Epilepsy is a complex neurologic condition which affects over 50 million people worldwide. Pharmacotherapy, primarily involving the use of anti-seizure drugs (ASDs), is an essential part of controlling seizures. However, nearly 30% of patients develop drug-resistant epilepsy, clinically defined as the persistence of seizure following trials of two ASDs (Kwan et al., 2010). Although several hypotheses have been proposed to explain this phenomenon, the mechanism of drug-resistant epilepsy still remains unclear. However, a growing body of evidence has demonstrated that blood-brain barrier (BBB) dysfunction represents an important hallmark of the epileptic brain (Salar et al., 2014; Gorter et al., 2019). As previously reported, initial brain injury or seizure may trigger disruption of the BBB, resulting in the immediate release of glutamate. Excess glutamate results in cell stress, inflammatory and cell adhesion molecule activation, and leukocyte infiltration into the brain. Finally, neuronal death, rewiring, gliosis, neurogenesis and angiogenesis, and upregulation/downregulation of receptors, transporters, and ion channels may take place within weeks to months of initial injury. These structural and functional BBB changes may trigger further risk of future seizures and anomalies (Gorter et al., 2019). Evidently, the association between drug-resistant epilepsy and impairment of the BBB function cannot be ignored.
One potential explanation for this association is the biotransformation of ASDs at the BBB in drug-resistant epilepsy. As a majority of ASDs drugs are administered orally, the drugs encounter multiple “hurdles” on the route to the epileptic brain tissue (Figure 1). Following passage through the gastrointestinal tract, drugs are absorbed and partially metabolized by monooxygenases (cytochrome P450s) and several other liver enzymes. This effect, deemed “first pass metabolism", decreases the amount of drug released into the systemic circulatory system. Once in circulation, the drug encounters the BBB, a metabolically active, semi-permeable interface that regulates drug entry into the brain parenchyma. However, the biotransformation of drugs at the pathological BBB prevents the ASDs from reaching the target epileptic brain tissue. Additionally, the overactivity of drug efflux transporters and metabolizing enzymes at the neuronal level further prevents ASDs from reaching the desired destination.
Figure 1.
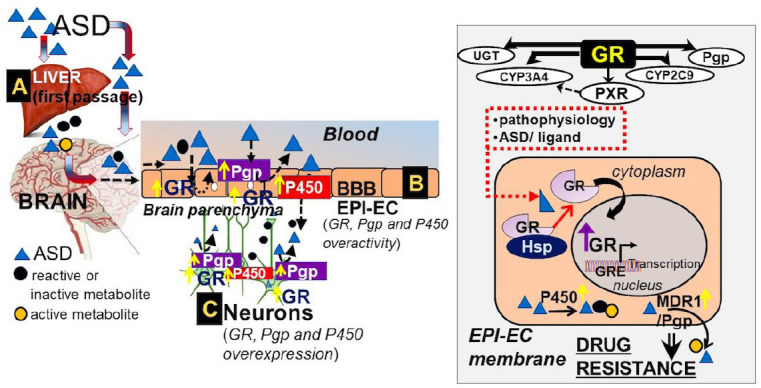
Schematic representation of ASD encountering “multiple hurdles” to reach the target human regions in pharmacoresistant epilepsy with specific relevance of the BBB.
(A) The “first passage” of the ASD through the liver is responsible for primary drug metabolism. (B) The BBB epileptic endothelial cells play an important role in the barrier interface that governs physiological and metabolic properties. As demonstrated by the right inset, the interaction of GR and heat shock proteins becomes critical for GR maturation (Hossain et al., 2020). Upon activation, GR is responsible for controlling important downstream events including the function of efflux transporters (Pgp), phase 1 and 2 drug metabolizing enzymes (CYPs, uridine 5′-diphospho-glucuronosyltransferase, etc.), and other nuclear receptors (PXR) that are found to be overactive in epileptic brain endothelial cells. (C) Finally, the ASD that permeates through the BBB reaches the brain parenchyma. The GR-CYP-Pgp drug regulatory mechanism at the BBB endothelial cells may affect the neurons. ASD: Antiseizure drug; BBB: blood-brain barrier; CYP P450: cytochrome P450s; EPI-EC: epileptic brain endothelial cells; GR: glucocorticoid receptor; GRE: glucocorticoid-response element; Hsp: heat shock protein; Pgp: P-glycoprotein.
As described, the BBB plays an important role in regulating drug bioavailability at the target epileptic brain tissue. This complex and highly regulated pathway will be the focus of discussion, starting from the enzymes involved in drug biotransformation.
Drug biotransformation at the drug-resistant epileptic BBB: It is hypothesized that drug biotransformation at the diseased BBB is mostly conducted by Phase 1 drug metabolizing enzymes, such as cytochrome P450 (CYP) enzymes, and/or phase 2 drug metabolizing enzymes, such as uridine 5′-diphospho-glucuronosyltransferase and glutathione S-transferases. Although Phase 2 enzymes undergo glucuronidation, CYP enzymes, a superfamily of monooxygenases containing a heme cofactor, mainly undergo oxidation and some reduction reactions that primarily occur in the liver. However, significantly elevated levels of functionally active CYP enzymes were also observed at the human brain endothelial cells of patients with drug-resistant epilepsy, in contrast to control BBB endothelium. These enzymes included 11 of 16 cytochrome P450 isoforms analyzed, namely CYP1A1, CYP1B1, CYP2A6, CYP2B6, CYP2C, CYP2C9, CYP2E1, CYP2J2, CYP3A4, CYP4A11, and CYP11b. Upregulation of CYP3A4 is of particular importance as CYP3A4 is responsible for the metabolism of several clinically-used drugs, including a majority of ASDs. Exposure to shear stress was found to increase the expression of endothelial brain CYP3A4 function. CYP-mediated drug-drug interaction was also identified at the BBB (Hossain et al., 2020). An upregulated and active neurovascular drug biotransformation machinery was evident in human epilepsies with a specific link of CYP enzymes to seizure frequency and ASD therapies used by individual subjects before surgery (Williams et al., 2019). These results demonstrate that BBB dysfunction may upregulate the expression of CYP enzymes (for example CYP3A4, CYP2C9) which function in the metabolism of most ASDs, thereby decreasing the bioavailability and efficacy of these drugs to the target tissue.
Cytochrome P450s and drug efflux transporter system regulated by glucocorticoid receptor: In addition to the BBB disease-state and shear stress condition, it has recently been identified that glucocorticoid receptors (GR) play a pivotal role in the regulation of CYP enzyme activity and the drug efflux transporter [e.g. MDR1/P-glycoprotein/P-glycoprotein (Pgp)] system at the BBB endothelium and neurons (Ghosh et al., 2017). Glucocorticoids are steroid hormones involved in a multitude of metabolic, inflammatory and homeostatic functions (Kadmiel and Cidlowski, 2013). In a well-established pathway, glucocorticoids diffuse through the plasma membrane of a given cell and bind to the GR present in the cytoplasm. In the absence of glucocorticoid, GR remains in the inactive state bound to a chaperone protein, such as heat shock proteins (Figure 1). However, the binding of the glucocorticoid ligand triggers GR to undergo a conformational change. The active glucocorticoid/GR complex then translocates to the nucleus, where it may homodimerize and bind to glucocorticoid response elements (GRE), acting directly on the cell's DNA through transcriptional activation and repression (Kadmiel and Cidlowski, 2013).
In a previous study, we demonstrated that levels of GR were increased in human epileptic brain endothelial cells compared to control brain endothelial cells. The subsequent increase in GR signaling was associated with an increase in the expression of CYP3A4, CYP2C9, CYP2E1, and a decrease in the expression of CYP2D6 and CYP2C19. Moreover, silencing of GR resulted in decreased expression of pregnane-X receptors (another type of nuclear receptor), CYP2C9, and CYP3A4, the enzyme responsible for the metabolism of several ASDs (Ghosh et al., 2017). GR upregulation was also associated with increased expression of Pgp, the protein product of the MDR1 gene. Pgp is an adenosine triphosphate (ATP)-binding cassette transporter involved in drug efflux activity on the luminal membrane of brain capillary endothelial cells. More specifically, Pgp pumps drugs back into the bloodstream by using energy from ATP hydrolysis and prevents the drug from crossing the BBB endothelial cells. This significantly decreases the concentration of drug across the BBB (Loscher and Potschka, 2005). Therefore, through these two distinct mechanisms, the overall GR regulatory process becomes significant in determining the net permeability of ASDs into the brain.
Mechanistic regulation of GR in the epileptic brain as heat shock proteins accelerate GR maturation in dysplastic BBB endothelial cells: GR is a ligand-dependent transcription factor residing in the cytoplasm in a resting state. Upon pathophysiological trigger, ligand binding induces a conformational change in the receptor, exposing a nuclear localization signal and exchanging interaction partners. The receptor then translocates into the nucleus. It has been well-established that heat shock proteins facilitate this process of GR maturation and nuclear translocation, although the mechanism remains unknown. Therefore, it was hypothesized that heat shock proteins exert an important regulatory function over brain endothelial cell GR activity in human epilepsies.
In a recent study, we found that GR, heat shock protein 70 (Hsp70), heat shock protein 90 (Hsp90), and heat shock protein 40 were upregulated in epileptic brain regions compared to non-epileptic brain regions. More specifically, increased GR and Hsp90 co-localization was observed in the microvessels, astrocytes, and neurons of epileptic tissue, while GR and Hsp70 co-localization was observed in the microvessels and neurons.
Moreover, decreased levels of Hsp70 and Hsp90 were bound to GR in human epileptic (dysplastic) brain tissue compared to non-epileptic (non-dysplastic) tissues. A similar pattern was found in human epileptic brain endothelial cells compared to non-epileptic brain endothelial cells. As unbound GR-Hsp is indicative of complete GR maturation, decreased GR-Hsp interaction in epileptic tissue reflects accelerated GR maturation. The corresponding increase in ATPase activity in epileptic tissue suggests increased chaperone activity, demonstrating the essential role of heat shock proteins in the acceleration of GR maturation in individuals with epilepsies (Hossain et al., 2020).
The study also showed that GR silencing in epileptic brain endothelial cells resulted in increased interaction of GR with heat shock proteins. This suggests that GR silencing slowed down the maturation process of GR. Therefore, overexpression of Hsp90 and Hsp70 as well as co-localization of these heat shock proteins and GR in human epileptic tissue may result in increased maturation and nuclear co-localization of GR, which has shown to have important regulatory effects on drug biotransformation and the drug efflux transport mechanism active at the BBB, as discussed above.
Conclusion and future directions: The eventual implications of these findings exist within clinical practice, such as therapy and/or treatment targeting patients with drug-resistant epilepsy, considering the vital role of the BBB. For example, in our recent study, we found that treatment with GR modulators/ligands such as dexamethasone (a steroid), rifampicin (an antibiotic), and phenytoin (an anti-seizure medication) significantly increased the rate of GR nuclear translocation in human epileptic brain endothelial cells in focal cortical dysplasia (Hossain et al., 2020) that could alter the downstream activity of several proteins linked to GR function and thereby contribute to pharmacoresistance. Additionally, the pharmacological inhibition of Hsp90 could prevent glutamate transporter GLT-1 degradation by disruption of Hsp90β and GLT-1 interaction and was reported as a therapy target for the treatment of epilepsy and excitotoxicity. Our earlier studies showed that modulation of epileptic endothelial cell GR was also found to improve drug penetration across the brain vasculature (Ghosh et al., 2018). Therefore, both GR and Hsp90 could be pertinent molecular clients for drug regulation in epilepsy therapy. Due to multiple downstream effects, heat shock proteins and GR may therefore be considered important druggable targets.
Although inhibiting GR in epileptic endothelial cells may allow for increased drug bioavailability across the BBB in pharmacoresistant epilepsy (Ghosh et al., 2018), manipulating GR for therapeutic purposes presents a unique challenge: both the extreme inhibition and activation of GR may result in adverse effects. For example, GR regulates the negative feedback of cortisol on the secretion of corticotropin-releasing hormone and adrenocorticotropic hormone through the hypothalamic-pituitary-adrenal axis (Karin et al., 2020). GR overactivity may be associated with a “stress response", which over time may promote the development of neurological disorders (Williams and Ghosh, 2020). On the contrary, inhibition of GR may prevent discontinuation of a prolonged stress response, resulting in cortisol elevation as observed in patients with depression (Karin et al., 2020). Hypercortisolism may also manifest through brain atrophy changes and white matter hyperintensities, as observed in patients with Cushing's syndrome (Chen et al., 2020). Future research and therapy development must therefore focus on maintaining the balance between the gain and loss of GR function. Moreover, understanding drug pharmacokinetics and pharmacodynamics is critical to characterize drug-drug interactions in the presence of CYP enzyme inducers or inhibitors focusing on the BBB. For example, certain CYP enzyme inhibitors may significantly increase the concentration of other CYP-mediated ASDs, potentially increasing drug-induced toxicity. Considering the “multiple hurdle” system, GR regulation at the BBB is therefore an important frontier in research yet to be fully apprehended in a multitude of genetic and neurological conditions, and we encourage better understanding of this regulatory pathway for optimum drug efficacy.
The present work is supported in part by the National Institute of Neurological Disorders and Stroke/National Institutes of Health grants R01NS095825 (to CG).
Footnotes
C-Editors: Zhao M, Zhao LJ, Qiu Y; T-Editor: Jia Y
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
References
- 1.Chen Y, Zhang J, Tan H, Li J, Yu Y. Detrimental effects of hypercortisolism on brain structure and related risk factors. Sci Rep. 2020;10:12708. doi: 10.1038/s41598-020-68166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghosh C, Hossain M, Solanki J, Najm IM, Marchi N, Janigro D. Overexpression of pregnane X and glucocorticoid receptors and the regulation of cytochrome P450 in human epileptic brain endothelial cells. Epilepsia. 2017;58:576–585. doi: 10.1111/epi.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosh C, Hossain M, Mishra S, Khan S, Gonzalez-Martinez J, Marchi N, Janigro D, Bingaman W, Najm I. Modulation of glucocorticoid receptor in human epileptic endothelial cells impacts drug biotransformation in an in vitro blood-brain barrier model. Epilepsia. 2018;59:2049–2060. doi: 10.1111/epi.14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorter JA, Aronica E, van Vliet EA. The roof is leaking and a storm is raging: repairing the blood-brain barrier in the fight against epilepsy. Epilepsy Curr. 2019;19:177–181. doi: 10.1177/1535759719844750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hossain M, Williams S, Ferguson L, Bingaman W, Ghosh A, Najm IM, Ghosh C. Heat shock proteins accelerate the maturation of brain endothelial cell glucocorticoid receptor in focal human drug-resistant epilepsy. Mol Neurobiol. 2020;57:4511–4529. doi: 10.1007/s12035-020-02043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol Sci. 2013;34:518–530. doi: 10.1016/j.tips.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karin O, Raz M, Tendler A, Bar A, Korem Kohanim Y, Milo T, Alon U. A new model for the HPA axis explains dysregulation of stress hormones on the timescale of weeks. Mol Syst Biol. 2020;16:e9510. doi: 10.15252/msb.20209510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshe SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 9.Loscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2:86–98. doi: 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salar S, Maslarova A, Lippmann K, Nichtweiss J, Weissberg I, Sheintuch L, Kunz WS, Shorer Z, Friedman A, Heinemann U. Blood-brain barrier dysfunction can contribute to pharmacoresistance of seizures. Epilepsia. 2014;55:1255–1263. doi: 10.1111/epi.12713. [DOI] [PubMed] [Google Scholar]
- 11.Williams S, Ghosh C. Neurovascular glucocorticoid receptors and glucocorticoids: implications in, neurological disorders and drug therapy. Drug Discov Today. 2020;25:89–106. doi: 10.1016/j.drudis.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams S, Hossain M, Ferguson L, Busch RM, Marchi N, Gonzalez-Martinez J, Perucca E, Najm IM, Ghosh C. Neurovascular drug biotransformation machinery in focal human epilepsies: brain CYP3A4 correlates with seizure frequency and antiepileptic drug therapy. Mol Neurobiol. 2019;56:8392–8407. doi: 10.1007/s12035-019-01673-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


