Abstract
In an attempt to establish a biosynthetic route towards isobutene, we faced the problem that the first intermediate isobutyl-monophosphate was not commercially available. In order to overcome this limitation we searched in the literature for protocols reporting the synthesis of phosphate monoesters from alcohols. Based on the suitability of the preceding developments for our purposes, we established a customized method for the fast, easy and affordable generation of the pursued molecule. Herein, a prompt and straightforward method for isobutyl-monophosphate (ammonium salt) is provided.
-
•
This is a customized method for the production of isobutyl-monophosphate (ammonium salt), using isobutanol as starting compound
-
•
Synthesis takes place in a one-pot fashion, under mild reaction conditions, in 2 h
-
•
The established sequential strategy requires 8 h at the most, including synthesis and purification steps to obtain the isolated product
Keywords: Monophosphorylation, Phosphate monoesters, Phosphate activation, 31P-NMR, One-pot synthesis
Graphical abstract
Specifications table
| Subject Area: | Chemistry |
| More specific subject area: | Organic chemistry |
| Method name: | Synthesis of isobutyl monophosphate (ammonium salt) |
| Name and reference of original method: | Lira, L. M. et al. (2013). One-pot synthesis of organophosphate monoesters from alcohols. Tetrahedron Letters. 54(13), 1690–1692. https://doi:10.1016/J.TETLET.2013.01.059 |
| Resource availability: | The available data is provided in the text and in the supplementary material related to this article |
Background
A straightforward method for the preparation of phosphate monoesters directly from alcohols was described in the reference method [1]. In such work, authors made a wise selection of preceding synthesis and purification strategies enabling good isolated product yields for 11 different phosphate monoesters, employing alcohols as starting materials. Authors proposed a mechanism for the formation of the phosphate monoester using trichloroacetonitrile and dihydrogenphosphate via a reactive mixed anhydride/phosphor group activation.
-
•
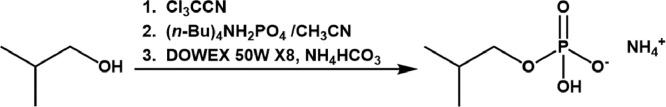
In this work, we customized the original method in order to produce substantial amounts of isobutyl-monophosphate (ammonium salt), (Fig. 1).
-
•
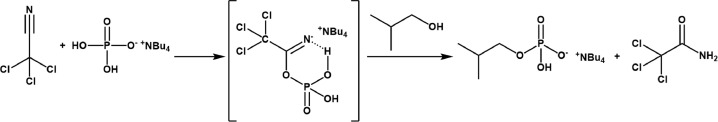
We assumed that the mechanism scope described in the original method could particularly work to synthetize isobutyl-monophosphate (ammonium salt), (Fig. 2).
-
•
Despite isobutyl-monophosphate biosynthesis is feasible via an enzymatic reaction (Fig. 3), as described in [2], the yield is very low. However, we employed this approach to obtain a reference molecule to direct the results shown in this work.
-
•
The positive results encouraged the development of an efficient working pipeline envisaged not only to save time but also to establish a friendly set-up for further users. Following the working pipeline shown in Fig. 4, the isolated product can be obtained in approximately 8 h.
Fig. 1.
Customized phosphorylation procedure for isobutyl monophosphate (ammonium salt) synthesis according to the original method [1].
Fig. 2.
Proposed mechanism for the formation of the phosphate monoester in the original method [1]. The foreseen mechanism implies using trichloroacetonitrile and dihydrogenphosphate to give place to a reactive mixed anhydride and phosphor group activation. In this work, it was assumed particularly for isobutyl-monophosphate.
Fig. 3.
Isobutyl-monophosphate biosynthesis via enzymatic reaction according to [2].
Fig. 4.
Pipeline for the customized method for isobutanol-monophosphate production (ammonium salt).
Method
Reagents and standard
Chemicals and solvents used in this work were obtained at the highest purity degree available from Sigma-Aldrich (St. Louis, US) and Carl Roth (Karlsruhe, DE). We used a home-made biosynthesized isobutyl monophosphate as standard, the biosynthesis was perform as previously described [2].
-
1.Synthesis strategy
-
▪Prepare solution A made of isobutanol (2 mmol) in acetonitrile (2 mL)
-
▪Transfer A to a two-necked flask TNF containing a magnetic stirrer
-
▪Add trichloroacetonitrile (4.8 mmol) into the TNF
-
▪Prepare solution B, made of tetrabutylammonium dihydrogenphosphate (4.0 mmol) in acetonitrile (10 mL)
-
▪By dropwise addition, transfer the total volume of solution B to the TNF
-
▪Stir the resulting reaction mixture RM at room temperature for two hours to ensure that the starting material is consumed
-
▪Afterwards, transfer the RM to a rotary evaporator flask and remove the solvent in vacuo to obtain the crude product 1 CP1
-
▪
-
2.
Purification strategy
2.1 Flash column
-
▪
Prepare mobile phase MP (isopropanol/ aq. NH4OH (30%)/H2O 7:2:1)
-
▪
Prepare a silica gel suspension in the MP
-
▪
Pack a column with silica suspension (20 cm tall and 1 cm in diameter)
-
▪
Suspend the CP1 in the MP
-
▪
Apply the CP1 in the column
-
▪
Elute the column with the MP and collect 5 mL fractions
-
▪
Spot 5 µL of each fraction on TLC-silica strips. There is no need to elute the TLCs, since the aim is only to detect the fractions showing the presence of phosphates
-
▪
Stain directly the TLC strips with molybdate reagent, let them dry and heat them at 70 °C
-
▪
In a rotary evaporator flask, combine all positive fractions for phosphates
-
▪
Remove the solvent in vacuo to obtain the crude product 2 CP2
2.2 DOWEX percolation
-
▪
Prepare percolation solution PS (NH4OH/H2O 3:1) and the buffer solution BS (0.025 M NH4HCO3)
-
▪
Pack a column with Dowex 50WX8 ion-exchange powder (8 cm tall and 1 cm diameter)
-
▪
Equilibrate the column with PS
-
▪
Flush the column with BS up to alkalinize it at pH 8.0
-
▪
Suspend CP2 in PS
-
▪
Apply the CP2 in the column
-
▪
Perform the percolation step with PS
-
▪
Collect the resulting ammonium organophosphate in a rotary evaporator flask
-
▪
Remove the solvent in vacuo, the obtained dry isolated product appears as a white solid
Structural confirmation by 1H-, 13C-, and 31P-NMR
To verify the structural characteristics of the synthetized Isobutyl-monophosphate, 1H-, 13C-, and 31P- nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance 500 spectrometer operating at 500.15, 125.76, and 202.46 MHz respectively. Spectra were recorded using D2O as solvent at room temperature. The chemical shifts (δ) are reported in parts per million (ppm) relative to the standard tetramethylsilane (TMS, δ = 0). For 31P-NMR H3PO4 was used as standard. Initially, the 31P-NMR spectra of the chemically synthetized Isobutyl-monophosphate (ammonium salt) was compared versus the standard generated via biosynthesis Fig. S1. Followed by the additional structural characterization by 1H-, 13C-, and 31P-NMR, to confirm the identity of the molecule, Figs. S2, S3, and S4, respectively.
Isobutyl-monophosphate (110.14 mg, 35.11%, white solid), synthetized with the method described herein. 1H-NMR (D2O, 500.15 MHz) δ = 3.45 (m, 2 H), 1.73 (m, 1 H), 0.78 (d, 6 H) ppm (Fig. S2).13C NMR (D2O, 125.76 MHz) δ = 71.51, 28.70, 18.25 ppm (Fig. S3). 31P-NMR (D2O, 202.46 MHz) δ = 3.68 ppm (Fig. S4). Chemical shifts are in agreement with previously reported NMR data [3].
Structural verification by LC-MS
To complement the structural characterization of the synthetized product, a HPLC-ESI-MS method was established for Isobutyl-monophosphate ion detection. An Agilent 6130 Quadrupole LC System, Santa Clara, US was operated, isothermally at 45 °C. Measurements were run at a flow rate of 1 mL min−1. The mobile phase was acetonitrile/ammonium carbonate (85:15, v/v) with isocratic elution. Sample concentration was 1 mM. Injection volume was 1 µL. The ESI-MS was run using the following parameters: API-ES spray chamber with a drying gas flow of 12 L min−1, nebulizer pressure of 35 psig, drying gas temperature 350 °C and a capillary voltage of 4000 V positive and negative. Mass detection was performed in negative SCAN mode from 50 to 400 m/z, and for negative SIM mode for 307.10 m/z, corresponding to [2M-H] of the isobutyl-monophosphate (exact mass 154.0). The recorded LC chromatograms and their respective MS spectra, for the isobutyl-monophosphate generated by the established customized method, are shown in Figs. S5 and S6.
Conclusion
Herein is described a customized approach for the production of isobutyl-monophosphate (ammonium salt), which implies a sequential strategy that requires 8 h at the most, including synthesis and purification steps to obtain the isolated product. We based on the original method describing the synthesis of organophosphate monoesters from alcohols [1]. In such work, the authors anticipate the applicability of their approach for a wide range of primary and secondary alcohols. Our findings extend the substrate scope of the aforesaid methodology, since isobutanol was not previously included in the starting alcohols panel, in the original method. In addition, we find some short cuts that enabled us to reduce the required time to perform the whole procedure, which are summarized in Fig. 4, where the key pipeline simply relies on simultaneously performing first plane tasks along with parallel activities. Furthermore, another point that substantially reduces the total time required for the complete procedure is that TLC elution is not necessary since the aim is to only detect the fractions showing the presence of phosphates. Thus, a fast spotting and staining assessment is enough to detect positive fractions containing phosphates. In summary, our method is an easy, quick, direct, mild, and affordable approach to generate isobutyl-monophosphate, even in labs that are not regularly performing chemical syntheses.
Acknowledgment
This research was supported by the European Union’s Horizon 2020 research and innovation program under grant agreement No 635536 in the course of the project EmPowerPutida 90030134. W. E.-H. specially thanks to the Science and Technology Council of Mexico (Consejo Nacional de Ciencia y Tecnología, CONACYT) for the granted financial support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.mex.2021.101455.
Appendix. Supplementary materials
References
- 1.Lira L.M., Vasilev D., Pilli R.A., Wessjohann L.A. One-pot synthesis of organophosphate monoesters from alcohols. Tetrahedron Lett. 2013;54:1690–1692. and references therein. [Google Scholar]
- 2.Crans D.C., Whitesides G.M. Glycerol kinase: substrate Specificity. J. Am. Chem. Soc. 1985;107:7008–7018. [Google Scholar]
- 3.Wu P.L., Chen J.H., Huang D.S. Oxidative cleavage of o-hydroxyphenyl phosphate by iodobenzene diacetate. J Chin. Chem. Soc. 1999;46:967–970. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.