Key Words: Axion-MEA, electrical activities, human disease model, Huntington's disease, HTT, monkey neuron, morphometric analysis, mouse neuron, neurodegenerative diseases, primary culture
Abstract
In vitro cultures of primary cortical neurons are widely used to investigate neuronal function. However, it has yet to be fully investigated whether there are significant differences in development and function between cultured rodent and primate cortical neurons, and whether these differences influence the utilization of cultured cortical neurons to model pathological conditions. Using in vitro culture techniques combined with immunofluorescence and electrophysiological methods, our study found that the development and maturation of primary cerebral cortical neurons from cynomolgus monkeys were slower than those from mice. We used a microelectrode array technique to compare the electrophysiological differences in cortical neurons, and found that primary cortical neurons from the mouse brain began to show electrical activity earlier than those from the cynomolgus monkey. Although cultured monkey cortical neurons developed slowly in vitro, they exhibited typical pathological features-revealed by immunofluorescent staining-when infected with adeno-associated viral vectors expressing mutant huntingtin (HTT), the Huntington's disease protein. A quantitative analysis of the cultured monkey cortical neurons also confirmed that mutant HTT significantly reduced the length of neurites. Therefore, compared with the primary cortical neurons of mice, cultured monkey cortical neurons have longer developmental and survival times and greater sustained physiological activity, such as electrophysiological activity. Our findings also suggest that primary cynomolgus monkey neurons cultured in vitro can simulate a cell model of human neurodegenerative disease, and may be useful for investigating time-dependent neuronal death as well as treatment via neuronal regeneration. All mouse experiments and protocols were approved by the Animal Care and Use Committee of Jinan University of China (IACUC Approval No. 20200512-04) on May 12, 2020. All monkey experiments were approved by the IACUC protocol (IACUC Approval No. LDACU 20190820-01) on August 23, 2019 for animal management and use.
Chinese Library Classification No. R459.9; R363; R364
Introduction
In vitro cultures of neuronal cells have been fundamental for advancing our understanding of the function of neuronal cells and for establishing cellular models of neurological diseases (Chen et al., 2020; Liu et al., 2020; Sahin et al., 2021). These cells retain morphological, neurochemical, and electrophysiological properties that are comparable to those of cortical neurons in the brain. Moreover, cultured primary neuronal cells are able to develop into mature cortical neurons and generate neurites that display the characteristics of distinct axons and dendrites (Bardy et al., 2015). Thus, cultured primary neuronal cells have been widely used to investigate neuronal function as well as pathological events caused by either insults or disease-related proteins.
Primary cultures of neuronal cells are normally established using neuronal cells isolated from fetal brains. For example, cultured rodent cortical neurons can be isolated at embryonic days 17 to 18 (Choi et al., 2013). Because of the easy breeding and fast generation time of rodents, as well as their low cost, cultured mouse neuronal cells have become a vitally important tool in neuroscience research. However, the development and maturation of rodent cortical neurons are considerably different from those of primate neuronal cells (Sokolov et al., 2018). It remains to be investigated whether species-dependent differences impact the growth, neuronal properties, and pathology of cultured cortical neurons from rodent and primate brains (Otani et al., 2016). The characteristics of cortical neurons cultured in vitro can be studied from a variety of perspectives, including morphology, molecular changes, and electrophysiological activity (Bateup et al., 2013).
Huntington's disease (HD) is an autosomal dominant neurological disorder characterized by motor dysfunction (Adegbuyiro et al., 2017), cognitive decline, and psychological dysfunction. The genetic mechanism of HD is the expansion of CAG triplicates (> 36 CAG repeats) (Tabrizi et al., 2019) in exon 1 of the HD gene, HTT, which encodes the huntingtin protein. This CAG triplicate is unstable and varies in length in different species (Neueder et al., 2017; Taran et al., 2020). A CAG repeat expansion (> 36 CAGs) in the HTT gene leads to a polyglutamine (polyQ) expansion that causes HTT to misfold and aggregate in the brains of patients (Yang et al., 2010). Because humans and monkeys are relatively closely related, the cortical neurons of monkeys are better able to mimic human disease characteristics than mouse cortical neurons; this is especially true for HD and other complex neurodegenerative diseases.
In the present study, we used cultured primary cortical neurons from rodents and monkeys, and compared their growth, maturation, and electrophysiological activity to explore the developing differences between mouse and monkey primary cortical neurons in vitro. We also compared pathological changes in these cultured cortical neurons expressing mutant HTT (Yang et al., 2020), which can cause HD (Yan et al., 2018). Our findings indicate that cultured monkey neurons develop more slowly but survive much longer than cultured mouse neurons, suggesting that cultured monkey neurons can be used to investigate time-dependent neurodegeneration and to evaluate the therapeutic effects of drugs that promote neuronal regeneration.
Materials and Methods
Animals
All mouse experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Jinan University of China (IACUC Approval No. IACUC-20200512-04) on May 12, 2020. Wild-type C57BL/6 mice (four female mice and four male mice) were purchased from the Guangdong Animal Medical Experimental Center (license No. SCXK (Yue) 2018-0002) and were used to produce fetal mice. Mice were kept in the animal facility of the Institute of Central Nerve Regeneration at Jinan University. The facility had a light period of 12 hours and a dark period of 12 hours, with a controlled temperature of 22 ± 2°C and humidity of 50 ± 10%. Animals were provided with a regular mouse diet and sterile water. The male and female mice were caged at 10 p.m., and any female mouse with a vaginal plug at 7 a.m. the next day was considered as being pregnant at 0.5 days. Fetuses at embryonic day 18 were obtained to isolate the brains for the primary neuronal cultures (Nakamichi et al., 2019).
A cynomolgus monkey fetus on gestational day 80 was used for the primary cultures of monkey cortical neurons. The monkey was kept at Guangdong Landao Biotechnology Co., Ltd. All monkey experiments were approved by the IACUC protocol (IACUC Approval No. LDACU 20190820-01) on August 23, 2019, for animal management and use. The fetal monkey was separated from a pregnant female monkey by cesarean section, and its brain was immediately isolated and placed on ice. Cortical tissue was dissected in Dulbecco's Modified Eagle's Medium containing 10% fetal bovine serum and 10% dimethyl sulfoxide. Next, the dissected cortical tissue was cut into small pieces and frozen to -80°C by gradient freezing, and was then stored in liquid nitrogen. The frozen monkey brain tissue was used for culturing cortical neurons using a previously described procedure (Negishi et al., 2002).
Culture of primary cortical neurons
Coverslips were coated with 0.1 mg/mL poly-L-lysine solution in a 24-well plate 1 day before plating, and were then washed three times with sterilized deionized water and dried before use. Cortical tissue from fetal mice or the fetal monkey was digested with 20 U/mL papain, plated onto the coated coverslips in neuronal culture medium (Neurobasal Medium with 2% B-27 supplement), and incubated in an incubator at 37°C, 5% CO2. To inhibit glial cell growth, cytosine was added to the medium at a concentration of 2.5 mg/mL (Xiong et al., 2007; Seibenhener and Wooten, 2012; Lu et al., 2016). Cortical neurons were maintained by changing 50% of the medium every other day until the growth of the cortical neurons was stable, and half of the medium was then changed every 3 or 4 days. The anti-β-tubulin III-positive and anti-glial fibrillary acidic protein (GFAP)-negative cells were considered to be neurons (the immunocytochemistry procedure is described in the following section).
Immunocytochemistry and morphometric analysis
To determine the developmental status of primary cortical neurons from the mouse and cynomolgus monkey fetal cortex, the cultured monkey cortical neurons (days 3, 7, 11, 14, and 81) and mouse cortical neurons (days 3, 7, 11, and 14) were analyzed by immunofluorescent staining. The cultured cells were washed with warm phosphate-buffered saline (PBS), fixed in freshly prepared 4% paraformaldehyde in PBS for 10 minutes, washed with PBS, and permeabilized with 0.1% Triton X-100 in PBS. They were then blocked with a blocking solution (3% bovine serum albumin + 0.3% Triton X-100 + 2% fetal bovine serum) for 30 minutes at room temperature. Afterwards, the cells were incubated overnight at 4°C with the following primary antibodies: rabbit anti-β-tubulin III antibody (1:1000; Abcam, Cambridge, UK; Cat# ab18207) and mouse anti-GFAP antibody (1:500; Sigma-Aldrich, St Louis, MO, USA; Cat# MAB360). The samples were then thoroughly rinsed three times with PBS before being incubated at 4°C for 2 hours with 4′,6-diamidino-2-phenylindole (DAPI; 1:1000; Beyotime Biotechnology, Shanghai, China; Cat# C1002) and the following secondary antibodies: goat anti-mouse Alexa Fluor 488 (1:1000; Thermo Fisher Scientific, Waltham, MA, USA; Cat# A-11001) and goat anti-rabbit Alexa Fluor 594 (1:1000; Thermo Fisher, Cat# A-11012). The cells were then washed three times with PBS and mounted onto slides. Photographs were taken with a Zeiss LSM880 confocal microscope and Zeiss Axio Observer A1 microscope (Zeiss, Oberkochen, Germany) (Reddy et al., 2018). Axon length was measured to indicate the state of cortical neurons. Under the same conditions, a longer axon length was taken to represent a better growth state of the cortical neurons. Data were measured using ImageJ 1.52q software (National Institutes of Health, Bethesda, MD, USA).
Functional analysis using electrophysiology
Cortical neuronal cultures (as described previously in the methods section) were plated in an M768-GLx 48-well plate (Axion Biosystems, Atlanta, GA, USA). Simultaneous recordings from 64 extracellular electrodes per well were made using the Maestro microelectrode array (MEA) system (Axion Biosystems) at a constant temperature of 37°C. Data were sampled at 12.5 kHz, and were digitized and analyzed using Axion Integrated Studio software (Axion Biosystems). Spike events were detected using an adaptive spike detection threshold of 5.5 SD for each electrode with 1 second binning.
Adeno-associated virus infection of cultured cortical neurons
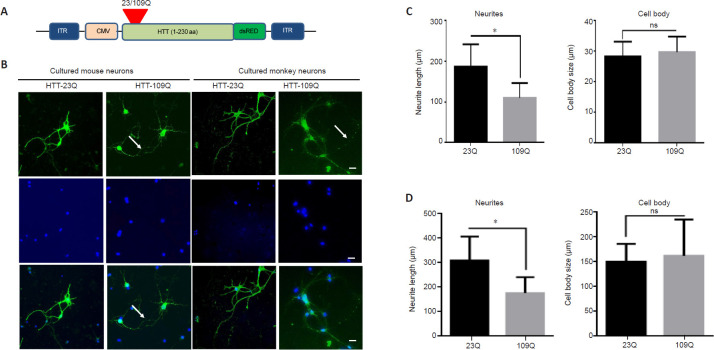
To investigate the effects of mutant HTT on cortical neurons at the cellular level, we used the adeno-associated virus (AAV) vector to express N-terminal HTT (1–230 amino acids) containing either 23Q or 109Q and a dsRED fluorescence protein (RFP). The AAV vectors were packaged and amplified by PackGene Biotech (Guangzhou, China). The vector contained the cytomegalovirus (CMV) promoter to express transgenes in cultured cortical neurons. On day 6 of culturing the cortical neurons, the culture medium was removed and replaced with fresh culture medium containing the AAV vectors. After infection for 24 hours, the medium was replaced with fresh culture medium. The transgenic expression of fluorescence was visible within 48 hours. Infected cells were then examined for their morphology and electrophysiological activity, as described earlier in the methods section. In addition, axon length was measured to indicate the state of cortical neurons. Data were measured using ImageJ 1.52q software.
Functional analysis using patch-clamp methods
A functional analysis was performed after the cortical neurons had been cultured for 21 days in vitro. First, the irrigation liquid needed for the electrophysiological recordings (Axon Axopatch 200B, Molecular Devices, San Jose, CA, USA) was prepared, and the 95%O2/5%CO2 gas was injected. Next, the coverslip with neurons was transferred to an electrophysiological recording tank, and the coverslip was fixed with a press. The target cells were recorded as RFP-positive cells. An electrode puller was then used to pull the glass electrode to a resistance of 5–12 MΩ, and a Multiclamp 700B (Molecular Devices) was used to complete the whole-cell recording. After the completion of high-resistance sealing and membrane rupture, the current-clamp mode was immediately switched on, and current was gradually injected into the cells to achieve depolarization. Next, the action potential release was recorded. The voltage-clamp mode was then switched on, voltage was clamped at –70 mV, and the sodium and potassium currents and changes in the action potential current were recorded from the cell. The sampling frequency of the experiment was 10 kHz, and Clampfit 9.0 software (Molecular Devices) was used for the data analysis.
Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM). Calculations were performed using GraphPad Prism 7 software (GraphPad, San Diego, CA, USA). More than three independent experiments were performed to obtain the morphometric analyses or micrograph results that were used for figure presentations. Representative results are shown in the figures. Statistical significance was assessed using the two-tailed Student's t-test to compare two groups. To analyze multiple groups, two-way analysis of variance was used, followed by Tukey's multiple comparisons test. A P-value < 0.05 was considered statistically significant.
Results
Neurites of cultured primary cortical neurons
The neurite growth of cultured mouse and monkey cortical neurons over time was compared using phase-contrast images, which can reveal all cell types. The primary cortical neurons of the mouse brain were able to quickly attach to the culture dish, and extended obvious neurites even on the 1st day in vitro. On day 3, primary mouse cortical neuron axons were markedly extended, and by day 7, most of them had established connections with neighboring cortical neurons. In contrast, the cultured primary monkey cortical neurons began to show apparent neurites after 3 days. On day 7, the neurite development of primary monkey cortical neurons was comparable to that of primary mouse cortical neurons on day 3. We also calculated the neurite lengths of the cultured cortical neurons. Cortical neurons were considered to begin to die when their neurite lengths began to shorten. We found that many of the cultured primary mouse cortical neurons started to die by day 14 and disappeared by day 21, while the monkey cortical neurons remained healthy and established noticeable neurite connections (Figure 1A). Quantification of neurite length confirmed that the cultured monkey cortical neurons continued to develop long neurites after day 21 (Figure 1B).
Figure 1.
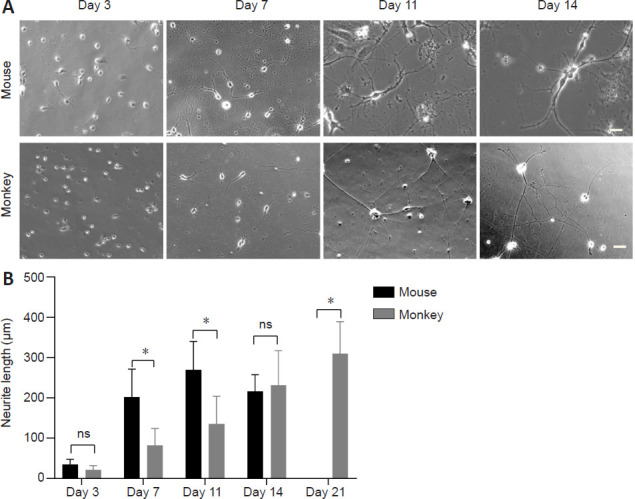
Comparison of growth between cultured mouse and monkey cortical neurons.
(A) Phase-contrast images (Zeiss Axio Observer A1 microscope system) of cultured mouse and monkey cortical neurons on different days in culture. Scale bars: 20 μm. (B) Quantitation of neurite length of the cultured cortical neurons. The cultured mouse cortical neurons died after day 14 in the absence of glial cells. The data were obtained by counting 51 cortical neurons per group. *P < 0.05. Data are presented as the mean ± SEM (two-way analysis of variance followed by Tukey's post hoc test) from three independent experiments (n = 3). ns: Not significant.
Immunocytochemistry and morphometric analysis of neuronal cells
To clearly observe the morphology of neuronal cells, cell culture medium was used to inhibit the growth of glial cells. To label cortical neurons, we performed immunofluorescence double staining with antibodies against β-tubulin III, a microtubule element of the tubulin family that is found almost exclusively in cortical neurons. To label glial cells, we used antibodies against GFAP, an astrocytic cell marker. Neuronal cells displayed long neurites or axons that were clearly stained by anti-β-tubulin III. Immunofluorescent staining revealed that very few astrocytes were labeled by anti-GFAP staining, while the majority of cells were labeled by anti-β-tubulin III, confirming that these were neuronal cells (as indicated by the phase-contrast images). Immunofluorescence staining of the cortical neurons on different days also revealed that cultured mouse cortical neurons started to die after day 14 (Figure 2A). In contrast, cultured monkey cortical neurons continued to develop long neurites after day 14 (Figure 2A and B). The elongation of neurites and the formation of neurite connections were sustained until day 81 (Figure 2B).
Figure 2.
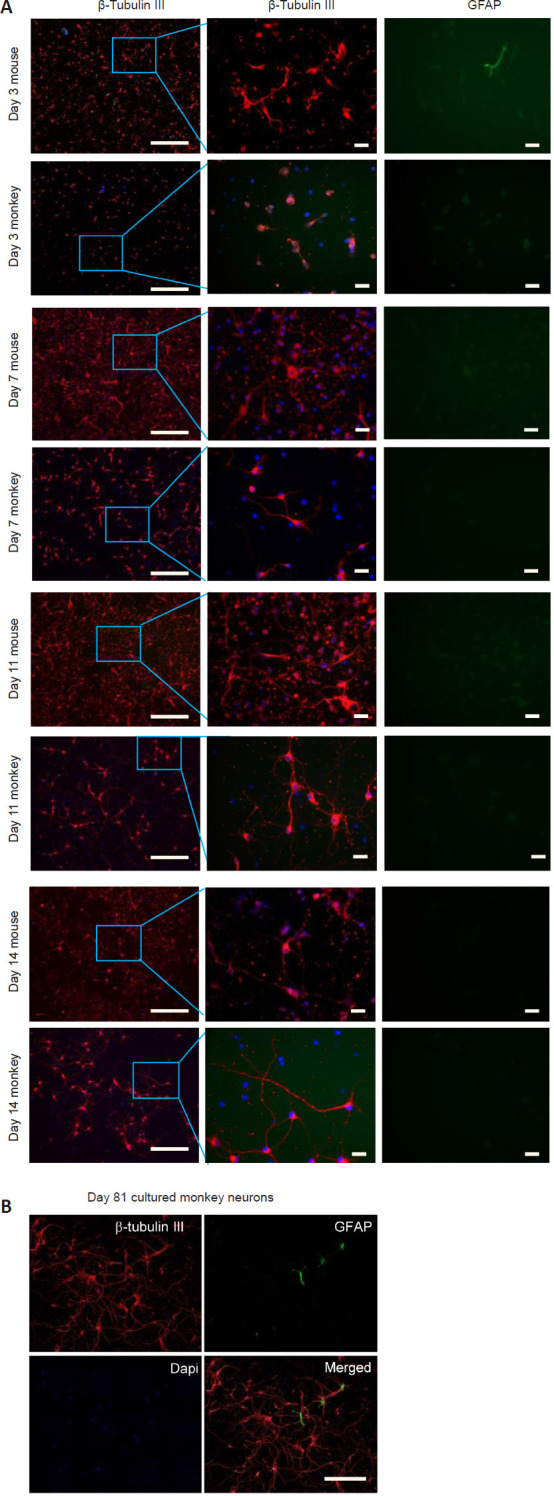
Immunofluorescence staining of cultured mouse and monkey cortical neurons at different time points.
(A) Double immunofluorescence staining of cultured mouse and monkey cortical neurons, using antibodies against GFAP and β-tubulin III, on days 3, 7, 11, and 14 (left column: magnification 10×, scale bars: 200 μm; middle and right columns: magnification 40×, scale bars: 20 μm; taken using a Zeiss Axio Observer A1 microscope system). The blue color indicates DAPI. (B) Double immunofluorescence staining of cultured monkey cortical neurons, using antibodies against GFAP and β-tubulin III, on day 81 (taken using a Zeiss LSM880 microscope system). Scale bar: 200 μm. DAPI: 4′,6-Diamidino-2-phenylindole; Gfap: glial fibrillary acidic protein.
Analysis of the electrophysiological properties of neuronal cultures
The MEA platform was used to assess the electrophysiological properties of neuronal cultures. Using the MEA system, the field potentials of monkey or mouse cortical neurons cultured in a 48-well dish were detected (Figure 3A). In Figure 3B, each square represents one well in the MEA electrode plate. There were many electrodes at the bottom of each well. (Figure 3C) represents a raster image of a single electrode at a certain time (within 30 minutes of the same time every day), in which one vertical line represents a spike. The number of spikes divided by the detection time is the firing rate of the cortical neurons, and was used to analyze the neuronal activity. Figure 3D represents raster images of a single well over a period of time (within 30 minutes of the same time every day), in which one vertical line represents a spike of one single discharge from a neuron.
Figure 3.
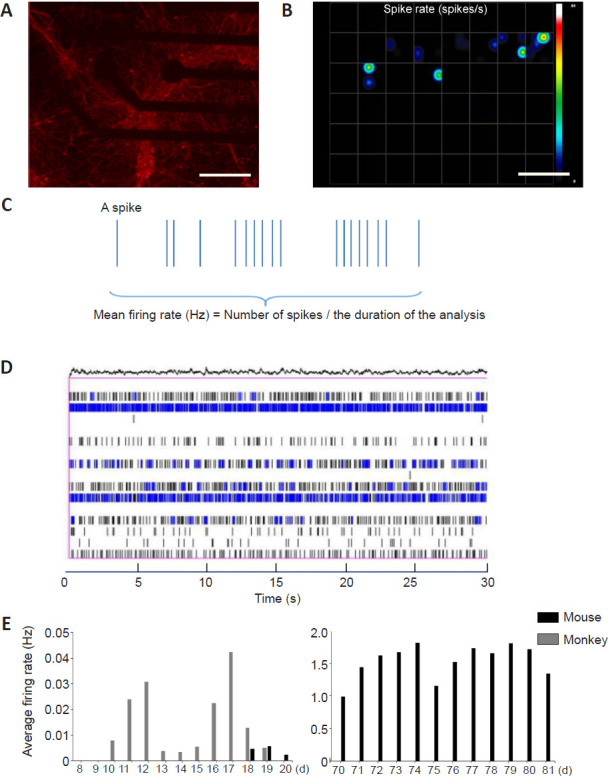
MEA analysis of the electrical activities of cultured neuronal cells.
(A) Image of cultured neuronal cells labeled by anti-β-tubulin III in a 48-well plate for MEA analysis (taken using a Zeiss Axio Observer A1 microscope system); the red fluorescence indicates cortical neurons. Scale bar: 200 μm. (B) Dynamic heat map analyzed by MEA. Each grid is an electrode hole in which cortical neurons were cultured. The colored dots in the figure represent the spike rate (spikes/time). The color indicator axis is on the right side; brighter colors represent higher discharge frequencies. Scale bar: 200 μm. (C) Raster image of a single electrode showing spikes over a period of time. (D) Raster image of spikes in a single cell over a period of time (30 seconds). (E) The average discharge frequency of cultured mouse and monkey cortical neurons from day 8 to day 20 (left) and from day 70 to day 81 (right). The absence of any discharge from the cultured mouse cortical neurons from day 19 to day 69 is because of their death in culture. Data were obtained by counting eight electrode holes per group. Axon MEA software was used for the data analysis.
Starting from day 8 of culture, we performed the MEA analysis of cultured mouse and monkey cortical neurons for 13 days. Quantification of the discharge frequency, which reflects the electrical activity, of cultured cortical neurons on different days revealed that cultured mouse cortical neurons began to emit electrical signals between day 9 and day 11, and that 50% of cortical neurons began to discharge on day 10. Cultured monkey cortical neurons showed electrical signals on days 18 and 20, with 50% of cortical neurons discharging on day 18 (Figure 3E). Because the cultured monkey cortical neurons survived for a longer time than the cultured mouse cortical neurons, we measured their MEA activity after 70 days in vitro. On days 70-81, the monkey cortical neurons were still able to discharge robustly at frequencies between 0.99-1.82 Hz (Figure 3E). Thus, cultured monkey cortical neurons become mature more slowly but retain electrical activity for a much longer time than cultured mouse cortical neurons.
Mutant HTT-mediated degeneration of cultured monkey and mouse cortical neurons
Cultured neuronal cells have been used to establish various cellular models of neurodegenerative diseases (D'Aiuto et al., 2018). To investigate whether cultured monkey cortical neurons show similar or different pathological events compared with cultured rodent cortical neurons, we used AAV vectors expressing N-terminal mutant HTT (amino acids 1-230) containing either 23 (control) or 109 (mutant) glutamines. We introduced either a mutated gene or a control fragment into cortical neurons via viral infection. Using this method, the effects of toxic proteins on cortical neurons can be identified, thus indicating the effectiveness of the neuron model in vitro. Using viral vectors to express mutant HTT, we revealed that cultured mouse cortical neurons were unable to survive for more than 7 days after infection, suggesting that the overexpression of mutant HTT can further reduce the relatively short survival time of cultured mouse cortical neurons. In contrast, when cultured monkey cortical neurons were infected with the same AAV vector on day 6, they were able to survive for more than 2 weeks. On day 21, immunofluorescence staining of the cultured monkey cortical neurons was performed using antibodies against anti-β-tubulin III (Wang et al., 2008a). The overexpression of control HTT (with a normal repeat; 23Q) did not affect neuronal survival; the AAV HTT-23Q-infected cortical neurons maintained elongated neurites that were clearly labeled by anti-β-tubulin III. In contrast, mutant HTT (109Q) overexpression led to the formation of fragmented neurites (Figure 4A and B). A quantitative analysis of the cultured mouse cortical neurons also confirmed that mutant HTT (109Q) significantly reduced the neurite length (Figure 4C). However, staining the soma of cultured monkey cortical neurons did not reveal any significant differences in the number or size of neuronal cells (Figure 4C). This result was similar to that observed in the monkey neuronal cells (Figure 4D).
Figure 4.
Degeneration of cultured cortical neurons overexpressing mutant HTT.
(A) AAV vector expressing N-terminal HTT. (B) Cultured monkey and mouse cortical neurons on day 6 were infected with AAV vectors expressing N-terminal HTT with 23Q (HTT-23Q) or 109Q (HTT-109Q). The infected cells were then examined on day 21 (mouse cortical neurons were examined on day 10). Cortical neurons were labeled by anti-β-tubulin III antibody (green) and the nuclei of cortical neurons were labeled by DAPI (blue). Cultured cortical neurons expressing mutant HTT (HTT-109Q) showed fragmentation of neurites (arrows). Images were taken using a Zeiss Axio Observer A1 microscope system (40×). Scale bars: 20 μm. (C) A quantitative analysis of neurite length and cell body size of cultured mouse cortical neurons revealed that HTT-109Q significantly reduced the neurite length. (D) Quantification of neurite length and neuronal cell body size of monkey cortical neurons. The data were obtained by counting 16 cortical neurons per group, and are presented as the mean ± SEM. *P < 0.05 (Student's t-test). Average of three independent experiments (n = 3). CMV: Cytomegalovirus promoter; dsRED: Discosoma sp. Red; ITR: inverted terminal repeat; HTT: huntingtin; ns: not significant.
For cultured mouse cortical neurons that were infected with AAV for 4 days, mutant HTT also formed aggregates and caused neurite fragmentation (Figure 4B). However, although the infected mouse cortical neurons showed similar morphological changes to the infected monkey cortical neurons, they only lived for 6–7 days after overexpressing mutant HTT. Thus, the use of cultured monkey cortical neurons allows pathological changes to be observed over a longer period of time compared with cultured mouse cortical neurons.
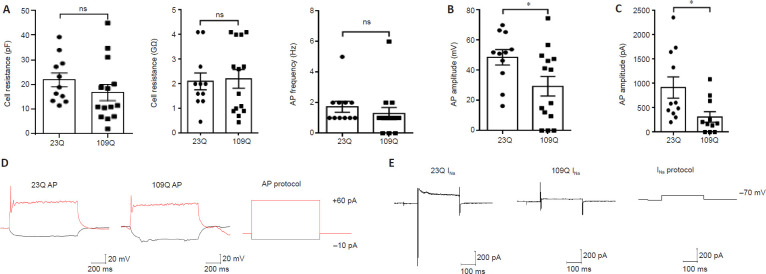
The electrophysiological properties of the cultured monkey cortical neurons expressing HTT-23Q or HTT-109Q were also tested. Although both groups had the same maturity (Figure 5A), their action potential amplitudes (Figure 5B) and sodium current amplitudes (Figure 5C) were significantly different. The action potential amplitude was related to calcium ion channels, and the sodium current amplitude was related to sodium ion channels. Both amplitudes indicated that the neuronal activity of HTT-109Q-expressing cells was lower than that of the HTT-23Q-expressing cells. This was represented visually by the action potential peak (Figure 5D) and sodium current peak (Figure 5E).
Figure 5.
Electrophysiological activities of cultured monkey cortical neurons expressing HTT-23Q or HTT-109Q.
(A) There were similar electrophysiological activities (cell resistance [pF and GΩ] and AP frequency [Hz]) in HTT-23Q- and HTT-109Q-expressing cortical neurons. (B) The action potential amplitude of HTT-109Q-expressing cortical neurons was significantly lower than that of HTT-23Q-expressing cortical neurons. (C) The sodium current amplitude of HTT-109Q-expressing cortical neurons was significantly lower than that of HTT-23Q-expressing cortical neurons. The data were obtained by counting 11–15 cortical neurons per group, and are presented as the mean ± SEM. *P < 0.05 (Student's t-test). Average of three independent experiments (n = 3). (D, E) Voltage-clamp analysis of action potentials (D) and sodium currents (E) revealed that sodium currents in HTT-109Q-expressing cortical neurons were lower than that those of HTT-23Q-expressing cortical neurons. Horizontal axis: time; vertical axis: voltage (D) and current (E). AP: Action potential; ms: millisecond; mV: millivolt; ns: not significant; pA: picoampere.
As a result, although the action potential peak (Figure 5D) was not significantly different between HTT-23Q- and HTT-109Q-expressing cortical neurons, the sodium current (Figure 5E) of HTT-109Q-expressing cortical neurons was markedly affected compared with HTT-23Q-expressing cortical neurons.
Discussion
Our experiments revealed that cultured mouse and monkey cortical neurons have significantly different development in vitro. Cultured mouse cortical neurons matured more rapidly than cultured monkey cortical neurons. In support of this idea, cultured mouse cortical neurons also displayed electrical activities much earlier than cultured monkey cortical neurons. These important differences offer valuable information when considering cultured neuronal cells as a model for investigating neuronal function and disease pathogenesis.
In our study, the neuronal cultures were deprived of glial cells by inhibiting the growth of such cells. It is known that the presence of glial cells can prolong the survival of neuronal cells in culture (Lange et al., 2018). However, the overgrowth of glial cells may also inhibit neuronal cell development and confound the investigation of neuronal differentiation and maturation. In the absence of glial cells, significant differences in the development and maturation of cultured mouse and monkey cortical neurons were able to be clearly observed. The differences noted in our studies appear to be consistent with the species-dependent features of cortical neurons in vivo. In non-human primate brains, it takes a much longer time for neuronal cells to mature and develop connections with other types of cells compared with rodent brains (Popova et al., 2017). Thus, although the neuronal cells were isolated for culture, our study suggests that the species-specific features of genetics and other physiological functions intrinsically determine the development, differentiation, and maturation of neuronal cells in vitro.
Species-dependent differences in cultured cortical neurons should be considered when primary cultured cortical neurons are used for an investigation. Cultured rodent cortical neurons provide considerable advantages over cultured non-human primate cortical neurons for a number of reasons. First, they are easily obtained from mice that can be bred fast at a relatively low cost. Second, cultured mouse cortical neurons have been well characterized, and many tools and reagents for studying mouse cortical neurons are available. Third, mouse cortical neurons show similar fundamental physiological functions to those observed in human cortical neurons. In contrast to the easy accessibility of cultured mouse cortical neurons, cultured non-human primate cortical neurons require the use of pregnant monkeys, which come at a much higher cost and are more time-consuming. However, the advantage of using cultured monkey cortical neurons is also clear; their development and maturation are closer to those of human cortical neurons in the brain (Buckner and Margulies, 2019). Thus, any neuronal functions or pathological changes observed in cultured monkey cortical neurons are likely to be more similar to those that occur in human cortical neurons (Insausti, 2013).
Cultured monkey cortical neurons can survive in vitro for a much longer time than cultured mouse cortical neurons, and this prolonged period of survival offers at least two important advantages. First, it gives a wider window of time to investigate how pathological events occur and progress when cortical neurons are under pathological conditions or express disease-related proteins. In our study, mutant HTT-expressing cultured monkey cortical neurons survived for a longer time than cultured mouse cortical neurons. Because mutant HTT infection can kill cultured mouse cortical neurons in a short period of time (Saudou et al., 1998; Wang et al., 2008b; Kaemmerer and Grondin, 2019), it is reasonable to expect that the short lifespan and rapid maturation of mouse cortical neurons render them more susceptible to the toxicity of overexpressed disease proteins. Second, cultured monkey cortical neurons are likely to be an important model for examining the therapeutic effects of genetic manipulations or drug treatments because they can live longer in vitro, which is useful for time-course studies.
To further enhance our understanding of the functional differences between mouse and monkey cortical neurons, the MEA technique (Segev et al., 2001) was used to detect the field potentials of mouse and monkey cortical neurons at different culture times (Guo, 2020). This method was used because electrophysiological function is the most fundamental function of cortical neurons, and may help to explain actual physiological activity of neurons. The detection and analysis of electrophysiological signals revealed a trend that was consistent with the growth process; that is, the primary cortical neurons of fetal mice grew rapidly, but the life cycle was short. In contrast, the primary cortical neurons of fetal monkeys grew slowly, but had a long cycle. Based on these comparisons, fetal monkey cortical neurons are therefore more suitable than fetal mouse cortical neurons for the establishment of human neuropathy-related models.
Because of the growth and dominance of fetal monkey cortical neurons, we explored the characteristics of a human disease, HD, using fetal monkey cortical neurons. We successfully established 23Q and 109Q HTT transgenic monkey cortical neurons and found that, similar to in human HD patients, there were abnormal HTT aggregates, and axon length was significantly shorter than that of controls. These findings suggest that the accumulation of aggregates in cortical neurons may indeed lead to a series of pathological changes in cortical neurons. This provides a good explanation for the pathogenesis of HD at the level of cortical neurons, and also provides a cellular basis for various treatments.
However, there are some limitations to our study. First, it remains important to verify the electrophysiological differences between cultured mouse and monkey neurons by conducting in vivo experiments. Second, it is difficult to acquire monkey fetal tissue for in vitro cultures of primary neurons. In addition, the culture conditions of primary monkey neurons are strict, and the cells need to be well maintained in vitro for their prolonged survival. However, despite these limitations, the prolonged survival period of cultured monkey neurons will allow the investigation of the mechanisms of time-dependent neurodegeneration, as well as of neuronal damage repair by regeneration.
The use of human-derived cells is advantageous in that it can be used to directly analyze a specific patient's genes. Furthermore, results from human-derived samples are closer to what occurs in the clinical situation, and are therefore more favorable for drug screening. However, ethical concerns and sample availability limit the research scope of human-derived samples for investigating neurodegenerative diseases. In the present study, we demonstrated that, compared with mouse-derived cell models, a monkey-derived cell model of Huntington's disease more closely reflects the pathological process that occurs in human patients. For the monkey model, it is possible to obtain fresh fetal brain tissue from specific days for primary neuronal cultures, and the culture results are relatively stable; in contrast, for human samples, we can only obtain aborted fetal tissue, which may affect neurodevelopment.
In our study, we first set up in vitro culture systems of primary cortical neurons from mice and monkeys, and then confirmed the general activity and purity of neuron growth. The in vitro culture system was used to compare the development of cortical neurons from a range of different perspectives, such as morphology, molecular biology, and electrophysiology (using the MEA technique). These comparisons confirmed that the primary cortical neurons of fetal monkeys were more capable of simulating human cortical neuron development in an in vitro culture system. The in vitro development, maturation, survival, and electrophysiological function maintenance of monkey nerve cells lasted longer compared with mouse nerve cells. Finally, we infected cortical neurons with mutant HTT to explore the advantages of using fetal monkey cortical neurons to establish a human disease model; our results provide a basis for the study of other neurological diseases, and especially neurodegenerative diseases.
Acknowledgments:
We are thankful to Axion BioSystems Company (Atlanta, USA) which provided us with the multi-electrode array (MEA) platform (Maestro Pro MEA system) and corresponding technical guidance.
Footnotes
C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Gardner B, Song LP; T-Editor: Jia Y
Conflicts of interest:The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support:This work was supported by the National Natural Science Foundation of China, No. 81922026 (to SY); the National Key Research and Development Program of China Stem Cell and Translational Research, No. 2017YFA0105104 (to SY); Key Field Research and Development Program of Guangdong Province, No. 2018B030337001 (to XJL); Guangdong Key Laboratory of Non-human Primate Models of Brain Diseases, No. 2020B121201006 (to XJL); Guangzhou Key Research Program on Brain Science, No. 202007030008 (to SY); the Fundamental Research Funds for the Central Universities, 21619104 (to SY). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:All mouse experiments and protocols were approved by the Animal Care and Use Committee of Jinan University of China (IACUC Approval No. 20200512-04) on May 12, 2020. All monkey experiments were approved by the IACUC protocol (IACUC Approval No. LDACU 20190820-01) on August 23, 2019 for animal management and use. The experimental procedures followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Funding:This work was supported by the National Natural Science Foundation of China, No. 81922026 (to SY); the National Key Research and Development Program of China Stem Cell and Translational Research, No. 2017YFA0105104 (to SY); Key Field Research and Development Program of Guangdong Province, No. 2018B030337001 (to XJL); Guangdong Key Laboratory of Non-human Primate Models of Brain Diseases, No. 2020B121201006 (to XJL); Guangzhou Key Research Program on Brain Science, No. 202007030008 (to SY); the Fundamental Research Funds for the Central Universities, No. 21619104 (to SY).
References
- 1.Adegbuyiro A, Sedighi F, Pilkington AWt, Groover S, Legleiter J. Proteins containing expanded polyglutamine tracts and neurodegenerative disease. Biochemistry. 2017;56:1199–1217. doi: 10.1021/acs.biochem.6b00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardy C, van den Hurk M, Eames T, Marchand C, Hernandez RV, Kellogg M, Gorris M, Galet B, Palomares V, Brown J, Bang AG, Mertens J, Bohnke L, Boyer L, Simon S, Gage FH. Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro. Proc Natl Acad Sci U S A. 2015;112:E2725–2734. doi: 10.1073/pnas.1504393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bateup HS, Johnson CA, Denefrio CL, Saulnier JL, Kornacker K, Sabatini BL. Excitatory/inhibitory synaptic imbalance leads to hippocampal hyperexcitability in mouse models of tuberous sclerosis. Neuron. 2013;78:510–522. doi: 10.1016/j.neuron.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckner RL, Margulies DS. Macroscale cortical organization and a default-like apex transmodal network in the marmoset monkey. Nat Commun. 2019;10:1976. doi: 10.1038/s41467-019-09812-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen ST, Lai WJ, Zhang WJ, Chen QP, Zhou LB, So KF, Shi LL. Insulin-like growth factor 1 partially rescues early developmental defects caused by SHANK2 knockdown in human neurons. Neural Regen Res. 2020;15:2335–2343. doi: 10.4103/1673-5374.285002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi WS, Kim HW, Xia Z. Preparation of primary cultured dopaminergic neurons from mouse brain. Methods Mol Biol. 2013;1018:61–69. doi: 10.1007/978-1-62703-444-9_6. [DOI] [PubMed] [Google Scholar]
- 7.D'Aiuto L, Naciri J, Radio N, Tekur S, Clayton D, Apodaca G, Di Maio R, Zhi Y, Dimitrion P, Piazza P, Demers M, Wood J, Chu C, Callio J, McClain L, Yolken R, McNulty J, Kinchington P, Bloom D, Nimgaonkar V. Generation of three-dimensional human neuronal cultures: application to modeling CNS viral infections. Stem Cell Res Ther. 2018;9:134. doi: 10.1186/s13287-018-0881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo L. Principles of functional neural mapping using an intracortical ultra-density microelectrode array (ultra-density MEA) J Neural Eng. 2020;17:036018. doi: 10.1088/1741-2552/ab8fc5. [DOI] [PubMed] [Google Scholar]
- 9.Insausti R. Comparative neuroanatomical parcellation of the human and nonhuman primate temporal pole. J Comp Neurol. 2013;521:4163–4176. doi: 10.1002/cne.23431. [DOI] [PubMed] [Google Scholar]
- 10.Kaemmerer WF, Grondin RC. The effects of huntingtin-lowering: what do we know so far. Degener Neurol Neuromuscul Dis. 2019;9:3–17. doi: 10.2147/DNND.S163808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lange J, Haslett LJ, Lloyd-Evans E, Pocock JM, Sands MS, Williams BP, Cooper JD. Compromised astrocyte function and survival negatively impact neurons in infantile neuronal ceroid lipofuscinosis. Acta Neuropathol Commun. 2018;6:74. doi: 10.1186/s40478-018-0575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu N, Zhang XL, Jiang SY, Shi JH, Cui JH, Liu XL, Han LH, Gong KR, Yan SC, Xie W, Zhang CY, Shao G. Neuroprotective mechanisms of DNA methyltransferase in a mouse hippocampal neuronal cell line after hypoxic preconditioning. Neural Regen Res. 2020;15:2362–2368. doi: 10.4103/1673-5374.285003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamichi N, Matsumoto Y, Kawanishi T, Ishimoto T, Masuo Y, Horikawa M, Kato Y. Maturational characterization of mouse cortical neurons three-dimensionally cultured in functional polymer FP001-containing medium. Biol Pharm Bull. 2019;42:1545–1553. doi: 10.1248/bpb.b19-00307. [DOI] [PubMed] [Google Scholar]
- 14.Negishi T, Ishii Y, Kawamura S, Kuroda Y, Yoshikawa Y. Cryopreservation and primary culture of cerebral neurons from cynomolgus monkeys (Macaca fascicularis) Neurosci Lett. 2002;328:21–24. doi: 10.1016/s0304-3940(02)00433-0. [DOI] [PubMed] [Google Scholar]
- 15.Neueder A, Landles C, Ghosh R, Howland D, Myers RH, Faull RLM, Tabrizi SJ, Bates GP. The pathogenic exon 1 HTT protein is produced by incomplete splicing in Huntington's disease patients. Sci Rep. 2017;7:1307. doi: 10.1038/s41598-017-01510-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obien ME, Deligkaris K, Bullmann T, Bakkum DJ, Frey U. Revealing neuronal function through microelectrode array recordings. Front Neurosci. 2014;8:423. doi: 10.3389/fnins.2014.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otani T, Marchetto MC, Gage FH, Simons BD, Livesey FJ. 2D and 3D stem cell models of primate cortical development identify species-specific differences in progenitor behavior contributing to brain size. Cell Stem Cell. 2016;18:467–480. doi: 10.1016/j.stem.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popova D, Karlsson J, Jacobsson SOP. Comparison of neurons derived from mouse P19, rat PC12 and human SH-SY5Y cells in the assessment of chemical- and toxin-induced neurotoxicity. BMC Pharmacol Toxicol. 2017;18:42. doi: 10.1186/s40360-017-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy PH, Yin X, Manczak M, Kumar S, Pradeepkiran JA, Vijayan M, Reddy AP. Mutant APP and amyloid beta-induced defective autophagy, mitophagy, mitochondrial structural and functional changes and synaptic damage in hippocampal neurons from Alzheimer's disease. Hum Mol Genet. 2018;27:2502–2516. doi: 10.1093/hmg/ddy154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahin M, Oncu G, Yilmaz MA, Ozkan D, Saybasili H. Transformation of SH-SY5Y cell line into neuron-like cells: Investigation of electrophysiological and biomechanical changes. Neurosci Lett. 2021;745:135628. doi: 10.1016/j.neulet.2021.135628. [DOI] [PubMed] [Google Scholar]
- 21.Saudou F, Finkbeiner S, Devys D, Greenberg ME. Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell. 1998;95:55–66. doi: 10.1016/s0092-8674(00)81782-1. [DOI] [PubMed] [Google Scholar]
- 22.Segev R, Shapira Y, Benveniste M, Ben-Jacob E. Observations and modeling of synchronized bursting in two-dimensional neural networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2001;64:011920. doi: 10.1103/PhysRevE.64.011920. [DOI] [PubMed] [Google Scholar]
- 23.Slavik J, Skopalik J, Provaznik I, Hubalek J. Multi-electrode array with a planar surface for cell patterning by microprinting. Sensors (Basel) 2019;19:5379. doi: 10.3390/s19245379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sokolov AM, Seluzicki CM, Morton MC, Feliciano DM. Dendrite growth and the effect of ectopic Rheb expression on cortical neurons. Neurosci Lett. 2018;671:140–147. doi: 10.1016/j.neulet.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spira ME, Hai A. Multi-electrode array technologies for neuroscience and cardiology. Nat Nanotechnol. 2013;8:83–94. doi: 10.1038/nnano.2012.265. [DOI] [PubMed] [Google Scholar]
- 26.Tabrizi SJ, Leavitt BR, Landwehrmeyer GB, Wild EJ, Saft C, Barker RA, Blair NF, Craufurd D, Priller J, Rickards H, Rosser A, Kordasiewicz HB, Czech C, Swayze EE, Norris DA, Baumann T, Gerlach I, Schobel SA, Paz E, Smith AV, Bennett CF, Lane RM Phase 1-2a IONIS-HTTRx Study Site Teams. Targeting huntingtin expression in patients with Huntington's disease. N Engl J Med. 2019;380:2307–2316. doi: 10.1056/NEJMoa1900907. [DOI] [PubMed] [Google Scholar]
- 27.Taran AS, Shuvalova LD, Lagarkova MA, Alieva IB. Huntington's disease-an outlook on the interplay of the HTT protein, microtubules and actin cytoskeletal components. Cells. 2020;9:1514. doi: 10.3390/cells9061514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang CE, Zhou H, McGuire JR, Cerullo V, Lee B, Li SH, Li XJ. Suppression of neuropil aggregates and neurological symptoms by an intracellular antibody implicates the cytoplasmic toxicity of mutant huntingtin. J Cell Biol. 2008a;181:803–816. doi: 10.1083/jcb.200710158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang CE, Tydlacka S, Orr AL, Yang SH, Graham RK, Hayden MR, Li S, Chan AW, Li XJ. Accumulation of N-terminal mutant huntingtin in mouse and monkey models implicated as a pathogenic mechanism in Huntington's disease. Hum Mol Genet. 2008b;17:2738–2751. doi: 10.1093/hmg/ddn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong LZ, Wang Q, Liu MY, Peng Y, Li QB, Lu ZH, Lei C. Shenfu injection attenuates neurotoxicity of bupivacaine in cultured mouse spinal cord neurons. Chin Med J (Engl) 2007;120:1958–1962. [PubMed] [Google Scholar]
- 31.Yan S, Tu Z, Liu Z, Fan N, Yang H, Yang S, Yang W, Zhao Y, Ouyang Z, Lai C, Yang H, Li L, Liu Q, Shi H, Xu G, Zhao H, Wei H, Pei Z, Li S, Lai L, et al. A Huntingtin knockin pig model recapitulates features of selective neurodegeneration in Huntington's disease. Cell. 2018;173:989–1002. doi: 10.1016/j.cell.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang D, Wang CE, Zhao B, Li W, Ouyang Z, Liu Z, Yang H, Fan P, O'Neill A, Gu W, Yi H, Li S, Lai L, Li XJ. Expression of Huntington's disease protein results in apoptotic neurons in the brains of cloned transgenic pigs. Hum Mol Genet. 2010;19:3983–3994. doi: 10.1093/hmg/ddq313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang H, Yang S, Jing L, Huang L, Chen L, Zhao X, Yang W, Pan Y, Yin P, Qin ZS, Tang B, Li S, Li XJ. Truncation of mutant huntingtin in knock-in mice demonstrates exon1 huntingtin is a key pathogenic form. Nat Commun. 2020;11:2582. doi: 10.1038/s41467-020-16318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]