Abstract
Two types of secretory vesicles co-exist at some presynaptic terminals. Clear synaptic vesicles (CSV) release their contents at the synaptic active zone, upon single impulses, while dense-core vesicles (DCV) usually release their contents in the periphery of the terminal upon repetitive stimulation. Part of the transmitter released by DCV diffuses to produce paracrine effects, and part of it reaches the postsynaptic terminal, adding its effect to that of synaptic release. This article presents an analytical method to separate the contribution of CSV and DCV to the postsynaptic responses, based on the kinetics of postsynaptic currents (PSCs). Since stimulation with single presynaptic impulses usually triggers release only from CSV, the kinetics of the resulting PSC can be used as a template to model the postsynaptic response to release from CSV during stimulation trains, accounting for the variations in the amplitude of PSCs due to short-term synaptic plasticity. Subtraction of this model simulation to the total recorded PSC renders the response to DCV peri‑synaptic release, which has slower kinetics. The method can be further simplified by measuring only the amplitudes of the PSC peaks for synaptic release and the integral of the current for peri‑synaptic release.
-
•
The postsynaptic current in response to presynaptic release from clear synaptic vesicles is modeled using the kinetics of the PSC in response to single impulses.
-
•
The model synaptic response is subtracted from the total recorded PSC to obtain the response to peri‑synaptic release from dense-core vesicles.
Keywords: Synaptic compartmentalization, Clear synaptic vesicles, Dense-core vesicles, Presynaptic terminal, Synaptic modeling, Synaptic plasticity
Graphical abstract

Specifications table
| Subject Area: | Neurophysiology |
| More specific subject area: | Synaptic physiology |
| Method name: | Separation of synaptic and peri‑synaptic components in postsynaptic currents |
| Name and reference of original method: | N/A |
| Resource availability: | Igor Pro, Matlab |
Background
At some chemical synapses, neurotransmitters are contained in clear vesicles, which dock at the active zone, and also in peri‑synaptic dense-core vesicles that surround the clear vesicles [3,12,14,16,19]. Neurotransmitter release from clear synaptic vesicles produces fast and localized responses exclusively at the postsynaptic neuron, in what has been called “wired” transmission. On the other hand, release from dense core vesicles is slower [21] and only part of the neurotransmitter released from peri‑synaptic dense core vesicles reaches the postsynaptic terminal, while most of it diffuses in the extracellular fluid and produces modulatory effects on surrounding neurons, thus participating in paracrine communication, which has also been called “volume transmission” [9,11]. The interrelation between these two modes of transmission seems to play crucial roles in the regulation of emotions, cognitive and motor functions in the central nervous system [1,2,10]. Stimulation of the presynaptic neuron with single impulses produces release of neurotransmitter quanta from clear but not from dense core vesicles [14,17,21]. On the other hand, repetitive stimulation produces facilitation and/or depression of synaptic transmission [7,15] and evokes release from dense core vesicles [4,5,14,21]. If both types of vesicles contain the same transmitter, such as in the case of some serotonergic neurons [4], then the postsynaptic response to the repetitive stimulation of the presynaptic neuron is composed by the addition of synaptic and peri‑synaptic release. This method was developed in order to analyze the synaptic and the peri‑synaptic components of these compound responses.
Method
This article focuses on a method for the analysis of postsynaptic currents (PSCs) at any synapse where presynaptic terminals contain both clear synaptic vesicles and dense-core peri‑synaptic vesicles releasing the same neurotransmitter, which acts on the same receptors or on different receptors leading to similar (i.e. depolarizing or hyperpolarizing) effects. To analyze the contribution of each vesicle type to the synaptic response, simultaneous recordings of the pre- and the post-synaptic neurons must be performed. The aim of this article is not to detail the recording methods, which have thoroughly been described elsewhere [6,8]. Recordings can be done with sharp intracellular electrodes in the case of big neurons or with whole-cell patch clamp in smaller neurons. In the case of synapses between leech Retzius and Pressure-sensory (P) neurons in culture, we used a sharp intracellular electrode in each neuron. The presynaptic Retzius neuron was recorded and stimulated under current clamp conditions and the postsynaptic currents (PSCs) in the P cell were recorded under single-electrode discontinuous voltage clamp conditions, digitalized with an A/D converter and stored in a PC. Presynaptic stimulation is performed by injecting square current pulses through the electrode, of sufficient amplitude and duration so that each pulse produces one action potential (AP). In the case of Retzius neurons, we used 10-ms pulses and the amplitude was adjusted for each cell.
In order to use the analysis method described below, presynaptic stimulation consists in producing a single (test) action potential (AP), followed after 5 s by a train of ten AP (Fig. 1A, B) with a frequency within the physiological dynamic range of firing of the neuron studied. The interval between the test AP and the beginning of the AP train can be varied, in case of synaptic facilitation or depression, and it must be sufficiently long so that the postsynaptic current in response to the first impulse in the train is similar to that produced by the test AP. Each synapse can be tested with several trials, allowing at least two minutes (or the time necessary for plasticity phenomena to extinguish after the previous stimulation train) for recovery between trials. If trains at different frequencies are tested, they should be presented in a random order.
Fig. 1.
Schematic representation of the stimulation, recording and modeling method to separate the synaptic and peri‑synaptic components of PSCs. A) Scheme showing a pair of neurons forming a synapse (Pre = presynaptic; Post = postsynaptic) and recorded with intracellular electrodes. The asterisk depicts the presynaptic terminal, which is shown in more detail in the inset in C. The trace above (I Pre) represents the stimulation protocol, with square current pulses delivered to the presynaptic neuron. Stimulation consists of a single pulse, followed, after several seconds by a train of ten pulses. The duration and amplitude of each pulse must be adjusted so that each pulse produces an action potential in the presynaptic neuron (as shown in B; the time scales in A is different to show the current pulses amplified). B) Schematic representation of the membrane potential of a presynaptic neuron (V Pre), stimulated with the protocol shown in (A) and firing a single action potential (Test AP) and a train of ten APs. C) Schematic representation of the postsynaptic current and the results of the modeling. The gray trace shows the experimentally recorded (total) PSC. From the single PSC in response to the test presynaptic AP a model PSC is obtained with Eq. (1) (black trace superimposed on gray trace). This same model current is used to simulate each of the individual PSCs during the train. The amplitude of each of these simulations (colored red to pink in the upper scheme) is adjusted by multiplying the model PSC by the amplitude factor obtained from the measurement of the individual amplitudes in the recording. Then all these simulated currents with adjusted amplitudes are linearly summed to obtain the synaptic current simulation (blue trace). This simulation is subtracted from the total recorded current to obtain the peri‑synaptic current simulation (green trace). The inset is a schematic representation of the presynaptic terminal (marked with an asterisk in (A), containing pools of synaptic clear vesicles (blue) and peri‑synaptic dense-core vesicles (green). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 1C shows representative PSCs in response to a single presynaptic AP and to a train of ten impulses at 10 Hz, in a Retzius-P synapse.
The analysis method is based upon the fact that single APs usually produce release from synaptic clear vesicles only [3,6,13,18]. In contrast, repetitive stimulation at increasing frequencies recruit also peri‑synaptic dense-core vesicles and thus the PSCs in response to trains of APs may contain mixed responses to neurotransmitter release from synaptic clear vesicles and from peri‑synaptic dense-core vesicles [4,16,20]. Since it can be assumed that the PSC in response to a single AP is produced only by release from clear synaptic vesicles, then the shape of this current can be used as a template to simulate what purely synaptic release would look like during stimulation trains, taking synaptic plasticity into account.
The test PSC for each trial is modeled with the sum of three exponentials (one for the onset of the current and two for its decay) using the equation:
| (1) |
The parameters of the equation can be fit using any curve-fitting software. We used Igor Pro (Wavemetrics, Inc., Lake Oswego, OR, USA). Supplementary material includes an Igor Pro-file (experiment) with the fitting function and procedures to do the simulations described below. To use this file, the data points forming the test PSC (excluding the stimulation artifacts) are copied from the recording file and pasted as a uni-dimensional wave in Igor. Eq. (1) has been fed in the curve fitting dialog box, found under the Analysis menu.
In order to fit data using a user-written function, initial guesses must be set for each of the coefficients in the Coefficients tab. For a PSC with an amplitude of 0.1 nA and time to peak of 20 ms, the following numbers can be used: A1=0.2 A2=0.1 A3=0.1 tau1=15 Tau2=25 tau3=60. Once all the coefficients are set, the “Do It” button is hit. Igor will find a fit to the data using the given equation. If the fit is not good, the initial guess for the coefficients can be changed.
Once a good fit for the single PSC is found, the equation is used to simulate each of the PSCs in response to each of the APs in the stimulation train.
The amplitude of the PSC in response to each AP during stimulation trains can vary due to short-term plasticity phenomena such as synaptic facilitation and depression, but this only changes the amplitude of the current and not its kinetics. Supplementary Fig. 1 shows kinetics for PSCs recorded from leech Retzius-P synapses in culture (see also [7,15] for references from other synapses). Therefore, one can simulate PSCs with the same kinetics as the test PSC and vary their amplitude for each stimulation pulse. The variations in amplitude are directly measured from the amplitude of the current peaks recorded experimentally (Fig. 2B). These amplitudes can be measured manually (for example using Clampfit software) or using a peak-detection algorithm (the Igor file included in supplementary material has a procedure that automatically does this). Each of these amplitudes is then divided by the amplitude of the test PSC and the resulting factor is used to multiply the amplitude of the model simulation obtained from the test PSC, in order to simulate the individual synaptic responses to each impulse in the train. A delay must be added to each of these simulations, according to the position of each current in the train (see Fig. 1C, colored traces) before summing all the simulations. Then all the individual current simulations in a train are linearly summed. The resulting waveform (Fig. 1C, blue trace) is a simulation of the postsynaptic response to synaptic release during the whole stimulation train. This simulation will be referred to as “synaptic simulation” and represents what the postsynaptic current would look like if only release from synaptic clear vesicles were activated during the train.
Fig. 2.
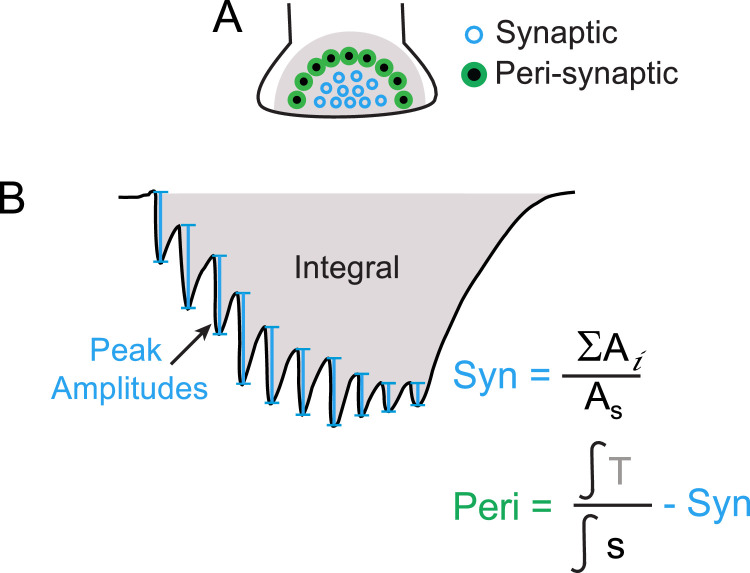
Schematic representation of the simplified analysis method. Release from synaptic clear vesicles (blue circles in A) is analyzed by measuring the amplitudes of individual postsynaptic current peaks in response to each action potential during trains of impulses (see blue vertical bars in B). The amplitudes of all peaks (Ai) are summed and divided by the amplitude of the single test PSC (As), recorded before the train (not shown here; see Fig. 1). Release from peri‑synaptic dense core vesicles (green and black circles in A) is analyzed by measuring the integral of the whole postsynaptic current in response to the stimulation train (T; shaded gray in B) and dividing it by the integral of the single (s) test PSC. Since the integral includes both synaptic and peri‑synaptic responses (gray shade in A), the synaptic component must be subtracted to the resulting ratio to obtain the peri‑synaptic component. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To obtain the response to peri‑synaptic release from dense-core vesicles during the stimulation train, the synaptic simulation (blue trace in Fig. 1C) for a given recording is subtracted from the total experimentally recorded postsynaptic current (gray trace in Fig. 1C). This renders a simulation of what the PSC would look like if only release from peri‑synaptic dense-core vesicles were activated during the stimulation train (Fig 1C, green trace).
All the simulations and analysis can be performed with custom-written routines in any programming plattform. Supplementary material contains a file for Igor Pro-software that includes an example of such routines, which readers can use. The file contains instructions for its use.
A simplified method to analyze the contribution of synaptic and peri‑synaptic release to PSCs
During stimulation trains the individual PSCs observed in response to each presynaptic impulse have similar kinetics to that in response to a single impulse, thus suggesting that the fast component of individual PSCs is produced by neurotransmitter release by synaptic (clear) vesicles. The amount of synaptic release is directly reflected in the amplitude of PSCs, and therefore the synaptic component can be measured from the amplitudes of the individual PSCs in response to each presynaptic action potential during stimulation trains, whose peaks are clearly identifiable in the recordings (see Fig. 2B, blue vertical bars). On the other hand, the peri‑synaptic component is a much slower response (Fig. 1C, green trace) that increases the area below the synaptic current. Therefore, the peri‑synaptic component is better reflected in the integral of the whole PSC in response to stimulation trains (Fig. 2A, B, gray shade).
With these considerations, the analysis of synaptic release can be simplified to measuring the amplitude of the individual PSCs following each presynaptic action potential. As shown above, these amplitudes (Fig. 2B) can be measured manually (for example using Clampfit software (Axon Instruments), or using a peak-detection automatic function. The amplitudes of the ten individual PSCs in a stimulation train are then summed and the sum is divided by the amplitude of the PSC in response to the single (test) AP preceding the train in the same synapse. This normalizes the data, rendering an index that reflects the average plasticity of synaptic release from clear vesicles during the train of impulses and allows the comparison of PSCs obtained from different synapses.
The normalized data obtained in this simplified manner for the synaptic component are shown in Fig. 3 plotted vs. those obtained with the modeling method described above. As can be seen, there is a linear correlation between the results obtained with both methods.
Fig. 3.
Validation of the simplified method. The model simulations and the simplified method were used to calculate the synaptic release in PSCs obtained from 23 stimulation trials with AP trains at different frequencies in synapses formed between Retzius and P neurons in culture. The amplitude of each individual postsynaptic current during the trains was divided by ten times the amplitude of the single test current and the average of these ratios for all the currents in a train are plotted in the abscissa. The ordinates show the release index of the synaptic simulation, calculated by dividing the integral of the modeled synaptic simulation of the response to the train by ten times the integral of the test current simulation. The linear relationship between the data obtained with the two methods shows that similar results are obtained with both of them.
To analyze the peri‑synaptic component from the area below the curve of the PSCs, the integral (which can be measured using Clampfit, Igor or any analysis software) of the whole PSC in response to the stimulation train (Fig. 2B, gray shade) is divided by the integral of the PSC in response to the test pulse. This renders a total release index, which also normalizes the data, allowing the comparison of PSCs obtained from different synapses. Since the integral of the PSC in response to trains of impulses includes the responses to both synaptic and peri‑synaptic release, the synaptic release index (obtained from the amplitudes as explained above) must then be subtracted from the total release index in order to estimate the peri‑synaptic component of release.
Since the results obtained with both methods are similar (Fig. 3), for the quantitative analysis of the total contribution of each component, the modeling presented in the first part of this article is not necessary. The first method, nevertheless, gives useful information about the kinetics of synaptic and peri‑synaptic release, which is not obtained with the simplified method.
Depending on the kinetics of the PSC, upon stimulation at high frequencies the peaks of the individual synaptic responses cannot be resolved because the rise time of the synaptic currents is longer than the interval between impulses. In these cases, the synaptic component of release can be estimated by dividing the amplitude of the total current in response to the stimulation train by the number of impulses in the train, and assigning equal amplitudes to the individual currents. This is valid when the sum of the postsynaptic responses is linear.
Declaration of Competing Interest
The Authors confirm that there are no conflicts of interest.
Acknowledgments
This work has been supported by CONACYT grant CB-252935.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.mex.2021.101374.
Appendix. Supplementary materials
References
- 1.Borroto-Escuela D.O., Ambrogini P., Chruścicka B., Lindskog M., Crespo-Ramirez M., Hernández-Mondragón J.C., Perez de la Mora M., Schellekens H., Fuxe K. The role of central serotonin neurons and 5-HT heteroreceptor complexes in the pathophysiology of depression: a historical perspective and future prospects. Int. J. Mol. Sci. 2021;22(4):1927. doi: 10.3390/ijms22041927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borroto-Escuela D.O., Perez De La Mora M., Manger P., Narváez M., Beggiato S., Crespo-Ramírez M., Navarro G., Wydra K., Díaz-Cabiale Z., Rivera A., Ferraro L., Tanganelli S., Filip M., Franco R., Fuxe K. Brain dopamine transmission in health and Parkinson’s disease: modulation of synaptic transmission and plasticity through volume transmission and dopamine heteroreceptors. Front. Synaptic Neurosci. 2018;10:20. doi: 10.3389/fnsyn.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruns D., Jahn R. Real-time measurement of transmitter release from single synaptic vesicles. Nature. 1995;377:62–65. doi: 10.1038/377062a0. [DOI] [PubMed] [Google Scholar]
- 4.Bruns D., Riedel D., Klingauf J., Jahn R. Quantal release of serotonin. Neuron. 2000;28:205–220. doi: 10.1016/s0896-6273(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 5.Consolo S., Baldi G., Russi G., Civenni G., Bartfai T., Vezzani A. Impulse flow dependency of galanin release in vivo in the rat ventral hippocampus. Proc. Natl. Acad. Sci. 1994;91:8047–8051. doi: 10.1073/pnas.91.17.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietzel I.D., Drapeau P., Nicholls J.G. Voltage dependence of 5-hydroxytryptamine release at a synapse between identified leech neurones in culture. J. Physiol. 1986;372:191–205. doi: 10.1113/jphysiol.1986.sp016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dittman J.S., Kreitzer A.C., Regehr W.G. Interplay between facilitation, depression, and residual calcium at three presynaptic terminals. J. Neurosci. 2000;20:1374–1385. doi: 10.1523/JNEUROSCI.20-04-01374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuchs P.A., Henderson L.P., Nicholls J.G. Chemical transmission between individual Retzius and sensory neurones of the leech in culture. J. Physiol. 1982;323:195–210. doi: 10.1113/jphysiol.1982.sp014068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuxe K., Agnati L.F. Two principle modes of electrochemical communication in the brain: volume versus wiring transmission. In: Fuxe K., Agnati L.F., editors. Volume Transmission in the Brain: Novel Mechanisms of Neuronal Transmission. Raven Press; New York: 1991. pp. 1–9. [Google Scholar]
- 10.Fuxe K., Borroto-Escuela D.O. Volume transmission and receptor-receptor interactions in heteroreceptor complexes: understanding the role of new concepts for brain communication. Neural Regen. Res. 2016;11:1220–1223. doi: 10.4103/1673-5374.189168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuxe K., Borroto-Escuela D.O., Romero-Fernandez W., Zhang W., Agnati L.F. Volume transmission and its different forms in the central nervous system. Chin. J. Integr. Med. 2013;19:323–329. doi: 10.1007/s11655-013-1455-1. [DOI] [PubMed] [Google Scholar]
- 12.Golding D.W., Bayraktaroglu E. Exocytosis of secretory granules – a probable mechanism for the release of neuromodulators in invertebrate neuropiles. Experientia. 1984;40:1277–1285. [Google Scholar]
- 13.Henderson L.P., Kuffler D.P., Nicholls J., Zhang R. Structural and functional analysis of synaptic transmission between identified leech neurones in culture. J. Physiol. 1983;340:347–358. doi: 10.1113/jphysiol.1983.sp014766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hökfelt T., Barde S., Xu Z.-.Q.D., Kuteeva E., Rüegg J., Le Maitre E., Risling M., Kehr J., Ihnatko R., Theodorsson E., Palkovits M., Deakin W., Bagdy G., Juhasz G., Prud’homme H.J., Mechawar N., Diaz-Heijtz R., Ögren S.O. Neuropeptide and small transmitter coexistence: fundamental studies and relevance to mental illness. Front. Neural Circ. 2018:12. doi: 10.3389/fncir.2018.00106. https://www.frontiersin.org/article/10.3389/fncir.2018.00106/full Available at: [Accessed April 20, 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreitzer A.C., Regehr W.G. Modulation of transmission during trains at a cerebellar synapse. J. Neurosci. 2000;20:1348–1357. doi: 10.1523/JNEUROSCI.20-04-01348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuffler D.P., Nicholls J., Drapeau P. Transmitter localization and vesicle turnover at a serotoninergic synapse between identified leech neurons in culture. J. Comp. Neurol. 1987;256:516–526. doi: 10.1002/cne.902560404. [DOI] [PubMed] [Google Scholar]
- 17.Lundberg J.M., Rudehill A., Sollevi A., Fried G., Wallin G. Co-release of neuropeptide Y and noradrenaline from pig spleen in vivo: importance of subcellular storage, nerve impulse frequency and pattern, feedback regulation and resupply by axonal transport. Neuroscience. 1989;28:475–486. doi: 10.1016/0306-4522(89)90193-0. [DOI] [PubMed] [Google Scholar]
- 18.Stewart R.R., Adams W.B., Nicholls J.G. Presynaptic calcium currents and facilitation of serotonin release at synapses between cultured leech neurones. J. Exp. Biol. 1989;144:1–12. doi: 10.1242/jeb.144.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Thureson-Klein A.K., Klein R.L. Exocytosis from neuronal large dense-cored vesicles. Int. Rev. Cytol. 1990;121:67–126. doi: 10.1016/s0074-7696(08)60659-2. [DOI] [PubMed] [Google Scholar]
- 20.Trueta C., De-Miguel F.F. 2021. Synaptic versus extrasynaptic exocytosis of serotonin by a single neuron: frequency-dependecy and the amounts of transmitter released. Sometido.
- 21.Xia X., Lessmann V., Martin T.F.J. Imaging of evoked dense-core-vesicle exocytosis in hippocampal neurons reveals long latencies and kiss-and-run fusion events. J. Cell Sci. 2009;122:75–82. doi: 10.1242/jcs.034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.