Graphical abstract
Keywords: Sono/photodynamic treatment, Shrimp surimi, Microbial community, Curcumin
Highlights
-
•
Sono/photodynamic treatment inhibited bacteria growth in shrimp surimi.
-
•
Sono/photodynamic treatment altered microbial community during storage.
-
•
Sono/photodynamic treatment preserved the quality of shrimp surimi.
Abstract
Shrimp surimi is widely acknowledged as a value-added shrimp product due to its delicious taste, rich flavor, and nutrition. However, the refrigerated shrimp surimi is prone to deterioration due to rapid microbial growth during storage. The present study sought to assess the effects of curcumin-mediated sono/photodynamic treatment on bacterial spoilage and shrimp surimi quality stored at 4 °C. The total viable count (TVC), microbiota composition, and quality parameters, including the total volatile basic nitrogen (TVB-N), thiobarbituric acid reactive substance (TBARs), and pH were investigated. The results showed that the spoilage bacteria in shrimp surimi rapidly increased with a surge on day 2 during refrigeration storage. The Psychrobacter and Brochothrix were identified as the Specific Spoilage Organisms (SSOs), which were also positively correlated with TVB-N and TBARs. The results further elucidated that the sono/photodynamic treatment could significantly inhibit the growth of SSOs on the surface and interior of shrimp surimi and delay shrimp surimi quality deterioration. In conclusion, the sono/photodynamic treatment as a non-thermal sterilization method could be a reliable and potential method for inactivating spoilage microorganisms and preserving shrimp surimi quality.
1. Introduction
In recent years, shrimp surimi has extensively attracted the attention of consumers as a value-added food due to its delicious taste, high-protein, and low-fat content. The shrimp surimi consists of shrimp with egg white, starch, and other ingredients, repeatedly grounded to form the creamy appearance and excellent gelling properties [1]. However, shrimp surimi is vulnerable to microbial contamination and thus becomes spoilage-prone [2]. So far, frozen storage is the most widely adopted preservation method that enables the long-term storage of shrimp surimi. However, this method significantly deteriorates shrimp quality and taste [1]. Similarly, refrigerated storage between 0 and 4 °C has been widely adopted for short-term shrimp surimi storage, which maintains its flavor, color, texture, and nutrients [3], thus making the refrigerated shrimp surimi increasingly popular. For instance, most hot-pot restaurants prefer refrigerated shrimp surimi due to its better taste and popularity among consumers. However, the very short shelf life of refrigerated shrimp surimi increases tremendous challenges for inventory management, and sometimes results great waste. Besides, this increases the outbreak risk of safety issues. Thus, developing safe and effective approaches for the preservation of refrigerated shrimp surimi is highly necessitated.
Sono/photodynamic treatment (SPDT) is a novel non-thermal sterilization method based on photodynamic treatment (PDT) and sonodynamic therapy (SDT). PDT takes advantages of the bactericidal activity of reactive oxygen species (ROS) generated by light irradiating photosensitizers (PSs). However, the depth of visible light penetration to activate PS is limited, restricting the sterilization of surimi due to the rapid growth of spoilage bacteria in the interior of surimi. SDT shares a similar bactericidal mechanism but replacing light with a low-frequency ultrasonic wave to activate the sensitizers [4]. Particularly, certain sensitizers could be activated simultaneously by light and ultrasound to produce ROS. Therefore, the sono/photodynamic treatment (SPDT) might combine the advantages of both PDT and SDT as a novel sterilization method. For instance, N. Grevika [5] compared the killing efficacy of PDT, SDT, and SPDT against Staphylococcus aureus biofilm and found the highest microbicidal efficacy by SPDT. Similarly, M. Pourhajibagher [6] found that the cell viability, metabolic activity, and biofilm growth in Aggregatibacter actinomycetemcomitans could be effectively reduced by SPDT using curcumin-decorated nanophytosomes.
Curcumin, as a food additive approved by the European Food Safety Authority, is a yellow pigment isolated from Curcuma longa [7], [8], [9], [10]. At present, several studies have reported that curcumin, activated by ultrasonic sound and blue LED light, showed desirable bactericidal activity against a range of bacteria, such as Staphylococcus aureus [11], [12], Pseudomonas aeruginosa [13], and Escherichia coli [14], [15]. Therefore, the antibacterial properties and the changes in shrimp surimi quality during the curcumin-based sono/photodynamic sterilization at 4 °C storage were evaluated in this study based on the adequate penetration activity of ultrasound.
2. Materials and methods
2.1. Photo/sonosensitizer, light and ultrasonic sound source
Curcumin (IUPAC name: (1E,6E)-1,7-Bis(4-hydroxy-3-methox-yphenyl) hepta-1,6-diene-3,5-dione) was purchased from Sigma-Aldrich (St. Louis, MO, USA). A stock solution of curcumin (25 mM) was prepared in ethanol and then diluted in sterile water to obtain the concentrations to be tested. A light-emitting diode (LED) (M425L, Zolix Instruments Co., Ltd, Beijing, China) with peak emission wavelength of 425 nm was used as the light source. Ultrasonic generator XH300B (Beijing Xianghao Tech CO., LTD, Beijing, China) was used to generate ultrasound at 24 KHz.
2.2. Sample treatment
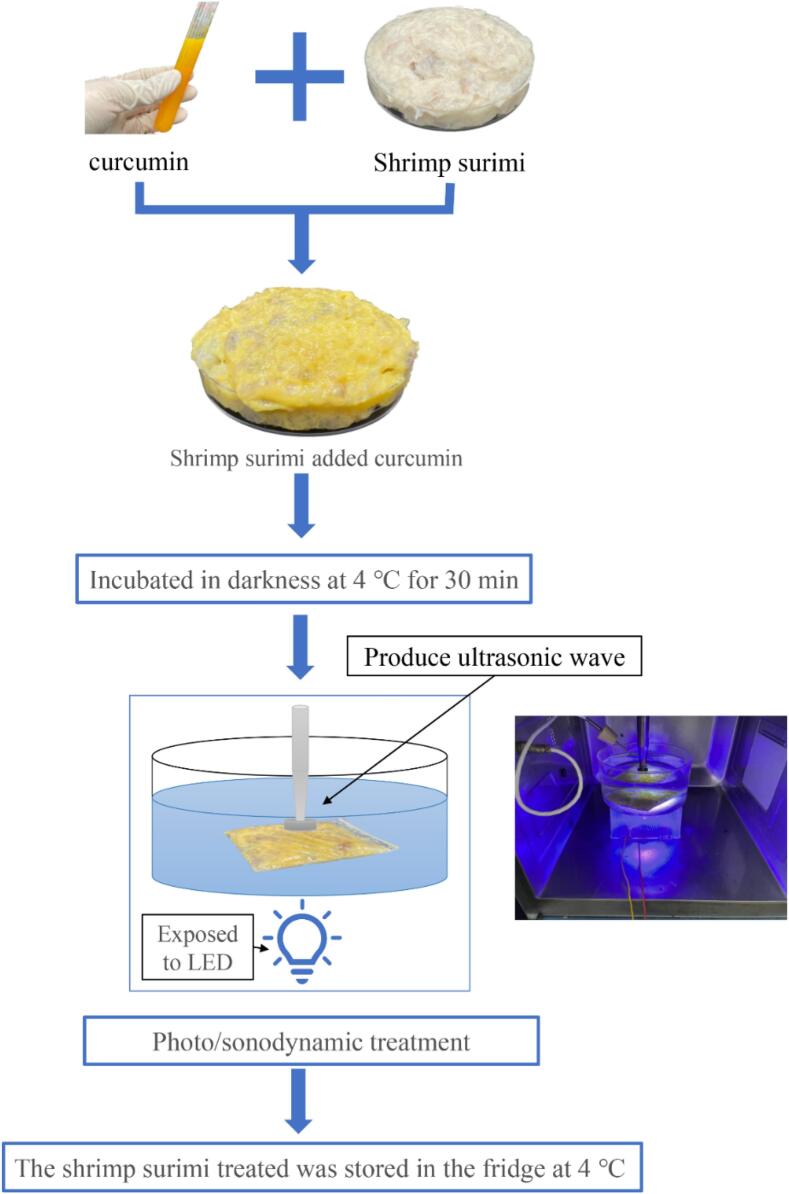
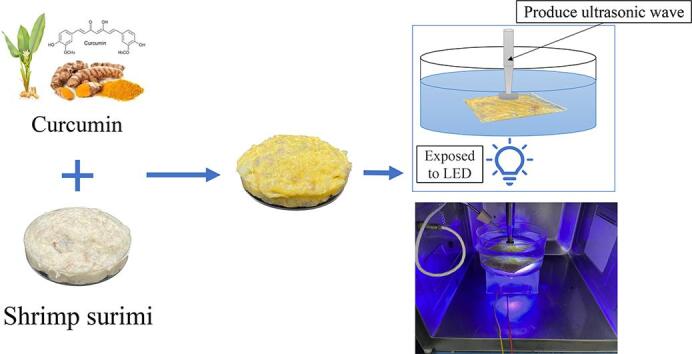
Shrimp surimi was provided by Haixin Foods Co., Ltd, which was composed of shrimp (>90% w/w), starch and egg white. The procedure of sono/photodynamic treatment of shrimp surimi samples was illustrated in Fig. 1. Briefly, the shrimp surimi samples were mixed with curcumin and then processed with ultrasound and blue lights. For each batch, 25 g shrimp surimi was treated in water (volume 6 × 7.52 × π cm3) in a crystallizing dish, and the distance between the ultrasonic probe and the shrimp surimi was 2 cm. Then the shrimp surimi samples were packed in airtight PE bag (7 × 15 cm, 0.12 mm; JGQUA, Zhejiang, China) and stored at 4℃ for subsequent quality and bacterial analyses.
Fig. 1.
The process of sono/photodynamic treatment for shrimp surimi.
2.3. Orthogonal test
An orthogonal L9(33) test design was used for the optimization of sono/photodynamic treatment (Table 1). Total viable counts (TVC) of surimi samples upon SPDT treatment at different parameters (curcumin concentration at 200, 400 or 600 nmol/g, ultrasonic power at 300, 600 or 900 W, and sono/photodynamic treatment for 15, 30 or 45 min) were determined.
Table 1.
Orthogonal test factors and levels.
| Levels | Factors |
||
|---|---|---|---|
| A Curcumin concentration (nmol/g) | B Ultrasonic power /W | C Sono/photodynamic treatment time/min | |
| 1 | 200 | 300 | 15 |
| 2 | 400 | 600 | 30 |
| 3 | 600 | 900 | 45 |
2.4. Determination of TVC
TVC was determined according to the method of Li [16]. Briefly, 10 g of shrimp surimi were homogenized by a food processer (JYL-C012, Joyoung Co., Ltd. China) in 90 mL stroke-physiological saline solution. This initial sample suspension (1/10) and its subsequent dilutions were prepared in accordance with the procedures described previously, and inoculated on aerobic plate count agar (PCA) (CM0325, Oxoid, Hampshire, UK). After incubation at 30 °C for 72 h, the number of colony-forming units (CFU) was counted. Each assay was performed in triplicates.
2.5. 16S microbiome analysis
Samples for microbiota analysis were collected from shrimp surimi samples on days 0 (immediately after treatment), 1, 3 and 6 during the refrigerated storage. For microbial DNA extraction, E.Z.N.A.® bacteria DNA Kit (Omega Bio-tek, Norcross, GA, U.S.) was used according to the manufacturer protocol. Then, the V3-V4 hypervariable regions of the bacteria 16S rRNA gene were amplified with primers 338F (5′- ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) by thermocycler PCR system (GeneAmp 9700, ABI, USA). The PCR products was extracted from a 2% agarose gel and further purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) and quantified using QuantiFluor™-ST (Promega, USA) according to the manufacturer’s protocol. Samples with bright strip between 400 and 450 bp were chose for further analyses. The amplicon library was prepared with a Sample Preparation Kit (Illumina, USA) and sequenced on an Illumina Miseq platform according to standard protocols. Sequences with 97% similarity were defined as an operational taxonomic unit (OTU) and the representative sequence reads of various OTUs were hierarchically classified into different taxa. Beta diversity was calculated to evaluate species richness and genetic diversity of the microbial community according to Y. Zhang [17].
2.6. Determination of TVB-N, TBARs, and pH
TVB-N value was determined according to the method by Jia [18] with minor modification. Briefly, 4 g of shrimp surimi was dispersed in 40 mL distilled water and homogenized by a food processer for 3 min. And then the TVB-N was determined by Kjeldahl Apparatus (KDY9820, Beijing, China). TBARs was determined as described by Deyang Li [19] with minor modification. Briefly, TBARs was calculated by multiplying the absorbance with a coefficient of 7.8 and expressed as mg malondialdehyde (MDA) eq/kg sample. Meanwhile, the pH of the supernatant was measured using a digital pH meter (Mettler Toledo FE20/EL20, Shanghai, China).
2.7. Electronic tongue evaluation
Difference in taste of the shrimp surimi was measured by electronic tongue evaluation system according to the method of Pattarapon [20]. The shrimp surimi was diluted and stirred with purified water at the ratio of 1:5, and then centrifuged. The supernatant was taken for testing with the taste analysis system TS-5000Z (INSENT, Japan). Reference solution (simulated saliva) was selected as 30 mM KCl + 0.3 mM tartaric acid. The tasteless points for the taste components were observed to be −13 for sourness, −6 for saltiness, and 0 for others [21].
2.8. Statistical analysis
The differences in TVC, TVB-N, TBARs, and pH among samples were assessed using one-way ANOVA analysis of variance in SPSS 20. Differences were considered significant if P < 0.05. Data graphs were drawn using GraphPad Prism 8.0 software. Microbiota analysis was performed using Majorbio Cloud Platform (www.majorbio.com). The correlation among physicochemical indicators and microbial populations were conducted by Pearson correlation analysis using R software version 3.5.1. and RStudio (R Package, USA), and the significance was verified via Student’s t-test.
3. Results
3.1. The effects of sono/photodynamic treatment on TVC of shrimp surimi
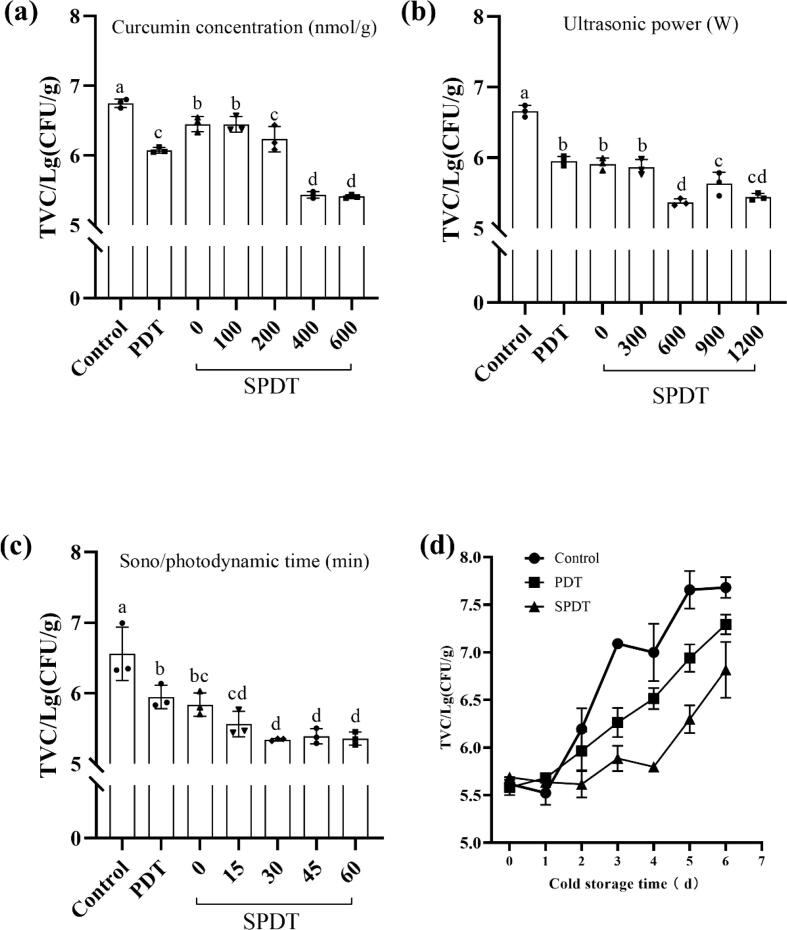
As depicted in Fig. 2, the TVC of shrimp surimi showed a continuous increase with a surge on day 2 during storage at 4 °C. This result revealed that the refrigerated shrimp surimi could only last 2–3 days. A previous study suggested that the edibility and market value of shrimp products are severely jeopardized when their TVC reaches 106 CFU/g [22]. The results further demonstrated both PDT and SPDT treatment could significantly decline the increase in TVC, with SPDT showing better bactericidal effects. The TVC of SPDT treated samples were kept below 106 CFU/g till day 4.
Fig. 2.
Bactericidal effects of SPDT on shrimp surimi. (a-c) The effects of curcumin concentration, ultrasonic power and treatment duration on the total viable count (TVC) of shrimp surimi samples after refrigerated storage for 3 days. (d) The changes in total viable count (TVC) of shrimp surimi samples during the refrigerated storage for 6 days. Control: the samples without treatment before refrigerated storage; PDT: the samples with photodynamic treatment for 30 min with 400 nmol/g curcumin before storage; SPDT: the samples with sono/photodynamic treatment for 30 min at 600 W with 400 nmol/g curcumin before storage.)
The single-factor analyses suggested that the desirable bactericidal effects (>1.3 lg (CFU/mL) decrease) could be achieved when curcumin concentration was above 400 nmol/g, the ultrasonic power > 600 W, and the sono/photodynamic treatment time was longer than 30 min.
Based on the single-factor analyses, curcumin concentration, ultrasonic power, and treatment time were further optimized using an orthogonal L9 (33) test design. As summarized in Table 2, the results indicated that the minimum TVC (5.89 lg CFU/g) of shrimp surimi was obtained when the curcumin concentration, ultrasonic power, and sono/photodynamic treatment time were 400 nmol/g, 300 W, 30 min (A2B1C2), respectively. It is noteworthy that curcumin concentration plays an essential factor in determining TVC. Thus, this optimized parameter was selected for further experiments.
Table 2.
Orthogonal test results.
| No. | A curcumin concentration | B ultrasonic power | C sono/photodynamic treatment | TVC |
|---|---|---|---|---|
| 1 | 200 | 600 | 45 | 6.13 |
| 2 | 200 | 900 | 30 | 6.27 |
| 3 | 200 | 300 | 15 | 6.66 |
| 4 | 400 | 900 | 45 | 6.03 |
| 5 | 400 | 600 | 15 | 6.37 |
| 6 | 400 | 300 | 30 | 6.20 |
| 7 | 600 | 900 | 15 | 6.27 |
| 8 | 600 | 300 | 45 | 6.05 |
| 9 | 600 | 600 | 30 | 5.89 |
| K1 | 19.06 | 18.44 | 18.39 | |
| K2 | 18.61 | 18.69 | 18.20 | |
| K3 | 24.49 | 18.75 | 19.30 | |
| R | 5.88 | 0.32 | 1.10 | |
| The optimal level | A2 | B1 | C2 | |
| Primary and secondary factors | A > C > B | |||
| Optimal combination | A2B1C2 | |||
3.2. The effects of sono/photodynamic treatment on microbial community during storage
High-throughput sequencing analysis based on the Illumina HiSeq2500 platform was performed to assess diversity in microbial communities of shrimp surimi during cold storage and identify the specific spoilage organisms (SSOs) responsible for spoilage of refrigerated shrimp surimi. A total of 1,760,599 sequences (after quality trimming) from the 32 shrimp surimi samples were yielded (raw data can be accessed from NCBI's Sequence Read Archive with SRP324256). The coverage rates of all groups were more than 99%, indicating the reliability of the sequencing data for further study.
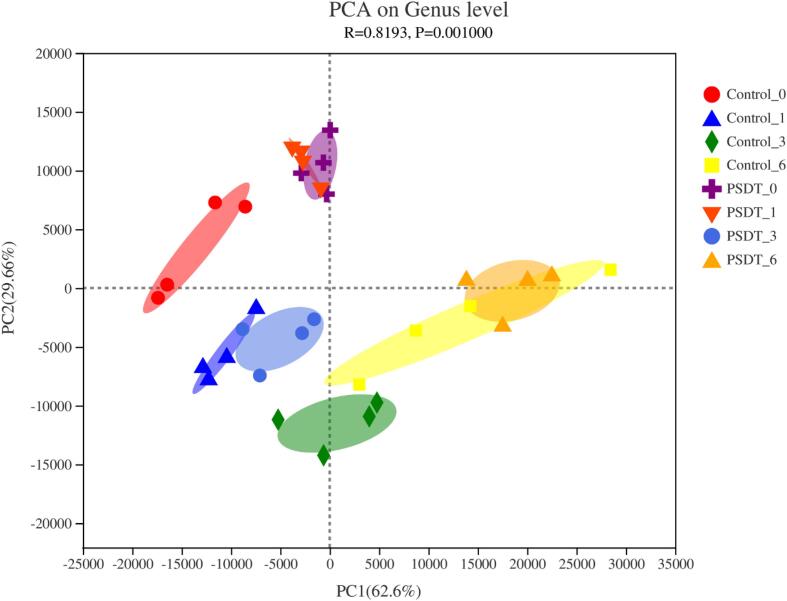
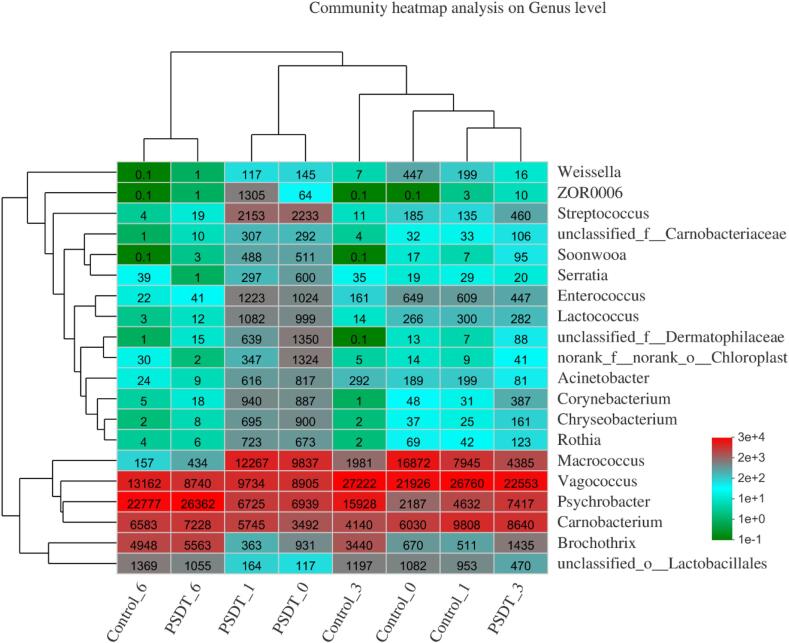
β-Diversity visualized by the principal component analysis (PCA) also supported that SPDT could decline the changes in microbial community during the storage (Fig. 3). The maximum variations in the microbiota of different shrimp surimi samples were 62.6% (PC1) and 29.66% (PC2), suggesting a strong separation by region. As shown in Fig. 4d, the untreated control samples showed significant differences at different time points, while the samples on day 0 and day 1 (SPDT 0 and SPDT 1) were similar in the treatment group. Moreover, the PCA results showed similar microbiota composition in control 1, and SPDT 3 samples, which was consistent with the heat-map generated clustering results.
Fig. 3.
The principal coordinates analysis (PCA) of the microbiota from the control group and the sono/photodynamic treatment group during the refrigerated storage at 4 °C.
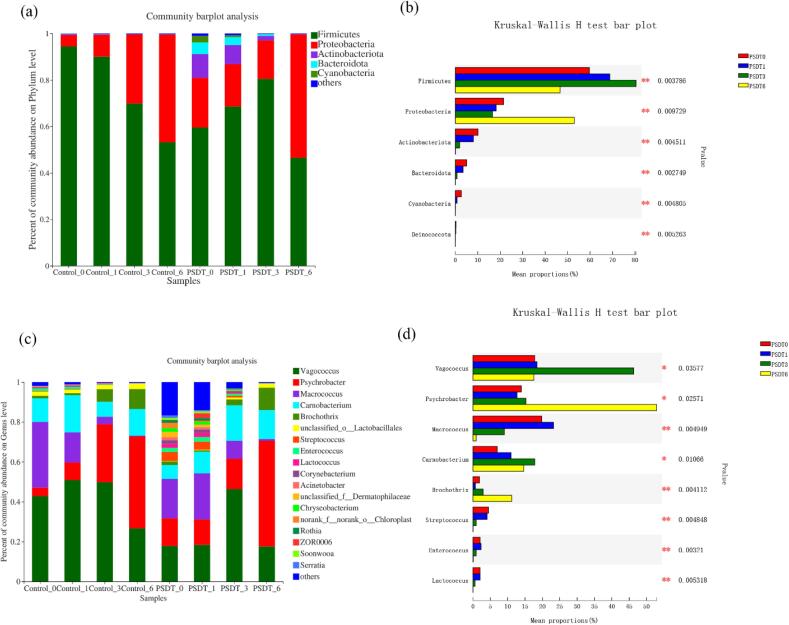
Fig. 4.
Relative abundance of the bacteria of shrimp surimi samples during refrigerated based on Illumina-MiSeq sequencing. The statistically significant differences were analyzed using Kruskal-Wallis H test.
At the phylum level (Fig. 4a, Fig. 4b), the shrimp surimi bacterial profile of on day 0 (Con 0) was dominated by Firmicutes (94.44%). As expected, the SPDT treated shrimp surimi (SPDT 0) had a similar bacterial community profile as the control group (CON 0) on day 0. Notably, proteobacteria rapidly increased in the untreated control group, reaching 46.36% on the sixth day, while proteobacteria showed no significant changes before the third day in the SPDT treated samples, and Firmicutes remained as the dominant bacteria. However, on the sixth day, proteobacteria also dominated the microbial community in SPDT treated samples.
At the genus level (Fig. 4c, Fig. 4d), the shrimp surimi was dominated by Vagococcus, Psychrobacter, Macrococcus, and Carnobacterium. In the control group, Psychrobacter showed a significant increase from 4.27% on day 0 to 46.15% on day 6, which was similar to the increasing trend of Brochothrix from 1.31% to 10.02%. In contrast, Macrococcus decreased from 39.94% on day 0 to 0.31% on day 6. Similarly, Vagococcus showed a slight increase in the first 3 days (from 42.81% to 49.79%) but rapidly decreased to 26.67% on day 6. These changes were consistent with the treated group, i.e., Psychrobacter became dominant during the storage. Notably, the Psychrobacter and Brochothrix remained steady up to three days storage, but increased to 52.99% and 11.18%, respectively, on day 6.
A heat-map was generated to analyze and compare the relative abundances and dynamic changes in the microbial community at genus levels (top 20 genera are shown) in all samples at different time points during storage (Fig. 5). According to the clustering results, the bacterial communities of SPDT-treated samples on day 3 shared the most similarity with the untreated samples on day 1. This result suggested that SPDT treatment could retard the bacterial changes in the first 3–4 days during the refrigerated storage. The changes in the relative abundance of microorganisms elucidated that Psychrobacter and Brochothrix had a significant increase from 2187 and 670 on day 0 to 22777, and 4948 on day 6, respectively, in the control group.
Fig. 5.
The heat-map of microbiota composition at the genus level in the control group and the sono/photodynamic treatment group during the refrigerated storage at 4 °C.
3.3. Changes of quality in shrimp surimi treated with/without SPDT
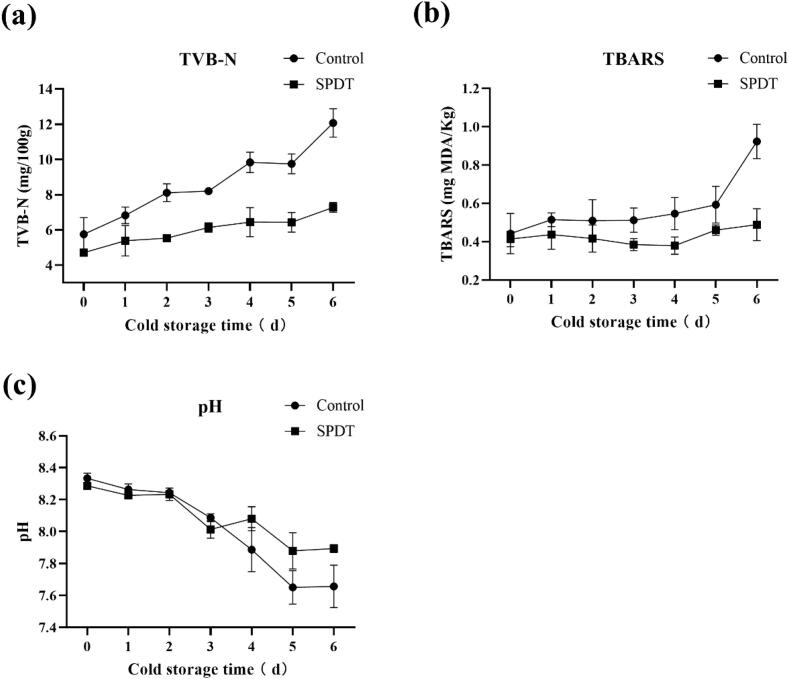
TVB-N is one of the most important indicators to assess food deterioration. Hence, TVB-N was measured to further evaluate the preservative effects of sono/photodynamic treatment on shrimp surimi. As shown in Fig. 6a, the sono/photodynamic treatment could effectively inhibit the increase in TVB-N. The TVB-N values of all samples at day 0 were below 6 mg/ 100 g. In the control samples, TVB-N increased continually to over 12.1 mg/ 100 g after 6 days of refrigerated storage, while the samples with sono/photodynamic treatment showed significantly lower TVB-N values (7.28 mg/ 100 g). Similarly, TBARs also showed a time-dependent increase during the 6 days of storage (Fig. 6b), while sono/photodynamic treatment significantly inhibited this upward trend. Fig. 6c depicts the changes in pH of the shrimp surimi stored at 4 °C. In the control group, the pH decreased from 8.34 to 7.65. The sono/photodynamic treatment prevented the shrimp surimi from being sour. On the 4th day of storage, the pH of the treatment group was significantly higher than in the control group (P < 0.05) and remained 7.88 for the next two days of storage.
Fig. 6.
The effects of sono/photodynamic treatment on the changes in TVB-N, TBARs, and pH of shrimp surimi samples during the refrigerated storage at 4 °C.
Further, the electronic tongue system was employed to evaluate surimi taste by measuring eight basic senses of taste: sourness, bitterness, astringency, aftertaste-B, aftertaste-A, umami, richness, and saltiness, as presented in Table 3. The electronic tongue showed that the SPDT treated samples stored for 6 days had higher scores in umami, saltiness, aftertaste-A, and bitterness, while the untreated surimi had higher sourness and astringency after 6 days. Additionally, the aftertaste-B showed no significant difference in all the samples. This observation suggested that sono/photodynamic treatment could retain the umami and saltiness in the surimi samples but develop some bitterness and aftertaste A. On the contrary, this treatment also decreased sourness and astringency.
Table 3.
The effect of sono/photodynamic treatment on the eight flavors by electronic tongue.
| Cold storage time | Sourness | Bitterness | Astringency | Aftertaste-B | Aftertaste-A | Umami | Richness | Saltiness | |
|---|---|---|---|---|---|---|---|---|---|
| Control | 0 | −50.17 ± 0.40a | 9.00 ± 0.03a | 2.96 ± 0.11a | −0.99 ± 0.19a | −1.16 ± 0.02b | 19.46 ± 0.08b | −5.46 ± 0.30d | 27.15 ± 0.03a |
| 3 | −49.94 ± 0.36a | 8.13 ± 0.09c | 2.45 ± 0.14b | −1.14 ± 0.10a | −1.25 ± 0.04c | 19.20 ± 0.02d | −4.95 ± 0.17c | 25.67 ± 0.08c | |
| 6 | −48.18 ± 0.43b | 6.52 ± 0.06e | 1.98 ± 0.11c | −1.05 ± 0.14a | −1.23 ± 0.01c | 18.11 ± 0.04e | −4.09 ± 0.18a | 22.65 ± 0.01f | |
| 0 | −50.72 ± 0.42a | 8.61 ± 0.06b | 2.28 ± 0.13b | −0.98 ± 0.13a | −1.07 ± 0.02a | 19.80 ± 0.04a | −4.67 ± 0.20bc | 26.37 ± 0.14b | |
| SPDT | 3 | −50.46 ± 0.41a | 8.05 ± 0.10c | 1.96 ± 0.15c | −0.99 ± 0.05a | −1.11 ± 0.02ab | 19.46 ± 0.02b | −4.47 ± 0.23abc | 25.08 ± 0.04d |
| 6 | −50.64 ± 0.33a | 7.59 ± 0.02d | 1.61 ± 0.13d | −0.98 ± 0.09a | −1.13 ± 0.03ab | 19.3 ± 0.04c | −4.32 ± 0.24ab | 24.42 ± 0.04e | |
3.4. Pearson correlation analysis among TVB-N, TBRAS, taste, and microbial
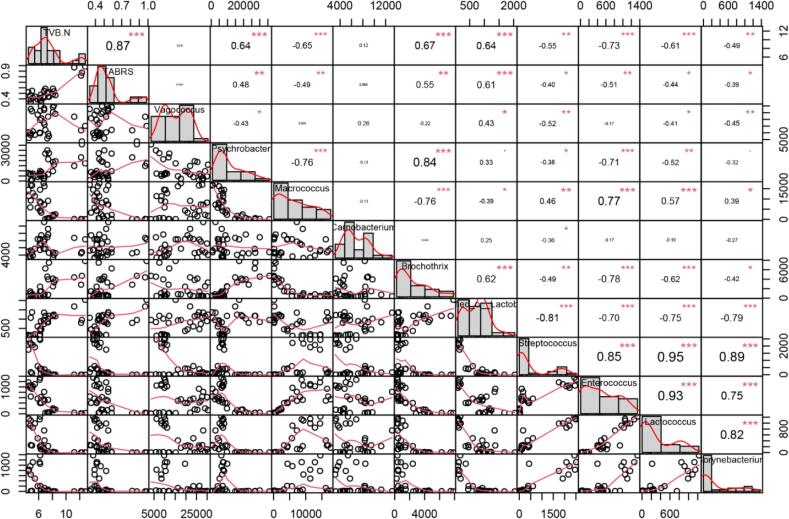
As depicted in Fig. 7, the Pearson correlation analysis was employed to analyze the correlation of TBRAS, TVB-N values with the top 10 abundant microbial composition at the genus level as inputs. Notably, TVB-N and TBARs as deteriorative indicators showed a significant positive correlation. Moreover, Psychrobacter and Brochothrix showed a significantly positive correlation with TVB-N and TBARs, and a significantly negative correlation with Micrococcus, Streptococcus, Enterococcus, Lactococcus, and Corynebacterium. Additionally, the results revealed a correlation between bacteria. For instance, Psychrobacter and Brochothrix might promote each other’s growth (positive correlation), while Psychrobacter and Enterococcus might share a competitive relationship (negative correlation).
Fig. 7.
The correlation between microorganisms in shrimp surimi samples and deteriorative indicators (TVB-N and TBARs). The correlation was calculated by Pearson Correlation Analysis. Significant correlations were indicated by *(P < 0.05), **(P < 0.01), ***(P < 0.001).
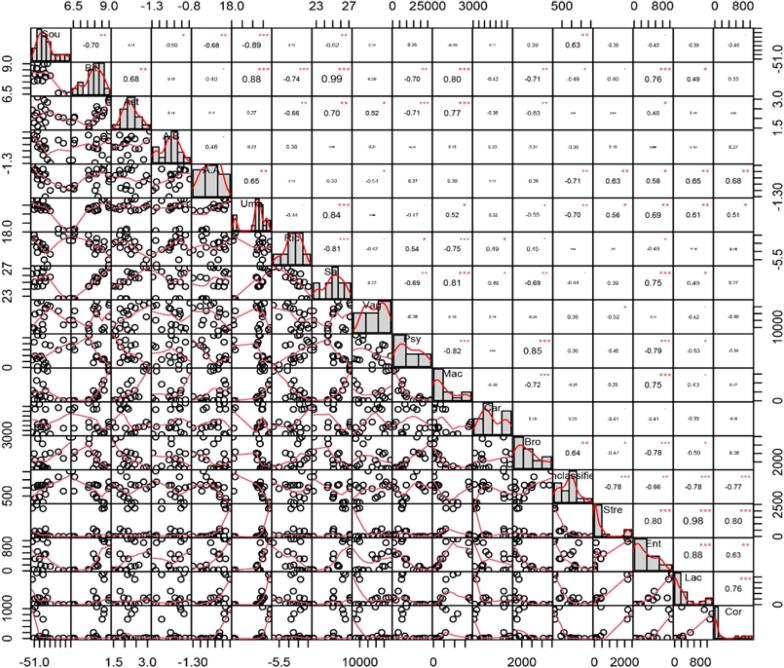
Similarly, the Pearson correlation analysis was used to analyze the correlation of electronic tongue taste attributes and microbial composition (Fig. 8). Bitterness was found to have a significantly positive correlation with Macrococcus, Streptococcus, and Enterococcus, and a significantly negative correlation with Psychrobacter, Brochothrix, and Carnobacterium. Astringency was showed a significantly positive correlation with Vagococcus, Macroccoccus, and Carnobacterium, and have a significantly negative correlation with Psychrobacter and Brochothrix. Afertaste-A showed a significantly positive correlation with Vagococcus while a significantly negative correlation with Streptococcus, Enterococcus, Lactococcus, and Corynebacterium. Umami showed a significantly positive correlation with Micrococcus, Streptococcus, Enterococcus, Lactococcus, and Corynebacterium, and a significantly negative correlation with Psychrobacter and Brochothrix. Richness showed a significantly positive correlation with Psychrobacter, Corynebacterium, and Brochothrix, and a significantly negative correlation with Vagococcus, Macroccoccus, and Enterococcus. Saltness showed a significantly positive correlation with Macroccoccus, Enterococcus, and Lactococcus and a significantly negative correlation with Psychrobacter, Corynebacterium, and Brochothrix.
Fig. 8.
The correlation between microorganisms in shrimp surimi samples and taste attributes. The correlation was calculated by Pearson Correlation Analysis. Significant correlations were indicated by *(P < 0.05), **(P < 0.01), ***(P < 0.001).
4. Discussion
Sono/photodynamic, a non-thermal sterilization method, utilizes the activated sono/photosensitizer to inactivate foodborne bacterial pathogens [23]. Herein, the preservative effects of curcumin-mediated sono/photodynamic treatment on the quality and microbiota composition of shrimp surimi during refrigerated storage were assessed. The obtained results demonstrated that fresh shrimp surimi rapidly deteriorated during cold storage. The spoilage by rapid microbial growth occurred after 2–3 days of storage at 4 °C, limiting the shelf life of shrimp surimi. This finding was consistent with the previous study results reported by M. Wang et al., demonstrating that the shelf of Hypophthalmichthys molitrix surimi was less than 2 days during cold storage and the TVC increased significantly within 24 h of refrigeration [1].
Additionally, the present study results elucidated that curcumin-mediated sono/photodynamic treatment inhibited the growth of spoilage bacteria and declined the deterioration of shrimp surimi. According to the CFU measurements, the sono/photodynamic treatment might double the shelf life of shrimp surimi at optimum treatment conditions (curcumin at 400 nmol/g, ultrasonic power at 300 W, and treatment for 30 min). Indeed, Bhavya [24] showed that curcumin can be used as the sonosensitizer to sterilize orange juice infected with Escherichia coli and Staphylococcus aureus and prolong its shelf life. Accumulating studies have also revealed that the sono/photodynamic treatment could effectively inhibit the growth of spoilage bacteria, such as Staphylococcus aureus [25] and Aggregatibacter actinomycetemcomitans [6], which was consistent with our study results. Therefore, sono/photodynamic could be a potential method to inhibit food-borne microbial growth and used for food preservation.
The changes in the microbial community of shrimp surimi during the refrigerated storage were determined to analyze the antimicrobial effects of curcumin-mediated SPDT in shrimp surimi. Psychrobacter and Brochothrix were identified as the SSO of shrimp surimi during storage, which was consistent with the previously reported results [26]. For instance, Psychrobacter was found to be the SSO in refrigerated fish [27], [28], [29]; while Brochothrix, a Gram-positive bacterium, was considered as the predominant spoilage microbiota of shrimp and fish [30]. Zhang et al. [31] isolated Psychrobacter glacincola 38–1, Brochothrix thermosphacta 38–2, and Pseudomonas fragi 38–8 from fish balls and found that their rapid growth could inhibit the growth of other bacteria during the cold storage.
Moreover, the Pearson correlation also revealed the possible associations between microbial growth and surimi quality deterioration. Both Psychrobacter and Brochothrix were positively correlated with the increase in TVB-N and TBARs, which was also consistent with the previous reports. TVB-N is usually associated with protein macromolecules degraded by microorganisms [32]. A previous study has reported that Psychrobacter was one of the dominant microbiota of brown shrimp during aerobic storage and associated with volatile compounds formation [33]. In another study, Psychrobacter was reported as one of the dominant microorganisms in the spoiled cuttlefish stored at 2 °C and associated with TVB-N accumulation [34]. Meanwhile, Brochothrix, as a facultative anaerobe, was also found to be associated with TVB-N accumulation in fresh chicken burgers [35].
Additionally, the electronic tongue system results also supported that the sono/photodynamic could delay the quality deterioration of shrimp surimi during cold storage. Besides, microbial growth plays a vital role in the flavor quality of foods as the changes in the food taste are deeply influenced by the changes in taste substances (including sugar, amino acids, inorganic salts and aicds). Our study results also suggested that microorganisms, such as Psychrobacter and Brochothrix could attribute to the change in flavor by decomposing protein, carbohydrate, fat, and other nutrients of shrimp surimi.
5. Conclusion
In conclusion, the TVC of refrigerated shrimp surimi rapidly increased and approached 106 CFU/g by the end of day 2, while the sono/photodynamic effectively inhibited bacterial growth in shrimp surimi. The sono/photodynamic showed some preservative effects on the quality parameters, such as TVB-N and TBARs of shrimp surimi. This phenomenon might also be associated with the inhibition of shrimp surimi SSOs (Psychrobacter and Brochothrix) identified by sono/photodynamic. Overall, the sono/photodynamic treatment could be a potential non-thermal sterilization method for preserving shrimp surimi and other refrigerated surimi products.
CRediT authorship contribution statement
Dehuang Wang: Methodology, Formal analysis, Data curation, Writing - original draft. Feng Zhou: Investigation. Danning Lai: Validation. Yi Zhang: Supervision, Funding acquisition. Jiamiao Hu: Supervision, Funding acquisition, Writing - review & editing. Shaoling Lin: Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The study was financially supported by the National Natural Science Foundation of China (31801649), Natural Science Foundation of Fujian Province (2020I0010; 2020I0012; 2021N5001), Science Fund for Distinguished Young Scholars of Fujian Agriculture and Forestry University (xjq201918; CXZX2018063) and Fujian Science and Technology Economic Integration Service Platform of Fujian Association for Science and Technology (2020K02).
Contributor Information
Jiamiao Hu, Email: jiamiao.hu@fafu.edu.cn.
Shaoling Lin, Email: shaoling.lin@fafu.edu.cn.
References
- 1.Wang M. Research and the quality change of flavor fish paste in the process of storage. Huazhong Agricultural University. 2017:70. [Google Scholar]
- 2.Guan W., Ren X., Li Y., Mao L. The beneficial effects of grape seed, sage and oregano extracts on the quality and volatile flavor component of hairtail fish balls during cold storage at 4 °C. LWT. 2019;101:25–31. [Google Scholar]
- 3.Pan C., Chen S., Hao S., Yang X. Effect of low-temperature preservation on quality changes in Pacific white shrimp, Litopenaeus vannamei: a review. J. Sci. Food Agric. 2019;99:6121–6128. doi: 10.1002/jsfa.9905. [DOI] [PubMed] [Google Scholar]
- 4.Pang X., Li D., Zhu J., Cheng J., Liu G. Beyond antibiotics: photo/sonodynamic approaches for bacterial theranostics. Nano-Micro Lett. 2020;12:144. doi: 10.1007/s40820-020-00485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.N. Drantantiyas, S. Astuti, A. Nasution, Comparison microbial killing efficacy between sonodynamic therapy and photodynamic therapy, 2016.
- 6.Pourhajibagher M., Bahador A. Attenuation of Aggregatibacter actinomycetemcomitans virulence using curcumin-decorated nanophytosomes-mediated photo-sonoantimicrobial chemotherapy. Sci. Rep. 2021;11:6012. doi: 10.1038/s41598-021-85437-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J., Hou W., Cao B., Zuo T., Xue C., Leung A.W., Xu C., Tang Q.-J. Virucidal efficacy of treatment with photodynamically activated curcumin on murine norovirus bio-accumulated in oysters. Photodiagn. Photodyn. Ther. 2015;12:385–392. doi: 10.1016/j.pdpdt.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 8.EFSA Refined exposure assessment for curcumin (E 100) EFSA J. 2014;12:3876. [Google Scholar]
- 9.EFSA Scientific Opinion on the re-evaluation of curcumin (E 100) as a food additive. EFSA J. 2010;8:1679. [Google Scholar]
- 10.Ma R.-H., Ni Z.-J., Zhu Y.-Y., Thakur K., Zhang F., Zhang Y.-Y., Hu F., Zhang J.-G., Wei Z.-J. A recent update on the multifaceted health benefits associated with ginger and its bioactive components. Food Funct. 2021;12:519–542. doi: 10.1039/d0fo02834g. [DOI] [PubMed] [Google Scholar]
- 11.Li Y., Xu Y., Liao Q., Xie M., Tao H., Wang H.-L. Synergistic effect of hypocrellin B and curcumin on photodynamic inactivation of Staphylococcus aureus. Microb. Biotechnol. 2021;14:692–707. doi: 10.1111/1751-7915.13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X., Ip M., Leung A.W., Xu C. Sonodynamic inactivation of methicillin-resistant Staphylococcus aureus in planktonic condition by curcumin under ultrasound sonication. Ultrasonics. 2014;54:2109–2114. doi: 10.1016/j.ultras.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Abdulrahman H., Misba L., Ahmad S., Khan A.U. Curcumin induced photodynamic therapy mediated suppression of quorum sensing pathway of Pseudomonas aeruginosa: An approach to inhibit biofilm in vitro. Photodiagn. Photodyn. Ther. 2020;30:101645. doi: 10.1016/j.pdpdt.2019.101645. [DOI] [PubMed] [Google Scholar]
- 14.Wang X., Ip M., Leung A.W., Yang Z., Wang P., Zhang B., Ip S., Xu C. Sonodynamic action of curcumin on foodborne bacteria Bacillus cereus and Escherichia coli. Ultrasonics. 2015;62:75–79. doi: 10.1016/j.ultras.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Tao R., Zhang F., Tang Q.-J., Xu C.-S., Ni Z.-J., Meng X.-H. Effects of curcumin-based photodynamic treatment on the storage quality of fresh-cut apples. Food Chem. 2019;274:415–421. doi: 10.1016/j.foodchem.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 16.Li Y., Yang Z., Li J. Shelf-life extension of Pacific white shrimp using algae extracts during refrigerated storage. J. Sci. Food Agric. 2017;97:291–298. doi: 10.1002/jsfa.7730. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y., Yao Y., Gao L., Wang Z., Xu B. Characterization of a microbial community developing during refrigerated storage of vacuum packed Yao meat, a Chinese traditional food. LWT. 2018;90:562–569. [Google Scholar]
- 18.Jia S., Liu Y., Zhuang S., Sun X., Li Y., Hong H., Lv Y., Luo Y. Effect of ε-polylysine and ice storage on microbiota composition and quality of Pacific white shrimp (Litopenaeus vannamei) stored at 0 °C. Food Microbiol. 2019;83:27–35. doi: 10.1016/j.fm.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Li D., Xie H., Liu Z., Li A., Li J., Liu B., Liu X., Zhou D. Shelf life prediction and changes in lipid profiles of dried shrimp (Penaeus vannamei) during accelerated storage. Food Chem. 2019;297 doi: 10.1016/j.foodchem.2019.124951. [DOI] [PubMed] [Google Scholar]
- 20.Pattarapon P., Zhang M., Bhandari B., Gao Z. Effect of vacuum storage on the freshness of grass carp (Ctenopharyngodon idella) fillet based on normal and electronic sensory measurement. J. Food Process. Preserv. 2018;42(2):e13418. [Google Scholar]
- 21.D. Liu, S. Li, N. Wang, Y. Deng, L. Sha, S. Gai, H. Liu, X. Xu, Evolution of taste compounds of Dezhou-braised chicken during cooking evaluated by chemical analysis and an electronic tongue system. [DOI] [PubMed]
- 22.Chen S., Tao F., Pan C., Hu X., Ma H., Li C., Zhao Y., Wang Y. Modeling quality changes in Pacific white shrimp (Litopenaeus vannamei) during storage: Comparison of the Arrhenius model and Random Forest model. J. Food Process. Preserv. 2021;45 [Google Scholar]
- 23.Pourhajibagher M., Rokn A.R., Barikani H.R., Bahador A. Photo-sonodynamic antimicrobial chemotherapy via chitosan nanoparticles-indocyanine green against polymicrobial periopathogenic biofilms: Ex vivo study on dental implants. Photodiagn. Photodyn. Ther. 2020;31:101834. doi: 10.1016/j.pdpdt.2020.101834. [DOI] [PubMed] [Google Scholar]
- 24.Bhavya M.L., Hebbar H.U. Sono-photodynamic inactivation of Escherichia coli and Staphylococcus aureus in orange juice. Ultrason. Sonochem. 2019;57:108–115. doi: 10.1016/j.ultsonch.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Nike Dwi Grevika D., Suryani Dyah A., Aulia M.T.N. Comparison microbial killing efficacy between sonodynamic therapy and photodynamic therapy. Proc. SPIE. 2016 [Google Scholar]
- 26.Møretrø T., Langsrud S. Residential bacteria on surfaces in the food industry and their implications for food safety and quality. Compr. Rev. Food Sci. Food Saf. 2017;16:1022–1041. doi: 10.1111/1541-4337.12283. [DOI] [PubMed] [Google Scholar]
- 27.Jiang X., Meng L., Feng J., Dai Z. Analysis of quality change and microbial assessment of chub mackerel in storage. Journal of Chinese Institute of Food Science and Technology. 2019;19:197–205. [Google Scholar]
- 28.Sun X.D., Holley R.A. Antimicrobial and antioxidative strategies to reduce pathogens and extend the shelf life of fresh red meats. Compr. Rev. Food Sci. Food Saf. 2012;11:340–354. [Google Scholar]
- 29.GonzÁLez C., Santos J., GarcÍA-LÓPez M.-L., Otero A. Psychrobacters and related bacteria in freshwater fish. J. Food Protect. 2000;63:315–321. doi: 10.4315/0362-028x-63.3.315. [DOI] [PubMed] [Google Scholar]
- 30.Mamlouk K., Macé S., Guilbaud M., Jaffrès E., Ferchichi M., Prévost H., Pilet M.-F., Dousset X. Quantification of viable Brochothrix thermosphacta in cooked shrimp and salmon by real-time PCR. Food Microbiol. 2012;30:173–179. doi: 10.1016/j.fm.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y., Wei J., Yuan Y., Chen H., Dai L., Wang X., Yue T. Bactericidal effect of cold plasma on microbiota of commercial fish balls. Innov. Food Sci. Emerg. Technol. 2019;52:394–405. [Google Scholar]
- 32.Hong H., Luo Y., Zhou Z., Shen H. Effects of low concentration of salt and sucrose on the quality of bighead carp (Aristichthys nobilis) fillets stored at 4°C. Food Chem. 2012;133:102–107. [Google Scholar]
- 33.Broekaert K., Noseda B., Heyndrickx M., Vlaemynck G., Devlieghere F. Volatile compounds associated with Psychrobacter spp. and Pseudoalteromonas spp., the dominant microbiota of brown shrimp (Crangon crangon) during aerobic storage. Int. J. Food Microbiol. 2013;166:487–493. doi: 10.1016/j.ijfoodmicro.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Parlapani F.F., Michailidou S., Anagnostopoulos D.A., Sakellariou A.K., Pasentsis K., Psomopoulos F., Argiriou A., Haroutounian S.A., Boziaris I.S. Microbial spoilage investigation of thawed common cuttlefish (Sepia officinalis) stored at 2 °C using next generation sequencing and volatilome analysis. Food Microbiol. 2018;76:518–525. doi: 10.1016/j.fm.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Assanti E., Karabagias V.K., Karabagias I.K., Badeka A., Kontominas M.G. Shelf life evaluation of fresh chicken burgers based on the combination of chitosan dip and vacuum packaging under refrigerated storage. J. Food Sci. Technol. -Mysore- 2021;58(3):870–883. doi: 10.1007/s13197-020-04601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]