Abstract
Honey is a supersaturated sugar solution produced from plant nectar, with its composition influenced by geographic and floral origins, and with several properties contributing to its health-related abilities. This study aimed to determine the bioactive composition, antioxidant characteristics, antibacterial activity, and physicochemical properties of commercial Australian honeys. In total, 42 commercial Australian honeys were selected, and categorised according to front-label descriptions. Honeys were analysed: quality (Hydroxymethylfurfural); colour (colour intensity, L*,a*,b*); bioactive composition (phenolic, flavonoid, and carotenoid content); antioxidant characteristics (DPPH, CUPRAC, FRAP); antibacterial activity (MIC50); physicochemical properties (pH, TSS, viscosity, aw). Colour intensity correlated with each assessed bioactive compound and antioxidant characteristic (p ≤ 0.001). MIC50 (S. aureus) was associated with FRAP and aw, suggesting mechanisms of action for honey's antibacterial activity. Manuka-type honeys had higher colour intensity (1440 (98.5) mAU) than other categories (p ≤ 0.05), and consistently higher bioactive and antioxidant properties. This provides the potential to inform antioxidant-related health outcomes.
Keywords: Australian commercial honey, Bioactive, Antioxidant, Antibacterial, Physicochemical
Graphical abstract
Highlights
-
•
Commercial Australian honey Hydroxymethylfurfural levels below recommendations.
-
•
Colour intensity of commercial honey associated with antioxidant characteristics.
-
•
Honey MIC50 for S. aureus associated with antioxidant properties and water activity.
-
•
Commercial Manuka honeys tended to have the highest antioxidant characteristics.
1. Introduction
Honey is used as a food source and for medicinal purposes across a variety of different cultures (Hunter et al., 2020; Mandal and Mandal, 2011), and is produced as a combination of nectars and bees salivary secretions (Codex Alimentarius, 2001). Honey is a supersaturated sugar solution consisting predominantly of fructose (~38%) and glucose (~31%) (Pascual-Maté et al., 2018), and a variety of over 200 other substances (da Silva et al., 2016). An array of these compounds are considered antioxidants, including phenols, flavonoids, carotenoids, and other enzymatic and non-enzymatic compounds (Bueno-Costa et al., 2016; da Silva et al., 2016). Honey's composition, including antioxidant content, is dependent on its floral source and geographic origins (Kavanagh et al., 2019; Liu et al., 2013), which also influence the appearance of honey's physicochemical properties (García et al., 2020).
The in vitro physiological and health-related benefits of some of honey's compounds are well established (Bogdanov et al., 2008). The effects include honey's antioxidant activity, which can be attributed to honey's composition, including its bioactive compounds (Naumovski, 2015). Honey's antioxidant compounds can reduce the effects of oxidative reactions, such as reducing cellular damage caused by free radical production in vitro (Liu et al., 2013). Additionally, the bioactive compounds, notably phenols, have been demonstrated to contribute to the antibacterial activity of honey (Combarros-Fuertes et al., 2020). The antibacterial properties of honey can also be attributed to its physicochemical properties, such as pH and osmolarity (Combarros-Fuertes et al., 2020), and compounds toxic to bacterial cells in vitro, including hydrogen peroxide, and methylglyoxal in Manuka honey (Bang et al., 2003; Deng et al., 2018). Honey is also associated with decreases in inflammation in vitro through the simultaneous effects of its antioxidant and antibacterial properties (Ruiz-Ruiz et al., 2017).
In Australia, honey is sold as a pure product and must not contain additional substances, including added sugars (Food Standards Australia New Zealand, 2015). Commercial honeys are subjected to heating (45 °C for 8 h) (Chen et al., 2012) and filtration, which contributes to maintaining product quality and consistency (Kortesniemi et al., 2018). However, commercial treatments have potential to negatively alter honey's physical properties, in addition to its antibacterial potential (Chen et al., 2012). Further, as some antioxidants degrade during thermal processing (Munialo et al., 2019), potential alterations to honey antioxidant quality may also occur. However contradictory evidence identifies that appropriate processing methods can increase honey's total antioxidant activity (Escriche et al., 2014).
The bioactive, antioxidant, antibacterial, and physicochemical properties of different types of honey have previously been described in literature, considering their locations and botanic origins (Bodó et al., 2020; Dżugan et al., 2020). However, the analysis of Australian commercial honeys is relatively unexplored (Aazza et al., 2014; Beretta et al., 2005; Saxena et al., 2010). In Australia, commercial honeys represent a considerable market proportion, with 70% of honeys reportedly purchased in supermarkets (Batt and Liu, 2012). To the best of our knowledge, there are no published studies examining the bioactive, antioxidant, antibacterial, and physicochemical properties of honeys of this type. Therefore, this study aimed to determine the bioactive composition, antioxidant characteristics, antibacterial activity, and physicochemical properties of a relatively large selection of commercially available Australian honeys.
2. Materials and methods
2.1. Chemicals and reagents
All chemicals used were of analytical grade. Ethanol, Methanol, (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), Acetone, Aluminium Chloride, Ammonium Acetate, Catechin, Copper (II) Chloride Dehydrate, Ferric Chloride, Folin-Ciocalteur Reagent, Gallic Acid, Glacial Acetic Acid, Hexane, Hydrochloric Acid, Neocuprine, Potassium Hexacyanoferrate (II), Sodium Acetate Trihydrate, Sodium Carbonate, Sodium Hydrogen Sulphite, Sodium Hydroxide, Sodium Nitrate, and Zinc Acetate were purchased from Sigma-Aldrich (Castle Hill, Sydney, Australia). Nutrient Agar Plates and Nutrient Broth were purchased from Thermo Fisher Scientific (Thebarton, South Australia, Australia). Deionised (DI) water was collected following purification (Millipore Australia, North Ryde, NSW), with a resistivity higher than 18 MΩcm−1.
2.2. Samples
Honey samples (n = 42) were purchased in 2017 from chain supermarkets (Aldi®, Coles®, Independent Grocers Australia®, Woolworths®) across the Australian Capital Territory and surrounding regions based on availability at time of purchase. All samples were stored in darkness at room temperature (26 ± 3 °C) as per Beretta et al. (2005). Honeys were then sorted alphabetically and assigned an identification letter ranging from A to AP for reporting. Limited information regarding the floral and geographical origins of the commercial honeys were provided on the label, except for stating the honeys were of Australian origin. As these honey samples were purchased directly from supermarket retailers, only the information provided on the labels was available for use. However, from the front-label packaging information provided, the samples were categorised as: (1) Manuka (including honeys stated to contain Manuka) honey, (2) Organic honey, (3) Generic Brand honey, (4) honeys with specified Floral origins, (5) honeys from a specific Region of Australia, and (6) Pure (otherwise unspecified) honeys.
2.3. Preparation of samples
Honeys were diluted with DI water to predetermined concentrations, with values converted to 100% concentrations following analysis. Diluted samples were sonicated (FXP4, Ultrasonics Australia, Brookvale, NSW) on the highest sonication power until a clear, sediment-free solution was obtained (Beretta et al., 2005). This allowed for the production of an evenly distributed honey-water solution. Samples did not exceed 50 °C to prevent potential temperature-related degradations of bioactive compounds (Beretta et al., 2005).
2.4. Honey quality analysis
The Hydroxymethylfurfural (HMF) content of the honeys was determined following the White method (White, 1979). A clarified honey sample was compared to a reference standard in which HMF molecules were destroyed by Sodium Bisulphate (0.2% w/v). Both samples were measured spectrophotometrically (GeneQuant 1300, GE Healthcare, Silverwater, NSW, Australia) at 284 nm and 336 nm against a DI water blank, with HMF content reported in mg/kg of honey, and determined by the following:
(Note: W = weight of sample (grams); Factor = (126*100*1000*100)/(16830*1000) = 74.87 (126 = honey molecular weight; 16830 = molar absorptivity of HMF at 284 nm) (White, 1979)).
2.5. Bioactive composition
2.5.1. Total phenolic content
Total phenolic content (TPC) was determined using the Folin-Ciocalteur method (Kähkönen et al., 1999) in triplicate, modifying for honey analysis. Absorbance was measured at 765 nm against a DI water blank, using a UV spectrophotometer (Multiskan Go, Thermo Scientific, MA, USA). Results were expressed as milligram Gallic Acid equivalents (GAE) per gram of honey (mg GAE/g).
2.5.2. Total flavonoid content
Total flavonoid content (TFC) was determined according to Wu and Ng (2008). The absorbance was measured at 510 nm against a DI water blank (Multiskan Go, Thermo Scientific, MA, USA), with results expressed as microgram Catechin equivalents (CE) per gram of honey (μg CE/g).
2.5.3. Carotenoid content (β-carotene and lycopene)
Carotenoid equivalents were determined using a modified version of the method described by Ferreira et al. (2009). Initially, 2 mL of each 50% (w/v) honey sample was combined with 10 mL hexane-acetone mixture (6:4), sonicated immediately for 10 min, and filtered (Whatman No. 1 filter paper). Absorbance was determined spectrophotometrically (Novaspec Plus, Visible Spectrophotometer, Amersham Biosciences, UK) at 453 nm, 505 nm, and 663 nm against a DI water blank. The concentrations of β-carotene (β-CE) and lycopene (LE) equivalents were determined using the following equations, with results expressed as milligram of carotenoid per kilogram of honey (mg β-CE/kg; mg LE/kg).
2.6. Antioxidant characteristics
2.6.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay
The antioxidant radical scavenging capacity was measured using the DPPH assay according to Thaipong et al. (2006), with modifications according to honey analysis. Absorbance was measured in triplicate at 515 nm (Multiskan Go, Thermo Scientific, MA, USA). Results were expressed as millimoles of Trolox equivalents (TE) per gram of honey (mmol TE/g) using the following (note: MTrolox = 250.29 g/mol) (Thaipong et al., 2006):
The percentage of inhibition of antioxidant activity was determined as follows:
2.6.2. Cupric reducing antioxidant capacity (CUPRAC) assay
The cupric reducing antioxidant capacity (CUPRAC) was determined according to Apak et al. (2004). The absorbance was measured in triplicate at 450 nm (Multiskan Go, Thermo Scientific, MA, USA) and expressed as millimoles of Trolox equivalents per gram of honey (mmol TE/g).
2.6.3. Ferric reducing antioxidant power (FRAP) assay
The FRAP assay was completed according to Thaipong et al. (2006). The absorbance was measured in triplicate at 593 nm (Multiskan Go, Thermo Scientific, MA, USA), with results expressed as millimoles of Trolox equivalents per gram of honey (mmol TE/g).
2.7. Colour analysis
2.7.1. Colour intensity
The colour intensity was measured using the method of Beretta et al. (2005). The 50% (w/v) honey samples were filtered (Whatman No. 1 filter paper), and absorbance was determined spectrophotometrically (Multiskan Go, Thermo Scientific, MA, USA) at 450 nm and 720 nm. The colour intensity is reported as the difference in spectrophotometric absorbance at the two wavelengths against a DI water blank and expressed as mAU.
2.7.2. L*, a*, b*
The International Commission on Illumination (CIE) L*, a*, b* colour measurements of the honey samples were determined by evenly distributing 50 g of each sample around a clear plastic Petri dish 8.5 cm in diameter (Bertoncelj et al., 2007). Measurements were taken in quintuplicate against a white coloured background and reported as per the values provided by the equipment (Colour Reader CR-20, Konica Minolta, Japan).
2.8. Antibacterial activity
The antibacterial activity was measured using the minimal inhibitory concentration (MIC50) method, as per Patton et al. (2006). Serial dilutions (n = 13) of unfiltered honey were prepared using nutrient broth. Honey-nutrient broth solutions were inoculated with test culture (standardised to McFarland 3.0) of Gram-positive Staphylococcus aureus or Gram-negative Pseudomonas aeruginosa to give 5% (v/v) concentrations, mixed, and aliquoted into wells. Immediately following this, spectrophotometric absorbance was determined in triplicate at 620 nm (T0) (Multiskan Go, Thermo Scientific, MA, USA) against a control well containing inoculated broth without honey. Samples were incubated in darkness for 24 h at 37 °C, and absorbance was measured again at T24 at 620 nm. To calculate the MIC50, absorbance at T0 was subtracted from T24, with the control well assigned a value of 100% growth. The percent inhibition of growth by the honey samples was determined using the formula:
When the difference between T0 and T24 recorded a negative value, the percent of bacterial growth was calculated:
The MIC50 was calculated using GraphPad Prism version 9 (San Diego, California, USA), and is reported as the concentration of honey (%; w/v) required to inhibit 50% of bacterial growth.
2.9. Physicochemical properties
2.9.1. pH
The pH of the undiluted honey samples was determined in triplicate using a pH meter (Mettler Toledo, Port Melbourne, Australia) at room temperature (22.5 °C ± 0.660) (Aumeeruddy et al., 2019).
2.9.2. Total soluble solids
The total soluble solids (TSS) were determined using a handheld digital refractometer (Opti Brix 54, Bellingham = Stanley, Kent, United Kingdom) (Chan et al., 2017), modified for the use of 50% honey dilutions to allow for equipment requirements. Measurements were completed in triplicate and are expressed as °Brix.
2.9.3. Viscosity
The viscosity of the undiluted honey samples (Pa s) was determined using a viscometer (Smart Series, Fungilab, Barcelona, Spain) using an R6 spindle at 5, 10 or 20 rpm, depending on the % torque of the sample (Yücel & Sultanoğlu, 2013).
2.9.4. Water activity (aw)
The aw of the undiluted honey samples was determined at room temperature (24.6 °C ± 0.702) using an aw measuring instrument (LabSwift-aw, Novasina, Switzerland) according to manufacturer's instructions.
2.10. Statistical analysis
Statistical analysis was completed using IBM SPSS Statistics version 25 (Armonk, NY: IBM Corp). Variables were examined to determine suitability for parametric or non-parametric methods using histograms, and the Shapiro-Wilk test for normality. Normally distributed variables are presented as mean ± standard deviation, while not normally distributed measures are presented as median (interquartile range). Due to the inclusion of not normally distributed variables, we utilised Kendall's Tau coefficient of correlation to determine relationships between each of the parameters. To identify the influence of each variable of honey, a partial correlation was also completed. A One-Way ANOVA or Kruskall-Wallis ANOVA with Bonferroni correction as post hoc analysis was completed depending on the distribution of variables, to determine the differences in honey's properties between all pre-determined categories. Significance was accepted at p ≤ 0.05.
3. Results and discussion
3.1. Honey quality analysis
The formation of HMF results from sugar molecule dehydration in honey during the Maillard reaction that occurs during thermal treatments or storage (Pasias et al., 2017). The presence of HMF in honey is indicative of degradation and overall loss of honey quality (Önür et al., 2017), and has been identified to be potentially carcinogenic in vitro (Capuano and Fogliano, 2011). Therefore, the restriction of this compound is essential and should not exceed 40 mg/kg (Codex Alimentarius, 2001). All honey samples contained a HMF content lower than internationally recommended levels, with HMF levels ranging from 0.150 (0.150) mg/kg (honey S) to 21.0 (1.50) mg/kg (honey Y).While excessive degradation of the honey samples (Codex Alimentarius, 2001) was not observed, storage duration prior to sample purchase is unknown, potentially contributing to HMF production (Önür et al., 2017).
3.2. Bioactive compounds
A summary of the total phenolic content (TPC) of the commercially available Australian honey samples is presented in Table 1 (complete data presented in Supplementary Table S1). For the analysed samples, the highest reported TPC was 0.663 (0.078) mg GAE (honey Y), with the lowest polyphenolic profile being 0.309 (0.125) mg GAE (honey AB). Similar phenol levels were reported in other international honey studies where Liu et al. (2013) reported TPC of honey ranging from 0.307 ± 0.01 to 0.822 ± 0.03 mg GAE/g. In contrast, Aazza et al. (2014) reported a range of 136.82 ± 13.60 to 923.70 ± 13.60 mg GAE/kg for a selection of commercial Moroccan honeys. The wider range of phenolic content observed for these samples compared to the commercial Australian honeys could be due to the difference in geographical flora utilised in the production of both honey samples.
Table 1.
Summary of the bioactive composition, antioxidant characteristics, colour analysis, antibacterial activity, and physicochemical properties of a selection of commercially available Australian honeys.
| Highest Ranked Honeys | Lowest Ranked Honeys | |||||
|---|---|---|---|---|---|---|
| Bioactive Composition | ||||||
| TPC (mg GAE)a | Y1 | Z1 | T1 | AB6 | AO3 | A3 |
| 0.663 (0.078) |
0.635 (0.049) |
0.604 (0.124) |
0.309 (0.125) |
0.344 (0.025) |
0.360 (0.019) |
|
| TFC (μg CE)a | Z1 | Y1 | S5 | A3 | B4 | G6 |
| 96.4 (7.37) |
92.5 (12.1) |
84.6 (11.8) |
41.6 (1.48) |
44.9 (2.38) |
47.0 (4.70) |
|
| β-carotene (mg/kg)a | Z1 | Y1 | X1 | A3 | K5 | Q6 |
| 69.6 (4.95) |
69.5 (17.9) |
51.5 (3.68) |
17.1 (4.58) |
17.4 (2.32) |
19.3 (15.6) |
|
| Lycopene (mg/kg)a | R5 | Y1 | Z1 | A3 | K5 | AF3 |
| 24.9 (17.8) |
24.8 (10.0) |
23.5 (3.76) |
7.43 (3.62) |
7.74 (2.60) |
7.98 (1.79) |
|
| Antioxidant Characteristics | ||||||
| DPPH (mmol TE) | Y1 | AN2 | AI4 | AE3 | J4 | H4 |
| 0.713 ± 0.041 |
0.600 ± 0.088 |
0.596 ± 0.046 |
0.207 ± 0.045 |
0.242 ± 0.020 |
0.315 ± 0.021 |
|
| DPPH Inhibition (%) | Y1 | AN2 | AI4 | AE3 | J4 | H4 |
| 69.0 ± 3.97 |
58.1 ± 8.46 |
57.8 ± 4.38 |
20.5 ± 4.32 |
23.9 ± 1.94 |
30.8 ± 2.03 |
|
| CUPRAC (mmol TE)a | Y1 | Z1 | O1 | J4 | AO3 | AJ5 |
| 21.8 (1.36) |
20.8 (0.910) |
19.8 (0.803) |
8.28 (1.31) |
8.51 (0.368) |
10.4 (0.151) |
|
| FRAP (mmol TE) | Y1 | O1 | Z1 | J4 | AE3 | AO3 |
| 3.76 ± 0.158 |
3.08 ± 0.635 |
2.84 ± 0.266 |
0.670 ± 0.390 |
0.841 ± 0.297 |
1.05 ± 0.168 |
|
| Colour Analysis | ||||||
| Colour Intensity (mAU)a | Z1 | Y1 | X1 | G6 | K5 | Q6 |
| 1440 (98.5) |
1196 (42.9) |
905 (77.5) |
241 (5.30) |
261 (4.00) |
303 (6.70) |
|
| L*a | O1 | A3 | B4 | Y1 | Z1 | AP3 |
| 33.6 (0.800) |
25.1 (1.20) |
23.8 (1.90) |
11.7 (0.800) |
12.0 (0.800) |
12.6 (0.850) |
|
| a* | O1 | AA1 | AN2 | G6 | K5 | AO3 |
| 7.90 ± 0.212 |
7.50 ± 0.436 |
7.36 ± 1.78 |
0.980 ± 0.327 |
1.98 ± 0.455 |
2.30 ± 0.596 |
|
| b*a | O1 | A3 | P4 | Y1 | Z1 | L6 |
| 24.7 (0.400) |
18.2 (2.05) |
16.7 (3.10) |
5.30 (0.800) |
6.00 (1.05) |
6.20 (0.900) |
|
| Antibacterial Activity | ||||||
| S. aureus MIC50 (% dilution; w/v) | L6 | P4 | AK2 | Q6 | R5 | AM2 |
| 5.82 |
4.76 |
4.75 |
0.078b |
0.122 |
0.124b |
|
| Physicochemical Properties | ||||||
| pHa | A3 | AI4 | P4 | AF3 | AP3 | AO3 |
| 4.64 (0.160) |
4.60 (0.200) |
4.43 (0.170) |
3.74 (0.020) |
3.81 (0.050) |
3.82 (0.160) |
|
| TSS (°Brix) | H4 | B4 | E4 | AC4 | AB6 | AN2 |
| 60.2 ± 0.001 |
59.3 ± 0.115 |
59.3 ± 0.115 |
55.3 ± 0.115 |
55.8 ± 0.001 |
56.2 ± 0.001 |
|
| Viscosity (Pa s)a | O1 | H4 | A3 | P4 | C2 | AK2 |
| 158.0 (1.32)† |
76.4 (2.14)† |
65.0 (26.1)† |
11.0 (0.275)††† |
12.8 (0.362)††† |
16.8 (0.776)†† |
|
| aw | A3 | B4 | C2 | AO3 | AN2 | AM2 |
| 0.583 | 0.572 | 0.568 | 0.454 | 0.459 | 0.461 | |
Note: The three highest and lowest ranked honeys are presented. MIC50 was calculated from triplicate data; L*, a*, b* measurements were taken in quintuplicates, aw was analysed once for each sample, and all remaining variables were completed in triplicate. Normally distributed variables are presented as mean ± standard deviation, and not normally distributed variables are identified by ‘a’ and reported as median (interquartile range). ND = no data (insufficient sample volume); † = 5 rpm; †† = 10 rpm; ††† = 20 rpm; b = honey samples that recorded Ps. aeruginosa inhibition at 25% dilution concentrations (w/v). Letters represent honey sample ID; superscripts next to the honey identification letter are related to front-label category descriptions of the honey: 1 = Manuka Honey; 2 = Organic Honey; 3 = Generic Brand Honey; 4 = Australian Floral Honey; 5 = Regional Honey; 6 = Pure Honey.
A specific group of polyphenol includes flavonoids, and the TFC values of these honey samples are shown in Table 1 and Table S1. Honey sample Z (96.4 (7.37) μg CE) contained the greatest amount of the compound, and in comparison, sample A (41.6 (1.48) μg CE) had the lowest amount. The results obtained in this study are similar to findings in previously published literature, with total flavonoid content ranging from 27.07 ± 0.35 to 71.78 ± 0.84 mg CE/kg (Khalil et al., 2012). The variation observed in both the TPC and TFC of different types of honey investigated within this study could be due to a variety of factors, including the honey's floral source, in addition to potential quality degradation resulting from commercial treatments.
The carotenoid content of the samples was determined using β-carotene and lycopene equivalents (Ferreira et al., 2009) (Table 1; Table S1). Honey sample Z (69.6 (4.95) mg β-CE/kg) contained the highest β-carotene equivalents, with honey A (17.1 (4.58) mg β-CE/kg) reporting the lowest. Additionally, the lycopene equivalents range from honey R (24.9 (17.8) mg LE/kg) reporting the highest, to honey A (7.43 (3.62) mg LE/kg). The composition of carotenoids in honey is dependent on a variety of factors, including the floral and geographic origins (Bueno-Costa et al., 2016), which could be the cause of large ranges observed in the β-carotene and lycopene values.
3.3. Antioxidant characteristics
The DPPH assay was utilised to determine the antiradical scavenging capacity of the samples, and both the Trolox equivalent and percentage of inhibition were determined (Table 1; Table S1). It was revealed that honey sample possessing the greatest radical scavenging capacity was Y (0.713 ± 0.041 mmol TE; 69.0% ± 3.97). Alternatively, honey AE (0.207 ± 0.045 mmol TE; 20.5% ± 4.32) presented the lowest scavenging ability. Values derived in this study are comparable to other commercial samples, where inhibition was reported between 1.95% ± 0.87–19.12% ± 1.34 (Devarajan and Venugopal, 2012), with Australian samples presenting greater antioxidant potential.
The specific determination of the antioxidant reducing ability of the samples was completed through the CUPRAC assay (Table 1; Table S1), which assesses the ability of antioxidant compounds to reduce the Copper (II) Cupric ion (Apak et al., 2004). Honey sample Y (21.8 (1.36) mmol TE) showed the greatest cupric ion reducing ability, while sample J (8.28 (1.31) mmol TE) demonstrated the weakest ability. Previous findings demonstrate lower honey CUPRAC values (124.8 ± 63–532 ± 19 μmol TE/g) (Ulusoy et al., 2010), which may indicate a higher mineral content within honey analysed in this research, contributing to a higher antioxidant reducing ability of the Australian commercial samples.
The FRAP methodology determines the reducing power of honey by determining the ability of antioxidants present in samples to reduce the molecules Fe3+/Fe2+ (Petretto et al., 2015). The greatest ferric reducing ability (Table 1; Table S1) was identified in sample Y (3.76 ± 0.158 mmol TE), with sample J (0.670 ± 0.390 mmol TE) reporting the weakest power. Similar values were recorded for unifloral honeys, with a mean range of 1033.33–2779.17 mmol TE/kg (Sowa et al., 2017).
3.4. Relationships between bioactive compounds and antioxidant characteristics
The completion of a Kendall's Tau correlation identified relationships between each analysed variable (Table 2). The TPC and TFC values showed a significant correlation (τ = 0.566, p ≤ 0.001), and have also reported positive correlations with each antioxidant characteristic assay (p ≤ 0.001). However, it was observed that when TPC was controlled for, TFC and each antioxidant characteristic were no longer associated (p ≥ 0.05). In contrast, when TFC values were controlled, the antioxidant characteristics remain associated with TPC (p ≤ 0.05), suggesting honey's TFC content does not significantly contribute to honey's phenolic content, or the antioxidant potential of phenolic content. This is supported by the TFC for these samples being between 10.1% and 17.9% of the total polyphenolic content, with flavonoid content reported to be ~12.85% of the total polyphenols of honey (Blasa et al., 2006).
Table 2.
Kendall's Tau correlations between the bioactive content, antioxidant characteristics, colour, antibacterial activity, and physicochemical properties of a selection of commercially available Australian honeys.
| TPC | TFC | β-Carotene | Lycopene | DPPH | CUPRAC | FRAP | Colour Intensity | L* | a* | b* | MIC50 (S. aureus) | pH | TSS | Viscosity | aw | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC | 1 | |||||||||||||||
| TFC | 0.566* | 1 | ||||||||||||||
| β-Carotene | 0.494* | 0.501* | 1 | |||||||||||||
| Lycopene | 0.310* | 0.322* | 0.580* | 1 | ||||||||||||
| DPPH | 0.443* | 0.361* | 0.368* | 0.194 | 1 | |||||||||||
| CUPRAC | 0.700* | 0.508* | 0.42** | 0.224** | 0.417* | 1 | ||||||||||
| FRAP | 0.559* | 0.561* | 0.359* | 0.222** | 0.489* | 0.659* | 1 | |||||||||
| Colour Intensity | 0.596* | 0.547* | 0.600* | 0.287* | 0.498* | 0.566* | 0.489* | 1 | ||||||||
| L* | −0.396* | −0.449* | −0.538* | −0.321* | −0.319* | −0.358* | −0.375* | −0.538* | 1 | |||||||
| a* | 0.397* | 0.315* | 0.334* | 0.094 | 0.406* | 0.403* | 0.355* | 0.594* | −0.202 | 1 | ||||||
| b* | −0.321* | −0.359* | −0.455* | −0.284* | −0.170 | −0.282* | −0.303* | −0.356* | 0.728* | −0.033 | 1 | |||||
| MIC50 (S. aureus) | 0.159 | 0.101 | 0.038 | −0.052 | 0.150 | 0.143 | 0.220** | 0.103 | 0.015 | 0.092 | −0.063 | 1 | ||||
| pH | 0.228** | 0.013 | 0.057 | −0.100 | 0.102 | 0.289* | 0.132 | 0.144 | 0.105 | 0.245** | 0.176 | 0.083 | 1 | |||
| TSS | −0.089 | −0.214** | −0.169 | 0.004 | −0.112 | −0.056 | −0.124 | −0.209 | 0.241** | −0.077 | 0.168 | 0.089 | −0.067 | 1 | ||
| Viscosity | −0.019 | −0.044 | −0.072 | 0.074 | −0.005 | −0.047 | −0.126 | −0.156 | 0.311* | −0.009 | 0.288* | −0.100 | 0.091 | 0.204 | 1 | |
| aw | 0.119 | −0.105 | −0.096 | −0.089 | −0.065 | 0.145 | 0.086 | −0.012 | 0.073 | 0.035 | −0.069 | 0.222** | 0.221** | 0.237** | −0.036 | 1 |
Note: * Correlation is significant at 0.01; ** Correlation is significant at 0.05.
The β-carotene and lycopene carotenoid equivalents were positively correlated (τ = 0.580, p ≤ 0.001), and were associated with both TPC and TFC (β-carotene: p ≤ 0.001; lycopene: p ≤ 0.05). While strong associations were observed for β-carotene and the antioxidant characteristics (p ≤ 0.001), lycopene did not correlate with DPPH (p = 0.070), and weakly correlated with CUPRAC and FRAP (p ≤ 0.05). When controlling for both TPC and TFC, neither β-carotene or lycopene correlated with any of the antioxidant characteristics (p ≥ 0.05), suggesting these carotenoid equivalents do not contribute to honey's antioxidant potential in isolation.
3.5. Colour analysis
Honey's colour is predominantly related to its pigmented contents, such as flavonoids and carotenoids, which are determined by honeys floral origins (Bertoncelj et al., 2007). Honey samples with more pigmented compounds appear darker, in comparison to honeys with lower levels of these compounds (Bueno-Costa et al., 2016). Of the included samples (Table 1; complete data presented in Supplementary Table S2), honey Z (1440 (98.5) mAU) possessed the highest colour intensity, with honey G (241 (5.30) mAU) reporting the lowest. These findings are contradicted by Roshan et al. (2017) who determined a lower range of colour intensity of 41–471 mAU for their selection of unifloral Australian honeys. Observed colour variations of the commercial samples may be due to either floral variation of Australian vegetation, or commercial treatments (Segato et al., 2019).
The lightest honey, as determined by L* values, was O (33.6 (0.800)), with the darkest honey being Y (11.7 (0.800)) (Table 1; Table S2). Considering that an L* value of 100 represents white, the honeys in this study are relatively dark, with similar studies reporting L* values of between 42.12 ± 2.27 to 64.40 ± 0.63 (Bertoncelj et al., 2007). Additionally, a* values range between 7.90 ± 0.212 (honey O) and 0.980 ± 0.327 (honey G), with b* values ranging from 24.7 (0.400) (honey O) to 5.30 (0.800) (honey Y). Positive a* and b* values reported for all honeys indicate that the samples present as red and yellow in colour (Szabó et al., 2016), while some honeys present a green profile (Kuś et al., 2014).
3.6. Relationships between colour, bioactive compounds, and antioxidant characteristics
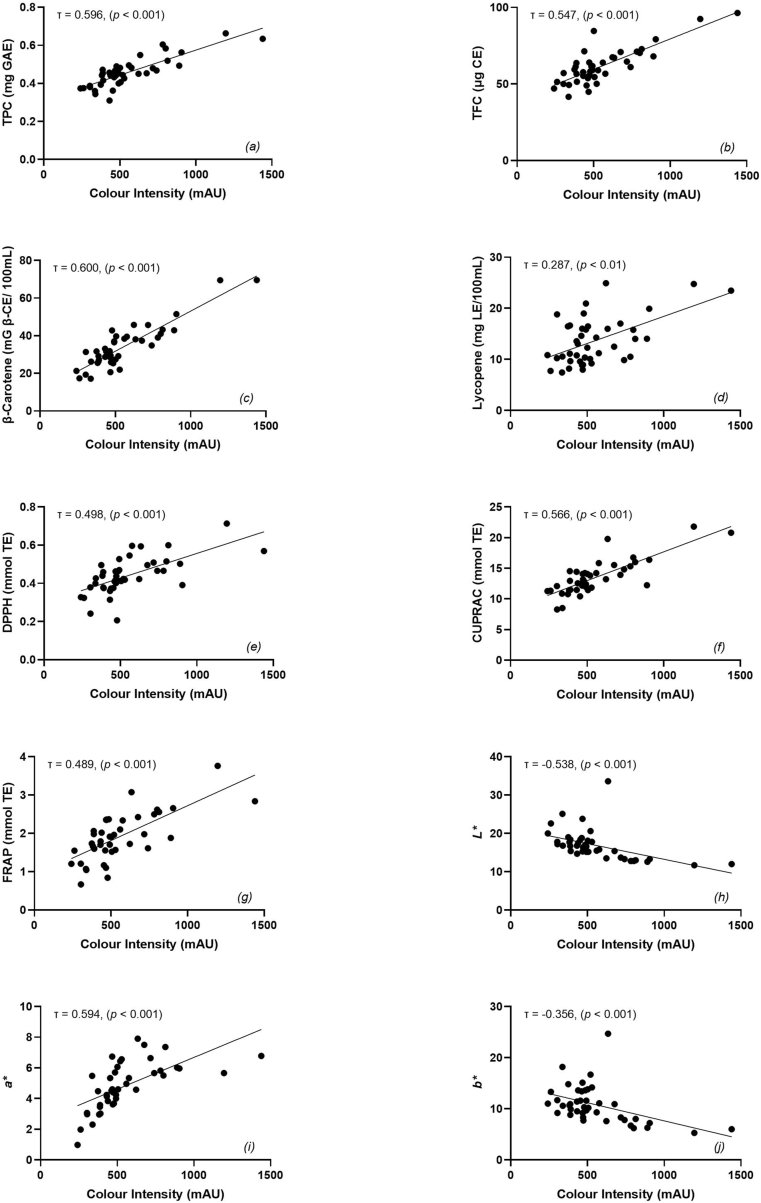
An inverse relationship was observed between colour intensity and L*, confirming the samples' lightness (Fig. 1h; τ = −0.538, p ≤ 0.001), as supported by previous research (Bertoncelj et al., 2007). Similarly, an inverse relationship was observed between colour intensity and b* (Fig. 1j; τ = −0.356, p ≤ 0.001), with a strong correlation between L* and b* (τ = 0.728, p ≤ 0.001). As positive b* values represent honey's yellowness, these correlations highlight that yellow is characteristic of light-coloured honeys. In contrast, the association between colour intensity and a* (Fig. 1i; τ = 0.594, p ≤ 0.001) indicates that darker honeys are characterised by red.
Fig. 1.
Correlation between Colour Intensity (mAU) and (a) Total Phenolic Content (mg GAE), (b) Total Flavonoid Content (μg CE), (c) β-carotene equivalents (mg/100 mL), (d) Lycopene equivalents (mg/100 mL), (e) DPPH (mmol TE), (f) CUPRAC (mmol TE), (g) FRAP (mmol TE), (h) L*, (i) a*, (j) b*, of a selection of commercially available Australian honey samples. Statistical significance is p ≤ 0.01.
Colour intensity positively correlated (p ≤ 0.01) with each bioactive and antioxidant variable (Table 2, Fig. 1). Carotenoid content of honey reportedly contributes to its colour through providing pigmentation (Bueno-Costa et al., 2016), with Fig. 1c depicting strong correlations between colour intensity and β-carotene content (τ = 0.600, p ≤ 0.001). However, weak correlations between colour and lycopene were observed (Fig. 1d; τ = 0.287, p ≤ 0.01). The significant correlation between colour intensity and TPC, TFC, DPPH, CUPRAC, and FRAP (Fig. 1; all p ≤ 0.001) indicates increases in honey colour relates to increases in antioxidant scavenging capacity, which is supported by the inverse correlation between L* values and all bioactive and antioxidant characteristics (p ≤ 0.01). Previously, pale-coloured honeys were reported to have a lower antioxidant activity than darker variations (Cimpoiu et al., 2013; Saxena et al., 2010).
The relationship between colour intensity, bioactive content, and antioxidant characteristics suggests that the darker the honey, the greater its antioxidant potential. Consumers consider the health benefits and nutritive value of honey when selecting honey products (Arvanitoyannis and Krystallis, 2006), including Australian consumers (Batt and Liu, 2012). Therefore, based on our findings and consumer values, there is potential for the utilisation of the relationship between colour intensity and the antioxidant characteristics to increase marketability of honey products. However, in this case, it should be considered that the completion of commercial treatments may also result in the darkening of honeys (Segato et al., 2019).
3.7. Antibacterial activity
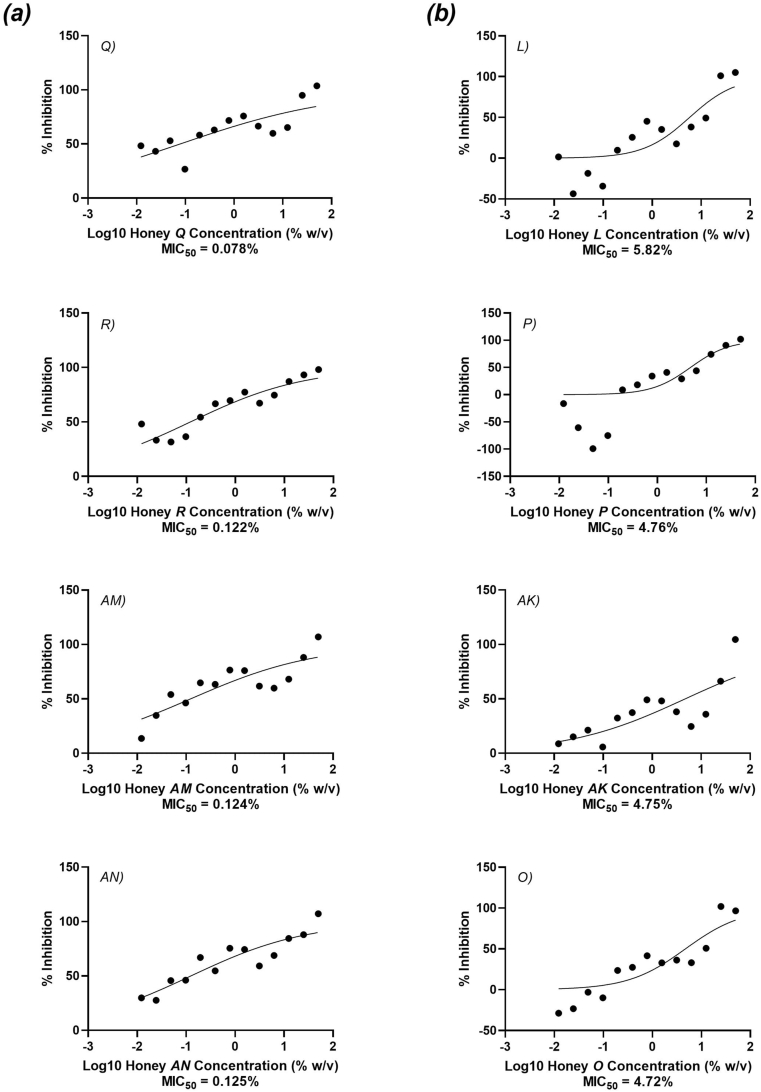
The antibacterial activity of the samples was determined through the MIC50 method, where the concentration of honey (% dilution; w/v) required to inhibit 50% of bacterial growth was determined for S. aureus and Ps. aeruginosa. All samples were effective as an inhibitory agent against S. aureus (Table 1; Table S2; Fig. 2; complete figure in Supplementary Fig. S1). The most effective MIC50 concentration was 0.078% (Honey Q; Fig. 2a), with the weakest for this species being 5.82% (Honey L; Fig. 2b). Inhibitory activity of honey against this bacterial species is consistently supported by similar research (Mahmoodi-Khaledi et al., 2017; Patton et al., 2006). Bucekova et al. (2020) demonstrated the MIC of commercially available honey samples purchased in Slovakia against S. aureus. While their data cannot be compared to the MIC50 values of the Australian samples, they concluded that the antibacterial activity of commercial honey is not uniform (Bucekova et al., 2020), which is reflected by the MIC50 values presented in this study.
Fig. 2.
The MIC50 (%, w/v) of a selection of commercially available Australian honeys against S. aureus; (a) represents the four lowest honey concentrations recorded of the studied samples, (b) represents the four highest honey concentrations recorded.
All honeys effectively inhibited Ps. aeruginosa growth at 50% (w/v) concentrations. However, only 9 honeys (21%) inhibited any growth at 25% (w/v) concentrations (honeys identified in Table 1; Table S2), with no further dilutions observed to inhibit growth. Interestingly, these were not the same honeys to demonstrate the lowest MIC50 values for the inhibition of S. aureus, indicating differences in honey effectiveness based on bacterial species. Therefore, MIC50 values could not be determined, with the analysed commercial samples not being an effective antibacterial agent against Ps. aeruginosa. It was previously determined that Ps. aeruginosa demonstrates antibiotic resistance to honey at weaker concentrations (Mahmoodi-Khaledi et al., 2017), which could be attributed to its gram-negative structure (Breijyeh et al., 2020).
3.8. Mechanisms for honey's antibacterial activity
3.8.1. Physicochemical properties
The pH of honey is typically accepted as between 3.2 and 4.5 (da Silva et al., 2016), contributing to antimicrobial activity by creating an undesirable pathogenic environment (Lund et al., 2020). Results indicated (Table 1; Table S2) the lowest pH from these samples was 3.74 (0.020) (honey AF), with the highest being 4.64 (0.160) (honey A). The pH was found to be positively associated with TPC and CUPRAC (p ≤ 0.05), suggesting that honey's bioactive composition is responsible for its acidity levels, and therefore the pH contribution to antibacterial activity.
The TSS of honey often indicates the sugar compounds present, which contributes to low moisture content and osmotic stress for select microorganisms (Combarros-Fuertes et al., 2020). Of the assessed honeys (Table 1; Table S2), honey H (60.2 ± 0.001 °Brix) reported the greatest TSS content, while honey AC (55.3 ± 0.115 °Brix) reported the least. Our values are lower than previous findings, where soluble solid levels ranged from 76.3 to 85.3 °Brix (Oroian and Ropciuc, 2017). Differences in soluble solid content could be ascribed to different analysis techniques, or as a consequence of commercial treatments applied to our samples (Braghini et al., 2020).
There is a large variation observed for the honey's viscosity (Table 1; Table S2), with a range of 11.0 (0.275) Pa s (honey P) to 158 (1.32) Pa s (honey O), in addition to a range of rotation speeds (5 rpm, 10 rpm and 20 rpm) required due to the varied consistency. It is established that factors influencing honey's viscosity are its high sugar concentration and low moisture content (Jiang et al., 2020; Yücel & Sultanoğlu, 2013). Interestingly, no associations (p ≥ 0.05) were observed between sample viscosity and TSS, representative of the sugar content, or aw, which indicates honey's moisture.
The aw of honey is affected by factors such as sugar composition, degree of crystallisation, and water-sugar interactions, and typically measures below 0.60, preventing microorganism growth (Subbiah et al., 2020). The analysed honeys reported a lower aw, ranging from 0.454 (honey AO) to 0.583 (honey A) (Table 1; Table S2). The sample aw was associated with the TSS (τ = 0.237; p ≤ 0.05) (Table 2), however this was not anticipated due to the influence of sugar content on the water activity (Oroian and Ropciuc, 2017). The aw was also associated with the MIC50 for S. aureus (τ = 0.222; p ≤ 0.05) (Table 2). Interestingly, other assessed physicochemical properties did not correlate with MIC50 (p ≥ 0.05) despite reports of these properties contributing to honey's antibacterial activity (Combarros-Fuertes et al., 2020; Mandal and Mandal, 2011).
3.8.2. Relationships between antibacterial activity and antioxidant characteristics
The MIC50 values of the assessed honeys against S. aureus also correlated with FRAP (τ = 0.220; p ≤ 0.05) (Table 2), indicating the contribution of honey's antioxidant compounds to its antibacterial activity. This is further supported by Leyva-Jimenez et al. (2019), where the extracted phenolic content of honey had similar antibacterial potency as whole honey samples for a range of bacterial species (Leyva-Jimenez et al., 2019). Despite no correlation being found between MIC50 and TPC, when the phenolic content is controlled for, the MIC50 of the samples is no longer associated with FRAP (p = 0.198). This change in significance highlights the contribution of the phenolic content to honey's antibacterial activity.
3.9. Characteristics of honey categories
Manuka samples (Category 1) reported among the largest means for each antioxidant assay, indicating samples in this category had the highest antioxidant capacity. Specifically, honeys Y and Z frequently reported among the highest for each bioactive (TPC, TFC, β-carotene, lycopene) and antioxidant (DPPH, CUPRAC, FRAP) (Table 1) method. In contrast, Generic Brand (Category 3) and Australian Floral honeys (Category 4) reported the lowest values for nearly all parameters. It was anticipated that Manuka honeys would possess the greatest antibacterial activity, based on previous effectiveness compared with other honey types, particularly for S. aureus (Gośliński et al., 2020). However, in our study, a Pure (Category 6) and Regional (Category 5) honey (Table 1) reported the highest antibacterial ability. For the physicochemical properties, no categories consistently reported the highest or lowest values, indicating that honey type does not influence these factors.
The difference between each category was determined for each of the assays using One-Way ANOVA or Kruskal-Wallis, depending on the normality, with differences observed only between Manuka honeys and some other categories. These were observed for FRAP between Manuka and Generic Branded (p = 0.001), Australian Floral (p = 0.001), Regional (p = 0.009) and Pure (p = 0.02) honeys. Similarly, TFC was significantly higher in Manuka honey than Generic Branded (p = 0.001), Australian Floral (p = 0.001), and Pure (p = 0.002) honeys, and Manuka honey had a greater colour intensity than Australian Floral honeys (p = 0.003).
Many studies that determine the antioxidant and antibacterial properties of honey report distinct floral and geographic origins of their samples. These studies have reported the antioxidant characteristics, specifically the phenolic composition, is due to these factors (Escuredo et al., 2013; M. I. Khalil et al., 2011). However, this information is not available for commercial samples unless provided by the manufacturer on the packaging. Despite this, the honeys investigated in this study are representative of what is available to consumers on the Australian commercial market.
3.10. Future directions and limitations
To our knowledge, this is the first study examining the bioactive, antioxidant, antibacterial, and physicochemical properties of commercially available Australian honeys. Although consumer behaviour towards Australian honeys, including commercial honeys, has been identified (Batt and Liu, 2012), there has been no analysis determining which sensory values drive likeability and purchasing behaviour. Based on the observed antioxidant characteristics of the honeys, there is potential for further investigation into antioxidant-related health benefits of commercial Australian honey, including determining if these samples are efficient at reducing plasma antioxidant content in vivo. Additionally, given the observed association between the antioxidant characteristic FRAP and antibacterial activity, the synergistic anti-inflammatory potential should be investigated (Hunter et al., 2020).
The findings of this investigation are limited by the techniques used, with spectrophotometric analysis used for the quantification of bioactive and antioxidant compounds. The technique measures the refractive light absorption of solid compounds, and often detects different compounds at the same wavelength, resulting in an overestimation of content (Biehler et al., 2010). Further, an analysis of the polyphenolic profile of the samples was not completed, which could improve this research by allowing for the identification of compounds that most contribute to the sample's antioxidant characteristics. Finally, it has been reported that a considerable contributing variable to honey's antibacterial ability is the presence of hydrogen peroxide (Bang et al., 2003), however, this was not assessed, with its contribution to the MIC50 of the samples unknown.
4. Conclusions
In conclusion, the colour intensity of the honeys is related to a variety of bioactive properties and antioxidant characteristics. Additionally, the antibacterial activity was associated with the honey's water activity, and the antioxidant characteristic FRAP. The phenolic content of the samples contributed to both antioxidant and antibacterial properties of the honeys, as demonstrated by changes in significance when controlling for this variable. From all honeys tested in this study, Manuka honeys consistently reported higher results for some of the included assays, with a variety of generic branded and specific floral honeys reporting the lowest values. These findings can potentially inform honey producers and consumers on future directions of honey marketability through highlighting the potential antioxidant and antibacterial-related health benefits.
Funding
This research was supported by an Australian Government Research Training Program Scholarship for M.H. and C.G.; N.M.D. is supported by a Dementia Australia Research Foundation PhD Scholarship.
CRediT authorship contribution statement
Maddison Hunter: Conceptualization, Methodology, Validation, Investigation, Formal analysis, Visualization, Writing – original draft, Visualization, Writing – review & editing. Reena Ghildyal: Methodology, Validation, Formal analysis, Writing – review & editing. Nathan M. D'Cunha: Validation, Writing – review & editing. Caroline Gouws: Validation, Writing – review & editing. Ekavi N. Georgousopoulou: Formal analysis, Writing – review & editing. Nenad Naumovski: Conceptualization, Methodology, Visualization, Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no know competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Ms. Jacqui Richardson and Mr. Peter Ogilvie for their assistance with the acquisition of materials and training for the MIC50 assay. Graphical abstract created with BioRender (https://biorender.com).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2021.08.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aazza S., Lyoussi B., Antunes D., Miguel M.G. Physicochemical characterization and antioxidant activity of 17 commercial Moroccan honeys. Int. J. Food Sci. Nutr. 2014;65:449–457. doi: 10.3109/09637486.2013.873888. [DOI] [PubMed] [Google Scholar]
- Alimentarius Codex. Revised Codex standard for honey. 2001. http://www.fao.org/input/download/standards/310/cxs_012e.pdf Retrieved from.
- Apak R., Güçlü K., Özyürek M., Karademir S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004;52:7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- Arvanitoyannis I., Krystallis A. An empirical examination of the determinants of honey consumption in Romania. Int. J. Food Sci. Technol. 2006;41:1164–1176. doi: 10.1111/j.1365-2621.2006.01174.x. [DOI] [Google Scholar]
- Aumeeruddy M.Z., Aumeeruddy-Elalfi Z., Neetoo H., Zengin G., Blom van Staden A., Fibrich B., Lambrechts I.A., Rademan S., Szuman K.M., Lall N., Mahomoodally F. Pharmacological activities, chemical profile, and physicochemical properties of raw and commercial honey. Biocatal. Agric. Biotechnol. 2019;18:101005. doi: 10.1016/j.bcab.2019.01.043. [DOI] [Google Scholar]
- Bang L.M., Buntting C., Molan P. The effect of dilution on the rate of hydrogen peroxide production in honey and its implications for wound healing. J. Alternative Compl. Med. 2003;9:267–273. doi: 10.1089/10755530360623383. [DOI] [PubMed] [Google Scholar]
- Batt P.J., Liu A. Consumer behaviour towards honey products in Western Australia. Br. Food J. 2012;114:285–297. doi: 10.1108/00070701211202449. [DOI] [Google Scholar]
- Beretta G., Granata P., Ferrero M., Orioli M., Maffei Facino R. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal. Chim. Acta. 2005;533:185–191. doi: 10.1016/j.aca.2004.11.010. [DOI] [Google Scholar]
- Bertoncelj J., Doberšek U., Jamnik M., Golob T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007;105:822–828. doi: 10.1016/j.foodchem.2007.01.060. [DOI] [Google Scholar]
- Biehler E., Mayer F., Hoffmann L., Krause E., Bohn T. Comparison of 3 spectrophotometric methods for carotenoid determination in frequently consumed fruits and vegetables. J. Food Sci. 2010;75:C55–C61. doi: 10.1111/j.1750-3841.2009.01417.x. [DOI] [PubMed] [Google Scholar]
- Blasa M., Candiracci M., Accorsi A., Piacentini M.P., Albertini M.C., Piatti E. Raw Millefiori honey is packed full of antioxidants. Food Chem. 2006;97:217–222. doi: 10.1016/j.foodchem.2005.03.039. [DOI] [Google Scholar]
- Bodó A., Radványi L., Kőszegi T., Csepregi R., Nagy D.U., Farkas Á., Kocsis M. Melissopalynology, antioxidant activity and multielement analysis of two types of early spring honeys from Hungary. Food Biosci. 2020;35:100587. doi: 10.1016/j.fbio.2020.100587. [DOI] [Google Scholar]
- Bogdanov S., Jurendic T., Sieber R., Gallmann P. Honey for nutrition and health: a review. J. Am. Coll. Nutr. 2008;27:677–689. doi: 10.1080/07315724.2008.10719745. [DOI] [PubMed] [Google Scholar]
- Braghini F., Biluca F.C., Gonzaga L.V., Vitali L., Costa A.C.O., Fett R. Effect thermal processing in the honey of Tetragonisca angustula: profile physicochemical, individual phenolic compounds and antioxidant capacity. J. Apicult. Res. 2020;60:1–7. doi: 10.1080/00218839.2020.1737362. [DOI] [Google Scholar]
- Breijyeh Z., Jubeh B., Karaman R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 2020;25:1340. doi: 10.3390/molecules25061340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucekova M., Bugarova V., Godocikova J., Majtan J. Demanding New honey qualitative standard based on antibacterial activity. Foods. 2020;9:1263. doi: 10.3390/foods9091263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno-Costa F.M., Zambiazi R.C., Bohmer B.W., Chaves F.C., Silva W.P.d., Zanusso J.T., Dutra I. Antibacterial and antioxidant activity of honeys from the state of Rio Grande do Sul, Brazil. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2016;65:333–340. doi: 10.1016/j.lwt.2015.08.018. [DOI] [Google Scholar]
- Capuano E., Fogliano V. Acrylamide and 5-hydroxymethylfurfural (HMF): a review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2011;44:793–810. doi: 10.1016/j.lwt.2010.11.002. [DOI] [Google Scholar]
- Chan B.K., Haron H., Talib R.A., Subramaniam P. Physical properties, antioxidant content and anti-oxidative activities of Malaysian stingless kelulut (Trigona spp.) Honey. J. Agric. Sci. 2017;9:32–40. doi: 10.5539/jas.v9n13p32. [DOI] [Google Scholar]
- Chen C., Campbell L., Blair S.E., Carter D.A. The effect of standard heat and filtration processing procedures on antimicrobial activity and hydrogen peroxide levels in honey. Front. Microbiol. 2012;3:265. doi: 10.3389/fmicb.2012.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimpoiu C., Hosu A., Miclaus V., Puscas A. Determination of the floral origin of some Romanian honeys on the basis of physical and biochemical properties. Spectrochim. Acta Mol. Biomol. Spectrosc. 2013;100:149–154. doi: 10.1016/j.saa.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Combarros-Fuertes P., Fresno J.M., Estevinho M.M., Sousa-Pimenta M., Tornadijo M.E., Honey L.M. Estevinho. Another alternative in the fight against antibiotic-resistant bacteria? Antibiotics. 2020;9:774. doi: 10.3390/antibiotics9110774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva P.M., Gauche C., Gonzaga L.V., Costa A.C.O., Honey R. Fett. Chemical composition, stability and authenticity. Food Chem. 2016;196:309–323. doi: 10.1016/j.foodchem.2015.09.051. [DOI] [PubMed] [Google Scholar]
- Deng J., Liu R., Lu Q., Hao P., Xu A., Zhang J., Tan J. Biochemical properties, antibacterial and cellular antioxidant activities of buckwheat honey in comparison to manuka honey. Food Chem. 2018;252:243–249. doi: 10.1016/j.foodchem.2018.01.115. [DOI] [PubMed] [Google Scholar]
- Devarajan S., Venugopal S. Antioxidant and α-amylase inhibition activities of phenolic compounds in the extracts of Indian honey. Chin. J. Nat. Med. 2012;10:255–259. doi: 10.1016/S1875-5364(12)60051-X. [DOI] [Google Scholar]
- Dżugan M., Grabek-Lejko D., Swacha S., Tomczyk M., Bednarska S., Kapusta I. Physicochemical quality parameters, antibacterial properties and cellular antioxidant activity of Polish buckwheat honey. Food Biosci. 2020;34:100538. doi: 10.1016/j.fbio.2020.100538. [DOI] [Google Scholar]
- Escriche I., Kadar M., Juan-Borrás M., Domenech E. Suitability of antioxidant capacity, flavonoids and phenolic acids for floral authentication of honey. Impact of industrial thermal treatment. Food Chem. 2014;142:135–143. doi: 10.1016/j.foodchem.2013.07.033. [DOI] [PubMed] [Google Scholar]
- Escuredo O., Míguez M., Fernández-González M., Carmen Seijo M. Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chem. 2013;138:851–856. doi: 10.1016/j.foodchem.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Ferreira I.C.F.R., Aires E., Barreira J.C.M., Estevinho L.M. Antioxidant activity of Portuguese honey samples: different contributions of the entire honey and phenolic extract. Food Chem. 2009;114:1438–1443. doi: 10.1016/j.foodchem.2008.11.028. [DOI] [Google Scholar]
- Food Standards Australia New Zealand Australia New Zealand food standards code - standard 2.8.2 - honey. 2015. https://www.legislation.gov.au/Details/F2015L00407 Retrieved from.
- García S., Troncoso J.M., Rondanelli-Reyes M. Study of honey according to botanical origin and physicochemical parameters in the Biobío Region, Chile. Chil. J. Agric. Res. 2020;80:675–685. doi: 10.4067/S0718-58392020000400675. [DOI] [Google Scholar]
- Gośliński M., Nowak D., Kłębukowska L. Antioxidant properties and antimicrobial activity of manuka honey versus Polish honeys. J. Food Sci. Technol. 2020;57:1269–1277. doi: 10.1007/s13197-019-04159-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter M., Kellett J., D'Cunha N.M., Toohey K., McKune A., Naumovski N. The effect of honey as a treatment for oral ulcerative lesions: a systematic review. Explor. Res. Hypothesis Med. 2020;5:27–37. doi: 10.14218/erhm.2019.00029. [DOI] [Google Scholar]
- Jiang M., Zhu W., Ruan S., Jia Y., Bai X., Sun J. Effect of ultrasonic power and frequency on rheological properties of Chinese honey. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2020;137:110425. doi: 10.1016/j.lwt.2020.110425. [DOI] [Google Scholar]
- Kähkönen M.P., Hopia A.I., Vuorela H.J., Rauha J.-P., Pihlaja K., Kujala T.S., Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- Kavanagh S., Gunnoo J., Marques Passos T., Stout J.C., White B. Physicochemical properties and phenolic content of honey from different floral origins and from rural versus urban landscapes. Food Chem. 2019;272:66–75. doi: 10.1016/j.foodchem.2018.08.035. [DOI] [PubMed] [Google Scholar]
- Khalil M.I., Alam N., Moniruzzaman M., Sulaiman S.A., Gan S.H. Phenolic acid composition and antioxidant properties of Malaysian honeys. J. Food Sci. 2011;76:C921–C928. doi: 10.1111/j.1750-3841.2011.02282.x. [DOI] [PubMed] [Google Scholar]
- Khalil M.I., Moniruzzaman M., Boukraâ L., Benhanifia M., Islam M., Sulaiman S.A., Gan S.H. Physicochemical and antioxidant properties of Algerian honey. Molecules. 2012;17:11199–11215. doi: 10.3390/molecules170911199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortesniemi M., Rosenvald S., Laaksonen O., Vanag A., Ollikka T., Vene K., Yang B. Sensory and chemical profiles of Finnish honeys of different botanical origins and consumer preferences. Food Chem. 2018;246:351–359. doi: 10.1016/j.foodchem.2017.10.069. [DOI] [PubMed] [Google Scholar]
- Kuś P.M., Congiu F., Teper D., Sroka Z., Jerković I., Tuberoso C.I.G. Antioxidant activity, color characteristics, total phenol content and general HPLC fingerprints of six Polish unifloral honey types. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2014;55:124–130. doi: 10.1016/j.lwt.2013.09.016. [DOI] [Google Scholar]
- Leyva-Jimenez F.J., Lozano-Sanchez J., Borras-Linares I., Cadiz-Gurrea M.d.l.L., Mahmoodi-Khaledi E. Potential antimicrobial activity of honey phenolic compounds against Gram positive and Gram negative bacteria. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2019;101:236–245. doi: 10.1016/j.lwt.2018.11.015. [DOI] [Google Scholar]
- Liu J.R., Ye Y.L., Lin T.Y., Wang Y.W., Peng C.C. Effect of floral sources on the antioxidant, antimicrobial, and anti-inflammatory activities of honeys in Taiwan. Food Chem. 2013;139:938–943. doi: 10.1016/j.foodchem.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Lund P.A., De Biase D., Liran O., Scheler O., Mira N.P., Cetecioglu Z., Fernández E.N., Bover-Cid S., Hall R., Sauer M. Understanding how microorganisms respond to acid pH is central to their control and successful exploitation. Front. Microbiol. 2020;11:2233. doi: 10.3389/fmicb.2020.556140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoodi-Khaledi E., Lozano-Sánchez J., Bakhouche A., Habibi-Rezaei M., Sadeghian I., Segura-Carretero A. Physicochemical properties and biological activities of honeys from different geographical and botanical origins in Iran. Eur. Food Res. Technol. 2017;243:1019–1030. doi: 10.1007/s00217-016-2811-0. [DOI] [Google Scholar]
- Mandal M.D., Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011;1:154–160. doi: 10.1016/S2221-1691(11)60016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munialo C.D., Naumovski N., Sergi D., Stewart D., Mellor D.D. Critical evaluation of the extrapolation of data relative to antioxidant function from the laboratory and their implications on food production and human health: a review. Int. J. Food Sci. Technol. 2019;54:1448–1459. doi: 10.1111/ijfs.14135. [DOI] [Google Scholar]
- Naumovski N. Plant Bioactive Compounds for Pancreatic Cancer Prevention and Treatment. Nova Publishers; 2015. Bioactive composition of plants and plant foods; pp. 81–115. [Google Scholar]
- Önür İ., Misra N.N., Barba F.J., Putnik P., Lorenzo J.M., Gökmen V., Alpas H. Effects of ultrasound and high pressure on physicochemical properties and HMF formation in Turkish honey types. J. Food Eng. 2017;219:129–136. doi: 10.1016/j.jfoodeng.2017.09.019. [DOI] [Google Scholar]
- Oroian M., Ropciuc S. Honey authentication based on physicochemical parameters and phenolic compounds. Comput. Electron. Agric. 2017;138:148–156. doi: 10.1016/j.compag.2017.04.020. [DOI] [Google Scholar]
- Pascual-Maté A., Osés S.M., Marcazzan G.L., Gardini S., Fernández Muiño M.A., Teresa Sancho M. Sugar composition and sugar-related parameters of honeys from the northern Iberian Plateau. J. Food Compos. Anal. 2018;74:34–43. doi: 10.1016/j.jfca.2018.08.005. [DOI] [Google Scholar]
- Pasias I.N., Kiriakou I.K., Proestos C. HMF and diastase activity in honeys: a fully validated approach and a chemometric analysis for identification of honey freshness and adulteration. Food Chem. 2017;229:425–431. doi: 10.1016/j.foodchem.2017.02.084. [DOI] [PubMed] [Google Scholar]
- Patton T., Barrett J., Brennan J., Moran N. Use of a spectrophotometric bioassay for determination of microbial sensitivity to manuka honey. J. Microbiol. Methods. 2006;64:84–95. doi: 10.1016/j.mimet.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Petretto G.L., Cossu M., Alamanni M.C. Phenolic content, antioxidant and physico‐chemical properties of Sardinian monofloral honeys. Int. J. Food Sci. Technol. 2015;50:482–491. doi: 10.1111/ijfs.12652. [DOI] [Google Scholar]
- Roshan N., Rippers T., Locher C., Hammer K.A. Antibacterial activity and chemical characteristics of several Western Australian honeys compared to manuka honey and pasture honey. Arch. Microbiol. 2017;199:347–355. doi: 10.1007/s00203-016-1308-3. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ruiz J.C., Matus-Basto A.J., Acereto-Escoffié P., Segura-Campos M.R. Antioxidant and anti-inflammatory activities of phenolic compounds isolated from Melipona beecheii honey. Food Agric. Immunol. 2017;28:1424–1437. doi: 10.1080/09540105.2017.1347148. [DOI] [Google Scholar]
- Saxena S., Gautam S., Sharma A. Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem. 2010;118:391–397. doi: 10.1016/j.foodchem.2009.05.001. [DOI] [Google Scholar]
- Segato S., Merlanti R., Bisutti V., Montanucci L., Serva L., Lucatello L., Mirisola M., Contiero B., Conficoni D., Balzan S., Marchesini G., Capolongo F. Multivariate and machine learning models to assess the heat effects on honey physicochemical, colour and NIR data. Eur. Food Res. Technol. 2019;245:2269–2278. doi: 10.1007/s00217-019-03332-x. [DOI] [Google Scholar]
- Sowa P., Grabek‐Lejko D., Wesołowska M., Swacha S., Dżugan M. Hydrogen peroxide‐dependent antibacterial action of Melilotus albus honey. Lett. Appl. Microbiol. 2017;65:82–89. doi: 10.1111/lam.12749. [DOI] [PubMed] [Google Scholar]
- Subbiah B., Blank U.K.M., Morison K.R. A review, analysis and extension of water activity data of sugars and model honey solutions. Food Chem. 2020;326:126981. doi: 10.1016/j.foodchem.2020.126981. [DOI] [PubMed] [Google Scholar]
- Szabó R.T., Mézes M., Szalai T., Zajácz E., Kovács-Weber M. Colour identification of honey and methodical development of its instrumental measuring. Columella J. Agric. Environ. Sci. 2016;3:29–36. doi: 10.18380/szie.colum.2016.3.1.29. [DOI] [Google Scholar]
- Thaipong K., Boonprakob U., Crosby K., Cisneros-Zevallos L., Hawkins Byrne Comparison of Abts D. DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006;19:669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- Ulusoy E., Kolayli S., Sarikaya A.O. Antioxidant and antimicrobial activity of different floral origin honeys from Turkiye. J. Food Biochem. 2010;34:321–335. doi: 10.1111/j.1745-4514.2009.00332.x. [DOI] [Google Scholar]
- White J.W. Spectrophotometric method for hydroxymethylfurfural in honey. J. Assoc. Off. Anal. Chem. 1979;62:509–514. [PubMed] [Google Scholar]
- Wu S.J., Ng L.T. Antioxidant and free radical scavenging activities of wild bitter melon (Momordica charantia Linn. var. abbreviata Ser.) in Taiwan. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2008;41:323–330. doi: 10.1016/j.lwt.2007.03.003. [DOI] [Google Scholar]
- Yücel Y., Sultanoğlu P. Characterization of honeys from Hatay Region by their physicochemical properties combined with chemometrics. Food Biosci. 2013;1:16–25. doi: 10.1016/j.fbio.2013.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.