Spinal cord injury (SCI) is currently an incurable condition which induces sensorimotor impairments below the injury level. Mainly, SCI are the consequence of physical damages which occur on spinal cord due to traffic accidents or sports and recreation injuries. To date, nor treatment of therapy could be proposed to patients with SCI (Wilson et al., 2012).
Knowledge regarding the sequence of the basic cellular and molecular events which take place after SCI is now well documented. SCI causes axonal disruption, neuronal death, demyelination, release of myelin debris and massive microglial/macrophage reactivity. These primary events induce the formation of a scar which is composed of several cellular populations. Even if there are still some debates or speculations about the precise cellular origin of the main components of the scar, it is now admitted that the scar is composed of a fibrotic core which is surrounded by a glial scar (Sabelström et al., 2014). The fibrotic core exerts an inhibitory effect on axonal regrowth whereas the neighboring glial scar restricts the inflammation and secrets permissive molecules. Thereby, this new knowledge about the opposite and specific properties of the fibrotic and glial components of the scar has allowed considering spinal scar modulation as a potential therapy after SCI (Anderson et al., 2016; Dias et al., 2018).
This is in this context that we proposed to investigate the effects of repetitive magnetic stimulation (RMS) as a non-invasive therapy to modulate the spinal scar after SCI (Chalfouh et al., 2020). Indeed, RMS is a noninvasive form of nervous system stimulation which is already used clinically as treatment for some neuropsychiatric disorders even if the mechanisms behind its effects are still not fully understood. According to the size of the coils used, RMS induces a focal magnetic field allowing selectively stimulating neuronal populations in brain. In our study, we used RMS to specifically stimulate spinal cord at lesion site. To do so, figure of eight double coil was positioned in close contact to the back of the animals to induce trans-spinal magnetic stimulation (rTSMS). Our stimulation protocol was based on a 10-minute stimulation period during 14 consecutive days. First of all, as a proof of concept, we tested rTSMS on scar modulation after SCI. SCI were performed on mice, then, the day after SCI half of the mice were treated using rTSMS. In these mice, 15 days after SCI we performed histological analyses to investigate the spinal scar. In rTSMS treated animals the fibrotic core of the scar was significantly decreased and at the opposite the glial scar was significantly increased 15 days after SCI. In order to confirm these results, based on the same lesion and stimulation paradigms, we performed histological analyses 90 days after SCI. Our results showed that rTSMS also induces scar modulation 90 days after SCI in decreasing fibrosis and enhancing gliosis (Chalfouh et al., 2020).
As described before, SCI induces sensorimotor impairments leading to the paralysis of the lower body including legs (called paraplegia) and/or the full body including arms and legs (called tetraplegia). That is why we tested the effects of rTSMS on sensorimotor functions. To do so, we used a foot misplacement apparatus which allows visualizing and recording the displacement of the mice into a flat ladder. These experiments reveal that rTSMS increases sensorimotor recovery in mice. Indeed, treated animals were able to cross the corridor faster with less hindlimb misplacements in comparison to the untreated mice (Chalfouh et al., 2020).
Before transferring this treatment to the clinic, it is of primary importance to validate it in a more clinically relevant context. In fact, the main causes of SCI are traumatic accidents. Thereby, rTSMS treatment could not be applied to paraplegic or tetraplegic patients the day after injury. In order to skirt around this issue, we have tested the effects of rTSMS in beginning the treatment 10 days after SCI. Based on this specific context, sensorimotor and histological experiments have been performed as described previously. Our results demonstrate that when rTSMS treatment is applied 10 days after SCI, it enhances functional recovery and modulates the spinal scar.
These promising results encouraged us to validate rTSMS treatment in the two categories of persons which are the more concerned by SCI: teenagers and elderly. SCI were performed on juvenile (post-natal 30 days) and elderly (18 months) mice and 10 days after they were treated using rTSMS. Our results show that rTSMS induces modulation in both juvenile and aged mice. It appears also, that rTSMS enhances strongly functional recovery in juvenile mice but moderately in aged mice (Chalfouh et al., 2020).
The precise mechanisms underlying RMS effects are not fully understood. In fact, it is mainly reported that RMS exerts its effects by stimulating or inhibiting neuronal action potential triggering. However, some recent studies have described that RMS can play a direct role on other cellular populations (Cullen et al., 2019). That is why to better understand the precise mechanisms which are induced by rTSMS after SCI we performed proteomic analyses. These analyses reveal that rTSMS upregulates a large amount of proteins implicated in axonal regrowth and proliferation of neural cells and downregulates proteins implicated in apoptosis. Firstly, to validate these results, we performed histological analyses and BDA tract-tracing experiments 15 days after SCI. Our results confirm proteomic data by demonstrating that rTSMS increases axonal regrowth and neuronal survival. Secondly, we would to investigate the role that rTSMS could play on neural cell proliferation. To do so, we analyzed the proliferation and the reactivity of the endogenous spinal cord stem cells; ependymal cells, in vitro and in vivo respectively. Indeed, it has been described by several teams that ependymal cells constituted the endogenous spinal cord stem cell population (Barnabé-Heider et al., 2010). Based on neurosphere assay and fate mapping experiments, our analyses reveal, for the first time, that rTSMS increases stem cell self-renewal potential in vitro and enhances ependymal cells proliferation in vivo (Chalfouh et al., 2020). The main results of our study are summarized in Figure 1.
Figure 1.
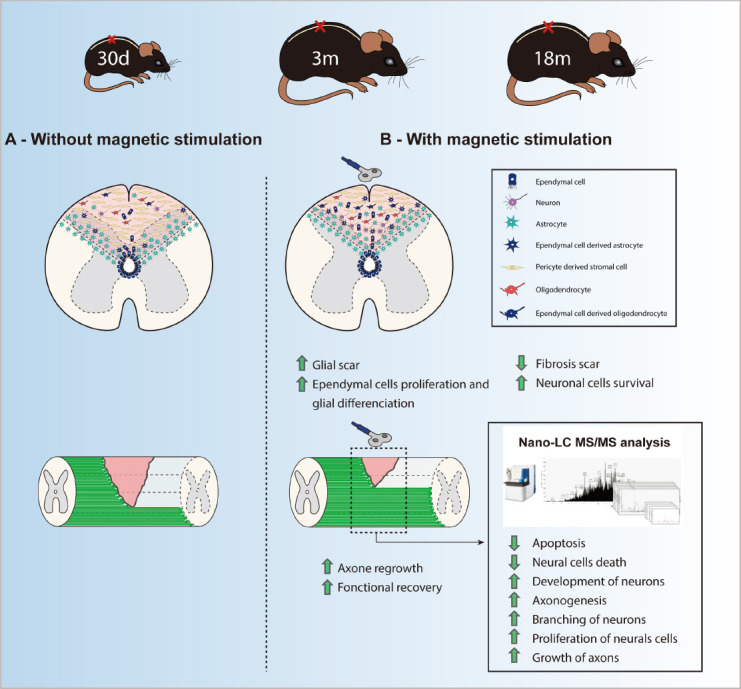
Summary of the main results of the study of Chalfouh et al. (2020).
m: Month.
Nevertheless, even if our results are very promising, several questions remain unanswered. Indeed, it has been recently reported that after SCI, mice present some spontaneous recovery due to differentiation of oligodendrocyte precursors to Schwann cells like P0 positive cells (Duncan et al., 2018). To date, this phenomenon has not been documented in humans after SCI.
Moreover, in our study we used a commercial stimulation coil which is already used in clinic. The size of the magnetic field generated is around 1.5 cm2, meaning that in mice the anatomical zone stimulated is vertebral segments 3 to 4. Before transferring this therapy to humans, it is of primary importance to design a specific stimulation coil that can generate a magnetic field of 10 cm2 allowing stimulating the lesion site and the uninjured neighboring parts of the spinal cord. Furthermore, in our study we did not try several patterns of stimulation. In fact, a recent paper as described the main role played by stimulation frequencies and patterns and demonstrated that these parameters are more important than the number of pulses per se (Dufor et al., 2019).
Another limitation of our study is the fact that the precise mechanisms implicated in the roles played by rTSMS in each cellular type are not clearly described. In effect, proteomic analyses revealed molecular pathways upregulated after rTSMS treatment such as axonal regrowth or downregulation such as apoptosis. Nevertheless, we do not know in which cells these pathways are specifically expressed. It could be very interesting to conduct for example single cell analysis to define precisely the molecular mechanisms involved in each cell type following rTSMS. Furthermore, we have conducted axonal tract tracing experiments showing that rTSMS induces a robust infiltration of BDA positive axons. However, our results could not determine if rTSMS promotes axonal regrowth or axonal survival. Further experiments should be conducted to define precisely the exact mechanisms involved during this process.
A last limitation of our work is the fact that we did not find the precise group of proteins or the specific receptor(s) by which rTSMS acts on the cells present into the spinal cord. In fact, in our study, rTSMS non-invasively modulates the spinal scar; however, we did not know precisely how rTSMS plays its role in the different cellular populations such as astrocytes, fibroblasts, oligodendrocyte precursors, neurons, microglia, or ependymal cells. A recent study describes the main role played by the protein cryptochrome as regulator of the effects of magnetic stimulation. Indeed, in a model of brain injury, the authors describe that cryptochrome acts as “cellular magnetoreceptor” and that the effects of magnetic stimulation are cryptochrome dependant (Dufor et al., 2019). It could be of primary interest to confirm the role played by this protein in the effects observed after rTSMS treatment.
Finally, it is important to compare rTSMS treatment and its effects with those described after other therapies. Different strategies have been already applied after SCI: cellular transplantation (Mayeur et al., 2013), virus injections to convert astrocytes into neurons for example (Chen et al., 2020), peripheral nerve grafts or biomaterials depots filled or not with neurotrophic factors (Anderson et al., 2018). It has been proven that all of these strategies are effective and induce robust axonal regrowth and/or functional recovery. Nevertheless, they are invasive making them difficult to consider as future potential treatments after SCI in humans.
To conclude, recently, increasing knowledge regarding the spinal scar has offered new perspective about the possibility to treat SCI in modulating the endogenous cell populations. In particular, we hypothesized that modulation of specific cells such as, ependymal cells, pericytes or astrocytes, which constitute the different components of the spinal scar after SCI, can be seen respectively as keystones for future treatments. Such treatment opens the way for repairing the spinal cord non-invasively. Taking into account these different considerations, it appears that not only rTSMS but all non-invasive forms of nervous system stimulations constitute very promising strategies which should be further investigated.
This work was supported by ADIR association and IRME association. NG is supported by European Union and Normandie Regional Council. Europe gets involved in Normandie with European Regional Development Funds (ERDF).
Additional files: Open peer review reports 1 (84.6KB, pdf) and 2 (85.2KB, pdf) .
Footnotes
P-Reviewers: Rai SNN, Mulherkar S; C-Editors: Zhao M, Liu WJ, Wang L; T-Editor: Jia Y
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open peer reviewers:Sachchida Nand Nand Rai, University of Allahabad, India; Shalaka Mulherkar, Washington University in St Louis School of Medicine, USA.
References
- 1.Anderson MA, Burda JE, Ren Y, Ao Y, O'Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson MA, O'Shea TM, Burda JE, Ao Y, Barlatey SL, Bernstein AM, Kim JH, James ND, Rogers A, Kato B, Wollenberg AL, Kawaguchi R, Coppola G, Wang C, Deming TJ, He Z, Courtine G, Sofroniew MV. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature. 2018;561:396–400. doi: 10.1038/s41586-018-0467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnabé-Heider F, Göritz C, Sabelström H, Takebayashi H, Pfrieger FW, Meletis K, Frisén J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7:470–482. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Chalfouh C, Guillou C, Hardouin J, Delarue Q, Li X, Duclos C, Schapman D, Marie JP, Cosette P, Guérout N. The regenerative effect of trans-spinal magnetic stimulation after spinal cord injury: mechanisms and pathways underlying the effect. Neurotherapeutics. 2020;17:2069–2088. doi: 10.1007/s13311-020-00915-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen YC, Ma NX, Pei ZF, Wu Z, Do-Monte FH, Keefe S, Yellin E, Chen MS, Yin JC, Lee G, Minier-Toribio A, Hu Y, Bai YT, Lee K, Quirk GJ, Chen G. A NeuroD1 AAV-based gene therapy for functional brain repair after ischemic injury through in vivo astrocyte-to-neuron conversion. Mol Ther. 2020;28:217–234. doi: 10.1016/j.ymthe.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cullen CL, Senesi M, Tang AD, Clutterbuck MT, Auderset L, O'Rourke ME, Rodger J, Young KM. Low-intensity transcranial magnetic stimulation promotes the survival and maturation of newborn oligodendrocytes in the adult mouse brain. Glia. 2019;67:1462–1477. doi: 10.1002/glia.23620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dias DO, Kim H, Holl D, Werne Solnestam B, Lundeberg J, Carlén M, Göritz C, Frisén J. Reducing pericyte-derived scarring promotes recovery after spinal cord injury. Cell. 2018;173:153–165.e122. doi: 10.1016/j.cell.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dufor T, Grehl S, Tang AD, Doulazmi M. Neural circuit repair by low-intensity magnetic stimulation requires cellular magnetoreceptors and specific stimulation patterns. Sci Adv. 2019;5:eaav9847. doi: 10.1126/sciadv.aav9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan GJ, Manesh SB, Hilton BJ, Assinck P. Locomotor recovery following contusive spinal cord injury does not require oligodendrocyte remyelination. Nat Commun. 2018;9:3066. doi: 10.1038/s41467-018-05473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayeur A, Duclos C, Honoré A, Gauberti M, Drouot L, do Rego JC, Bon-Mardion N, Jean L, Vérin E, Emery E, Lemarchant S, Vivien D, Boyer O, Marie JP, Guérout N. Potential of olfactory ensheathing cells from different sources for spinal cord repair. PLoS One. 2013;8:e62860. doi: 10.1371/journal.pone.0062860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabelström H, Stenudd M, Frisén J. Neural stem cells in the adult spinal cord. Exp Neurol. 2014;260:44–49. doi: 10.1016/j.expneurol.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Wilson JR, Cadotte DW, Fehlings MG. Clinical predictors of neurological outcome, functional status, and survival after traumatic spinal cord injury: a systematic review. J Neurosurg Spine. 2012;17:11–26. doi: 10.3171/2012.4.AOSPINE1245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


