Abstract
The aim of this work is the development of a suitable method to extract and detect zinc-bound compounds from cabbage, broccoli and kale (family Brassicaceae, species oleracea) using solid phase extraction (SPE) and size-exclusion chromatography inductively coupled plasma mass spectrometry (SEC-ICP-MS). Tris [2-Amino-2-(hydroxymethyl)-1,3-propanediol]/hydrochloric acid (Tris/HCl) or ammonium nitrate were used as extractants added to the freeze-dried samples, which were then sonicated and centrifuged. An enzymatic mixture was added to the extracts and then incubated for 5- and 18 h prior to analysis by SEC-ICP-MS. Results showed a good coefficient of variation (CV) of the elution time (0.06-0.9%), concentration (4.7–16.9%) and molecular size (0.4–5.4%). The limit of detection (LOD) and the limit of quantitation (LOQ) were 0.9 μg L−1 and 2.8 μg L−1, respectively. The proposed method is reliable and robust and can be applied to samples with difficult matrices like vegetables and soil.
-
•
Good precision, stability and reproducibility.
-
•
Easy to execute and suitable for analysis of vegetables and other samples with complex matrices, eg. soil.
-
•
This method contributed to good maintenance of the instrument and to minimal cleaning time.
Keywords: SEC-ICP-MS, Vegetables, Extraction, Zinc, Enzyme
Graphical abstract

Specifications table
| Subject Area: | Agricultural and Biological Sciences |
| More specific subject area: | Analytical chemistry, Food science |
| Method name: | Detection of zinc in vegetables |
| Name and reference of original method: | Persson, D. P., Hansen, T. H., Laursen, K. H., Schjoerring J. K., Husted, S. (2009). Simultaneous iron, zinc, sulfur and phosphorus speciation analysis of barley grain tissues using SEC-ICP-MS and IP-ICP-MS. Metallomics, 1, 418–426. |
| Resource availability: | N/A |
Background
Zinc is an essential trace mineral for multiple aspects of metabolism [1] and can play an important role in the immune system and the gene expression [2,3]. Zinc is important for a normal pregnancy and child growth [4], and deficiency is associated with diarrhoea and respiratory infections in children [2]. There is evidence that zinc has an effect on depression and mood, suggesting its supplementation as adjunct to antidepressants therapy for individuals with a diagnosis of major depressive disorder and as a therapy in its own in pre-menopausal women with zinc deficiency [5]. The complex and different matrix of vegetables makes the extraction of Zn-bound compounds difficult even from vegetables within the same species [6]. Different solvents have been used to extract compounds binding zinc from fruit and vegetables. Water, methanol, ethanol, isopropyl alcohol, dimethyl sulfoxide and acetone were used to extract different parts of fruit and vegetables [7,8,9,10,11,12,13]. However, these extraction methods are complex and require multiple steps. The natural deep eutectic solvents (NADES) is a promising solvent only used to extract minerals from barley [14,15]. Different conditions for the SEC-ICP-MS were used, but not for vegetables [16,17]. In this study two different buffers, Tris/HCl and ammonium nitrate, were used to extract zinc-bound compounds from cabbage, broccoli and kale which are the most consumed vegetables in the UK and then analysed by an adapted method suitable for SEC-ICP-MS.
Materials and reagents
Ammonium nitrate, (Sigma-Aldrich, Poole, Dorset, UK)
Tris [2-Amino-2-(hydroxymethyl)-1,3-propanediol], (Sigma-Aldrich, Poole, Dorset, UK)
Phytase from wheat, (Sigma-Aldrich, Poole, Dorset, UK)
Protease, (Type XIV from Streptomyces griseus), (Sigma-Aldrich, Poole, Dorset, UK)
Xylanase, (Thermomyces lanuginosus), (Sigma-Aldrich, Poole, Dorset, UK)
Cellulase, (Trichoderma reesei), (Sigma-Aldrich, Poole, Dorset, UK)
Tri-glycine, (Sigma-Aldrich, Poole, Dorset, UK)
Vitamin B12, (Sigma-Aldrich, Poole, Dorset, UK)
Cytochrome, (Sigma-Aldrich, Poole, Dorset, UK)
Apoferritin, (Sigma-Aldrich, Poole, Dorset, UK)
Blue dextran, (Sigma-Aldrich, Poole, Dorset, UK)
Freeze-dried savoy cabbage (Brassica oleracea var. sabauda), freeze-dried broccoli (Brassica oleracea var. italica) and freeze-dried curly kale (Brassica oleracea var. acephala), (Norwich Medical School, University of East Anglia, Norwich Research Park, Norwich, UK)
Elemental solution (Zn), Fisher Scientific (Loughborough, Leicestershire, UK)
Method details
1. Sample preparation
Savoy cabbage (Brassica oleracea var. sabauda), broccoli (Brassica oleracea var. italica) and curly kale (Brassica oleracea var. acephala) were purchased from different supermarkets. About 400 g of savoy cabbage (chopped in wedges) and broccoli (chopped in heads) were boiled in 1.2 L Milli-Q (18.2 MQ) water for about 15 min, while 360 g of curly kale were boiled in about 1.6 L Milli-Q (18.2 MQ) water for 8 min. After cooking, the vegetables were drained for 15 min at room temperature, placed in bags, frozen at minus 20 °C, freeze-dried (Coolsafe 110-4 pro, ScanVac), and finely ground. The ground samples were stored, at the Norwich Medical School, in the dark in sealed bags at 4 °C in a desiccator, with silica gel beads to prevent moisture.
Two batches of samples (100 mg) were extracted twice by solid phase extraction with 2 mL of buffer (200 mM ammonium nitrate pH 7.6 or 50 mM Tris/HCl pH 7.5) in plastic tubes, sonicated at 20% power for 2 min. The extracts within the same batch and from the same vegetable were combined and centrifuged at 4000 g/min at 4 °C for 10 min.
2. Enzymatic digestion
The enzymatic mixture (3 mL) (Table 1) was prepared in a plastic tube and added to 2 mL of the extract to have a final volume of 5 mL. The samples were incubated for 5 h at 37 ᴼC with gentle shaking (120 rpm) in an orbital incubator. After 5 h, 1 mL of the incubated samples was taken and kept in a refrigerator for SEC-ICP-MS. The rest of the incubated samples was put back in the incubator for another 13 h to have a total of 18 h. After 13 h, all the incubated samples were centrifuged at 4000 g/min for 10 min and analyzed by SEC-ICP-MS. All samples were diluted 1:10 prior to analysis.
Table 1.
Enzymatic mixture used for the digestion.
| Enzyme | Origin | Activity (U/g) |
|---|---|---|
| Phytase (30 U/g) | wheat | 20 |
| Protease (3500 U/g) | Streptomyces griseus | 175 |
| Xylanase (2500 U/g) | Thermomyces lanuginosus | 280 |
| Cellulase (700 U/g d=1.2 g/mL) | Trichoderma reesei | 440 |
3. Analytical instrumentation
Separation of samples was performed via size-exclusion chromatography [20] using a high-performance liquid chromatography (HPLC) (PerkinElmer LC 200 Series HS, Seer Green, Bucks, UK), a Flexar ultraviolet/visible (UV/VIS) detector operating at 280 nm and a high-pressure peristaltic pump equipped of PEEK tubing (0.17 mm id) and operating at a flow rate of 0.6 mL min−1. Detection and quantification of elements were made using Chromera software (PerkinElmer v. 4.1.0). The column was a Superdex Peptide 10/300 GL (10Å~300 mm, GE Healthcare Bio-Sciences, Uppsala, Sweden) with an optimum separation range between 100 and 7000 Da. Samples were analyzed using an ICP-MS (PerkinElmer NexION300XX, Seer Green, Bucks, UK) equipped with a glass Meinhard nebulizer, a quadrupole mass spectrometer and a collision cell. The SEC-ICP-MS settings were as in Table 2. Ammonium nitrate (200 mM, pH 7.6), as mobile phase, was prepared daily by dissolving 16 g of ammonium nitrate in 1 L of Purite ultrapure water and the pH was adjusted with ammonium hydroxide before degassing by vacuum filtration using 0.2 μm filters (Millipore, Watford, UK). Samples (100 μL) were injected into the column at room temperature and the analysis was performed in isocratic mode. After five runs a series of five repetitive 20 µL aliquots of water was injected over 20 min, without delay between injections, followed by one 100 µL aliquot of ammonium nitrate (200 mM at pH 7.6) to re-equilibrate the column. The same series of five repetitive 20 µL aliquots of water was injected, at the end of the batch, using water as mobile phase. An average of seven replicates per sample was carried out.
Table 2.
Instrumentation settings.
| ICP-MS | |
| Gas flow | 1 L/min |
| Auxiliary gas flow | 1.2 L/min |
| Plasma flow | 18 L/min |
| RF power | 1600 Watts |
| Cell gas flow (He) | 3.9 mL/min |
| Mode | Collision cell |
| HPLC | |
| Column | Superdex Peptide 10/300 GL |
| Eluent | NH4NO3 (200 Mm, pH 7.6) |
| Flow rate | 0.6 mL/min |
| Mode | isocratic |
| Sample volume | 100 µL |
| Wavelength | 280 nm |
4. Quality control
Zinc is a common contaminant which can be problematic for trace analysis. In this study, plastic bottles were used for the solvents and metal-free glass vials were used for the samples to avoid metal contamination which was checked by injecting a blank made of 2% HNO3. External calibration for quantification provided a linear range with an excellent correlation coefficient (R = 1) and was performed by injecting six concentrations of zinc standard solution (0, 0.050, 0.5, 5, 10 and 100 mg L−1), dissolved in 2% HNO3, into the ICP-MS via HPLC but without the column, which was detected as Zn64 isotope. The standards for mass calibration were triglycine (0.189 kDa), vitamin B12 (1.35 kDa), cytochrome (12.4 kDa), apoferritin (443 kDa) and blue dextran (2000 kDa) dissolved in an appropriate amount of mobile phase and filtered. A log-linear regression curve, with a correlation coefficient R = 0.99, was obtained by plotting the logarithm of the molecular size versus the coefficient of distribution (Kd) (Fig 1). Data are presented as the average of seven intra- and inter-day analyses. The limit of detection (0.9 μg L−1) and the limit of quantitation (2.8 μg L−1) were determined using LOD=3.3*(SD/m) and LOQ=10*(SD/m) where SD is the standard deviation of the intercept and m is the slope of the calibration curve. Reproducibility and precision were measured by the coefficient of variation (CV) of the retention time of the smallest peaks eluted at about 28 and 32 min (Table 3). Determination of the accuracy is difficult because it is impossible to obtain a sample with a known amount of zinc. For other kind of matrices, like plasma for example, the accuracy can be determined by spiking a zinc-free plasma with known concentrations; for vegetables this is not possible because most of the vegetables contain a certain amount of zinc and therefore zinc-free samples are not available. It is impossible to obtain a sample with a known amount of zinc because of the large variation of its amount in vegetables which depends on different factors, e.g., environment, soil, geographic area and so on.
Fig. 1.
Calibration curve for a) molecular mass and b) quantification.
Table 3.
Coefficient of variation of the retention time, concentration and molecular size of the peaks eluted after 28 and 32 min.
| CV (%) |
||||||
|---|---|---|---|---|---|---|
| 28 min |
32 min |
|||||
| RT | concentration | MW | RT | concentration | MW | |
| Cabbage | 0.12 | 5.5 | 0.4 | 0.12 | 8.0 | 0.4 |
| Broccoli | 0.06 | 4.7 | 0.8 | 0.25 | 11.1 | 1.0 |
| Kale | 0.17 | 8.9 | 3.3 | 0.92 | 16.9 | 5.4 |
Extraction
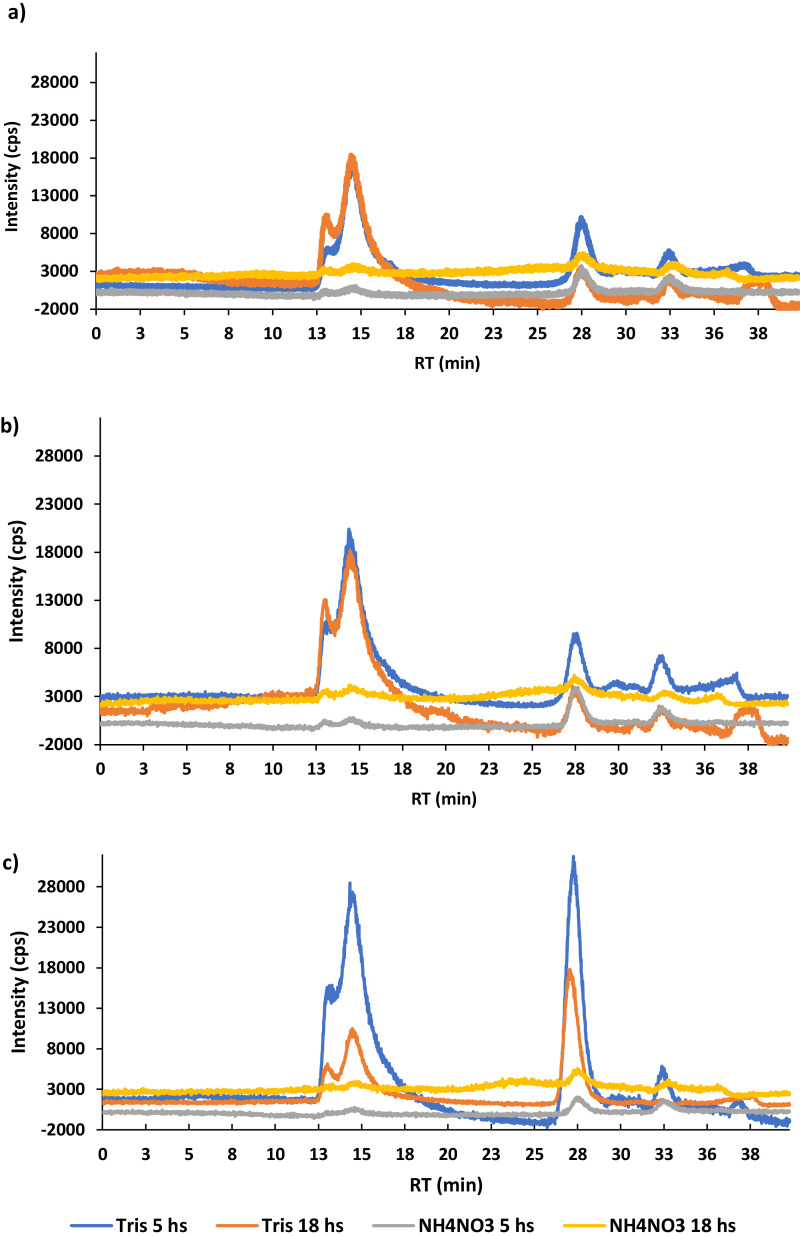
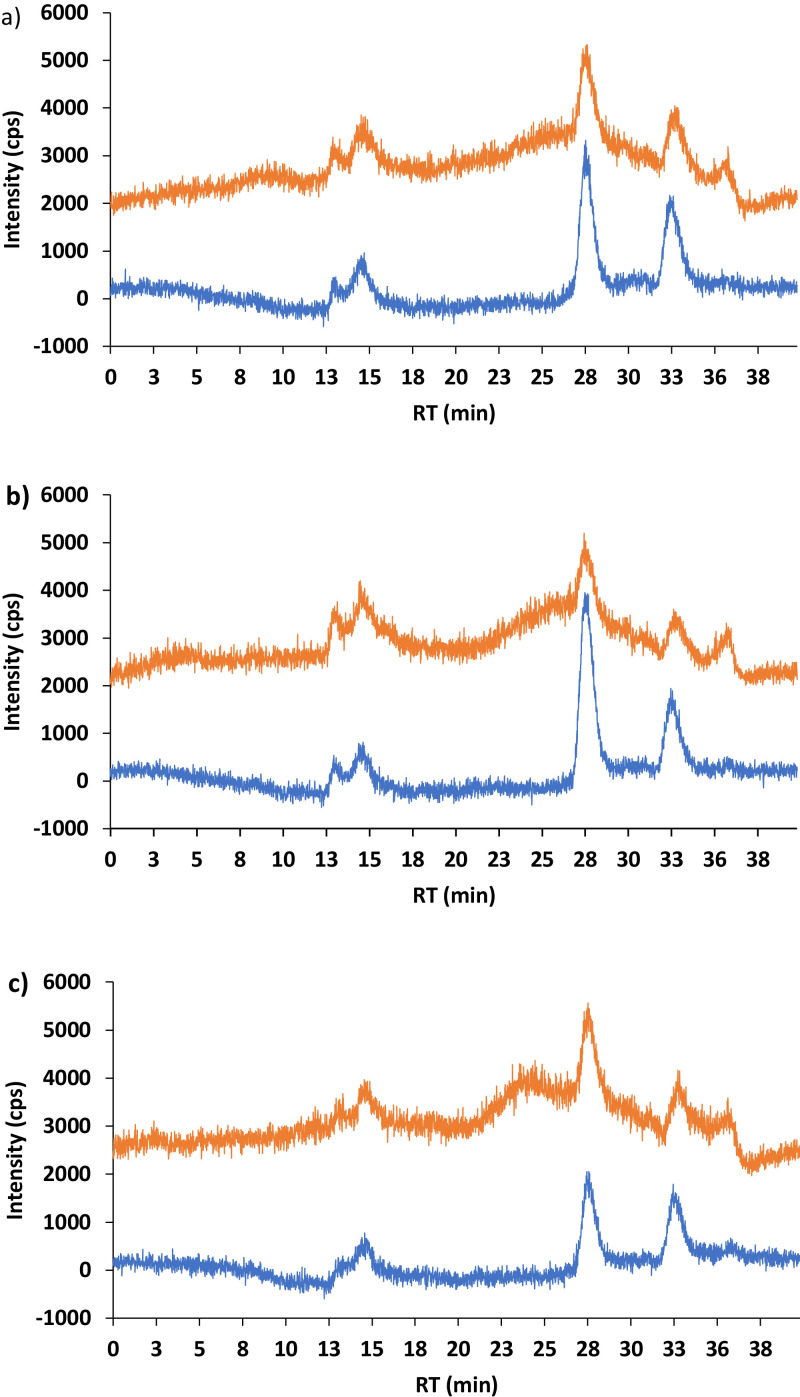
Tris/HCl buffer works better for the extraction of species containing zinc (Fig. 2) than ammonium nitrate which gave a high noise background threshold (Fig. 3). Tris/HCl is a common buffer used to extract metal-bound species from different genotypes of wheat [18,24], probably for its lysis properties which can explain its high extraction ability. Ammonium nitrate is used as a fertilizer in agriculture to provide a source of nitrogen which stimulates the plant growth, and it was chosen because it was considered a suitable solvent to extract water-soluble plant available trace elements from soil [21,22] and toxic elements from contaminated grassland soils to assess metal mobility in soil [19]. It was expected ammonium nitrate to extract Zn-bound species better than Tris/HCl, because the ammonia [23] formed by its dissociation leads to the formation of soluble metal ammine complexes, mainly occurring in the soil, which are available for plants [19]. On the contrary, in this study ammonium nitrate showed a very low level of extraction compared to Tris/HCl buffer.
Fig. 2.
Comparison between the samples extracted with Tris/HCl and NH4NO3 and digested for 5 h and 18 h. SEC-ICP-MS: mobile phase ammonium nitrate (200 mM, pH 7.6), flow rate of 0.6 mL min−1, l = 280 nm. a) cabbage, b) broccoli, c) kale.).
Fig. 3.
Comparison between 5 h (blue line) and 18 h (orange line) digestion of (a) cabbage, (b) broccoli and (c) kale with NH4NO3 (low intensity chromatograms in Fig.2). SEC-ICP-MS: mobile phase ammonium nitrate (200 mM, pH 7.6), flow rate of 0.6 Ml. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
SEC-ICP-MS
Ammonium nitrate is not a good extractant, but it is excellent as mobile phase for its stability, reproducibility and precision. The challenge of this study was to find a method suitable for the ICP and the HPLC instruments at the same time. Ammonium nitrate has a good buffer capacity at a pH range of 7.6–7.8, it is very soluble in water and does not precipitate in the column or in the ICP system. The five repetitive 20 µL aliquots injections are a push-through way to rinse the column, and the tubing, chamber and injector of the ICP. Precipitate and dirt accumulation on the cone, injector, nebulizer and torch of the ICP are common along with blockage of the tubing. This method is reliable for samples with difficult and complex matrices and showed consistency of the results not only in vegetables but also in soil.
Acknowledgments
Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council and this work was supported by BBSRC grant (DRINC/L025515/1). The author would like to thank the Norwich Medical School, University of East Anglia, for providing the raw vegetable samples.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.King J.C., Brown K.H., Gibson R.S., Krebs N.F., Lowe N.M., Siekmann J.H. Biomarkers of nutrition for development (BOND)—zinc review. J. Nutr. 2016;146:858S–885S. doi: 10.3945/jn.115.220079. (Suppl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey R.L., West K.P., Black R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015;66(2):22–33. doi: 10.1159/000371618. [DOI] [PubMed] [Google Scholar]
- 3.Saunders A.V., Craig W.J., Baines S.K. Zinc and vegetarian diets. Med. J. Aust. 2012;199(2):17–21. doi: 10.5694/mja11.11493. [DOI] [PubMed] [Google Scholar]
- 4.Wessells K.R., Brown K.H. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. Plos ONE. 2012;7:11. doi: 10.1371/journal.pone.0050568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lomagno K.A., Hu F., Riddell L., Booth A., Szymlek-Gay E.A., Nowson C., K.Byrne L. Increasing iron and zinc in pre-menopausal women and its effects on mood and cognition: a systematic review. Nutrients. 2014;6(11):5117–5141. doi: 10.3390/nu6115117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dell'Aquila C. Qualitative in vitro study on the degradation of mineral complexes. Food Chem. 2019 doi: 10.1016/j.foodchem.2019.125655. [DOI] [PubMed] [Google Scholar]
- 7.Panja P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2017;23:173–182. [Google Scholar]
- 8.Dorta E., Lobo M.G, González M. Reutilization of mango byproducts: study of the effect of extraction solvent and temperature on their antioxidant properties. J. Food Sci. 2012;77(1):C80–C88. doi: 10.1111/j.1750-3841.2011.02477.x. [DOI] [PubMed] [Google Scholar]
- 9.Dorta E., Lobo M.G, González M. Improving the efficiency of antioxidant extraction from mango peel by using microwave-assisted extraction. Plant Foods Hum. Nutr. 2013;68(2):190–199. doi: 10.1007/s11130-013-0350-4. [DOI] [PubMed] [Google Scholar]
- 10.Dorta E., Lobo M.G, González M. Optimization of factors affecting extraction of antioxidants from mango seed. Food Bioproc. Tech. 2013;6(4):1067–1081. [Google Scholar]
- 11.Saini A., Panesar P.S., Bera M.B. Valorization of fruits and vegetables waste through green extraction of bioactive compounds and their nanoemulsions-based delivery system. Bioresour. Bioprocess. 2019;6(1):26. [Google Scholar]
- 12.Soquetta M.B., de Marsillac Terra L., Bastos C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. J. Food. 2018;16(1):400–412. [Google Scholar]
- 13.Sagar N.A., Pareek S., Sharma S., Yahia E.M., Lobo M.G. Fruit and vegetable waste: bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018;17(3):512–531. doi: 10.1111/1541-4337.12330. [DOI] [PubMed] [Google Scholar]
- 14.Osowska N., Ruzik L. New potentials in the extraction of trace metal using natural deep eutectic solvents (NADES) Food Anal. Methods. 2019;12:926–935. [Google Scholar]
- 15.Osowska N., Ruzik L., Paduszyński K., Matczuk M. New solvents for metal extraction-NADES. Prediction and optimization of efficient extraction of selected metals by ICP-MS/MS. J. Anal. At. Spectrom. 2021;36:946–953. [Google Scholar]
- 16.Xue Y.F., Eagling T., He J., Zou C.Q., McGrath S.P., Shewry P.R., Zhao F.J. Effects of nitrogen on the distribution and chemical speciation of iron and zinc in pearling fractions of wheat grain. J. Agric. Food Chem. 2014;62:4738–4746. doi: 10.1021/jf500273x. [DOI] [PubMed] [Google Scholar]
- 17.Md Noh M.F., Gunasegavan R.D.N, Khalid N.M., Balasubramaniam V., Mustar S., Abd Rashed A. Recent techniques in nutrient analysis for food composition database. Molecules. 2020;25:4567. doi: 10.3390/molecules25194567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eagling T., Neal A.L., McGrath S.P., Fairweather-Tait S.J., Shewry P.R., Zhao F.J. Distribution and speciation of iron and zinc in grain of two wheat genotypes. J. Agric. Food Chem. 2014;62(3):708–716. doi: 10.1021/jf403331p. [DOI] [PubMed] [Google Scholar]
- 19.Qasim B., Motelica-Heino M., Joussein E., Soubrand M., Gauthier A. Potential toxic element fractionation and phytoavailability assessment in technosoils from former smelting and mining areas. Environ. Sci. Pollut. Res. 2015;22(8):5961–5974. doi: 10.1007/s11356-014-3768-9. [DOI] [PubMed] [Google Scholar]
- 20.Dell'Aquila C., Neal A.L., Shewry P.R. Development of a reproducible method of analysis of iron, zinc and phosphorus in vegetables digests by SEC-ICP-MS. Food Chem. 2019 doi: 10.1016/j.foodchem.2019.125652. [DOI] [PubMed] [Google Scholar]
- 21.Agrelli D., Duri L.G., Fiorentino N., Cozzolino E., Fagnano M., Adamo P. Potentially toxic element availability and risk assessment of cadmium dietary exposure after repeated croppings of brassica juncea in a contaminated agricultural soil. Agronomy. 2020;10(6):880. [Google Scholar]
- 22.Clarke C.E., Stone W., Hardie A.G., Quinton J.N., Blake L.I, Johnson K.L. Better together: water treatment residual and poor-quality compost improves sandy soil fertility. J. Environ. Qual. 2019;48(6):1781–1788. [Google Scholar]
- 23.Zhang C., Zhang X.Y., Zou H.T., Kou L., Yang Y., Wen X.F., Li S.G., Wang H.M., Sun X.M. Contrasting effects of ammonium and nitrate additions on the biomass of soil microbial communities and enzyme activities in subtropical China. Biogeosciences. 2017;14:4815–4827. [Google Scholar]
- 24.Bittencourt L.M., Lana D.A.P.D., de A.M., Pimenta C., dos Santos A.V., Gonçalves A.P.F., Augustic R., Costa L.M. Determination of metal associated with proteins of wheat seed samples after sequential extraction procedure. J. Braz. Chem. Soc. 2014;25(2):264–270. [Google Scholar]