Abstract
Lithium has been used in the treatment of bipolar disorders for decades, but the exact mechanisms of action remain elusive to this day. Recent evidence suggests that lithium is critically involved in a variety of signaling pathways affecting apoptosis, inflammation, and neurogenesis, all of which contributing to the complex pathophysiology of various neurological diseases. As a matter of fact, preclinical work reports both acute and long-term neuroprotection in distinct neurological disease models such as Parkinson’s disease, traumatic brain injury, Alzheimer’s disease, and ischemic stroke. Lithium treatment reduces cell injury, decreases α-synuclein aggregation and Tau protein phosphorylation, modulates inflammation and even stimulates neuroregeneration under experimental conditions of Parkinson’s disease, traumatic brain injury, and Alzheimer’s disease. The therapeutic impact of lithium under conditions of ischemic stroke was also studied in numerous preclinical in vitro and in vivo studies, giving rise to a randomized double-blind clinical stroke trial. The preclinic data revealed a lithium-induced upregulation of anti-apoptotic proteins such as B-cell lymphoma 2, heat shock protein 70, and activated protein 1, resulting in decreased neuronal cell loss. Lithium, however, does not only yield postischemic neuroprotection but also enhances endogenous neuroregeneration by stimulating neural stem cell proliferation and by regulating distinct signaling pathways such as the RE1-silencing transcription factor. In line with this, lithium treatment has been shown to modulate postischemic cytokine secretion patterns, diminishing microglial activation and stabilizing blood-brain barrier integrity yielding reduced levels of neuroinflammation. The aforementioned observations culminated in a first clinical trial, which revealed an improved motor recovery in patients with cortical stroke after lithium treatment. Beside its well-known psychiatric indications, lithium is thus a promising neuroprotective candidate for the aforementioned neurological diseases. A detailed understanding of the lithium-induced mechanisms, however, is important for prospective clinical trials which may pave the way for a successful bench-to-bedside translation in the future. In this review, we will give an overview of lithium-induced neuroprotective mechanisms under various pathological conditions, with special emphasis on ischemic stroke.
Key Words: Alzheimer's disease, apoptosis, bench-to-bedside translation, inflammation, ischemic stroke, lithium, neurogenesis, neuroprotective agent, Parkinson's disease, traumatic brain injury
Introduction
Lithium has been commonly used in the treatment of bipolar disorders for decades. However, evidence suggests that lithium also exerts neuroprotection in distinct neurological diseases such as Parkinson´s disease (PD), traumatic brain injury (TBI), Alzheimer´s disease (AD), and ischemic stroke (IS) (Ren et al., 2003; Zhu et al., 2010; Forlenza et al., 2014; Zhao et al., 2019). The latter is one of the leading causes of death and the most common cause of disability worldwide (Campbell und Khatri, 2020). Since stroke therapy is limited to acute intervention and restricted to only a small number of patients, a variety of research groups strives to find new therapeutic approaches that modulate the disease progress and lead to acute and long-term neuroprotection (Zhou et al., 2018). However, the translational approach of neuroprotective stroke agents from experimental research to clinical application has failed until recently (Neuhaus et al., 2017). Moretti and colleagues identified three main reasons for this, i.e., (1) fundamental differences between animal models and humans, (2) the poor quality of many experimental studies, and (3) the lack of clinical studies covering human variables such as comorbidities and age (Moretti et al., 2015). The advantage of applying a well-known drug like lithium as an adjuvant neuroprotective drug are therefore evident. In this review, we will give an overview on observed neuroprotective effects of lithium under conditions of different neurological diseases with a special focus on IS.
Search Strategy and Selection Criteria
The literature included in the present review was found on PubMed and Google Scholar databases between October and November 2020, using one of the following search terms: neuroprotection stroke, lithium Parkinson’s disease, lithium traumatic brain injury, lithium Alzheimer’s disease, lithium stroke and lithium cerebral ischemia.
The History of Lithium, Current Clinical Applications, and Known Signaling Pathways
Lithium is an elementary light metal, which is only used as lithium salt because of its high intrinsic reactivity. First evidence of using lithium as therapeutic drug goes back to the 19th century (Shorter, 2009). It was not before the 20th century, however, that the Australian psychiatrist John F. Cade came off with the first randomized double-blind study that suggested therapeutic benefits of lithium as a mood stabilizer (Shorter, 2009). Since then lithium is used as a first-line medication in the treatment of bipolar disorders (Manji und Lenox, 1998). Further indications known for lithium imply various psychiatric and neurological diseases such as acute depression, manic episodes, and cluster headache.
Although lithium is a commonly used drug, the precise mechanisms of action remain elusive. To our current understanding, lithium acts as a pleiotropic substance, which regulates a variety of cellular regulation proteins (Malhi et al., 2013). In particular, the lithium-induced inhibition of the glycogen synthase kinase 3 beta (GSK3β) was identified as a central aspect of the mood-stabilizing effect. The GSK3β pathway is, among other, involved in the regulation of apoptosis and neuronal plasticity (Jaworski et al., 2019). Regarding its relevance in bipolar disorders, de Sousa and colleagues demonstrated a correlation of inactivated GSK3β and symptoms during a depressive episode (de Sousa et al., 2015). The importance of this pathway is further illustrated by the fact that the selective inhibition of the GSK3β pathway showed anti-depressive and anti-maniac effects (Martinowich et al., 2009). Moreover, long-term lithium treatment has been shown to elevate the levels of the brain-derived neurotrophic factor (Emamghoreishi et al., 2015; Abdanipour et al., 2019). The expression levels of brain-derived neurotrophic factor, in turn, correlate with the severity of symptoms in patients suffering from depression and bipolar disorders (Fernandes et al., 2015). With regard to neurotransmitters, lithium treatment affects both the GABAergic and the dopaminergic system, which play a pivotal role in patients with psychiatric disorders (Ago et al., 2012; Wakita et al., 2015). Given the fact that lithium affects multiple signaling pathways, a plethora of studies were conducted to open new fields for lithium treatment. As such, novel evidence suggests lithium to have a therapeutic potential beyond the current clinical applications.
Preclinic Neuroprotective Effects of Lithium
In the last decade, a variety of preclinic studies have demonstrated the neuroprotective potential of lithium in distinct neurological settings such as PD, TBI, and AD (Figure 1). Herein, we will only give a brief overview of the current knowledge related to lithium and these diseases.
Figure 1.
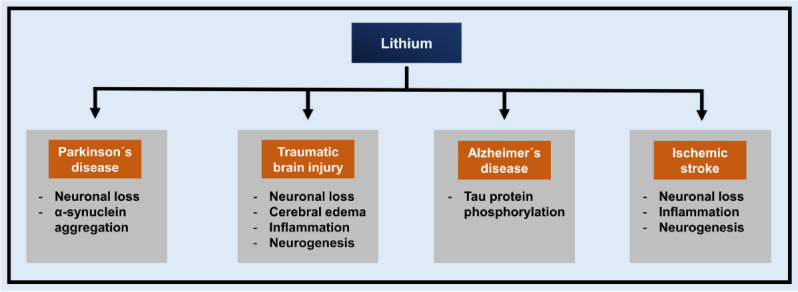
Overview of preclinical evidence for lithium-induced neuroprotective mechanisms in distinct neurological diseases.
Preclinical studies have shown the neuroprotective potential of lithium in neurological diseases such as Parkinson’s disease, traumatic brain injury, Alzheimer’s disease, and ischemic stroke by affecting individual downstream signaling cascades.
Using a rodent PD model, Zhao et al. (2019), for instance, observed a decrease of aggregated α-synuclein due to lithium treatment, yielding improved behavioral test performance. In addition, the loss of neurons located in the pars compacta was significantly reduced (Zhao et al., 2019). The authors also identified modulated miRNA expression patterns, which might serve as a biological equivalent of lithium-induced neuroprotection in preclinical PD models. Moreover, the combination of valproate and lithium ameliorates motoric behavior and has been shown to enhance the number of dopaminergic neurons as well as dopamine metabolites in PD mice (Li et al., 2013). Transplanting lithium-preincubated stem cells in PD rodents, Qi et al. (2017) demonstrated a regulation of the Wnt signaling pathway that gave rise to a significant incline of cognitive functions and motor coordination in these animals.
Similar to preclinical PD models, lithium pre-treatment in TBI mice leads to a reduction of tissue injury and an increase of memory performance and spatial learning (Zhu et al., 2010). These interesting observations were associated with a decreased expression of the pro-inflammatory interleukin-1β (Zhu et al., 2010). Similar results were obtained by Yu et al. (2011), showing that lithium treatment results in reduced neurodegeneration and neuroinflammation. The latter was associated with better behavioral outcomes as indicated by minimized anxiety-like behavior and ameliorated short-term and long-term motor coordination (Yu et al., 2011). Likewise, Ciftci and colleagues observed neuroprotective effects after lithium treatment, leading to reduced depressed-like behavior in mice after cold-induced TBI (Ciftci et al., 2020). Since the GSK3β pathway is also activated in TBI, inhibition of that pathway due to lithium likely contributed to the aforementioned cellular protection (Shim und Stutzmann, 2016).
Extracellular beta-amyloid plaques and neurofibrillary tangles consisting of hyperphosphorylated tau proteins are key factors in the pathophysiology of AD (Weller und Budson, 2018). Interestingly, several preclinical studies showed a lithium-induced decrease of tau protein hyperphosphorylation via inhibition of GSK3β, leading to diminished cognitive impairment in rodents (Nakashima et al., 2005; Engel et al., 2006; Sudduth et al., 2012). Cognitive deficits such as spatial learning and memory functions were also found to be reduced due to lithium-regulated levels of β-catenin (De Ferrari et al., 2003). Beside these preclinic data, a meta-analysis suggests a protective effect of lithium treatment in patients with AD and mild cognitive impairment (Matsunaga et al., 2015). Nevertheless, the significance of the latter is limited due to the low number of patients enrolled. As a matter of fact, these data stress the outstanding potential of lithium treatment for various neurological diseases.
Lithium and Ischemic Stroke
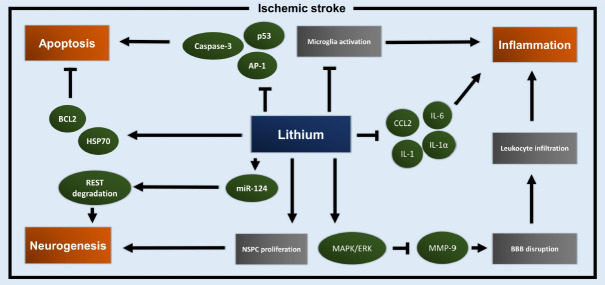
Beside the neurological diseases mentioned in the previous paragraph, lithium has also shown itself to be a promising candidate for the treatment of IS. The complex pathophysiology of IS is characterized by both acute and delayed neuronal damage. In the acute stage, both hypoxia and the lack of nutrients lead to neuronal loss by acute cell death, triggering mechanisms that result in neuroinflammation and apoptosis. The latter leads to delayed brain injury, additionally affected by subsequent vessel reperfusion. The current therapy of IS at the acute stage of the disease is limited to systemic thrombolysis and mechanical thrombectomy. Hence, researchers have been working on finding neuroprotective adjuvant treatment paradigms in order to modulate the long-term post-stroke disease progression. This approaches have, however, failed until recently (Neuhaus et al., 2017). We herein provide an overview of promising preclinical and clinical studies and also give brief information on the current understanding of mechanism underlying lithium-induced neuroprotection in IS. A schematic summary of lithium-induced signaling pathways under IS conditions is provided in Figure 2.
Figure 2.
Neuroprotective signaling pathways affected by lithium in ischemic stroke.
Lithium triggers and inhibits distinct signaling pathways and effector proteins, leading to acute and long-term neuroprotection under ischemic stroke conditions. Thereby, lithium mainly affects three key players in the pathophysiology of ischemic stroke, i.e., apoptosis, inflammation, and neurogenesis. AP-1: Activated protein 1; BBB: blood-brain barrier; BCL-2: B-cell lymphoma 2; CCL2: CC-chemokine-ligand-2; ERK: extracellular signal-regulated kinases; HSP70: heat shock protein 70; IL: interleukin; MAPK: mitogen-activated protein kinase; miR-124: microRNA 124; MMP-9: matrix metalloproteinase 9; NSPC: neural stem/progenitor cell; REST: RE1-silencing transcription factor.
Preclinical stroke studies
Nonaka and Chuang for the first time demonstrated that lithium pre-treatment leads to decreased infarct size and reduced neurological deficits in stroke rats (Nonaka und Chuang, 1998). The authors suggested an inhibition of activated NMDA receptors by lithium treatment to be an important mechanism in this context (Nonaka und Chuang, 1998). These observations were confirmed in a follow-up study by Xu et al. (2003) who claimed anti-apoptotic mechanisms to be responsible for the lithium effect. In detail, apoptosis was diminished in the penumbra of the ischemic cortex, where caspase-3 activity and activated protein 1 expression were significantly decreased (Xu et al., 2003). The relevance of lithium-regulated apoptosis was further confirmed in another study by Bian et al. who observed a decreased pro-apoptotic p53 expression and an up-regulated anti-apoptotic B-cell lymphoma 2 as well as an increased heat shock protein 70 (HSP70) expression in the ischemic brain (Bian et al., 2007). Interestingly, the importance of HSP70 as a therapeutic target in IS has just been recognized in recent years after the aforementioned publication by Bian and colleages (Kim et al., 2018). Although the majority of preclinical stroke studies applies models of unilateral focal cerebral ischemia, some studies rather focus on global cerebral ischemia models. Analyzing the therapeutic potential of lithium under the latter conditions confirmed the robust neuroprotective effect of lithium (Yan et al., 2007b). As a matter of fact, pre-treatment of lithium yielded better neurological recovery and enhanced levels of neurogenesis in the hippocampal region, albeit neuronal and astrocytic differentiation patterns were not affected by lithium directly (Yan et al., 2007b). The aforementioned effects do not solely depend on the GSK3β pathway, but involve other signaling pathways such as the pro-survival mitogen-activated protein kinase/extracellular signal-regulated kinases pathway as well (Yan et al., 2007b). The mitogen-activated protein kinase/extracellular signal-regulated kinases pathway, in turn, has already been shown to play an important role in the pathophysiology of IS in various preclinical models (Sawe et al., 2008). Another study by Yan and colleagues that focused on the behavioral outcome after transient global cerebral ischemia revealed an improved motor recovery and enhanced memory performance after lithium pre-treatment (Yan et al., 2007a). These observations were further confirmed by Bian and colleagues who noticed reduced ischemia-induced behavioral deficits in lithium-treated gerbils (Bian et al., 2007).
Whereas the aforementioned studies focused on clinically irrelevant lithium pre-treatment, additional studies were conducted investigating the therapeutic potential of post-stroke lithium administration. Likewise, lithium post-treatment lead to decreased infarct volume and reduced neurological deficits in a rat ischemia/reperfusion model (Ren et al., 2003). Moreover, this neuroprotection was again associated with decreased levels of apoptosis and an up-regulation of HSP70 (Ren et al., 2003). Additionally, lithium-reduced spatial learning and memory deficits in ischemia-reperfusion mice (Fan et al., 2015). Decreased memory deficits and lower oxidative-nitrosative stress levels in the prefrontal cortex and hippocampus were observed in a rat model of global cerebral ischemia (Ozkul et al., 2014). Whereas other studies analyzing the impact of lithium on post-stroke outcome refrained from long-term observations, Li et al. (2011) studied survival periods of up to seven weeks after stroke induction. Better outcome of neonatal rodents treated with lithium was attributed to reduced levels of activated microglia and decreased concentrations of both interleukin-1β and CC-chemokine-ligand-2 (Li et al., 2011). Surprisingly, lithium treatment did not only stimulate neural stem/progenitor cell proliferation in the ischemic brain but also in the non-ischemic brain. The latter may be an important factor for long-term neuroprotection under stroke conditions (Li et al., 2011).
Lithium is not only effective when given during the acute stage of IS, but also when administered at subacute time points. Evidence for successful subacute treatment paradigms under IS conditions comes from Xie et al. (2014), who exposed neonatal rats to cerebral ischemia followed by a delayed lithium treatment paradigm starting on day five for two consecutive weeks. The authors demonstrated that lithium decreased tissue loss and normalized motor hyperactivity as well as anxiety-like behavior twelve weeks after cerebral ischemia. From a mechanistic point of view, the authors observed a regulation of post-stroke neuroinflammation, as indicated by normalized serum levels of the pro-inflammatory cytokines interleukin-1α, interleukin-1β, and interleukin-6 (Xie et al., 2014).
Although the aforementioned authors switched their experimental paradigm from prestroke to poststroke treatment, the therapeutic window of lithium was unknown until recently. Hence, our group systematically investigated this aspect, demonstrating that long-term neuroprotection for as long as eight weeks was achieved when mice received lithium no later than six hours after stroke onset (Doeppner et al., 2017). The long-term neuroprotection was assessed by improved neurological outcome in distinct neurobehavioral tests focusing on motor recovery (Doeppner et al., 2017). Interestingly, application of lithium under these experimental settings not only modified postischemic microglial activity, but also implied a novel GSK3β independent mechanism by which miR-124 expression levels were increased, resulting in enhanced RE1-silencing transcription factor degradation (Doeppner et al., 2017).
To this date, the detailed mechanisms underlying lithium-induced regulation of poststroke inflammation, apoptosis, and neuroregeneration still remain largely unknown. However, recent evidence suggests that lithium might not only affect the brain parenchyma but also the endothelium itself. Previous in vitro experiments under non-hypoxic conditions showed a lithium-induced stabilization of endothelial cells (ECs) due to decreased myosin light chain phosphorylation (Bosche et al., 2016a, b). The same authors also suggested that lithium prevents the early posthypoxic Ca2+ overload in ECs by inhibiting the inositol-3-phosphate-sensitive Ca2+ release from the endoplasmic reticulum, which is an important factor for ischemic/hypoxic EC injury (Bosche et al., 2013). As such, we recently addressed the question whether or not lithium might indeed affect EC injury under stroke conditions in vivo (Haupt et al., 2020). Since ECs are a key compound of the blood-brain barrier (BBB), a lithium-induced impact on such ECs might reverse the early poststroke breakdown of the BBB. The latter is known to significantly contribute to cell injury during the first hours of cerebral ischemia (Jiang et al., 2018). Indeed, lithium treatment resulted in a stabilization of the BBB in stroke mice, leading to a modulated leukocyte infiltration into the brain parenchyma (Haupt et al., 2020). These observations were a consequence of a downstream inhibition of MMP-9 activity as the central aspect of lithium-induced BBB stabilization (Haupt et al., 2020).
Clinical stroke studies
In addition to the aforementioned preclinical studies, data from two clinical trials are available investigating the retrospective risk of patients treated with lithium (Lan et al., 2015) as well as examining the prospective motor recovery in stroke patients receiving lithium treatment (Abdollahi et al., 2014). The latter remains the only prospective randomized double-blind clinical trial for which eighty stroke patients were enrolled. Patients with a first-time ever stroke were randomly allocated to the treatment or control group. Starting with the treatment paradigm two days after stroke, the patients received 300 mg of lithium twice daily or placebo on thirty consecutive days (Abdollahi et al., 2014). The authors of the study, however, observed no significant difference with regard to motor recovery, as measured by the Modified National Institutes of Health Stroke Scale and hand subsection of Fugl-Meyer Assessment. Interestingly, a subgroup of patients that suffered from cortical stroke displayed a significantly better motor recovery when treated with lithium in comparison to the placebo group (Abdollahi et al., 2014). In detail, the absolute difference between the lithium group and the placebo group were about 10% of full function regaining based on hand subsection of Fugl-Meyer Assessment. This suggests an improved neurological recovery after cortical stroke due to early lithium treatment under clinical conditions in man. Likewise, a population-based retrospective study on 1885 patients with bipolar disorders that were treated with or without lithium observed a significant decrease of stroke risk in the lithium group (Lan et al., 2015). Interestingly, the risk reduction was associated with both the cumulative lithium dosage and the exposure time. Although these two studies are in part promising, the data included is highly limited with a low number of patients in total.
Conclusion and Perspective
Lithium has been shown to exert pleiotropic neuroprotective effects in distinct neurological diseases by triggering a variety of pro-survival mechanisms. The downstream targets of these mechanisms predominantly affect the regulation of apoptosis, inflammation, and neurogenesis. The individual signaling pathways depend on the pathophysiology of each disease. The preclinical data for IS suggests an implication of a variety of mechanisms that may be responsible for lithium-induced neuroprotection. Although some of these signaling pathways have already been unveiled, a great deal of questions remains unanswered. However, gaining a deeper understanding of these mechanisms is fundamental for a successful bench-to-bedside translation. Likewise, obtaining more clinical evidence under settings of IS and other neurological diseases is important in order to verify the therapeutic potential of lithium beyond its current indications.
Footnotes
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
References
- 1.Abdanipour A, Moradi F, Fakheri F, Ghorbanlou M, Nejatbakhsh R. The effect of lithium chloride on, NT3, and their receptor mRNA levels in the spinal contusion rat models. Neurol Res. 2019;41:577–583. doi: 10.1080/01616412.2019.1588507. [DOI] [PubMed] [Google Scholar]
- 2.Abdollahi F, Moqaddam MM, Majdinasab N, Sadr F, Sajedi SA, Mohammadianinejad SE. The effect of lithium in post-stroke motor recovery. Clin Neuropharmacol. 2014;37:73–78. doi: 10.1097/WNF.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 3.Ago Y, Tanaka T, Kita Y, Tokumoto H, Takuma K, Matsuda T. Lithium attenuates methamphetamine-induced hyperlocomotion and behavioral sensitization via modulation of prefrontal monoamine release. Neuropharmacology. 2012;62:1634–1639. doi: 10.1016/j.neuropharm.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Bian Q, Shi T, Chuang DM, Qian Y. Lithium reduces ischemia-induced hippocampal CA1 damage and behavioral deficits in gerbils. Brain Res. 2007;1184:270–276. doi: 10.1016/j.brainres.2007.09.054. [DOI] [PubMed] [Google Scholar]
- 5.Bosche B, Molcanyi M, Noll T, Rej S, Zatschler B, Doeppner TR, Hescheler J, Müller DJ, Macdonald RL, Härtel FV. A differential impact of lithium on endothelium-dependent but not on endothelium-independent vessel relaxation. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016a;67:98–106. doi: 10.1016/j.pnpbp.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Bosche B, Molcanyi M, Rej S, Doeppner TR, Obermann M, Müller DJ, Das A, Hescheler J, Loch Macdonald R, Noll T, Härtel FV. Low-dose lithium stabilizes human endothelial barrier by decreasing MLC phosphorylation and universally augments cholinergic vasorelaxation capacity in a direct manner. Front Physiol. 2016b;7:593. doi: 10.3389/fphys.2016.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell BCV, Khatri P. Stroke. Lancet. 2020;396:129–142. doi: 10.1016/S0140-6736(20)31179-X. [DOI] [PubMed] [Google Scholar]
- 8.Ciftci E, Karacay R, Caglayan A, Altunay S, Ates N, Altintas MO, Doeppner TR, Yulug B, Kilic E. Neuroprotective effect of lithium in cold- induced traumatic brain injury in mice. Behav Brain Res. 2020;392:112719. doi: 10.1016/j.bbr.2020.112719. [DOI] [PubMed] [Google Scholar]
- 9.De Ferrari GV, Chacón MA, Barría MI, Garrido JL, Godoy JA, Olivares G, Reyes AE, Alvarez A, Bronfman M, Inestrosa NC. Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by β-amyloid fibrils. Mol Psychiatry. 2003;8:195–208. doi: 10.1038/sj.mp.4001208. [DOI] [PubMed] [Google Scholar]
- 10.de Sousa RT, Zanetti MV, Talib LL, Serpa MH, Chaim TM, Carvalho AF, Brunoni AR, Busatto GF, Gattaz WF, Machado-Vieira R. Lithium increases platelet serine-9 phosphorylated GSK-3β levels in drug-free bipolar disorder during depressive episodes. J Psychiatr Res. 2015;62:78–83. doi: 10.1016/j.jpsychires.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Doeppner TR, Kaltwasser B, Sanchez-Mendoza EH, Caglayan AB, Bähr M, Hermann DM. Lithium-induced neuroprotection in stroke involves increased miR-124 expression, reduced RE1-silencing transcription factor abundance and decreased protein deubiquitination by GSK3β inhibition-independent pathways. J Cereb Blood Flow Metab. 2017;37:914–926. doi: 10.1177/0271678X16647738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emamghoreishi M, Keshavarz M, Nekooeian AA. Acute and chronic effects of lithium on BDNF and GDNF mRNA and protein levels in rat primary neuronal, astroglial and neuroastroglia cultures. Iran J Basic Med Sci. 2015;18:240–246. [PMC free article] [PubMed] [Google Scholar]
- 13.Engel T, Hernández F, Avila J, Lucas JJ. Full reversal of Alzheimer’s disease-like phenotype in a mouse model with conditional overexpression of glycogen synthase kinase-3. J Neurosci. 2006;26:5083–5090. doi: 10.1523/JNEUROSCI.0604-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan M, Jin W, Zhao H, Xiao Y, Jia Y, Yin Y, Jiang X, Xu J, Meng N, Lv P. Lithium chloride administration prevents spatial learning and memory impairment in repeated cerebral ischemia-reperfusion mice by depressing apoptosis and increasing BDNF expression in hippocampus. Behav Brain Res. 2015;291:399–406. doi: 10.1016/j.bbr.2015.05.047. [DOI] [PubMed] [Google Scholar]
- 15.Fernandes BS, Molendijk ML, Köhler CA, Soares JC, Leite CM, Machado-Vieira R, Ribeiro TL, Silva JC, Sales PM, Quevedo J, Oertel-Knöchel V, Vieta E, González-Pinto A, Berk M, Carvalho AF. Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker in bipolar disorder: a meta-analysis of 52 studies. BMC Med. 2015;13:289. doi: 10.1186/s12916-015-0529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forlenza O V, De-Paula VJR, Diniz BSO. Neuroprotective effects of lithium: Implications for the treatment of Alzheimer’s disease and related neurodegenerative disorders. ACS Chem Neurosci. 2014;5:443–450. doi: 10.1021/cn5000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haupt M, Zechmeister B, Bosche B, Lieschke S, Zheng X, Zhang L, Venkataramani V, Jin F, Hein K, Weber MS, Hermann DM, Bähr M, Doeppner TR. Lithium enhances post-stroke blood-brain barrier integrity, activates the MAPK/ERK1/2 pathway and alters immune cell migration in mice. Neuropharmacology. 2020;181:108357. doi: 10.1016/j.neuropharm.2020.108357. [DOI] [PubMed] [Google Scholar]
- 18.Jaworski T, Banach-Kasper E, Gralec K. GSK-3β at the intersection of neuronal plasticity and neurodegeneration. Neural Plast. 2019;2019:4209475. doi: 10.1155/2019/4209475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang X, Andjelkovic A V, Zhu L, Yang T, Bennett MVL, Chen J, Keep RF, Shi Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol. 2018;163–164:144–171. doi: 10.1016/j.pneurobio.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JY, Han Y, Lee JE, Yenari MA. The 70-kDa heat shock protein (Hsp70) as a therapeutic target for stroke. Expert Opin Ther Targets. 2018;22:191–199. doi: 10.1080/14728222.2018.1439477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan CC, Liu CC, Lin CH, Lan TY, McInnis MG, Chan CH, Lan TH. A reduced risk of stroke with lithium exposure in bipolar disorder: a population-based retrospective cohort study. Bipolar Disord. 2015;17:705–714. doi: 10.1111/bdi.12336. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Li Q, Du X, Sun Y, Wang X, Kroemer G, Blomgren K, Zhu C. Lithium-mediated long-term neuroprotection in neonatal rat hypoxia-ischemia is associated with antiinflammatory effects and enhanced proliferation and survival of neural stem/progenitor cells. J Cereb Blood Flow Metab. 2011;31:2106–2115. doi: 10.1038/jcbfm.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li XZ, Chen XP, Zhao K, Bai LM, Zhang H, Zhou XP. Therapeutic effects of valproate combined with lithium carbonate on mptp-induced parkinsonism in mice: Possible mediation through enhanced autophagy. Int J Neurosci. 2013;123:73–79. doi: 10.3109/00207454.2012.729234. [DOI] [PubMed] [Google Scholar]
- 24.Malhi GS, Tanious M, Das P, Coulston CM, Berk M. Potential mechanisms of action of lithium in bipolar disorder: Current understanding. CNS Drugs. 2013;27:135–153. doi: 10.1007/s40263-013-0039-0. [DOI] [PubMed] [Google Scholar]
- 25.Manji HK, Lenox RH. Lithium: a molecular transducer of mood- stabilization in the treatment of bipolar disorder. Neuropsychopharmacology. 1998;19:161–166. doi: 10.1016/S0893-133X(98)00021-9. [DOI] [PubMed] [Google Scholar]
- 26.Martinowich K, Schloesser RJ, Manji HK. Bipolar disorder: From genes to behavior pathways. J Clin Invest. 2009;119:726–736. doi: 10.1172/JCI37703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsunaga S, Kishi T, Annas P, Basun H, Hampel H, Iwata N. Lithium as a treatment for Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2015;48:403–410. doi: 10.3233/JAD-150437. [DOI] [PubMed] [Google Scholar]
- 28.Moretti A, Ferrari F, Villa RF. Neuroprotection for ischaemic stroke: current status and challenges. Pharmacol Ther. 2015;146:23–34. doi: 10.1016/j.pharmthera.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima H, Ishihara T, Suguimoto P, Yokota O, Oshima E, Kugo A, Terada S, Hamamura T, Trojanowski JQ, Lee VMY, Kuroda S. Chronic lithium treatment decreases tau lesions by promoting ubiquitination in a mouse model of tauopathies. Acta Neuropathol. 2005;110:547–556. doi: 10.1007/s00401-005-1087-4. [DOI] [PubMed] [Google Scholar]
- 30.Neuhaus AA, Couch Y, Hadley G, Buchan AM. Neuroprotection in stroke: the importance of collaboration and reproducibility. Brain. 2017;140:2079–2092. doi: 10.1093/brain/awx126. [DOI] [PubMed] [Google Scholar]
- 31.Nonaka S, Chuang DM. Neuroprotective effects of chronic lithium on focal cerebral ischemia in rats. Neuroreport. 1998;9:2081–2084. doi: 10.1097/00001756-199806220-00031. [DOI] [PubMed] [Google Scholar]
- 32.Ozkul A, Sair A, Akyol A, Yenisey C, Dost T, Tataroglu C. Effects of lithium and lamotrigine on oxidative-nitrosative stress and spatial learning deficit after global cerebral ischemia. Neurochem Res. 2014;39:853–861. doi: 10.1007/s11064-014-1281-7. [DOI] [PubMed] [Google Scholar]
- 33.Qi L, Tang Y, He W, Pan H, Jiang W, Wang L, Deng W. Lithium chloride promotes neuronal differentiation of rat neural stem cells and enhances neural regeneration in Parkinson’s disease model. Cytotechnology. 2017;69:277–287. doi: 10.1007/s10616-016-0056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren M, Senatorov V V, Chen RW, Chuang DM. Postinsult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model. Proc Natl Acad Sci U S A. 2003;100:6210–6215. doi: 10.1073/pnas.0937423100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawe N, Steinberg G, Zhao H. Dual roles of the MAPK/ERK1/2 cell signaling pathway after stroke. J Neurosci Res. 2008;86:1659–1669. doi: 10.1002/jnr.21604. [DOI] [PubMed] [Google Scholar]
- 36.Shim SS, Stutzmann GE. Inhibition of glycogen synthase kinase-3: An emerging target in the treatment of traumatic brain injury. J Neurotrauma. 2016;33:2065–2076. doi: 10.1089/neu.2015.4177. [DOI] [PubMed] [Google Scholar]
- 37.Shorter E. The history of lithium therapy. Bipolar Disord. 2009;11:4–9. doi: 10.1111/j.1399-5618.2009.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sudduth TL, Wilson JG, Everhart A, Colton CA, Wilcock DM. Lithium treatment of APPSwDI/NOS2-/- mice leads to reduced hyperphosphorylated tau, increased amyloid deposition and altered inflammatory phenotype. PLoS One. 2012;7:e31993. doi: 10.1371/journal.pone.0031993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wakita M, Nagami H, Takase Y, Nakanishi R, Kotani N, Akaike N. Modifications of excitatory and inhibitory transmission in rat hippocampal pyramidal neurons by acute lithium treatment. Brain Res Bull. 2015;117:39–44. doi: 10.1016/j.brainresbull.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Weller J, Budson A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Res. 2018;7 doi: 10.12688/f1000research.14506.1. F1000 Faculty Rev-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie C, Zhou K, Wang X, Blomgren K, Zhu C. Therapeutic benefits of delayed lithium administration in the neonatal rat after cerebral hypoxia-ischemia. PLoS One. 2014;9:e107192. doi: 10.1371/journal.pone.0107192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu J, Culman J, Blume A, Brecht S, Gohlke P. Chronic treatment with a low dose of lithium protects the brain against ischemic injury by reducing apoptotic death. Stroke. 2003;34:1287–1292. doi: 10.1161/01.STR.0000066308.25088.64. [DOI] [PubMed] [Google Scholar]
- 43.Yan XB, Wang SS, Hou HL, Ji R, Zhou JN. Lithium improves the behavioral disorder in rats subjected to transient global cerebral ischemia. Behav Brain Res. 2007a;177:282–289. doi: 10.1016/j.bbr.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 44.Yan XB, Hou HL, Wu LM, Liu J, Zhou JN. Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology. 2007b;53:487–495. doi: 10.1016/j.neuropharm.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 45.Yu F, Wang Z, Tchantchou F, Chiu CT, Zhang Y, Chuang DM. Lithium ameliorates neurodegeneration, suppresses neuroinflammation, and improves behavioral performance in a mouse model of traumatic brain injury. J Neurotrauma. 2011;29:362–374. doi: 10.1089/neu.2011.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Q, Liu H, Cheng J, Zhu Y, Xiao Q, Bai Y, Tao J. Neuroprotective effects of lithium on a chronic MPTP mouse model of Parkinson’s disease via regulation of α synuclein methylation. Mol Med Rep. 2019;19:4989–4997. doi: 10.3892/mmr.2019.10152. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Z, Lu J, Liu WW, Manaenko A, Hou X, Mei Q, Huang JL, Tang J, Zhang JH, Yao H, Hu Q. Advances in stroke pharmacology. Pharmacol Ther. 2018;191:23–42. doi: 10.1016/j.pharmthera.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Zhu ZF, Wang QG, Han BJ, William CP. Neuroprotective effect and cognitive outcome of chronic lithium on traumatic brain injury in mice. Brain Res Bull. 2010;83:272–277. doi: 10.1016/j.brainresbull.2010.07.008. [DOI] [PubMed] [Google Scholar]