified as low risk for prostate cancer (p <0.05). Mean tumor core length was 4.6 and 3.7 mm for fusion biopsy and standard 12-core biopsy, respectively (p <0.05). Magnetic resonance imaging/transrectal ultrasound fusion guided biopsy detected prostate cancer that was missed by standard 12-core biopsy in 14.3% of cases (15 of 105), of which 86.7% (13 of 15) were clinically significant. This biopsy upgraded 23.5% of cancers (4 of 17) deemed clinically insignificant on 12-core biopsy to clinically significant prostate cancer necessitating treatment.
Conclusions:
Magnetic resonance imaging/transrectal ultrasound fusion guided biopsy can improve prostate cancer detection. The results of this trial support the external validity of this platform and may be the next step in the evolution of prostate cancer management.
Keywords: prostate, prostatic neoplasms, biopsy, magnetic resonance imaging, ultrasonography
SINCE the 1980s, screening methods for CaP have been static, including serum PSA measurement and DRE on a periodic basis. If either is abnormal or suspicious for cancer, TRUS guided systematic prostate biopsy is performed. Although this biopsy uses ultrasound guidance and selects defined zones in the prostate, there is no certainty that the actual tumor is being biopsied. CaP is the only solid organ tumor still diagnosed by a nontargeted sampling method. Using this approach for the last 30 years has yielded limited and varied results.1,2 The USPSTF recently categorized PSA based CaP screening as a grade D recommendation in a goal to prevent over diagnosis, overtreatment, and physical and emotional suffering.3 The USPSTF recommendation regarding PSA based screening was based on the inability to effectively select patients whose benefit from treatment would outweigh the harms associated with screening, diagnosis and treatment.
Improvements in MRI quality, technique and technology have led to increased use in patients with or suspected of having CaP. Although prostate MRI is limited in its ability to detect low grade cancer and lesions less than 5 mm, it is ideal to select patients with intermediate and high risk CaP with greater than 90% negative and positive predictive values.4,5
A new methodology of screening men for CaP was reported by NIH investigators in which patients with increased PSA underwent MP prostate MRI and areas suspicious for CaP were identified.6 In the office/outpatient setting prostate MR images of suspicious lesions/targets are fused with real-time TRUS biopsy techniques to guide needles to suspicious areas in the prostate using electromagnetic tracking. To our knowledge we report the first application of this newly Food and Drug Administration cleared UroNav MR/TRUS fusion guided prostate biopsy system (Invivo, Gainesville, Florida) outside a research hospital setting (NIH).
MATERIALS AND METHODS
We performed a phase III trial, MRI/TRUS Fusion Guided Prostate BiopsydAn Improved Way to Detect and Quantify Prostate Cancer, which was approved by our institutional review board (ClinicalTrials.gov NCT01566045). Enrollment began May 2012 and the results of this trial have not been published previously. Subjects with increased PSA/abnormal DRE and MP-MRI with suspicious lesion(s) were included in study.
All study participants underwent MP prostate specific 3 Tesla MRI using a Magnetom Verio device. MRI was obtained with a 16-channel Sense cardiac coil (Invivo) placed on the anterior pelvis and a BPX-30 ERC (Medrad, Warrenton, Pennsylvania) filled with PFC-770 (3M™). An ERC was used to detect CaP because of a reported 36% increase in sensitivity compared to that in patients without ERC use.7 Prostate specific pulse sequences included a minimum of triplanar T2-weighted, axial diffusion weighted with ADC mapping (B values 0, 500, 1,000, 1,500 and 2,000) and dynamic contrast enhanced MRI sequences according to European Society of Urogenital Radiology guidelines.8 Three radiologists (ARR, EB-L and RV) identified and graded all lesions suspicious for cancer according to the NIH risk stratification systems, NIH prostate zones and nonvisual reporting (fig. 1).8,9
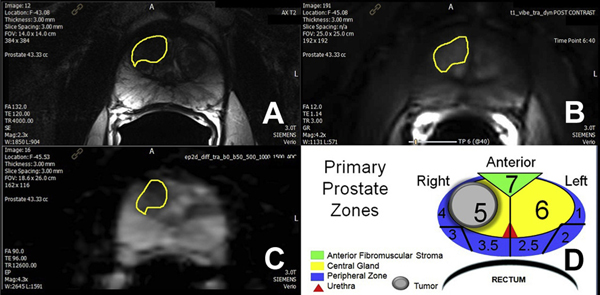
Figure 1.
Prostate MP-MRI in 66-year-old male with PSA 5.6 ng/ml. A, T2-weighted axial image shows anterior right central lesion with charcoal sign (yellow outline). B, dynamic contrast enhanced with type 3 focal enhancement curve. C, ADC map with ADC value 487 × 10−6 × mm2 per second. D, primary prostate zones with MR tumor volume (1.5 × 1.4 × 1.3 cm) = 1.4 cm3 and target core calculated volume (11 mm cancer) = 0.7 cm3.
The NIH MP-MRI scoring system was based on the number of positive sequences. A lesion was considered low risk if positive on 1 or 2 of the 3 sequences. If all 3 parameters were positive, the lesion was considered moderate/high risk.9 At the NIH a lesion is considered high risk if all 4 parameters are positive, including MR spectroscopy. MR spectroscopy was not performed in this trial due to cost, time and little impact on overall CDR, as previously reported by Turkbey et al.4 All lesions locations were recorded by T2 axial slice number and zone number. Each axial slice of the T2 sequence of the prostate was divided into 9 zones (fig. 1). All lesions are described as the respective zones of involvement for each MRI slice (3 mm).
Subjects with a positive MP-MRI entered the phase III trial. Demographics and common data elements, including prior prostate biopsy history, family history of CaP, PSA and prior imaging, were collected before protocol prostate biopsy. All data were collected prospectively.
The MR/TRUS fusion guided biopsy system is based on ultrasound guided rigid registration with visual correction using UroNav 3.0. All images were processed on a DynaCAD work station (Invivo) before biopsy. According to the protocol subjects underwent electromagnetically tracked MR/TRUS fusion guided biopsy of MRI suspicious lesions before standard 12-core TRUS guided biopsy (endfire iU22 Philips ultrasound). MR/TRUS fusion guided biopsy was completed first due to the significant edema that develops after 12-core biopsy, limiting fusion system performance. The principal investigator (ARR) was then blinded to target location by turning off the MR/TRUS fusion biopsy system. Standard 12-core biopsy was then performed under ultrasound guidance. All specimens were placed in separate pathology containers for each location. Our institutional pathologist (OY) reviewed all pathology slides.
Descriptive statistics were used to describe patient characteristics, including age, race, PSA, DRE, family history and previous biopsy results. All analyses were stratified by detection method, biopsy outcomes, NIH prostate scoring system, modified NIH prostate zones and the 5-point Likert scale using the chi-square test and Student t-test.8
RESULTS
During the study period 648 patients underwent MP-MRI for suspicion of CaP and 335 MRIs (52.4%) were positive for suspicious lesion(s). A total of 153 patients referred by primary urologists were consented for the study, of whom 32 were not included in analysis due to a prior CaP diagnosis. Also excluded were 16 men due to device failure (10), inclusion criteria not met (5) and inability to remain still for fusion biopsy (1). Thus, 105 consecutive subjects were included in this external validation cohort analysis. Table 1 lists demographics, and clinical and biopsy characteristics. MP-MRI detected a mean of 1.9 suspicious lesions per subject.
Table 1.
Cohort demographics, and clinical and biopsy characteristics
| Mean age (range) | 65.8 | (42–87) |
| Mean/median ng/ml PSA (range) | 9.2/7.52 | (0.6–62.0) |
| % Neg DRE (No. pts/total No.) | 80 | (84/105) |
| No. previous biopsy: | ||
| None | 35 | |
| Neg | 70 | |
| Mean No. suspicious lesions (range) | 1.9 | (1–3) |
| Mean No. cores: | ||
| Biopsy | 15.8 | |
| Targeted | 3.9 | |
| % CaP on biopsy (No. pts/total No.)* | 62.8 | (66/105) |
| % Clinically significant CaP on biopsy (No. pts/total No.)* | 48.6 | (51/105) |
| % Gleason grade (No. pts/total No.): | ||
| 6 | 28.8 | (19/66) |
| 7 | 54.5 | (36/66) |
| 8–10 | 16.7 | (11/66) |
Combined fusion and standard 12-core.
Did MRI Risk Stratification Score Impact CDR in Participants at Risk for CaP?
MP-MRI risk stratification systems resulted in a statistically significant difference in CDRs. Using the NIH scoring system the overall CDR, which included fusion biopsy and standard 12-core biopsy, was 47.5% (19 of 40) and 72.3% of cases (47 of 65) at low and moderate/high suspicion for cancer, respectively (p = 0.011). Overall CDRs for participants stratified by the 5-point Likert scale were 0%, 25.0% (2 of 8), 50.0% (23 of 46), 65.5% (19 of 29) and 100% (22 of 22) for grade 1 to 5 suspicious lesions, respectively (p <0.0001). The AUC using the 5-point Likert scale with respect to overall CDR was 0.74 (95% CI 0.66e0.83).
Did MR/TRUS Biopsy Outperform Standard Biopsy to Detect Clinically Significant CaP?
Clinically significant CaP was defined using the Epstein criteria as any Gleason pattern 4 or greater, or Gleason 3 + 3 disease with core length 50% or greater and/or more than 2 cores positive on standard 12-core TRUS guided biopy.10 Targeted core biopsy was considered clinically significant with any Gleason pattern 4 or greater, or MR lesion volume 0.2 cm3 or greater.11 The chi-square test was used to compare the CDR of MR/TRUS fusion guided biopsy and standard 12-core biopsy stratified by no cancer, no clinically significant CaP and clinically significant CaP (p <0.0001, table 2). Analysis was repeated in each subgroup stratified by NIH risk scores (p <0.0001, table 2). Some groups proposed using cancer core length or index tumor longest diameter to determine the clinical significance of the lesion. These 2 approaches could lead to overestimation or underestimation of actual tumor volume if the lesion is not symmetrical. All MR lesion volumes were calculated using all 3 dimensions by the ellipsoid formula, volume = length × width × height × 0.52, to calculate volume in cm3 (fig. 1). There was a 27.7% relative increase in the detection of clinically significant cancer using the fusion biopsy approach compared to the standard 12-core biopsy technique.
Table 2.
CaP risk by cancer biopsy method
| No. TRUS Guided 12-Core |
||||
|---|---|---|---|---|
| No. MRI/US Fusion Guided NIH Risk Suspicion (No. pts) | No Ca | Not Clinically Significant | Clinically Significant | Totals |
| Low (40):* | ||||
| No Ca | 21 | 5 | 1 | 27 |
| Not clinically significant | 2 | 0 | 0 | 2 |
| Clinically significant | 3 | 0 | 8 | 11 |
| Moderate/high (65):† | ||||
| No Ca | 18 | 4 | 3 | 25 |
| Not clinically significant | 0 | 4 | 0 | 4 |
| Clinically significant | 10 |
4 |
22 |
36 |
| Total No. | 54 | 17 | 34 | 105 |
p = 0.0001.
p <0.0001.
How Did CDR Results Stratified by Lesion and Overall CDR Compare to Initial NIH CDR?
Overall CDR and CDR per MRI suspicious lesions were comparable to those in the previously published cohort reported by Pinto et al (table 3).6 Coreby-core analysis resulted in a CDR of 37.9% (154 of 406 cases) for fusion guided biopsy and 12.5% (157 of 1,260) for standard 12-core biopsy (p <0.001). This was comparable to results in the NIH cohort, which showed an increased CDR per core by fusion with CaP revealed a statistically significant difference between standard 12-core and positive fusion biopsies (3.7 vs 4.6 mm, p = 0.01). Overall CDRs for lesions stratified by the 5-point Likert scale were 50% (1 of 2), 23.8% (5 of 21), 31.2% (29 of 93), 63.3% (38 of 60) and 96.3% (26 of 27) for grade 1 to 5 suspicious lesions, respectively (p <0.0001). Core length and lesion analysis using the 5-point Likert scale were not reported in the NIH cohort.
Table 3.
CDR in NIH and current validation cohorts
| % CDR (No. pts/total No.) |
||||
|---|---|---|---|---|
| MRI Suspicion | NIH | Validation | ||
| Overall participant level: | 54.4 | (55/101) | 62.9 | (66/105) |
| Low | 32.5 | (12/43) | 47.5 | (19/40) |
| Moderate/high | 74.1 | (43/58) | 72.3 | (47/65) |
| Fusion only participant level: | 43.6 | (44/101) | 50.5 | (53/105) |
| Low | 14.0 | (6/43) | 32.5 | (13/40) |
| Moderate/high | 65.5 | (38/58) | 61.5 | (40/65) |
| 12-Core only participant level: | 43.6 | (44/101) | 48.6 | (51/105) |
| Low | 18.6 | (8/43) | 35.0 | (14/40) |
| Moderate/high | 62.1 | (36/58) | 56.9 | (37/65) |
| Target lesion level: | 29.0 | (76/262) | 48.8 | (99/203) |
| Low | 14.6 | (23/158) | 32.6 | (28/86) |
| Moderate/high | 50.0 | (53/106) | 60.7 | (71/117) |
| Core positivity: | ||||
| 12-Core | 11.7 | (148/1,260) | 12.5 | (157/1,260) |
| Targeted biopsy | 20.6 | (108/524) | 37.9 | (154/406) |
What was Impact on CDR with MR/TRUS Biopsy Approach as Only Screening Method?
Cancer was detected in 50.5% of participants (53 of 105) when the fusion only biopsy approach was used (fig. 1). MR/TRUS fusion guided biopsy detected cancer in 14.3% of cases (15 of 105) missed by standard 12-core biopsy, including Gleason 8 in 4, Gleason 7 in 7, high volume Gleason 6 in 2 and low volume Gleason 6 in 2. MR/TRUS fusion guided biopsy also upgraded 23.5% of cancers (4 of 17) deemed clinically insignificant on 12-core biopsy to clinically significant CaP requiring treatment. However, the MR/TRUS fusion guided biopsy system missed 4 cases of clinically significant CaP. A Gleason 7 cancer was missed due to procedural error and 3 Gleason 7 cancers were missed due to small volume with a core length of 0.5, 2.5 and 2.5 mm, respectively. The remaining 9 cancers missed by the MR/TRUS fusion guided biopsy system were low volume, clinically insignificant Gleason 3 + 3 cancers.
DISCUSSION
This study is an external validation of this new, potentially office based technology that combines the increased sensitivity and specificity associated with prostate MP-MRI with the ease of use of the standard TRUS guided biopsy approach that most urologists use today.
The CDR of targeted biopsy was superior to that of standard 12-core biopsy and detected 12.4% more clinically significant cancers (13 of 105). It is possible that the performance of standard 12-core biopsy may have been overestimated since the same surgeon performed each biopsy. Although blinded, he had cognitive registration of the lesion location as well as the impact of MRI selection in patients undergoing biopsy.
Only 28.8% of subjects with CaP were diagnosed with low grade disease in our cohort. In contrast, the PLCO (Prostate, Lung, Colorectal and Ovarian) and ERSPSC (European Randomized Screening for Prostate Cancer) screening trials had a 57.3% to 60.3% incidence of low grade CaP.1,12
Our CDR per patient was higher than the published results by Pinto et al (62.8% vs 54.1% to 54.4%), although our cohort included more men with previous negative biopsy compared to the NIH cohort.6,13 However, multiple factors must be considered when comparing both MRI/TRUS fusion biopsy cohorts. MP-MRI quality is crucial when using these new technologies in regard to study technique and interpretation. Before the study the NIH molecular imaging program optimized our MR sequences for prostate imaging. Also, imaging conferences were held to discuss cases, review pathology findings and maintain quality control in regard to the interpretation and quality of prostate images. The increase in CDR compared to that in the NIH series could have been due to a higher threshold and/or selection bias for reporting a lesion as positive on MP-MRI. Of prostate MRIs interpreted at our institution 52% were considered positive, approximately 10% less than that reported in a systematic review of the literature on prostate MP-MRI.14 This is also evident in the difference in the mean number of lesions reported per patient in our study cohort vs the NIH cohort (1.9 vs 2.6). There was no randomization of subjects at either institution. Despite these limitations we implemented a MRI based screening program and similar results were achieved at these 2 centers.
Cost is a major limitation to implementing new diagnostic tests or technologies (genetic markers, PCA3 and/or imaging) for improved CaP risk stratification.15,16 Using 2013 Centers for Medicare and Medicaid Services data for processing prostate biopsy specimens if a fusion biopsy only approach was used, the cost saving from the decrease in the number of prostate biopsy specimens (which are reimbursed individually) submitted could offset 97% of the cost of prostate MP-MRI compared to standard 12-core biopsy. MRI also provides information on tumor volume, Gleason grade, and local and regional stage, which may lead to better treatment selection for patients or possibly no treatment at all. MP-MRI has a reported 92% accuracy for selecting patients for treatment vs active surveillance.17,18 Moreover, we are currently investigating prostate specific screening transabdominal MRI consisting of a single high resolution axial T2, a diffusion-weighted MRI with ADC maps and no ERC or contrast medium, which could have significantly decreased cost.
If a targeted only biopsy approach was used, 12.4% of CaP cases would have been missed (fig. 1), of which 3.8% (4 of 105) would have been clinically significant disease. This is far better than the performance of the standard 12-core biopsy, which misses 50% to 80% of clinically significant disease if present.19 Abd-Alazeez et al recently reported that MP-MRI had 89% to 100% negative predictive value to rule out clinically significant CaP in patients with negative MP-MRI who underwent template mapping biopsy.20 This supports the possible notion that biopsy may not be necessary in men with increased PSA and negative prostate MRI. In the near future if an image guided approach to CaP screening is implemented, the cost savings from eliminating unnecessary biopsies, over diagnosis and unnecessary treatment, biopsy complications and emotional distress in patients could offset the cost of the MR/TRUS fusion biopsy approach.
CONCLUSIONS
Given the limitations of PSA and the recent USPSTF recommendation against PSA based screening, a new paradigm for CaP screening and diagnosis is needed to improve patient selection for biopsy, treatment and/or observation. The combination of advances in MP-MRI and MRI/TRUS fusion guided prostate biopsy technologies have laid the foundation for a new approach to CaP detection and treatment by overcoming the inherent sampling errors and over diagnosis of low grade CaP associated with current strategies. This paradigm may aid in the accurate selection of men for CaP screening and possibly improve treatment decisions and outcomes.17
Figure 2.
Prostate CDRs of MRI/TRUS fusion guided biopsy by suspicion level on MRI using 5-point Likert scale and additional cancers detected only by 12-core TRUS guided biopsy.
ACKNOWLEDGMENTS
Invivo provided research equipment. NIH Molecular Imaging Program, Urological Oncology Branch and Center for Image Guided Oncology assisted with site setup.
Study received institutional review board approval.
Abbreviations and Acronyms
- ADC
apparent diffusion coefficient
- CaP
prostate cancer
- CDR
cancer detection rate
- DRE
digital rectal examination
- ERC
endorectal coil
- MP
multiparametric
- MR
magnetic resonance
- MRI
MR imaging
- NIH
National Institutes of Health
- PSA
prostate specific antigen
- TRUS
transrectal ultrasound
- USPSTF
United States Preventive Services Task Force
REFERENCES
- 1.Pinsky PF, Black A and Parnes HL: Prostate-cancer specific survival in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. Cancer Epidemiol 2012; 36: e401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schröder FH, Hugosson J and Roobol MJ: Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009; 360: 1320. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Preventive Services Task Force: Screening forProstate Cancer: U.S. Preventive Services Task Force Recommendation Statement. Release Date: May 2012. Rockville, Maryland: United States Preventive Services Task Force 2012. [Google Scholar]
- 4.Turkbey B, Mani H and Shah V: Multiparametric3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol 2011; 186: 1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haffner J, Lemaitre L and Puech P: Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int 2011; 108: E171. [DOI] [PubMed] [Google Scholar]
- 6.Pinto PA, Chung PH and Rastinehad AR: Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol 2011; 186: 1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turkbey B, Merino MJ and Gallardo EC: Comparison of endorectal coil and nonendorectal coil T2W and diffusion-weighted MRI at 3 Tesla for localizing prostate cancer: correlation with whole-mount histopathology. J Magn Reson Imaging 2013. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barentsz JO, Richenberg J, Clements R et al. : ESUR prostate MR guidelines 2012. European Society of Urogenital Radiology. Eur Radiol 2012; 22: 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rastinehad AR, Baccala AA Jr and Chung PH: D’Amico risk stratification correlates with degree of suspicion of prostate cancer on multiparametric magnetic resonance imaging. J Urol 2011; 185: 815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein JI, Walsh PC and Carmichael M: Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA 1994; 271: 368. [PubMed] [Google Scholar]
- 11.Ahmed HU, Hu Y and Carter T: Characterizing clinically significant prostate cancer using template prostate mapping biopsy. J Urol 2011; 186: 458. [DOI] [PubMed] [Google Scholar]
- 12.Schröder FH, Hugosson J and Roobol MJ: Prostate-cancer mortality at 11 years of follow-up. ERSPC Investigators. N Engl J Med 2012;366: 981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siddiqui MM, Rais-Bahrami S, Truong H et al. : Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol 2013; 64: 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore CM, Robertson NL and Arsanious N: Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol 2013; 63: 125. [DOI] [PubMed] [Google Scholar]
- 15.Gittelman MC, Hertzman B and Bailen J: PCA3 molecular urine test as a predictor of repeat prostate biopsy outcome in men with previous negative biopsies: a prospective multicenter clinical study. J Urol 2013; 190: 64. [DOI] [PubMed] [Google Scholar]
- 16.Stewart GD, Van Neste L and Delvenne P: Clinical utility of an epigenetic assay to detect occult prostate cancer in histopathologically negative biopsies: results of the MATLOC study. J Urol 2013; 189: 1110. [DOI] [PubMed] [Google Scholar]
- 17.Turkbey B, Mani H and Aras O: Prostate cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology 2013; 268: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stamatakis L, Siddiqui MM and Nix JW: Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer 2013; 119: 3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lecornet E, Ahmed HU and Hu Y: The accuracy of different biopsy strategies for the detection of clinically important prostate cancer: a computer simulation. J Urol 2012; 188: 974. [DOI] [PubMed] [Google Scholar]
- 20.Abd-Alazeez M, Kirkham A and Ahmed HU: Performance of multiparametric MRI in men at risk of prostate cancer before the first biopsy: a paired validating cohort study using template prostate mapping biopsies as the reference standard. Prostate Cancer Prostatic Dis 2014; 17: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]