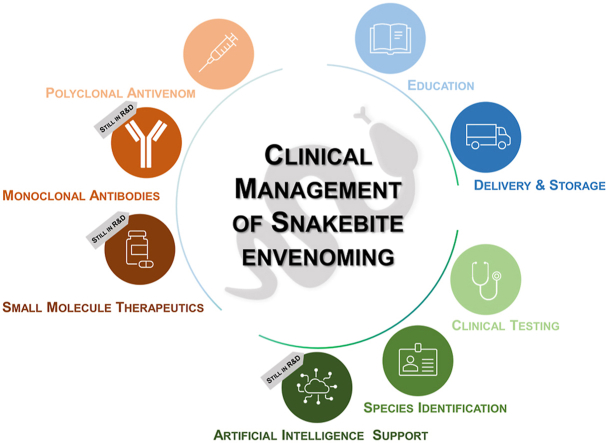
Abstract
Snakebite envenoming is a major cause of morbidity and mortality in rural communities throughout the tropics. Generally, the main clinical features of snakebites are local swelling, tissue necrosis, shock, spontaneous systemic hemorrhage, incoagulable blood, paralysis, rhabdomyolysis, and acute kidney injury. These clinical manifestations result from complex biochemical venom constituents comprising of cytotoxins, hemotoxins, neurotoxins, myotoxins, and other substances. Timely diagnosis of envenoming and identification of the responsible snake species is clinically challenging in many parts of the world and necessitates prompt and thorough clinical assessment, which could be supported by the development of reliable, affordable, widely-accessible, point-of-care tests. Conventional antivenoms based on polyclonal antibodies derived from animals remain the mainstay of therapy along with supportive medical and surgical care. However, while antivenoms save countless lives, they are associated with adverse reactions, limited potency, and are relatively inefficacious against presynaptic neurotoxicity and in preventing necrosis. Nevertheless, major scientific and technological advances are facilitating the development of new molecular and immunologic diagnostic tests, as well as a new generation of antivenoms comprising human monoclonal antibodies with broader and more potent neutralization capacity and less immunogenicity. Repurposed pharmaceuticals based on small molecule inhibitors (e.g., marimastat and varespladib) used alone and in combination against enzymatic toxins, such as metalloproteases and phospholipase A2s, have shown promise in animal studies. These orally bioavailable molecules could serve as early interventions in the out-of-hospital setting if confirmed to be safe and efficacious in clinical studies. Antivenom access can be improved by the usage of drones and ensuring constant antivenom supply in remote endemic rural areas. Overall, the improvement of clinical management of snakebite envenoming requires sustained, coordinated, and multifaceted efforts involving basic and applied sciences, new technology, product development, effective clinical training, implementation of existing guidelines and therapeutic approaches, supported by improved supply of existing antivenoms.
Keywords: Antivenoms, Diagnosis, Early adverse reactions, Monoclonal antibodies, Snakebite envenoming, Small molecule therapies
Graphical abstract
Highlights
-
•
Snakebite envenoming is a potentially fatal medical emergency requiring urgent clinical intervention.
-
•
Timely diagnosis of snakebite envenoming is essential, however, only few specific diagnostic tests are in use.
-
•
Current specific treatment of snakebite envenoming consists of antivenom comprising polyclonal animal-derived antibodies.
-
•
Recent possibilities for novel treatments include investigation of small molecule inhibitors and recombinant human antibodies.
-
•
Education and better antivenom supply and deployment strategies are needed to improve treatment outcomes in affected regions.
1. Background
Snakebite envenoming (SBE) is a major health problem among rural, agricultural workers throughout the tropics – especially in Africa, Asia, and Latin America. Some of the most important species that cause serious SBE globally include carpet vipers, Russell's vipers, Malayan and lancehead pit-vipers, rattlesnakes, puff adders, cobras, mambas, kraits, and taipans. These snakes possess venoms rich and diverse in composition, broadly comprising hemotoxins, neurotoxins, cytotoxins, and myotoxins (Gutierrez et al., 2017a). The resulting clinical features following bites may include swelling and necrosis of the bitten body part, rhabdomyolysis, local bleeding, systemic hemorrhage, incoagulable blood, anaemia, shock, and paralysis. Death results from progression of these features or following superadded complications, especially when untreated (Gutierrez et al., 2017a). For over a century, conventional antivenom has remained the mainstay of therapy against SBE (Pucca et al., 2019). These antivenoms consist of polyclonal (IgG) antibodies or antibody fragments (Fab or F(ab’)2) derived from the plasma of large animals following immunization with venom(s). Access to effective antivenoms has been a major challenge in the developing world, particularly in sub-Saharan Africa (Habib et al., 2020). Monovalent and polyvalent antivenoms are available for therapy based on empirical clinical judgement, which can be supported by identification of the biting snake. Several methods are used to determine the species of the biting snake (see next section). In many places, snake identification is most commonly achieved by either a description of the snake, if seen, a physical examination of the snake, if killed and brought by patients, or by identification of presentation of typical clinical syndromes. In addition to characteristic syndromes, in the tropics, bedside assays, such as examination of incoagulable blood determined by the 20-min Whole Blood Clotting Test (20WBCT), is often used to confirm envenoming by snakes with coagulopathic venom, e.g., carpet vipers, Russell's vipers, Malayan pit vipers, Green pit vipers, lanceheads, and Papuan taipans. However, the 20WBCT is limited by relative lack of sensitivity and lack of uniform standard application at the bedside for guiding treatment decisions (Ratnayake et al., 2017). In areas where multiple snakes that possess venom causing coagulopathy co-exist, or where the bite does not lead to incoagulable blood, the test may be of limited discriminatory value. However, in areas where both snakes that possess venom causing coagulopathy and snakes that do not, a negative test might exclude envenoming by the former. Thus, there are limitations to diagnosis as well as therapy and access-related challenges to the use of antivenom, though newer tests, therapeutic agents, and delivery platforms are being developed to mitigate illness and injury, and to guide treatment.
2. Current diagnosis and monitoring of envenoming
The diagnosis of SBE, including identification of the responsible snake and the severity of envenoming, is challenging, but critical, for deciding on when and how much antivenom to use. The proper identification of snake species or, when definitive identification is not possible, estimation of the most likely species is of prime importance for the management of SBE. Whenever possible, expert identification of the dead snake or a photo thereof is optimal. Otherwise, the species is inferred based on the patient's description and circumstances of the bite (e.g., a nocturnal bite while sleeping on ground suggests krait in South Asia or cobra in Africa) and the characteristic clinical syndrome (Ariaratnam et al., 2008a, Ariaratnam et al., 2009; Viravan et al., 1992; WHO, 2016; Bolon et al., 2020). In the absence of the culprit snake being brought in or identified, diagnosis is usually based on the circumstances of the bite, differentiating clinical manifestations, knowledge of species commonly found in a given geographical area, and from information gathered from the victim or a witness. This syndromic approach usually necessitates clinical toxinological knowledge and is difficult even in the best equipped settings in most regions.
Currently, no single, commonly available, laboratory investigation or bedside test exists to identify patients with systemic envenoming, and diagnosis involves combination of recognition of consistent clinical features and investigations (Ariaratnam et al., 2008b, Ariaratnam et al., 2008aa,b; Bolon et al., 2020). There are, however, simple tests and diagnostic tools (algorithms or checklists) that can be used to confirm the presence of important clinical signs of SBE, which indicate the need for early antivenom treatment and, in some cases, can help identify the most likely genus or species of snake responsible for the bite. Provided it does not cause a delay in initiating critical care (Isbister et al., 2013), evaluation of clinical severity and efficacy of antivenom treatment may be aided by low-tech bedside tests, such as the 20WBCT. The 20WBCT has been used as a simple inexpensive bedside test to identify coagulopathy worldwide, especially in many rural communities throughout the developing world (Costa et al., 2021; Habib and Warrell, 2013; Sano-Martins et al., 1994; Ratnayake et al., 2017; Dsilva et al., 2019). Where rapid, standardized laboratory testing is not available (e.g.,International Normalized Ratio, Prothrombin Time, Activated Partial Thromboplastin Time, Thrombin Time), the 20WBCT can be usefully applied as described in BOX 1. The test has performed well with reasonable sensitivity and specificity in several settings in the tropics, although its application needs to be standardized (Benjamin et al., 2018) [See Table 1].
BOX 1. 20 min Whole Blood Clotting Test (20WBCT) (WHO, 2016).
-
•
Place 2 mL of freshly sampled venous blood in a small, new, dry, glass vessel.
-
•
Leave undisturbed for 20 min at ambient temperature.
-
•
Tip the vessel once.
-
•
If the blood is still liquid (unclotted) and runs out, the patient has incoagulable blood (hypofibrinogenaemia) as a result of venom-induced consumption coagulopathy and is in need of antivenom treatment.
-
•
If the blood is clotted, the test is negative.
-
•
In Southeast Asia, incoagulable blood is diagnostic of a viper bite and rules out an elapid bite (kraits and cobras).
-
•
If there is any doubt, repeat the test in duplicate, including a “control” (blood from a healthy person).
Alt-text: BOX 1
Table 1.
Performance of the 20-min Whole Blood Clotting Test (20WBCT) compared to International Normalized Ratio (INR) or plasma fibrinogen level.
| Reference and year (country) | Sensitivity (%) | Specificity (%) | Patients (n) | Comparator |
Main responsible snake | Comments | |
|---|---|---|---|---|---|---|---|
| INR Threshold | Fibrinogen level Cut-off (g/L) | ||||||
| Isbister and Currie, 2003 (Australia) | 100 | 100 | 62 | (Prothrombin Time > 12 seconds) | Western brown snake | Only 2 coagulopathy patients included | |
| Pongpit et al., 2012 (Thailand) | 85.7 | 95.8 | 97 | ≥1.2 | <1.0 | Green pit viper | |
| 50 | 97.6 | 97 | <1.7 | Green pit viper | |||
| Isbister et al., 2013 (Sri Lanka) | 40 | 100 | 140 | >1.5 | Not stated; EIA confirmation of envenoming | Russell's viper | Conducted by non-trained staff. Test not standardized |
| Paiva et al., 2015 (Papua New Guinea) | 92.9 | 90.6 | ? | <0.5 | Papuan taipan | Unpublished; Presented at a conference | |
| Ratnayake et al., 2017 (Sri Lanka) | 82 | 98 | 987 | >1.4 | Not applicable; | Russell's viper | Trained staff. Test Standardized |
| Dsilva et al., 2019 (India) | 94 | 76 | 58 | ≥1.4 or clinical envenoming | Russell's and carpet viper | Envenoming defined clinically | |
| 100 | 74 | 58 | ≥1.4 | Russell's and carpet viper | |||
| 100 | 84 | 58 | ≥1.2 | Russell's and carpet viper | |||
| Thongtonyong and Chinthammitr, 2020 (Thailand) | 81 | 90.3 | 296 | <1.0 | Malayan pit viper | ||
| 98.5 | 78.6 | 296 | >1.4 | <1.0 | Malayan pit viper | ||
Given the challenges in diagnosis and known limitations of the 20WBCT, exploring alternative bedside tests, which are affordable, reliable, and quicker is a priority (Wedasingha et al., 2020). At present, there is only one commonly available commercial diagnostic test available: Seqirus’ Snake Venom Detection Kit. The success and acceptance of this Australian venom detection test suggests that this type of technology can be widely applied with satisfactory results (Currie, 2004; Isbister et al., 2013). This test uses polyclonal antibodies to differentiate snake venoms, but can only confirm contact with a particular genus of Australian elapid and does not confirm envenoming. Point-of-care tests such as this must be developed on a regional basis, so that they can be used to better inform the selection of antivenom, especially where species diagnosis is not clear and only monovalent antivenom is available.
In the case of species identification, if the dead snake has been brought to the hospital, it can sometimes be identified, but this requires skill and even experienced medical personnel may mistake harmless mimics for venomous snakes, or they may confuse different venomous species (Joseph et al., 2007; Williams et al., 2017; Cox et al., 2018). As a result, the patient may be given antivenom unnecessarily. This has been the case with hump-nosed pit viper (Hypnale hypnale) bites mistaken for saw-scaled viper (Echis carinatus) bites in Southwest India (Joseph et al., 2007; Williams et al., 2017); or mistaken for Russell's viper (Daboia russelii) bites in Sri Lanka, since currently available polyvalent antivenoms do not cover the venom of this species (Ariaratnam et al., 2008b). However, this may soon change as two newly-developed polyvalent antivenoms with activity against venom Hypnale hypnale are currently undergoing clinical trial in Sri Lanka.
Tests of blood coagulation, such as prothrombin time (PT), the International Normalized Ratio (INR), or activated partial thromboplastin time (aPTT), measurement of fibrin(ogen) related proteins also known as fibrin degradation products (FDP), or D-dimer, can also be used to diagnose snakebite envenoming. Thromboelastography (TEG) and thromboelastometry (TEM, ROTEG, and ROTEM) have also been used in developed countries as simple bedside methods for assessing coagulopathy in snakebite victims, but the equipment is expensive (Hadley et al., 1999). The need for bedside thromboelastography equipment is well recognized in the trauma setting, where such devices would benefit the bedside management of snakebite (Gonzalez et al., 2010).
In cases of neurotoxic envenoming, patients with neurological manifestations are monitored by serial general physical and neurological examination with special attention to cranial nerve function, airway, respiration, and bedside measures, such as Peak Expiratory Flow Rate (PEFR) and blood oxygen saturation (SpO2). The serial examinations assess the need for resuscitation and facilitate detection and monitor progression of neurological signs and symptoms. Indeed, the importance of airway management and artificial respiration has been well recognized for nearly 150 years (Brunton and Fayrer, 1873-4). Anticholinesterase inhibitors (e.g. edrophonium or tensilon) have been used to assess and provide immediate ancillary treatment, especially following envenomings by snakes causing post-synaptic neurotoxic envenoming (Banerjee et al., 1972; Watt et al., 1986; Warrell, 2010; Gutierrez et al., 2017a) and are generally not useful following envenoming by elapid snakes causing pre-synaptic neurotoxicity (Brunton and Fayrer, 1873-4, Banerjee et al., 1972, Anil et al., 2010). Other useful assessments, where available, can be generically described as ancillary bedside testing, but include simple tests such as visual acuity, neck and grip strength, ability to swallow, and electrophysiological methods based on twitch amplitude using instruments commonly available in basic anesthesiology suites to assess residual neuromuscular blockade (e.g. train of four) and have previously been used in the setting of snakebite for research purposes (reviewed by Ranawaka et al., 2013).
3. Challenges and limitations of existing antivenom therapy
Antivenoms currently available are produced via hyperimmunization of large animals, such as horses, sheep, donkeys, and camels (WHO, 2010; Laustsen et al., 2018b, Laustsen et al., 2018a). Consequently, antivenoms are heterologous and polyclonal immunobiologicals that are complex and costly to produce and come with a range of drawbacks in regard to effectiveness, safety, and availability.
Although an antivenom can be demonstrated to neutralize venom toxins in vitro or in laboratory animals (Gutiérrez et al., 2017b), clinical evidence of efficacy from well-designed randomized clinical trials is scarce and ethically challenging to obtain. Lack of standardization of the venom pools used to immunize the animals for antivenom manufacturing, associated with inter- or intraspecific venom variability, is one of the causes of poor effectiveness (Calvete et al., 2011; Kalita et al., 2018; Senji Laxme et al., 2021). In addition, the ‘gold standard’ in the preclinical assessment and quality control of antivenoms is the neutralization of venom-induced lethality, caused by neuromuscular paralysis, mostly by elapid venoms, or of thrombosis and/or cardiovascular collapse, mostly by viperid venoms (Gutiérrez et al., 2017b). Thus, other toxic actions of snake venoms, such as local effects from viperid envenomings, are usually not assessed in preclinical and clinical studies.
Antivenoms consist of heterologous proteins, and early adverse reactions (EARs) to antivenom therapy are expected undesirable events. It is noteworthy that ~70 % of the immunoglobulin content in some antivenoms is not directed against venom components (Laustsen et al., 2018b, Laustsen et al., 2018a). Clinically, patients suffering from adverse reactions against the antivenom immunoglobulins may present with urticaria, itching, tachycardia, nausea, vomiting, abdominal colic, bronchospasm, hypotension, and angioedema. Although both the frequency and severity of EARs have decreased due to improvements in the antivenom purification process, the incidence of EARs is still around 20 % in Latin America (Pardal et al., 2004; Otero et al., 2006; Mendonça-da-Silva et al., 2017), and >50 % in some Asian countries, where antivenom-related anaphylaxis is still a common event (León et al., 2013; Sharma et al., 2019; Isbister et al., 2012). Although EARs are not predictable by prior skin testing (Malasit et al., 1986), premedication with sub-cutaneous adrenaline (syn. epinephrine) has been shown to prevent EARs in 43 %–68 % of cases, and it is therefore recommended to be used to mitigate against EARs (de Silva et al., 2011; Habib, 2011). Conversely, premedication with antihistamines has no proven ability to prevent EARs (Bucaretchi et al., 1994; Wen et al., 1999; de Silva et al., 2011; Habib, 2011). The possibility of EARs generates fear among many health professionals and is a major obstacle for antivenom decentralization (Monteiro et al., 2020). The frequency of late adverse events (such as serum sickness), resulting from the deposition of long-lived immune complexes in blood vessels, kidneys, and joints seems to be lower than the frequency of EARs, but the true frequency is unknown (Itkin and Trujillo, 2005).
4. Choosing the right antivenom: Syndromic diagnosis versus identification of the biting snake
In a few countries, the available antivenoms are monovalent, and their proper use is possible only if the biting species is known or after diagnosis is performed based on clinical manifestations (WHO, 2018). Most patients do not bring the biting snake to the hospital, and often do not provide any clues that can aid in its identification. The decision on which antivenom to use is therefore based on clinical and epidemiological criteria. Accuracy of the syndromic approach for SBE management is influenced by the overlap of clinical manifestations in envenomings by different species, and by the relative prevalence of the types of envenomings in different geographic areas. In the Brazilian Amazon, SBEs caused by Bothrops and Lachesis generally have the same clinical presentation, with pain and swelling, associated to coagulopathy (Monteiro et al., 2020). The positive predictive value of Bothrops envenoming diagnosis in this region reaches 97.8–100 % when using an immunoassay based on monoclonal antibodies (Pardal et al., 2004; Sachett et al., 2017) due to the high prevalence (~90 %) of the Bothrops genus (Monteiro et al., 2020). In Thailand, however, coagulotoxic and neurotoxic syndromes following snakebites can be caused by several species and, in the past, before the development of polyvalent antivenoms, the treatment was efficacious only with correct snake identification (Viravan et al., 1992). Similar conclusions were obtained in Sri Lanka, although this country has polyvalent antivenoms available (Ariaratnam et al., 2009). Syndromic diagnostic algorithms have also been used in Australia (Isbister et al., 2013; Knudsen et al., 2021) and in sub-Saharan Africa by Médecins Sans Frontières (MSF). A large number of studies have been published on the development of diagnostic methods for the detection of venom components in different biological samples, notably using enzymatic immunoassays (Knudsen et al., 2021). Yet these are rarely used in both research and clinical practice, with the sole exception of Australia (Isbister et al., 2013).
5. New methods for diagnosis of snakebite envenoming
Early treatment of SBE has been linked to improved patient outcome, faster recovery, and decreased resource consumption at treatment facilities (Johnston et al., 2017; Anderson et al., 2018; Alfred et al., 2019; Pinho et al., 2005; Mise et al., 2018). Therefore, researchers have long been developing supportive tools for the diagnosis of SBE, especially for differentiation of snake venoms (Knudsen et al., 2021). Supportive diagnostic tools could potentially bring about multiple benefits, perhaps the foremost of which is facilitation of early and correct treatment, including selection of the most appropriate antivenom. In addition to their impact on the treatment of individual patients, novel diagnostics could also support epidemiologists in the mapping of snakebite incidents, which in turn can aid healthcare systems in deciding which antivenoms to procure and how to optimize their distribution. Such studies could also inform the design of novel antivenoms and aid antivenom manufacturers in identifying which patients to include in clinical trials.
Snakebite diagnostics reported in the literature include assays based on infrared thermography (de Madeiros et al., 2017; Sabitha et al., 2021), measurement of enzymatic activity of toxins (Maduwage et al., 2014), molecular biology methods (Suntrarachun et al., 2001; Supikamolseni et al., 2015), and immunoassays (e.g., Faria et al., 2018; Lin et al., 2020; Hung et al., 2014; Liu et al., 2018) to mention only a few), of which enzyme-linked-immunosorbent assays (ELISAs) are the most frequently described. The pros and cons of these methods make them differentially suited for different uses. For instance, PCRs with their often extremely low limits of detection may be well-suited for forensic studies at well-equipped facilities, but not necessarily for point-of-care use, due to the requirement of specialized equipment and trained personnel (though this is likely to change with the implementation of more rapid, affordable, and user-friendly forms of PCR, see e.g. (Jiang et al., 2015; Neuzil et al., 2006; Vaagt et al., 2013)). Conversely, rapid, user-friendly, and affordable lateral flow assays (LFAs) may be well-suited for point-of-care applications, while not offering the same functionalities as more complex assay types. Other parameters that influence the utility of novel diagnostics include sensitivity, specificity, sampling method and sample matrix, stability, choice of analyte (e.g. venom toxins, RNA, or DNA), measurability, accuracy, and precision.
New snakebite diagnostics need not, however, come in the form of bioassays. In an entirely different avenue of diagnostics, it has been proposed that artificial intelligence can assist in snake identification and in linking clinical syndromes to snake species or genera (de Castañeda et al., 2019). Snake recognition apps may also make differentiation of dry bites and bites by non-venomous snakes easier (a property not necessarily possessed by bioassays). With the advent of ever-more affordable, sensitive, and user-friendly technologies and the growing attention to SBE, it seems plausible that more snakebite diagnostics may find their way to the clinic in the future.
6. Therapeutic application of monoclonal antibodies and antibody fragments
With the advent of recombinant DNA technology and modern approaches for the discovery of monoclonal antibodies, there is today an opportunity to develop novel types of antivenoms based on recombinantly expressed antibodies and antibody fragments (Laustsen et al., 2020). Given that it is well-established that animal-derived antibodies and fragments thereof are (relatively) effective in neutralizing snake venom toxins (Gutiérrez et al., 2017b, 2011), it seems almost self-evident that mixtures of recombinantly expressed human antibodies and antibody fragments could find therapeutic utility if properly developed and manufactured (Casewell et al., 2020; Kini et al., 2018). Compared to animal-derived antibodies, human antibodies are less immunogenic to human recipients (Laustsen et al., 2018a, 2016), and if carefully designed, mixtures of monoclonal antibodies, that target neutralizing epitopes of toxins that are medically relevant for human envenomings or disrupt toxin synergism, can likely be optimized to have better efficacy, less batch-to-batch variation, and possibly even lower cost of manufacture (Kini et al., 2018; Laustsen, 2016b, Laustsen, 2018a, Laustsen, 2018b; Laustsen et al., 2015, 2018a). Given these potential benefits, scientific efforts have in recent years focused on the development of both fully human monoclonal and oligoclonal antibodies (Laustsen et al., 2018b) and camelid antibody fragments (Bailon Calderon et al., 2020; Julve Parreño et al., 2017; Richard et al., 2013). Combined, these studies have demonstrated the feasibility of using phage display technology for the discovery of such molecules, as well as the potential utility of formulating these into so-called ‘recombinant antivenoms’ (Laustsen, 2016a). One of the challenges for these types of endeavors is, however, to develop sufficiently broadly-neutralizing monoclonal antibodies, which is a necessity for feasible recombinant antivenom manufacture, as the inclusion of antibodies that are able to cross-neutralize multiple toxins allow the manufacturer to limit the number of antibodies needed in a recombinant antivenom formulation (Jenkins and Laustsen, 2020; Ledsgaard et al., 2018). Therefore, recent studies have focused on devising simple, rational methods that allow for facile discovery of antibodies with such properties. This is exemplified by Ahmadi et al., who introduced a cross-panning strategy in phage display selection campaigns that allowed for proactively selecting for broadly-neutralizing antibodies prior to antibody screening, allowing the researchers to discover fully human antibody fragments that could cross-neutralize three-finger toxin (3FTx) cytotoxins from African cobras (Ahmadi et al., 2020). In the future, other strategies, such as the application of recombinant consensus toxins (de la Rosa et al., 2019), may also prove useful for the discovery of broadly-neutralizing monoclonal antibodies, as well as improved high-throughput analytical methods for assessing cross-reactivity will become essential tools for developing polyvalent recombinant antivenoms (Krause et al., 2020; Laustsen et al., 2018b, Laustsen et al., 2018a; Ledsgaard et al., 2018). Finally, the application of Big Data and machine learning approaches are expected to take up speed in the field of antibody discovery (Laustsen et al., 2021), which in turn may be exploited in the development of recombinant antivenoms. However, before any recombinant antivenom development project can commence, it is essential to have a clear understanding of the benefits and drawbacks of different antibody formats, as the choice of format will have major influence on the development strategy (Knudsen et al., 2019).
7. Advantages and disadvantages of different antibody formats
In contrast to many other disease areas, such as oncology and autoimmune diseases, where antibodies have successfully entered the clinic, SBE is fundamentally different in nature: It is acute, a very large number of targets of great molecular complexity must be neutralized, therapy only needs to be administered over a short period of time, and the majority of patients come from impoverished regions of the tropics (Laustsen, 2019). This puts certain constraints on what antibody formats are likely to be useful for treating this pathology, as well as feasible to develop and cost-competitive to manufacture (Jenkins and Laustsen, 2020; Knudsen et al., 2019; Laustsen et al., 2017; Laustsen and Dorrestijn, 2018). Generally, different antibody formats have different advantages and disadvantages (Table 2), but it is important that the antibody format of choice is compatible with the human immune system and therefore safe, that the format is versatile and allows for facile development of antibodies that can neutralize different toxin families, that the format is easy and low-cost to manufacture, and that the format can be engineered to bind toxins with very high affinity (Knudsen et al., 2019; Laustsen et al., 2018a). These requirements may easily restrict the utility of more advanced antibody formats, and instead favor simpler formats with a history of successful clinical use, such as human immunoglobulin G antibodies (IgGs) and single-domain antibodies, although smaller non-antibody-based scaffold proteins could potentially also find use in the clinical setting (Jenkins et al., 2019). Ultimately, however, it is not unlikely that a combination of different antibody formats in a recombinant antivenom may be useful for targeting different types of toxins and thereby providing more benefits than each format is capable of alone (Casewell et al., 2020). In the shorter term, it is also possible that monoclonal antibodies and their fragments could be used to fortify existing plasma-derived antivenoms to improve efficacy – especially against highly toxic components of low immunogenicity that do not give rise to high antibody titers during immunization (Kini et al., 2018; Knudsen et al., 2019).
Table 2.
Overview of the advantages and drawbacks of different antibody formats.
| Antibody format | Advantages | Disadvantages |
|---|---|---|
| Animal-derived antibodies | In vivo affinity maturation possible, manufacturable | Immunogenic, medium cost of manufacture |
| Human IgG | Safe, manufacturable, long half-life, clinically successful format | Medium cost of manufacture |
| Human F(ab)2 | Safe, medium half-life | Non-optimal for recombinant expression, high cost of manufacture |
| Human Fab | Safe, manufacturable, clinically successful format | Medium cost of manufacture, short half-life |
| Human scFv | Easy to discover, low cost of manufacture | Prone to aggregation and polymerization, short half-life |
| Human VH | Easy to discover, (very) low cost of manufacture, fast tissue penetration | Short half-life |
| Camelid VHH | Easy to discover, in vivo affinity maturation possible, (very) low cost of manufacture, fast tissue penetration | Short half-life, not fully human |
| Bispecific IgG | Smaller number of different antibody clones needed to target different toxins | Complex and low-yield manufacture, same amount of total antibody needed for neutralization |
| Bi/multispecific VH and VHH | Smaller number of different antibody clones needed to target different toxins, easy half-life extension options available | Same amount of total antibody needed for neutralization, short half-life |
| Antibody-like scaffolds | (Very) low cost of manufacture sometimes possible, fast tissue penetration | High affinity more complicated to achieve, blocking intellectual property rights may exist |
8. Repurposed molecules
Small molecule therapeutics (SMT) represent a potentially useful addition to antibody-based antivenoms. As a group, most SMTs used in pharmaceutical preparations are derived from natural bioactive compounds or synthetic molecules intended to act on specific targets. In the foreseeable future, the therapeutic approach to snakebite envenoming might include combinations of SMTs and antibody-derived therapeutics, capitalizing on their respective strengths as toxin-neutralizing interventions. Particular attention has been paid to inhibitors of metalloproteases, secreted phospholipase A2s (sPLA2), and serine proteases, but several other potential targets for SMTs exist with or without enzymatic activities (Clare et al., 2021).
Drug development is exceedingly expensive, but one approach to mitigating development costs is to search for molecules developed for other indications that have desired inhibitor properties. Several of such drug candidates exist that have reached advanced stages of clinical development for other indications in oncology (metalloprotease inhibitors) and for acute indications such as severe sepsis (sPLA2 inhibitors) (Steward, 1999; Zeiher et al., 2005). If proven safe and effective, these previously developed compounds could be catapulted into clinical use with the least cost possible, especially if supported by industry data packages (Bulfone et al., 2018). Multiple laboratories have spurred recent interest in testing these previously tested candidate molecules alone, in combinations with each other, or with antivenom (Lewin et al., 2018a, 2018b; Bittenbinder et al., 2018; Albulescu et al., 2020a, b). Discovering small molecules for specific blockade of non-enzymatic toxins, such as 3FTxs, remains challenging, but could be amenable to combinations of SMTs and monoclonal antibodies (Laustsen et al., 2015). No molecule has every required therapeutic attribute (e.g. nafamostat is used as a serine protease inhibitor, but has exceptionally short half-life, varespladib and most metalloprotease inhibitors dissolve at very different pH and would be challenging to co-formulate), and Proof of Concept in humans has yet to be established for any of these compound classes.
Examples of small molecules likely to advance to clinical trials in the near future include repurposed sPLA2 inhibitors, such as varespladib and its orally bioavailable prodrug varespladib-methyl and metalloprotease inhibitors such as marimastat (Lewin et al., 2016; Albulescu et al., 2020a). Significant preclinical work in the field now includes combination studies of varespladib with marimastat and even the addition of a third inhibitor of serine proteases, nafamostat (Xie et al., 2020a, 2020b; Still, 2020; Albulescu et al., 2020b). To advance these molecules to help modernize treatment of SBE, clinical trials including the existing standard of care must be carefully conducted with attention to measurable endpoints able to discriminate study drug effects from standard of care. While a species-by-species approach could be feasible with unlimited funding, it is neither cost-effective nor desirable. The ideal toxin-specific inhibitor should be broadly specific and robust against smaller changes to the tertiary and quaternary structures of the enzyme being inhibited. To that end, it may make sense to approach clinical trial sites with concentrations of SBEs exemplifying distinct syndromes and venom characteristics (Xie et al., 2020a, 2020b; Still, 2020; Albulescu et al., 2020a, b). For example, an inhibitor capable of blocking monomeric, dimeric, and more complex sPLA2s could be tested in sites experiencing bites from Russell's vipers, saw-scale vipers (monomeric sPLA2), cobras, and kraits, featuring both non-covalently bound and covalently bound dimeric sPLA2s. Trials demonstrating efficacy against the toxicities of structurally and functionally diverse sPLA2s could successfully make the case for broad approval (Gutiérrez et al., 2020) and then be combined in formulation or co-administered with other small molecule inhibitors, such as a metalloprotease inhibitor (Lomonte et al., 2009; Albulescu et al., 2020b, Albulescu et al., 2020a).
Formulation and solubility issues will ultimately determine the potential for co-formulated antidotes and dosage forms. Oral solid dosage forms (e.g. tablets and capsules) may be more amenable to co-formulation, but molecules such as varespladib favor alkaline environments, while protease inhibitors such as marimastat and prinomastat favor an acidic environment for solubility, suggesting significant pre-clinical evaluation and optimization will be needed before they are to be considered for co-administered by the intravenous route in the future. Rapid absorption of potent orally available inhibitors could make them amenable to administration in intubated patients providing viable routes to avoid the need for mechanical ventilation in resource-limited environments. There is much to learn in the next few years as SMTs emerge and are evaluated for their potential addition to the therapeutic armamentarium for the initial and adjunctive treatment of SBE.
The existing SMTs have already passed prior drug development steps and attained reasonable safety status for human use (Wojtowicz-Praga et al., 1997; Rosenson et al., 2010). However, clinical studies are urgently needed to evaluate safety, efficacy, optimum mode of administration, and duration of therapy for such repurposed molecules (e.g., marimastat, prinomastat and varespladib) among SBE patients. The results from such trials should be compared to existing antivenom therapy and standard of care, which in turn may help shine light on whether such SMTs could be utilized as adjuncts to antivenom in either the pre-hospital or hospital setting (Kini et al., 2018).
9. Improving education and training
The burden of snakebite is highest in developing countries with weak healthcare systems, resulting in substantial deaths and disabilities (Habib and Abubakar, 2011). The clinical management of SBE entails hospitalization, administration of effective antivenoms, and provision of supportive care by Health Care Workers (HCWs). Studies have shown low levels of education among HCWs in developing countries (Michael et al., 2018; Bala et al., 2020a, Bala et al., 2020b), and it has been established in northern Ghana that the outcome of care, including deaths from SBE, depends partly on the knowledge of HCWs and their compliance to standardized protocols, and on the availability of effective antivenom. There, the impact of training of HCWs was shown to reduce mortality from SBE (Visser et al., 2004). Harmful traditional beliefs and practices play important roles in delaying hospital presentation following SBE, and delayed presentation is a predictor of poor prognosis (Habib and Abubakar, 2011). Empowering HCWs with adequate knowledge on snakebite management, identification of common harmful snakes, and educating communities in pre-hospital care of snakebites is necessary to improve pre-hospital and hospital management of snakebite envenoming.
There is growing concern that the HCW training curriculum is deficient in clinical toxinology in general and in covering snakebite care and management in particular (White, 2013). This deficiency emphasizes the need to improve training and inclusion of practice-oriented courses in the curricula of HCWs for effective snakebite care and management. In relation to this, the World Health Organization (WHO) and other stakeholders could play greater roles in ensuring improved training and knowledge of HCWs and communities to achieve the target of reducing snakebite morbidity and mortality. To achieve this, there is a need to develop region-specific, evidence-based guidelines and other Information, Education, and Communication (IEC) materials for snakebite care and management. Regrettably, some countries conduct numerous preclinical and clinical studies on snakebite management, but the results and conclusions have not been included into their national snakebite management guidelines. Encouraging advances and demonstrations of efficacy in HCW education and application of teachings have recently been reported in clinical education programs piloted in many countries, suggesting renewed recognition of the importance of this topic (Visser et al., 2004; Michael et al., 2018; Qiu et al., 2019; Samuel et al., 2020; Sulaiman et al., 2020). Because countries with a high snakebite burden often lack country-specific medical textbooks on snakebite, it should be a priority for these countries to develop textbooks that can help guide clinical management of snakebite in their settings. It is also important that countries with preclinical and clinical studies on snakebite incorporate the findings of such studies to update national snakebite management guidelines.
10. Snakebite in the prehospital setting
A detailed description of snakebite management guidelines is beyond the scope of this review, but comments on education, the critical nature of initiating treatment in timely fashion, and basic principles are warranted. As described in the previous section, inadequate knowledge about SBE symptoms and the application of harmful first aid methods in the prehospital setting hamper care and endanger patients. Detrimental first aid methods include the use of suction devices, tourniquets, and “medicinal” stones resulting in tissue damage, delayed release of toxins, or fatal delays in definitive care (Alberts et al., 2004; Ameade et al., 2021; Mahmood et al., 2019; Singaravelu et al., 2021). Manipulation of the bite wound, such as by incision or fasciotomy, may introduce infections, including tetanus (Habib, 2003), and increase local bleeding. Ignorance, overconfidence, and outdated training are not exclusive to the lay community or practitioners of traditional medicine using herbal remedies, but also exist in professionalized settings, such as the military, expeditionary, and even clinical organizations (Chen et al., 2016; Bhargava et al., 2020). Evidence suggests that even in large organizations with diverse skill sets, these deficiencies can be overcome with educational programs focused on the basics of treatment in the field and hospital, though clinical experience remains the major determinant of appropriate confidence and skill (Michael et al., 2018; Qiu et al., 2019; Samuel et al., 2020; Sulaiman et al., 2020).
Many considerations must be integrated in the provision of emergency care to a patient suffering from snakebite envenoming. From airway control to reassurance of the patient that help is near or that the situation can be managed competently on site until transport with incipient hospital evaluation and treatment can be provided. Many productive procedures can be undertaken simultaneously and calmly if HCWs are appropriately educated and mentally prepared. Along with calm initiation of movement toward definitive care at a dispensary or hospital, the most fundamental actions are supportive and include attention to the airway, breathing, hemodynamic stability of the patient, and safest possible slowing of toxin spread, e.g. pressure-immobilization techniques (Parker-Cote and Meggs, 2018).
BOX 2 describes key, initial steps upon encountering the victim of a snakebite. Practical, effective, and safe measures are recommended, and there are superb, publicly available resources, such as those provided by the WHO, to guide even the most advanced caregivers (WHO, 2016):
BOX 2. Initial aid of the snakebite victim (adapted from WHO recommendations).
After a bite by a snake suspected of being venomous, follow these steps:
-
•
Immediately move away from the area where the bite occurred.
-
•
Remove anything tight from around the bitten part of the body to avoid harm if swelling occurs.
-
•
Reassure the victim, as most venomous snake bites do not cause immediate death.
-
•
Immobilize the person completely and transport the person to a health facility as soon as possible.
-
•
Applying pressure at the bite site with a pressure immobilization pad may be suitable in some cases.
-
•
Avoid traditional first aid methods, herbal medicines, suction devices, and tourniquets.
-
•
Paracetamol may be given for local pain (which can be severe).
-
•
Vomiting may occur, so place the person on their left side in the recovery position.
-
•
Closely monitor airway and breathing and be ready to resuscitate if necessary.
Bringing the patient to the facility most capable of administering antivenom and other standards of supportive care is essential and can be aided by local knowledge, emergency services, or with the help of internet or poison control centers where available.
Alt-text: BOX 2
Even the most experienced physicians might only rarely encounter snakebite victims during their practice of clinical medicine. Outcomes can be improved by awareness of the basic elements of first aid, resources for obtaining help (e.g. poison control centers and/or expert identification of snakes), and knowledge of what practices are either harmful or without utility. Maintenance of awareness and fundamental do's and don'ts is required and should be an obligation of experts and advocates in the field.
11. Repositioning conventional antivenom therapy
As new antivenoms are introduced into clinical care, establishing their safety and efficacy through conventional phase II studies poses major ethical challenges given the known risks of heterologous plasma-derived antibodies in humans. Innovative approaches have been explored to find alternative strategies, such as the ‘3 + 3’ dose escalation continual reassessment method (CRM), wherein an initial dose, derived from preclinical testing, is progressively doubled until pre-defined desirable safety and efficacy levels are achieved among cohort of few mildly envenomed patients (Abubakar et al., 2010). The approach was originally developed for oncologic drugs (Rosenberger and Haines, 2002), but has been used successfully to usher in new antivenoms (Abubakar et al., 2010). The method has, however, been criticized (Chippaux and Boyer, 2010), and modifications entailing commencement with a higher dose followed by subsequent de-escalation have been suggested, especially following presynaptic neurotoxic envenomings, where clinical manifestations may be irreversible and should be timely prevented. Recently, a Bayesian model-based adaptive design has been suggested to be more efficient and has the capacity to enroll larger patient sample sizes determined by simulations (Watson et al., 2020). This is also more flexible and has better operating characteristics than the CRM. For the adaptive design, data from sequentially enrolled patients is used to continually update the parameters of the dose-response model eventually leading to better dose-optimization. Both approaches are suitable for safety and efficacy assessments with binary outcomes. The adaptive design was developed to be used to determine the optimal safe and effective dose of a new antivenom used to treat Russell's viper envenoming in Myanmar and will probably be increasingly used to introduce antivenoms into clinical management (Watson et al., 2020).
Despite their limitations, antivenoms remain the mainstay of therapy of SBE. Although only a couple of randomized controlled trials (RCTs) with placebo controls have been conducted (Rojnuckarin et al., 2006; Gerardo et al., 2017), antivenoms were found to be effective in reducing mortality by about 75 % in observational studies following carpet viper hemotoxic envenoming in West Africa (Habib and Warrell, 2013). The main indications for antivenom administration include severe cytotoxicity, hemotoxicity, neurotoxicity, cardiotoxicity and rhabdomyolysis, usually manifesting clinically as one or more of the following: extensive local swelling, tissue necrosis, shock, spontaneous systemic hemorrhage, incoagulable blood, paralysis (e.g., respiratory paralysis, ptosis, and ophthalmoplegia), hemoglobinuria, myoglobinuria, cardiac failure, and acute kidney injury (Gutierrez et al., 2017a).
Current antivenoms are either liquid or freeze-dried formulations, which have longer shelf-lives than their liquid counterparts, but which also require reconstitution before use. Polyvalent antivenom is most useful where diverse snakes cause envenoming, while monovalent products are useful where one or an easily discernible snake causes envenoming. The same dose is used for adults and children as dosing is to match the amount of venom injected regardless of patient size (Le Geyt et al., 2021), though there is a lack of properly conducted clinical trials of dose finding for antivenoms in children. Although studies have been conducted on adults, there is a trend towards generally using lower antivenom doses, with an observational and three randomized studies showing that relatively lower volumes (lower protein content) may be associated with fewer EARs without compromising effectiveness following either hemotoxic or neurotoxic envenoming (Jorge et al., 1995; Paul et al., 2004; Allen et al., 2012; Alirol et al., 2017). It is possible that children with smaller extracellular fluid volume and high venom-to-body-mass ratio may exhibit more devastating consequences of envenoming but also higher rates of EAR than adults following administration of the same dose of antivenom. Therefore, similar studies are needed to determine the timing of antivenom administration, volume, issues of fluid overload, and specific signs of EAR in children.
While antivenoms reverse hemostatic abnormalities and protect against mortality (Habib and Warrell, 2013), they are thought to be relatively inefficacious in preventing against local pathologies and necrosis. For instance, it has been shown that presence of blisters or bullous wounds is associated with more marked envenoming and poorer outcome, even though higher doses of antivenom are required for restoration of clotting following carpet viper envenoming (Iliyasu et al., 2014). Thus, it is being hypothesized that in addition to inflammatory markers found in blister fluids, venom toxins detected in wound aspirates may exist as a depot and lead to gradual release of venom toxins with ensuing worsening pathology (Iliyasu et al., 2014; Lin et al., 2019). Indeed, a study in Thailand showed recurrence of venom antigenemia in about a quarter of patients, suggesting possible continuous absorption of venom from the wound blisters into systemic circulation (Ho et al., 1986). Recently, it has been shown that antivenom (IgG antibody or fragments) also diffuses into the blister fluid, though its functional ability to neutralize the venom is unclear (Gimenes et al., 2020). Therefore, studies are needed to determine the utility of antivenom and complementary local interventions (e.g., blister aspiration) against local pathologies as some indicators suggest aspiration might be more effective than deroofing treatment even in non-envenoming inflammatory blistering disease not containing venom toxins (Ro et al., 2018).
Antivenoms made of papain digested fragments (Fabs) have been shown to lead to recurrence of envenoming without durable resolution of symptoms (Williams et al., 2018), and some manufacturers have stopped their production, though Fab-based antivenoms remain in use in other areas. The development of new, enhanced antivenoms effective against a broader range of the most important snake species in multiple countries in a region, such as the planned pan-African polyvalent antivenom, will hopefully help improve future patient outcomes and ultimately promote utilization through education. This could lead to increased demand and stimulate mass production, ushering in better pricing and affordability through economies of scale, and provision of quality products (Habib et al., 2020).
At the global level, efforts towards enhancing safety, efficacy, potency, and affordability of antivenoms should be undertaken by strengthening the manufacturing laboratories, implementing Good Manufacturing Practices (GMP), improving the design of immunizing mixtures, optimizing the purification of plasma-derived antibodies, and implementing better quality control procedures. Additionally, the WHO's antivenom prequalification program should be introduced and strengthened. In this regard, the WHO Roadmap for control of SBE states that target product profiles (TPPs) and minimum specifications will be implemented by 2021 as essential prerequisites for the introduction of a formal WHO antivenom prequalification scheme (WHO Roadmap, 2019). However, the recent Covid-19 pandemic will likely cause delays in these activities.
12. New approaches to delivery and deployment of snakebite therapeutics
Antivenoms are administered parenterally via the intravenous route, which requires properly trained personnel usually within hospital settings. As snakebites mostly occur in remote rural areas with no skilled personnel (see BOX 3), delay in treatment is a recognized predictor of poor outcomes (Habib and Abubakar, 2011; Silva et al., 2020). Several studies have confirmed reversal of envenoming, in particular coagulopathy or neurotoxicity, following oral use of therapeutic agents and inhibitors (e.g., marimastat, varespladib) in small and large animal studies, although they have not been replicated in humans (Bhattacharya et al., 2014; Lewin et al., 2018a; Lewin et al., 2018b; Albulescu et al., 2020b, Albulescu et al., 2020a). However, it is probable that these agents will be studied in humans in the near future and repositioned as oral therapies for prehospital interventions. For now, intravenous antivenom administration remains the mainstay of envenoming therapy and may be performed slowly or as slow push, as there is no observed difference between these administration methods with regards to development of EAR rates (Isbister et al., 2012).
BOX 3. Challenges to Clinical Management of Snakebite Envenoming.
Access to prompt and timely care
Lack of reliable, standardized, widely available point-of-care diagnostic tests that discriminate between varied types of envenoming and responsible snakes
Lack of safe and effective antivenom and accessory medicines
Lack of adequate knowledge and skills among healthcare workers
Lack of post-discharge follow-up to detect/manage long-term sequelae
Lack of guidance for managing snakebite envenoming in children
Weak health systems and infrastructure lacking vital items for snakebite care in endemic areas e.g., airway support
Alt-text: BOX 3
Current antivenom deployment strategies mostly rely on providing small stocks of antivenoms to hospitals in endemic areas in resource-constrained settings. Hospital staff can then periodically request replenishment from headquarters in urban centers. This is often associated with frequent stock-outs and consequent increased mortality (Habib and Abubakar, 2011). To improve accessibility, a ‘hub-and-spoke’ distribution and utilization network has been suggested, wherein rural facilities serve as satellites or spokes and are linked to major hospitals in urban hubs for referrals of complicated cases, linkages, support, and more readily available antivenom supplies (Habib, 2013; Habib et al., 2020). This way, products, services, and training are cascaded down from the centralized facility to the remote, peripheral areas. A strategy of boosting antivenom supply with constant deployment and distribution of enough antivenom to facilities for use when needed should be adopted, as opposed to that of trying to source for and supply small stocks of antivenom when the demand arises (WHO, 2019). Whenever feasible, development and procurement of products with longer shelf-life (e.g., freeze-dried antivenoms) should be encouraged, especially in resource constrained settings (Habib et al., 2020).
Given the need to reduce delays in antivenom administration, new approaches of antivenom delivery are increasingly being considered for remote and inaccessible locations. Helicopters have been used to deliver life-saving antivenom and interventions for envenomed patients in industrialized countries. Similarly, it has been suggested that drones can be used to deliver antidotes for snake and dog bites and blood for transfusion in developing countries (Laksham, 2019; Shah et al., 2021). However, the high rates of EARs accompanied by a general lack of skilled personnel, medications, and knowledge of management of EARs pose major threats to antivenom administration in rural settings. Training and skills acquisition of lower cadre HCWs and nurse-led clinics have, however, been shown to be equally effective compared to medical doctors in treating snakebite (Yates et al., 2010). These measures should be promoted to improve access to antivenoms in remote areas. Furthermore, the use of web-based snake identification services and snakebite apps on smartphones for immediate recognition of snakes causing bites, advice on finding the nearest hospital stocking antivenom, antivenom selection, and antivenom mode of delivery are being explored (https://slma.lk/sbc/sbc_guidelines/).
13. Future perspectives
Most of the major clinical manifestations of SBE have been characterized across different regions of the globe. However, atypical presentations, chronic complications (e.g., chronic kidney failure), and long-term sequelae (e.g., neurologic and psychologic deficits) remain to be defined for different snakes and different settings; thus, it is important to reiterate that clinical management guidelines recommend post-discharge follow-up and periodic reevaluation as previously recommended (WHO SEARO, 2016). Clinical studies will be needed to establish the reliability of new diagnostics, as well as safety and efficacy for monoclonal antibody-based therapies and SMT-based therapies. Appropriate preclinical and clinical studies should be developed and validated for investigating new therapeutic agents. Particularly, appropriate endpoints, models, and surrogate markers for assessing necrosis and neurotoxicity have to be established and validated in a reproducible manner before such clinical studies can commence. In addition to pre-hospital interventions, the efficacy of administering SMTs, repurposed agents, and monoclonal antibodies alone and in combination with antivenom should be further investigated. Clinical trials of novel therapeutics could potentially be supported by new diagnostic tools, as the results from diagnostic tools can be used as inclusion criteria in clinical trials; ensuring that novel therapeutics are only administered to patients for whom they are indicated. When new therapeutics and diagnostics are developed, affordability should be a major priority given the economies of the countries most affected by SBE. Global best-practice frameworks for benchmarking, manufacturing, and standardizing both conventional and new therapies should be established, strengthened, and implemented. HCWs should be trained to avoid harmful practices, identify clinical presentations of envenoming, and implement standard treatment protocols and algorithms. Global and national efforts towards improving access to antivenom in endemic areas should be promoted – consistent with the WHO Roadmap (WHO, 2019). Further research to determine the most appropriate, affordable, simple, practical, and scalable approaches for delivery and deployment of snakebite care should also be conducted. By combining efforts from the laboratory, clinical settings, and logistics, an opportunity exists for improving the clinical management of SBE worldwide.
Credit author statement
AH provided the sections headers and all authors agreed on the manuscript outline. Each author (MH, CK, CG, WM, ML, AL, AH) provided the first draft of designated section(s): MH on Education and training; CK on New diagnostic approaches; CG on Current diagnosis and on Pre-hospital care; WM on Limitations of existing antivenoms and on Syndromic diagnosis; ML on Repurposed molecules and on Pre-hospital care; AL on Monoclonal antibodies, Antibody formats and Highlights; AH on Summary, Background, Repositioning conventional antivenom, New approaches to deployment and the Future perspectives. All compiled and developed the initial draft and participated in revising it. AL provided the graphical abstract. MH standardized the referencing. All authors edited, improved the manuscript, updated the referencing and approved the final initial and revised versions.
Ethics statement
As a review an ethics/IRB approval is not required but the work was conducted in accordance with existing ethical standards with all authors contributing actively into the final manuscript. As a review no identifiable person is cited or mentioned and thus no informed consent is required.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. MH and AGH are members of the African Snakebite Research Group (ASRG) project and the Scientific Research Partnership for Neglected Tropical Snakebite (SRPNTS) that are supported by NIHR(UK) and DfID (UK) respectively.
Handling Editor: Raymond Norton
References
- World Health Organization (WHO) reportExpert Committee on Specifications for Pharmaceutical Preparations. WHO guidelines for stability testing of pharmaceutical products containing well established drug substances in conventional dosage forms. WHO Expert Committee on Specifications for Pharmaceutical Preparations - WHO Technical Report Series, No. 908 - Thirty-seventh Report (2003). Available at: http://apps.who.int/medicinedocs/en/d/Js5517e/12.1.html. (Accessed: 30th June 2017).
- World Health Organization, Regional Office for South-East Asia . second ed. WHO Regional Office for South-East Asia; 2016. Guidelines for the Management of Snakebites.https://apps.who.int/iris/handle/10665/249547 [Google Scholar]
- World Health Organization . 2018. WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins.https://www.who.int/bloodproducts/snake_antivenoms/snakeantivenomguide/en/ pg 192. [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2019. [Roadmap] Snakebite Envenoming: a Strategy for Prevention and Control. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Abubakar S.B., Abubakar I.S., Habib A.G., Nasidi A., Durfa N., Yusuf P.O., Larnyang S., Garnvwa J., Sokomba E., Salako L., Laing G.D., Theakston R.D.G., Juszczak E., Alder N., Warrell D.A. Pre-clinical and preliminary dose-finding and safety studies to identify candidate antivenoms for treatment of envenoming by saw-scaled or carpet vipers (Echis ocellatus) in northern Nigeria. Toxicon. 2010;55:719–723. doi: 10.1016/j.toxicon.2009.10.024. [DOI] [PubMed] [Google Scholar]
- Ahmadi S., Pucca M.B., Jürgensen J.A. An in vitro methodology for discovering broadly-neutralizing monoclonal antibodies. Sci. Rep. 2020;10:10765. doi: 10.1038/s41598-020-67654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts M.B., Shalit M., LoGalbo F. Suction for venomous snakebite: a study of "mock venom" extraction in a human model. Ann. Emerg. Med. 2004;43(2):181–186. doi: 10.1016/S0196064403008138.PMID:14747805. [DOI] [PubMed] [Google Scholar]
- Albulescu L.O., Hale M.S., Ainsworth S. Preclinical validation of a repurposed metal chelator as an early-intervention therapeutic for hemotoxic snakebite. Sci. Transl. Med. 2020;12(542):8314. doi: 10.1126/scitranslmed.aay8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albulescu L.O., Xie C., Ainsworth S. A therapeutic combination of two small molecule toxin inhibitors provides broad preclinical efficacy against viper snakebite. Nat. Commun. 2020;11(1):6094. doi: 10.1038/s41467-020-19981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfred S., Bates D., White J. Acute kidney injury following eastern Russell's viper (Daboia siamensis) snakebite in Myanmar. Kidney Int. Reports. 2019;4:1337–1341. doi: 10.1016/j.ekir.2019.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirol E., Sharma S.K., Ghimire A. Dose of antivenom for the treatment of snakebite with neurotoxic envenoming: evidence from a randomized controlled trial in Nepal. PLoS Neglected Trop. Dis. 2017;11(5):5612. doi: 10.1371/journal.pntd.0005612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen George E., Brown Simon G.A., Buckley Nicholas A., O'Leary Margaret A., Page Colin B., Currie Bart J., White Julian, Isbister Geoffrey K. ASP Investigators Clinical effects and antivenom dosing in brown snake (Pseudonaja spp.) envenoming--Australian snakebite project (ASP-14) PloS One. 2012;7(12):53188. doi: 10.1371/journal.pone.0053188. Epub 2012 Dec 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameade E.P.K., Bonney I., Boateng E.T. Health professionals' overestimation of knowledge on snakebite management, a threat to the survival of snakebite victims-A cross-sectional study in Ghana. PLoS Neglected Trop. Dis. 2021;15(1):8756. doi: 10.1371/journal.pntd.0008756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson V.E., Gerardo C.J., Rapp-Olsson M. Early administration of Fab antivenom resulted in faster limb recovery in copperhead snake envenomation patients. Clin. Toxicol. 2018:1–6. doi: 10.1080/15563650.2018.1491982. [DOI] [PubMed] [Google Scholar]
- Anil A., Surjit S., Ashish B. Role of neostigmine and polyvalent antivenom in Indian common krait (Bungarus caeruleus) bite. J. Infect. Publ. Health. 2010;3(2):83–87. doi: 10.1016/j.jiph.2010.01.002. Epub 2010 May 26. [DOI] [PubMed] [Google Scholar]
- Ariaratnam C.A., Sheriff M.H., Theakston R.D. DA Distinctive epidemiologic and clinical features of common krait (Bungarus caeruleus) bites in Sri Lanka. Am. J. Trop. Med. Hyg. 2008;79(3):458–462. [PubMed] [Google Scholar]
- Ariaratnam C.A., Thuraisingam V., Kularatne S.A.M. Frequent and potentially fatal envenoming by hump-nosed pit vipers (Hypnale hypnale and H. nepa) in Sri Lanka: lack of effective antivenom. Trans. R. Soc. Trop. Med. Hyg. 2008;102:1120–1126. doi: 10.1016/j.trstmh.2008.03.023. PMID: 18455743. [DOI] [PubMed] [Google Scholar]
- Ariaratnam C.A., Sheriff M.H.R., Arambepola C. Syndromic approach to treatment of snake bite in Sri Lanka based on results of a prospective national hospital-based survey of patients envenomed by identified snakes. Am. J. Trop. Med. Hyg. 2009;81:725–731. doi: 10.4269/ajtmh.2009.09-0225. PMID: 19815895. [DOI] [PubMed] [Google Scholar]
- Bailon C H., Yaniro C V.O., Colque A E.G. Development of nanobodies against hemorrhagic and myotoxic components of Bothrops atrox snake venom. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala A.A., Jatau A.I., Yunusa I. Knowledge assessment of snake antivenom among healthcare practitioners involving educational intervention in northern Nigeria: a study protocol. Ther. Adv. Drug Saf. 2020;31:11. doi: 10.1177/2042098620935721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala A.A., Jatau A.I., Yunusa I. Development and Validation of Antisnake Venom Knowledge Assessment Tool (AKAT) for Healthcare Practitioners. Toxicon X. 2020;8 doi: 10.1016/j.toxcx.2020.100064. eCollection 2020 Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R.N., Sahni A.L., Chacko K.A. Neostigmine in the treatment of Elapidae bites. J. Assoc. Phys. India. 1972;20(7):503–509. [PubMed] [Google Scholar]
- Benjamin J.M., Chippaux J.P., Sambo B.T., Massoubodji A. Delayed double reading of whole blood clotting test (WBCT) results at 20 and 30 minutes enhances diagnosis and treatment of viper envenomation. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018;24:14. doi: 10.1186/s40409-018-0151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava S., Kumari K., Sarin R.K. First-hand knowledge about snakes and snake-bite management: an urgent need. Nagoya J. Med. Sci. 2020;82(4):763–774. doi: 10.18999/nagjms.82.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S., Mousumi C., Piyasi M. Viper and cobra venom neutralization by alginate coated multicomponent polyvalent antivenom administered by the oral route. PLoS Neglected Trop. Dis. 2014;8(8):3039. doi: 10.1371/journal.pntd.0003039. eCollection 2014 Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittenbinder M.A. Coagulotoxic cobras: clinical implications of strong anticoagulant actions of African spitting Naja venoms that are not neutralised by antivenom but are by LY315920 (varespladib) Toxins. 2018;10:516. doi: 10.3390/toxins10120516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolon I., Durso A.M., Botero Mesa S., Ray N., Alcoba G., Chappuis F. Identifying the snake: first scoping review on practices of communities and healthcare providers confronted with snakebite across the world. PLoS One. 2020;15(3):229989. doi: 10.1371/journal.pone.0229989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton TL and Fayrer J. On the Nature and Physiological Action of the Poison of Naja tripudians and other Indian Venomous Snakes, part II. Proceedings of the Royal Society of London, vol 22, 1873-1874 (pp. 68-133). https://www.jstor.org/stable/i207064.
- Bucaretchi F., Douglas J.L., Fonseca M.R.C.C. Snake bites in children: antivenom early reaction frequency in patients pretreated with histamine antagonists H1 and H2 and hydrocortisone. Rev. Inst. Med. Trop. Sao Paulo. 1994;36:451–457. doi: 10.1590/s0036-46651994000500010. [DOI] [PubMed] [Google Scholar]
- Bulfone T.C., Samuel S.P., Bickler P.E. Developing small molecule therapeutics for the initial and adjunctive treatment of snakebite. J. Trop. Med. 2018 doi: 10.1155/2018/4320175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete J.J., Sanz L., Pérez A. Snake population venomics and antivenomics of Bothrops atrox: paedomorphism along its transamazonian dispersal and implications of geographic venom variability on snakebite management. J. Proteom. 2011;74:510–527. doi: 10.1016/j.jprot.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Casewell N.R., Jackson T.N.W., Laustsen A.H. Causes and consequences of snake venom variation. Trends Pharmacol. Sci. 2020;41:570–581. doi: 10.1016/j.tips.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Gui L., Kan T. A survey of snakebite knowledge among field forces in China. Int. J. Environ. Res. Publ. Health. 2016;14(1):15. doi: 10.3390/ijerph14010015.PMID:28035960. ; PMCID: PMC5295266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux J.-P., Boyer L.V. The 3 þ 3 dose escalation design is not appropriate for antivenom dose finding. Toxicon. 2010 doi: 10.1016/j.toxicon.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Clare R.H., Hall S.R., Patel R.N., Casewell N.R. Small molecule drug discovery for neglected tropical snakebite. Trends Pharmacol. Sci. 2021 doi: 10.1016/j.tips.2021.02.005. https://www.sciencedirect.com/science/article/abs/pii/S0165614721000432?dgcid=author [DOI] [PubMed] [Google Scholar]
- Costa T.N.D., Silva A.M.D., Souza R.M., Monteiro W.M., Bernarde P.S. Efficacy of the 20-minute whole blood clotting test (WBCT20) in the diagnosis of coagulation alteration related to snakebites in a Western Brazilian Amazon hospital. Rev. Soc. Bras. Med. Trop. 2021;54:912021. doi: 10.1590/0037-8682-0091-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.D., Parker C.S., Cox E.C.E. Misidentification of copperhead and cottonmouth snakes following snakebites. Clin. Toxicol. 2018;56(12):1195–1199. doi: 10.1080/15563650.2018.1473583. [DOI] [PubMed] [Google Scholar]
- Currie B.J. Snakebite in Australia: the role of the venom detection kit. Emerg. Med. Australasia (EMA) 2004;16(5–6):384–386. doi: 10.1111/j.1742-6723.2004.00640. [DOI] [PubMed] [Google Scholar]
- de Castañeda R.R., Durso A.M., Ray N. Snakebite and snake identification: empowering neglected communities and health-care providers with AI. Lancet Digit. Heal. 2019;1:202–203. doi: 10.1016/S2589-7500(19)30086-X. [DOI] [PubMed] [Google Scholar]
- de la Rosa G., Olvera F., Archundia I.G. Horse immunization with short-chain consensus α-neurotoxin generates antibodies against broad spectrum of elapid venomous species. Nat. Commun. 2019;10:3642. doi: 10.1038/s41467-019-11639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Medeiros C.R., Brioschi M.L., de Souza S.N. Infrared thermography to diagnose and manage venomous animal bites and stings. Rev. Soc. Bras. Med. Trop. 2017;50:260–264. doi: 10.1590/0037-8682-0390-2016. [DOI] [PubMed] [Google Scholar]
- de Silva H.A., Pathmeswaran A., Ranasinha C.D. Low-dose adrenaline, promethazine, and hydrocortisone in the prevention of acute adverse reactions to antivenom following snakebite: a randomised, double-blind, placebo-controlled trial. PLoS Med. 2011;8(5):1000435. doi: 10.1371/journal.pmed.1000435. Epub 2011 May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dsilva A.A., Basheer A., Thomas K. Snake envenomation: is the 20 min whole blood clotting test (WBCT20) the optimum test for management? QJM. 2019;112(8):575–579. doi: 10.1093/qjmed/hcz077.PMID:30918965. [DOI] [PubMed] [Google Scholar]
- Faria R.A.D., Lins V. de F., Nappi G.U. Development of an impedimetric immunosensor for specific detection of snake venom. Bionanoscience. 2018;8:988–996. [Google Scholar]
- Gerardo C.J., Quackenbush E., Lewis B. The efficacy of crotalidae polyvalent immune Fab (ovine) antivenom versus placebo plus optional rescue therapy on recovery from copperhead snake envenomation: a randomized, double-blind, placebo-controlled, clinical trial. Ann. Emerg. Med. 2017;70(2):233–244. doi: 10.1016/j.annemergmed.2017.04.034. Epub 2017 Jun 13. [DOI] [PubMed] [Google Scholar]
- Gimenes S.N.C., Colombini M., dos Santos Ibiapina H.N., Costa A.G., Sachett J., Monteiro W.M., Wen F.H., Fox J.W., Moura da Silva A.M. Local damage in human envenomings by Bothrops atrox in Brazilian Amazon. Toxicon April. 2020;177(Suppl. 120) doi: 10.1016/j.toxicon.2019.10.037. - page s8. [DOI] [Google Scholar]
- Gonzalez E., Pieracci F.M., Moore E.E. Coagulation abnormalities in the trauma patient: the role of point-of-care thromboelastography. Semin. Thromb. Hemost. 2010;36(7):723–737. doi: 10.1055/s-0030-1265289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez J.M., León G., Lomonte B. Antivenoms for snakebite envenomings. Inflamm. Allergy - Drug Targets. 2011;10:369–380. doi: 10.2174/187152811797200669. [DOI] [PubMed] [Google Scholar]
- Gutierrez J.M., Calvete J.J., Habib A.G. Snakebite envenoming. Nat. Rev. Dis. Prim. 2017;3:17063. doi: 10.1038/nrdp.2017.63. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M., Solano G., Pla D. Preclinical evaluation of the efficacy of antivenoms for snakebite envenoming: state-of-the-art and challenges ahead. Toxins. 2017;9(5):163. doi: 10.3390/toxins9050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez J.M., Lewin M.R., Williams D.J. Varespladib (LY315920) and methyl varespladib (LY333013) abrogate or delay lethality induced by presynaptically acting neurotoxic snake venoms. Toxins. 2020 doi: 10.3390/toxins12020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib A.G. Tetanus complicating snake bite in northern Nigeria: clinical presentation and public health implications. Acta Trop. 2003;85:87–91. doi: 10.1016/s0001-706x(02)00234-6. [DOI] [PubMed] [Google Scholar]
- Habib A.G. Effect of pre-medication on early adverse reactions following antivenom use in snakebite: a systematic review and meta-analysis. Drug Saf. 2011;34:869–880. doi: 10.2165/11592050-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Habib A.G. Public health aspects of snakebite care in West Africa – perspectives from Nigeria. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013;19(1):27. doi: 10.1186/1678-9199-19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib A.G., Abubakar S.B. Factors affecting snakebite mortality in northeastern Nigeria. Int. Health. 2011;3:50–55. doi: 10.1016/j.inhe.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Habib A.G., Warrell D.A. Antivenom therapy of carpet viper (Echis ocellatus) envenoming: effectiveness and strategies for delivery in West Africa. Toxicon. 2013 doi: 10.1016/j.toxicon.2013.01.002. 2013; e-published ahead of print 19. [DOI] [PubMed] [Google Scholar]
- Habib A.G., Musa B.M., Iliyasu G. Challenges and prospects of snake antivenom supply in sub-Saharan Africa. PLoS Neglected Trop. Dis. 2020;14(8):8374. doi: 10.1371/journal.pntd.0008374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley G.P., McGarr P., Mars M. The role of thromboelastography in the management of children with snake-bite in southern Africa. Trans. R. Soc. Trop. Med. Hyg. 1999;93(2):177–179. doi: 10.1016/s0035-9203(99)90300-0. [DOI] [PubMed] [Google Scholar]
- Ho M., Warrell D.A., Looareesuwan S., Phillips R.E., Chanthavanich P., Karbwang J., Supanaranond W., Viravan C., Hutton R.A., Vejcho S. Clinical significance of venom antigen levels in patients envenomed by the Malayan pit viper (Calloselasma rhodostoma) Am. J. Trop. Med. Hyg. 1986 May;35(3):579–587. doi: 10.4269/ajtmh.1986.35.579. [DOI] [PubMed] [Google Scholar]
- Hung D.Z., Lin J.H., Mo J.F. Rapid diagnosis of Naja atra snakebites. Clin. Toxicol. 2014;52:187–191. doi: 10.3109/15563650.2014.887725. [DOI] [PubMed] [Google Scholar]
- Iliyasu G., Halliru S.T., Habib Z.G., Tiamiyu A.B., Dayyab F.M., Abubakar S.B., Habib A.G. Blister and Bulla following snakebite in Nigeria: a prospective cohort study. Int. J. Trop. Dis. Health. 2014;4(10):1069–1077. [Google Scholar]
- Isbister G.K., Brown S.G.A., Page C.B., McCoubrie D.L., Greene S.L., Buckley N.A. Snakebite in Australia: a practical approach to diagnosis and treatment. Med. J. Aust. 2013;199(11):763–768. doi: 10.5694/mja12.11172. [DOI] [PubMed] [Google Scholar]
- Isbister Geoff, Currie Bart. Suspected snakebite: One year prospective study of emergency department presentations. Emergency Medicine. 2003;15:160–169. doi: 10.1046/j.1442-2026.2003.00434.x. [DOI] [PubMed] [Google Scholar]
- Itkin Y.M., Trujillo T.C. Intravenous immunoglobulin-associated acute renal failure: case series and literature review. Pharmacother. 2005;25:886–892. doi: 10.1592/phco.2005.25.6.886. [DOI] [PubMed] [Google Scholar]
- Jenkins T.P., Laustsen A.H. Cost of manufacturing for recombinant snakebite antivenoms. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins T.P., Fryer T., Dehli R.I. Toxin neutralization using alternative binding proteins. Toxins. 2019;11:53. doi: 10.3390/toxins11010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., Yuan Y., Liu L. Homogeneous fluorescent specific PCR for the authentication of medicinal snakes using cationic conjugated polymers. Sci. Rep. 2015;5:1–7. doi: 10.1038/srep16260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C.I., Ryan N.M., O'Leary M.A. Australian taipan (Oxyuranus spp.) envenoming: clinical effects and potential benefits of early antivenom therapy–Australian Snakebite Project (ASP-25) Clin. Toxicol. 2017;55:115–122. doi: 10.1080/15563650.2016.1250903. [DOI] [PubMed] [Google Scholar]
- Jorge M.T., Cardoso J.L., Castro S.C. A randomized 'blinded' comparison of two doses of antivenom in the treatment of Bothrops envenoming in Sao Paulo, Brazil. Trans. R. Soc. Trop. Med. Hyg. 1995;89:111–114. doi: 10.1016/0035-9203(95)90678-9. [DOI] [PubMed] [Google Scholar]
- Joseph J.K., Simpson I.D., Menon N.C.S. First authenticated cases of life-threatening envenoming by the hump-nosed pit viper (Hypnale hypnale) in India. Trans. R. Soc. Trop. Med. Hyg. 2007;101:85–90. doi: 10.1016/j.trstmh.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Julve Parreño J.M., Huet E., Fernández-Del-Carmen A. A synthetic biology approach for consistent production of plant-made recombinant polyclonal antibodies against snake venom toxins. Plant Biotechnol. J. 2017 doi: 10.1111/pbi.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalita B., Stephen P.M., Ashis K.M. Proteomic analysis reveals geographic variation in venom composition of Russell's Viper in the Indian subcontinent: implications for clinical manifestations post-envenomation and antivenom treatment. Expert Rev. Proteomics. 2018;15(10):837–849. doi: 10.1080/14789450.2018.1528150. [DOI] [PubMed] [Google Scholar]
- Kini R.M., Sidhu S.S., Laustsen A.H. Biosynthetic oligoclonal antivenom (BOA) for snakebite and next-generation treatments for snakebite victims. Toxins. 2018;10:534. doi: 10.3390/toxins10120534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen C., Ledsgaard L., Dehli R.I. Engineering and design considerations for next-generation snakebite antivenoms. Toxicon. 2019;167:67–75. doi: 10.1016/j.toxicon.2019.06.005. [DOI] [PubMed] [Google Scholar]
- Knudsen C., Jürgensen J.A., Føns S., Haack A.M., Friis R.U.W., Dam S.H., Bush S.P., White J., Laustsen A.H. Snakebite envenoming diagnosis and diagnostics. Front. Immunol. 2021;12:661457. doi: 10.3389/fimmu.2021.661457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause K.E., Jenkins T.P., Skaarup C. An interactive database for the investigation of high-density peptide microarray guided interaction patterns and antivenom cross-reactivity. PLoS Neglected Trop. Dis. 2020;14:8366. doi: 10.1371/journal.pntd.0008366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laksham Karthik Balajee. Unmanned aerial vehicle (drones) in public health: a SWOT analysis. J. Fam. Med. Prim. Care. 2019;8(2):342–346. doi: 10.4103/jfmpc.jfmpc_413_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laustsen A.H. first ed. University of Copenhagen; Copenhagen, Denmark: 2016. Recombinant Antivenoms. [Google Scholar]
- Laustsen A.H. Toxin synergism in snake venoms. Toxin Rev. 2016;35:165–170. [Google Scholar]
- Laustsen A.H. Guiding recombinant antivenom development by omics technologies. New Biotechnol. Affinity Proteomics. 2018;45:19–27. doi: 10.1016/j.nbt.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Laustsen A.H. Toxin-centric development approach for next-generation antivenoms. Toxicon. 2018;150:195–197. doi: 10.1016/j.toxicon.2018.05.021. [DOI] [PubMed] [Google Scholar]
- Laustsen A.H. How can monoclonal antibodies be harnessed against neglected tropical diseases and other infectious diseases? Expet Opin. Drug Discov. 2019;14:1103–1112. doi: 10.1080/17460441.2019.1646723. [DOI] [PubMed] [Google Scholar]
- Laustsen A.H., Dorrestijn N. Integrating engineering, manufacturing, and regulatory considerations in the development of novel antivenoms. Toxins. 2018;10:309. doi: 10.3390/toxins10080309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laustsen A.H., Lohse B., Lomonte B., Engmark M., Gutierrez J.M. Selecting key toxins for focused development of elapid snake antivenoms and inhibitors guided by a Toxicity Score. Toxicon. 2015;104:43–45. doi: 10.1016/j.toxicon.2015.07.334. [DOI] [PubMed] [Google Scholar]
- Laustsen A.H., Engmark M., Milbo C. From fangs to pharmacology: the future of snakebite envenoming therapy. Curr. Pharm. Des. 2016;22:5270–5293. doi: 10.2174/1381612822666160623073438. [DOI] [PubMed] [Google Scholar]
- Laustsen A.H., Johansen K.H., Engmark M. Recombinant snakebite antivenoms: a cost-competitive solution to a neglected tropical disease? PLoS Neglected Trop. Dis. 2017;11:5361. doi: 10.1371/journal.pntd.0005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laustsen A.H., Gutiérrez JoséMarí, Knudsen C., Johansen K.H., Bermudez-Mendez E., Cerni F.A. Pros and cons of different therapeutic antibody formats for recombinant antivenom development. Toxicon. 2018;146:151–175. doi: 10.1016/j.toxicon.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Laustsen A.H., Karatt-Vellatt A., Masters E.W. In vivo neutralization of dendrotoxin-mediated neurotoxicity of black mamba venom by oligoclonal human IgG antibodies. Nat. Commun. 2018;9:3928. doi: 10.1038/s41467-018-06086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laustsen A.H., Ainsworth S., Lomonte B. Editorial: novel immunotherapies against envenomings by snakes and other venomous animals. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laustsen A.H., Greiff V., Karatt-Vellat A., Muyldermans S., Jenkins T.P. Animal immunization, in vitro display technologies, and machine learning for antibody discovery. Trends Biotechnol. 2021 doi: 10.1016/j.tibtech.2021.03.003. [DOI] [PubMed] [Google Scholar]
- Le Geyt J., Pach S., Gutiérrez J.M., Habib A.G., Maduwage K.P., Hardcastle T.C., Hernández Diaz R., Avila-Aguero M.L., Ya K.T., Williams D., Halbert J. Paediatric snakebite envenoming: recognition and management of cases. Arch. Dis. Child. 2021 Jan;106(1):14–19. doi: 10.1136/archdischild-2020-319428. [DOI] [PubMed] [Google Scholar]
- Ledsgaard L., Jenkins T.P., Davidsen K. Antibody cross-reactivity in antivenom research. Toxins. 2018;10 doi: 10.3390/toxins10100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León G., Herrera M., Segura Á. Pathogenic mechanisms underlying adverse reactions induced by intravenous administration of snake antivenoms. Toxicon. 2013;76:63–76. doi: 10.1016/j.toxicon.2013.09.010. Epub 2013 Sep. 20. [DOI] [PubMed] [Google Scholar]
- Lewin M., Samuel S., Merkel J. Varespladib (LY315920) appears to Be a potent, broad-spectrum, inhibitor of snake venom phospholipase A2 and a possible pre-referral treatment for envenomation. Toxins. 2016;8 doi: 10.3390/toxins8090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin M.R., Gutiérrez José María, Samuel Stephen P. Delayed oral LY333013 rescues mice from highly neurotoxic, lethal doses of papuan taipan (oxyuranus scutellatus) venom. Toxins. 2018;10(10):380. doi: 10.3390/toxins10100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin M.R., Gilliam Lyndi L., Gilliam John. Delayed LY333013 (oral) and LY315920 (intravenous) reverse severe neurotoxicity and rescue juvenile pigs from lethal doses of Micrurus fulvius (eastern coral snake) venom. Toxins. 2018;10(11):479. doi: 10.3390/toxins10110479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.-C., Wang P.-J., Liu C.-C. Venom concentrations in blisters and haemorrhagic bullae in a patient bitten by a Taiwan habu (Protobothrops mucrosquamatus) J. Brazilian Soc. Trop. Med. 2019;52:20180160. doi: 10.1590/0037-8682-0160-2018. [DOI] [PubMed] [Google Scholar]
- Lin J.H., Lo C.M., Chuang S.H. Collocation of avian and mammal antibodies to develop a rapid and sensitive diagnostic tool for Russell's vipers snakebite. PLoS Neglected Trop. Dis. 2020;14:1–19. doi: 10.1371/journal.pntd.0008701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.C., Yu J.S., Wang P.J. Development of sandwich ELISA and lateral flow strip assays for diagnosing clinically significant snakebite in Taiwan. PLoS Neglected Trop. Dis. 2018;12:1–23. doi: 10.1371/journal.pntd.0007014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonte B., León G., Angulo Y. Neutralization of Bothrops asper venom by antibodies, natural products and synthetic drugs: contributions to understanding snakebite envenomings and their treatment. Toxicon. 2009;54(7):1012–1028. doi: 10.1016/j.toxicon.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Maduwage K., O'Leary M.A., Isbister G.K. Diagnosis of snake envenomation using a simple phospholipase A2 assay. Sci. Rep. 2014;4:1–4. doi: 10.1038/srep04827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood M.A., Halliday D., Cumming R. Inadequate knowledge about snakebite envenoming symptoms and application of harmful first aid methods in the community in high snakebite incidence areas of Myanmar. PLoS Neglected Trop. Dis. 2019;13(2):7171. doi: 10.1371/journal.pntd.0007171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malasit P., Warrell D.A., Chanthavanich P. Prediction, prevention and mechanism of early (anaphylactic) antivenom reactions in victims of snakebites. Br. Med. J. 1986;292(6512):17–20. doi: 10.1136/bmj.292.6512.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça-da-Silva I., Magela Tavares A., Sachett J. Safety and efficacy of a freeze-dried trivalent antivenom for snakebites in the Brazilian Amazon: an open randomized controlled phase IIb clinical trial. PLoS Neglected Trop. Dis. 2017;11(11):6068. doi: 10.1371/journal.pntd.0006068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael G.C., Grema B.A., Aliyu I. Knowledge of venomous snakes, snakebite first aid, treatment, and prevention among clinicians in northern Nigeria: a cross-sectional multicentre study. Trans. R. Soc. Trop. Med. Hyg. 2018;112(2):47–56. doi: 10.1093/trstmh/try028. [DOI] [PubMed] [Google Scholar]
- Mise Y.F., Lira-da-Silva R., Carvalho F.M. Time to treatment and severity of snake envenoming in Brazil. Rev. Panam. Salud Públic. 2018;42 doi: 10.26633/RPSP.2018.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro W.M., Farias A.S., Val F. Providing antivenom treatment access to all Brazilian amazon indigenous areas: 'every life has equal value. Toxins. 2020;12(12):E772. doi: 10.3390/toxins12120772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzil P., Zhang C., Pipper J. Ultra fast miniaturized real-time PCR: 40 cycles in less than six minutes. Nucleic Acids Res. 2006;34 doi: 10.1093/nar/gkl416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero R., León G., Gutiérrez J. Efficacy and safety of two whole IgG polyvalent antivenoms, refined by caprylic acid fractionation with or without beta-propiolactone, in the treatment of Bothrops asper bites in Colombia. Trans. R. Soc. Trop. Med. Hyg. 2006;100:1173–1182. doi: 10.1016/j.trstmh.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Paiva O.K., Bande B., Power R., Williams D.J. Oxford University; Oxford: 2015. Clinical Evaluation of the 20 Minute Whole Blood Clotting Test (20WBCT) and Reliability at Different Temperatures and Types of Glassware. Paper Presented at the 18th World Congress of the International Society on Toxinology. [Google Scholar]
- Pardal P.P.O., Souza S.M., Monteiro M.R.C.C. Clinical trial of two antivenoms for the treatment of Bothrops and Lachesis bites in the north eastern Amazon region of Brazil. Trans. R. Soc. Trop. Med. Hyg. 2004;98:28–42. doi: 10.1016/s0035-9203(03)00005-1. [DOI] [PubMed] [Google Scholar]
- Parker-Cote J., Meggs W.J. First aid and pre-hospital management of venomous snakebites. Trav. Med. Infect. Dis. 2018;3(2):45. doi: 10.3390/tropicalmed3020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul V., Pratibha S., Prahlad K.A. High-dose anti-snake venom versus low-dose anti-snake venom in the treatment of poisonous snake bites--a critical study. J. Assoc. Phys. India. 2004;52:14–17. [PubMed] [Google Scholar]
- Pinho F.M.O., Zanetta D.M.T., Burdmann E.A. Acute renal failure after Crotalus durissus snakebite: a prospective survey on 100 patients. Kidney Int. 2005;67:659–667. doi: 10.1111/j.1523-1755.2005.67122.x. [DOI] [PubMed] [Google Scholar]
- Pongpit J., Limpawittayakul P., Juntiang J., Akkawat B., Rojnuckarin P. The role of prothrombin time (PT) in evaluating green pit viper (Cryptelytrops sp) bitten patients. Trans. R. Soc. Trop. Med. Hyg. 2012;106:415–418. doi: 10.1016/j.trstmh.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Pucca M.B., Cerni F.A., Janke R. History of envenoming therapy and current perspectives. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C., Qiu X.F., Liu J.J. An effective snakebite first aid training method for medics in the Chinese troops: a RCT. Mil Med Res. 2019;6(1):39. doi: 10.1186/s40779-019-0230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranawaka U.K., Lalloo D.G., de Silva H.J. Neurotoxicity in snakebite--the limits of our knowledge. PLoS Neglected Trop. Dis. 2013;7(10):2302. doi: 10.1371/journal.pntd.0002302. PMID: 24130909; PMCID: PMC3794919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard G., Meyers A.J., McLean M.D. In vivo neutralization of α-cobratoxin with high-affinity llama single-domain antibodies (VHHs) and a VHH-fc antibody. PLoS One. 2013;8:69495. doi: 10.1371/journal.pone.0069495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro H.S., Shin J.Y., Sabbagh M.D., Roh S.G., Chang S.C., Lee N.H. Effectiveness of aspiration or deroofing for blister management in patients with burns: a prospective randomized controlled trial. Medicine (Baltim.) 2018 Apr;97(17):563. doi: 10.1097/MD.0000000000010563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojnuckarin P., Chanthawibun W., Noiphrom J. A randomized, double-blind, placebo-controlled trial of antivenom for local effects of green pit viper bites. Trans. R. Soc. Trop. Med. Hyg. 2006;100(9):879–884. doi: 10.1016/j.trstmh.2005.10.006. Epub 2006 Feb 8. [DOI] [PubMed] [Google Scholar]
- Rosenberger W.F., Haines L.M. Competing designs for phase I clinical trials: a review. Stat. Med. 2002;21:2757–2770. doi: 10.1002/sim.1229. [DOI] [PubMed] [Google Scholar]
- Rosenson R.S., Hislop C., Elliott M., Stasiv Y., Goulder M., Waters D. Effects of varespladib methyl on biomarkers and major cardiovascular events in acute coronary syndrome patients. J. Am. Coll. Cardiol. 2010 Sep 28;56(14):1079–1088. doi: 10.1016/j.jacc.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Sabitha P., Bammigatti C., Deepanjali S. Point-ofcare infrared thermal imaging for differentiating venomous snakebites from non-venomous and dry bites. PLoS Neglected Trop. Dis. 2021;15(2):8580. doi: 10.1371/journal.pntd.0008580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachett J.A.G., da Silva I.M., Alves E.C. Poor efficacy of preemptive amoxicillin clavulanate for preventing secondary infection from Bothrops snakebites in the Brazilian Amazon: a randomized controlled clinical trial. PLoS Neglected Trop. Dis. 2017;11:5745. doi: 10.1371/journal.pntd.0005745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel S.P., Chinnaraju S., Williams H.F. Venomous snakebites: rapid action saves lives-A multifaceted community education programme increases awareness about snakes and snakebites among the rural population of Tamil Nadu, India. PLoS Neglected Trop. Dis. 2020;14(12):8911. doi: 10.1371/journal.pntd.0008911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano-Martins I.S., Fan H.W., Castro S.C. Reliability of the simple 20 minute whole blood clotting test (WBCT20) as an indicator of low plasma fibrinogen concentration in patients envenomed by Bothrops snakes. Butantan Institute Antivenom Study Group. Toxicon. 1994;32(9):1045–1050. doi: 10.1016/0041-0101(94)90388-3.PMID:7801340. [DOI] [PubMed] [Google Scholar]
- Senji Laxme R.R., Attarde S., Khochare S. Biogeographical venom variation in the Indian spectacled cobra (Naja naja) underscores the pressing need for pan-India efficacious snakebite therapy. PLoS Neglected Trop. Dis. 2021;15(2):9150. doi: 10.1371/journal.pntd.0009150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R., Dey D., Pietzonka T. Determinants of use of biotherapeutics insub-saharan Africa. Trends Pharmacol. Sci. 2021;42(No. 2):75–84. doi: 10.1016/j.tips.2020.11.012. [DOI] [PubMed] [Google Scholar]
- Sharma S.K., Alirol E., Ghimire A. Acute severe anaphylaxis in Nepali patients with neurotoxic snakebite envenoming treated with the VINS polyvalent antivenom. J. Trop. Med. 2019:2689171. doi: 10.1155/2019/2689171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A., Hlusicka J., Siribaddana N. Time delays in treatment of snakebite patients in rural Sri Lanka and the need for rapid diagnostic tests. PLoS Neglected Trop. Dis. 2020;14(11):8914. doi: 10.1371/journal.pntd.0008914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singaravelu K.P., Pandit V.R., Chinnakali P. Pre-hospital care and its association with clinical outcome of snakebite victims presenting at a tertiary care referral hospital in South India. Trop. Doct. 2021;51(1):77–80. doi: 10.1177/0049475520966958. [DOI] [PubMed] [Google Scholar]
- Steward W.P. Marimastat (BB2516): current status of development. Canc. Chemother. Pharmacol. 1999;43:56–60. doi: 10.1007/s002800051099. [DOI] [PubMed] [Google Scholar]
- Still K.B.M. Development of high-throughput screening assays for profiling snake venom phospholipase A2 activity after chromatographic fractionation. Toxicon. 2020 doi: 10.1016/j.toxicon.05.022. [DOI] [PubMed] [Google Scholar]
- Sulaiman S.S., Kharusha I.K., Samara A.M. An assessment of medical students' proficiency in the diagnosis and management of snakebites: a cross-sectional study from Palestine. J. Occup. Med. Toxicol. 2020;15:3. doi: 10.1186/s12995-020-00254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntrarachun S., Pakmanee N., Tirawatnapong T. Development of a polymerase chain reaction to distinguish monocellate cobra (Naja khouthia) bites from other common Thai snake species, using both venom extracts and bite-site swabs. Toxicon. 2001;39:1087–1090. doi: 10.1016/s0041-0101(00)00246-4. [DOI] [PubMed] [Google Scholar]
- Supikamolseni A., Ngaoburanawit N., Sumnotha M. Molecular barcoding of venomous snakes and species-specific multiplex PCR assay to identify snake groups for which antivenom is available in Thailand. Genet. Mol. Res. 2015;14:13981–13997. doi: 10.4238/2015.October.29.18. [DOI] [PubMed] [Google Scholar]
- Thongtonyong N., Chinthammitr Y. Sensitivity and specificity of 20-minute whole blood clotting test, prothrombin time, activated partial thromboplastin time tests in diagnosis of defibrination following Malayan pit viper envenoming. Toxicon. 2020;185:188–192. doi: 10.1016/j.toxicon.2020.07.020. [DOI] [PubMed] [Google Scholar]
- Vaagt F., Haase I., Fischer M. Loop-mediated isothermal amplification (LAMP)-based method for rapid mushroom species identification. J. Agric. Food Chem. 2013;61:1833–1840. doi: 10.1021/jf304824b. [DOI] [PubMed] [Google Scholar]
- Viravan C., Looareesuwan S., Kosakarn W. A national hospital-based survey of snakes responsible for bites in Thailand. Trans. R. Soc. Trop. Med. Hyg. 1992;86(1):100–106. doi: 10.1016/0035-9203(92)90463-m. [DOI] [PubMed] [Google Scholar]
- Visser L.E., Kyei-Faried S., Belcher D.W. Protocol and monitoring to improve snake bite outcomes in rural Ghana. Trans. R. Soc. Trop. Med. Hyg. 2004;98(5):278–283. doi: 10.1016/S0035-9203(03)00065-8. [DOI] [PubMed] [Google Scholar]
- Warrell D.A. Snake bite. Lancet. 2010;375(9708):77–88. doi: 10.1016/S0140-6736(09)61754-2. [DOI] [PubMed] [Google Scholar]
- Watson J.A., Lamb T., Holmes J., Warrell D.A., Thwin K.T., Aung Z.L. A Bayesian phase 2 model based adaptive design to optimize antivenom dosing: application to a dose-finding trial for a novel Russell's viper antivenom in Myanmar. PLoS Neglected Trop. Dis. 2020;14(11):8109. doi: 10.1371/journal.pntd.0008109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt G., Theakston R.D., Hayes C.G. Positive response to edrophonium in patients with neurotoxic envenoming by cobras (Naja naja philippinensis). A placebo-controlled study. N. Engl. J. Med. 1986;315(23):1444–1448. doi: 10.1056/NEJM198612043152303. [DOI] [PubMed] [Google Scholar]
- Wedasingha S., Isbister G., Silva A. Bedside coagulation tests in diagnosing venom-induced consumption coagulopathy in snakebite. Toxins. 2020;12(9):583. doi: 10.3390/toxins12090583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen F.H., Marcopito L., Cardoso J. Sequential randomised and double blind trial of promethazine prophylaxis against early anaphylactic reactions to antivenom for bothrops snake bites. BMJ. 1999;318:1451–1453. doi: 10.1136/bmj.318.7196.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. Clinical toxinology specialty training. Toxicon. 2013;69:120–125. doi: 10.1016/j.toxicon.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Williams H.F., Vaiyapuri R., Gajjeraman P. Challenges in diagnosing and treating snakebites in a rural population of Tamil Nadu, India: the views of clinicians. Toxicon. 2017;130:44–46. doi: 10.1016/j.toxicon.2017.02.025. Epub 24. PMID: 28238804. [DOI] [PubMed] [Google Scholar]
- Williams D.J., Habib A.G., Warrell D.A. Clinical studies of the effectiveness and safety of antivenoms. Toxicon. 2018;150:1–10. doi: 10.1016/j.toxicon.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Wojtowicz-Praga S.M., Dickson R.B., Hawkins M.J. Matrix metalloproteinase inhibitors. Invest. N. Drugs. 1997;15(1):61–75. doi: 10.1023/a:1005722729132. [DOI] [PubMed] [Google Scholar]
- Xie C. Varespladib inhibits the phospholipase A2 and coagulopathic activities of venom components from hemotoxic snakes. Biomedicines. 2020 doi: 10.3390/biomedicines8060165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C. Antivenom neutralization of coagulopathic snake venom toxins assessed by bioactivity profiling using nanofractionation analytics. Toxins. 2020 doi: 10.3390/toxins12010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates V.M., Lebas E., Orpiay R. Management of snakebites by the staff of a rural clinic: the impact of providing free antivenom in a nurse-led clinic in Meserani, Tanzania. Ann. Trop. Med. Parasitol. 2010;104(5):439–448. doi: 10.1179/136485910X12743554760306. [DOI] [PubMed] [Google Scholar]
- Zeiher B.G., Steingrub J., Laterre P.F. LY315920NA/S-5920, a selective inhibitor of group IIA secretory phospholipase A2, fails to improve clinical outcome for patients with severe sepsis. Crit. Care Med. 2005;33(8):1741–1748. doi: 10.1097/01.ccm.0000171540.54520.69. [DOI] [PubMed] [Google Scholar]
- Ratnayake I., Shihana F., Dissanayake D.M. Performance of the 20-min whole blood clotting test in detecting venom induced consumption coagulopathy from Russell's viper (Daboia russelii) bites. Thromb Haemost. 2017;117(3):500–507. doi: 10.1160/TH16-10-0769. Epub 2017 Feb 2. PMID: 28150853. [DOI] [PubMed] [Google Scholar]