Abstract
Background
Cyclooxygenase-2 (COX-2) inhibitors are prescribed for the management of osteoarthritis (OA)-associated pain and inflammation. However, the role of COX-2 in normal and osteoarthritic articular chondrocytes has not been well investigated. We hypothesize that COX-2 plays a role in articular chondrocytes under normal conditions and during OA progression.
Methods
In vivo COX-2 levels in articular cartilage of normal and papain-induced osteoarthritic rats were compared. The role of COX-2 in human articular chondrocytes (HACs) was tested in vitro by COX-2 overexpression or activity inhibition. The levels of COX-2 and marker gene for normal function or articular cartilage degeneration were evaluated: mRNA by qRT-PCR; proteins by western blotting or immunohistochemistry; and glycosaminoglycan (GAG) by Safranin O–fast green staining. Parathyroid hormone-related protein (PTHrP) promoter activity was detected with luciferase reporter assays.
Results
In the OA rat study, COX-2 and PTHrP were simultaneously increased in osteoarthritic rat chondrocytes, while increased PTHrP levels were reduced by celecoxib, a COX-2 selective inhibitor. The levels of normal cartilage matrices, GAG and type II collagen decreased, while markers of degeneration, collagen type X and MMP13 were elevated in osteoarthritic articular chondrocytes. Celecoxib rescued the loss of GAG and the increased collagen type X and MMP13 levels. In vitro, COX-2 overexpression in HACs significantly increased Col2a1, Col10a1, PTHrP and MMP13 mRNA expression, which was decreased when COX-2 activity was suppressed. More importantly, COX-2 overexpression upregulated the PTHrP transcription, mRNA expression and protein levels.
Conclusion
COX-2 plays a pathophysiological role by preventing terminal differentiation of articular chondrocytes by upregulating PTHrP expression at the early stage of OA progression.
The Translational potential of this article
COX2 up-regulates PTHrP expression in normal and OA articular chondrocytes.
Keywords: Cyclooxygenase-2 (COX-2), PTHrP, Articular chondrocytes, Terminal differentiation, Osteoarthritis (OA)
1. Introduction
Osteoarthritis (OA) is a highly prevalent degenerative joint disease characterized by articular cartilage degradation, osteophyte formation and secondary synovitis. Pain and inflammation are the major symptoms in OA patients [1]. Symptoms are commonly managed with nonsteroidal anti-inflammatory drugs (NSAIDs) that inhibit prostaglandin production by blocking cyclooxygenase-1/2 (COX-1 and COX-2) [2]. COX-2 inhibitors are preferentially used for long-term OA treatment due to their minimal gastrointestinal side effects [3,4]. However, while the predicted beneficial effects of selective COX-2 inhibitors alleviate symptoms, there is limited evidence for their disease-modifying effects [5]. Other than its inflammatory effect in OA joint tissues, especially synovium, the role of COX-2 in normal and OA articular chondrocytes has not been well investigated. In this study, a COX-2 inhibitor (celecoxib) was used to evaluate the pathophysiological role of COX-2 in OA and normal articular cartilage.
Articular cartilage is composed of chondrocytes and extracellular matrix, which primarily consists of sulfated glycosaminoglycan (GAG) and type II collagen (col2a1) [6,7]. Articular cartilage degradation is a hallmark of OA, along with elevated production of proteolytic enzymes, such as metalloproteinase 13 (MMP13), a collagenase of type II collagen, in chondrocytes [8,9]. OA articular chondrocytes have also been demonstrated to lose their differentiation phenotype and undergo changes similar to those that occur in terminal differentiation of chondrocytes, such as in the chondrocytes in the growth plate of long bone [6,10,11]. Degenerative articular chondrocytes undergo terminal differentiation characterized by hypertrophy; expression of markers, such as type X collagen (Col10a1), MMP13 and runt-related transcription factor 2 (Runx2); mineralization; and eventually apoptosis [[11], [12], [13]]. The hypertrophic changes in articular chondrocytes occur not only in animals with experimental animals but also in cartilage from OA patients, which may lead to OA progression [10]. Manipulation and inhibition of the terminal differentiation of chondrocytes may be a therapeutic target to slow OA progression [[14], [15], [16]].
In general, COX-2 is an inducible enzyme largely responsible for inflammation that catalyzes the production of prostaglandins (PGs) from arachidonic acid in inflamed joint tissues in arthritic diseases [17,18]. Overexpression of COX-2 is likely induced by proinflammatory mediators that are produced by OA synovium [19]. However, the pathophysiological role of COX-2 in articular cartilage is not known. On the other hand, the physiological role of COX-2 in growth plate chondrocytes has been studied. A previous study demonstrated that COX-2 acts as a transcriptional cofactor of Runx2 to enhance its DNA-binding capability and promote Col10a1 expression, thus inducing hypertrophy in growth plate chondrocytes [20]. COX-2 was also reported to intersect with bone morphogenic protein-2 (BMP-2) signaling to promote hypertrophic differentiation of growth plate chondrocytes during long bone development [21]. Accordingly, in this study, we propose that in addition to promoting inflammation in OA, COX-2 may play a physiological role in normal articular chondrocytes or a pathophysiological role during OA progression.
Previous investigations identified an important feedback control loop between parathyroid hormone-related protein (PTHrP) and Indian hedgehog (Ihh) that fine tunes the speed of chondrocyte terminal differentiation during the process of endochondral bone formation. Briefly, hypertrophic chondrocytes express Ihh, while proliferating chondrocytes express PTHrP, which leads to delayed hypertrophy/maturation and maintains the functions of chondrocytes [22,23]. Several studies have demonstrated that PTHrP expression is higher in human osteoarthritic articular cartilage than in normal cartilage [[24], [25], [26]]. Mechanical loading and other factors have been found to induce PTHrP expression in the superficial and middle layers of articular cartilage [27], and increased PTHrP expression might contribute to inhibition of the hypertrophic potential of chondrocytes residing in the deep layer of articular cartilage [28]. Accordingly, the use of analogs of PTHrP or PTH as chondroregenerative factors to treat cartilage injury and OA has been suggested [14,15,29,30]. However, the function and regulation of PTHrP in OA cartilage are still poorly understood.
In this study, we aimed to investigate the role of COX-2 in articular cartilage, especially at the early stage of OA progression when articular chondrocytes are undergoing terminal differentiation. Subsequently, we further investigate the regulatory relationship between COX-2 and PTHrP.
2. Methods
2.1. OA induction and celecoxib treatment
The animal experiments were approved by the Animal Care and Use Committee of Kaohsiung Medical University. Thirty-six eight-week-old male Sprague–Dawley rats were purchased from BioLASCO Taiwan and housed under standard laboratory conditions (24 °C, 12-h light–dark cycle) with food and water ad libitum. OA was induced using intra-articular (i.a.) injection of papain as described previously [14]. Briefly, rats were anesthetized with diethyl and 20 μl of 4% papain (type IV, double crystallized, 10 units/mg; Sigma–Aldrich, St Louis, MO) solution and 20 μl of 0.03 M l-cysteine (Sigma–Aldrich) were administered via i.a. injection into the right knee. The injections were given with a 26-gauge needle via the patellar tendon on days 1, 4, and 7 of the experiment, and the left knee was used as the contralateral control. After 1 week of papain-induced OA, the rats were randomly divided into two groups, with 18 animals in each group: the OA + Saline group (OA induction and daily oral administration of 0.9% normal saline) and the OA + celecoxib (OA + Celec) group (OA induction and daily oral administration of 10 mg/kg celecoxib in a 0.9% normal saline suspension). The rats in the OA + Saline and OA + Celec groups were treated with saline or celecoxib for 5 weeks, and rats from each group were killed by CO2 inhalation at 1, 3, and 5 weeks.
2.2. Histology and immunohistochemistry
The rat tibial plateaus with articular cartilage were collected and fixed with 10% neutral buffered formalin prior to histologic preparation. The decalcified samples were paraffin embedded, and 5-μm microsections of the coronary plane were prepared. GAG was stained with Safranin O–fast green (1% Safranin O counterstained with 0.75% hematoxylin and then 1% fast green; Sigma–Aldrich), and the total and red-stained areas in the articular cartilage of each proximal tibia were measured using Image-Pro Plus software, version 5.0. The ratio of the red-stained area to the total area (red:total) in each group was calculated.
For immunohistochemistry staining, the tibial articular sections were rehydrated, and endogenous peroxidase in the tissue was blocked with 3% hydrogen peroxide. Sections were then blocked with 10% FBS for 1 h and incubated with primary antibodies against type II collagen (1:2000; mouse monoclonal antibody; Chemi-Con, Temecula, CA), type X collagen (rat polyclonal antibody) (1:200; Cosmo Bio, Tokyo, Japan), COX-2 (rabbit polyclonal antibody) (1:50; Santa Cruz, Europe), PTHrP (rabbit polyclonal antibody) (1:100; OriGene, MD, USA) and MMP13 (1:200; rabbit polyclonal antibody) (1:100; Bioss, MA, USA) at 4 °C overnight. Then, the samples were incubated for 30 min with biotin-labeled goat anti-mouse/rabbit immunoglobulin and horseradish peroxidase-conjugated streptavidin (BioCare Medical, CA, USA) secondary antibodies. Staining with a 3,3′-diaminobenzidine solution (Dako) containing 0.01% hydrogen peroxide resulted in a brown color. Finally, sections were counterstained with hematoxylin and observed under a microscope. The relative density of immunostaining (density/area; mean ± SEM area 25.44 ± 2.77 mm2) was measured using Image-Pro Plus software, version 5.0.
2.3. Human articular chondrocyte cultures
Normal human articular chondrocytes (HACs) were obtained from the knee joint of a cadaver (provided by Kaohsiung Medical University Hospital) of a 23-year-old Asian male who died following a traffic accident and had previously provided donation consent. The cartilage samples were minced and digested sequentially with hyaluronidase (0.5 mg/mL), pronase (1 mg/mL), and collagenase (1 mg/mL) (Sigma–Aldrich, St. Louis, MO). The isolated chondrocytes were then cultured and expanded in a monolayer after 4 passages as previously described [31]. Normal human chondrocytes (HACs) from the knee used in the study also were purchased from Clonetics™ (Lonza Walkersville, Inc.). The cells cultured and maintained according to the manufacturer's protocol and expanded cell number until 8th passage in serial monolayer subculture. The expansion culture medium was Dulbecco's modified Eagle's medium (DMEM) containing 1% nonessential amino acids, 1% penicillin/streptomycin, 0.1% ITS (insulin, transferrin, and selenium) (Invitrogen), and 10% fetal bovine serum, and then, the cells were resuspended in alginate solution at a density of 1 × 106 cells/mL as previously described [31]. Briefly, after polymerization of alginate beads in a CaCl2 solution, fifteen beads were cultured in 5 mL of culture medium in each well of a 6-well plate. The beads were cultured in a humidified incubator at 37 °C and 5% CO2 for 7 days to allow redifferentiation before the experiment [14], and the culture medium was changed every 2 days.
2.4. Celecoxib and DFU treatment of articular chondrocytes in a 3D culture system
HACs were cultured in alginate beads and then treated with celecoxib and 5,5-dimethyl-3-(3-flurophyenyl)-4-(4-methylsulphonyl)phenyl-2(5H)-furan (DFU; an analog of rofecoxib) at 10−5 to 10−6 M (therapeutic concentration range) for 7 days. HACs were harvested on the 7th day after drug treatment. HACs were released from the alginate beads when the beads were dissolved in a 0.9% NaCl solution containing 0.05 M sodium citrate and 0.03 M sodium EDTA at pH 7.4. The cells were collected by low-speed centrifugation at 1500 rpm for 5 min.
2.5. Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from HACs using the TOOLSmart RNA Extractor (TOOLS, Taiwan). First-strand complementary DNA (cDNA) was generated from 1 μg of RNA using a TOOLS Easy Fast RT Kit (TOOLS, Taiwan). The levels of Col2a1, Col10a1, Runx2, MMP13, PTHrP and Ihh messenger RNA (mRNA) were measured via qRT-PCR with a Bio-Rad iQ5 real-time PCR detection system (Bio-Rad, Hercules, CA) using TOOLS 2X SYBR qPCR Mix (TOOLS, Taiwan). Reactions consisted of the cDNA, specific primers (Table 1) for each gene, and the TOOLS 2X SYBR qPCR Mix. Specific PCR products were detected with the double-stranded DNA-binding fluorescent dye SYBR Green [32]. The expression level of each target gene was then calculated as previously described [33]. All qRT-PCR amplifications were performed in triplicate, and the experiments were repeated at least three times.
Table 1.
Primer sequences used for real-time polymerase chain reaction.
| Gene | Forward (5’ → 3′) | Reverse (5’ → 3′) |
|---|---|---|
| COL2a1 | CAA CAC TGC CAA CGT CCA GAT | TCT TGC AGT GGT AGG TGA TGT TCT |
| Aggrecan | ACA GCT GGG GAC ATT AGT GG | GTG GAA TGC AGA GGT GGT TT |
| COL10a1 | AGC CAG GGT TGC CAG GAC CA | TTT TCC CAC TCC AGGAGGGC |
| PTHrP | ATG CAG CGG AGA CTG GTT CAG CAG T | GGG AGA GGG CTT GGA GTT AGG GG |
| Ihh | ATG TGC TCA TTT TCC TGG AC | CCA AGC TGT GAA AGA CTC TC |
| MMP13 | TCT TGA GCT GGA CTC ATT GT | AGC CTC TCA GTC ATG GAG CT |
| Runx2 PTGS2 |
AGA TGG GAC TGT GGT TAC TG TGA GCA TCT ACG GTT TGC TG |
GTA GCT ACT TGG GGA GGA TT TGC TTG TCT GGA ACA ACT GC |
| GAPDH | TCT CCT CTG ACT TCA ACA GCG AC | CCC TGT TGC TGT AGC CAA ATT C |
Cycling conditions: Denature: 95 °C for 30 s, 95 °C for 4 min, followed by 35 cycles of 95 °C for 10 s, 55–61 °C(shown in column of Annealing Temperature) for 15 s and 72 °C for 15 s
2.6. Western blot analysis
HACs were lysed in radioimmunoprecipitation assay buffer (Invitrogen) supplemented with protease inhibitor cocktail (Sigma–Aldrich). After lysis, total proteins were separated in a NuPAGE Novex 4–12% Bis-Tris Gel (Invitrogen) and transferred to polyvinylidene difluoride nylon membranes (Sigma–Aldrich). The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline and incubated overnight at 4 °C with primary antibodies against COX-2 (sc-7951; Santa Cruz Biotechnology, CA, USA), Col10a1 (bs0554 R; Bioss, MA, USA), PTHrP (TA334682; OriGene Technology, MD, USA) and GAPDH (MA5-15738; Thermo Scientific, MA, USA). Specific bands were detected with a horseradish peroxidase-conjugated secondary antibody and were read using an enhanced chemiluminescence western blot system from Pierce (Rockford, IL, USA).
2.7. Transfection with expression plasmids
The human COX-2 (Ptgst2) (NM_000963.3) cDNA sequence cloned in pUC57-Amp was purchased from GENWIZ, Inc. and subcloned into a pcDNA3.1+ expression vector (Invitrogen™). HACs were plated at 1 × 105 cells per well in 6-cm dishes. The cells were transiently transfected with COX-2 plasmid constructs combined with the ViaFect™ transfection reagent (Promega) according to the manufacturer's instructions. The cells were transfected with a total of 1 μg DNA in Opti-MEM (Invitrogen), and the control group was transfected with empty vector. After 24 h and 72 h of incubation with transfection mixtures, the cell supernatant was collected and replaced with culture medium.
2.8. Reporter plasmids and luciferase assay
The 5′-flanking region of the PTHrP gene was obtained from HACs using a DNeasy Tissue Kit (QIAGEN, Dusseldorf, Germany). Moreover, the 5′-PTHrP promoter was amplified by PCR with the following primer pair (forward) PTHrP/-944XhoI, 5′-CTCGAGACCTTGAGGTCAGCTGGCAA-3′, and (reverse) PTHrP/+50 HindIII, 5′-GGGAACAGAAGTCTCCTTTCAAGAA-3’. The XhoI/HindIII double-digested PCR products were subcloned into the promoter-less pGL3-basic vector. For the reporter assay, HACs were transiently cotransfected with pcDNA3.1-basic or pcDNA3.1-COX-2 cDNA + pGL3-empty or pGL3-PTHrP promoter. The cells were transfected using ViaFect (Promega) according to the manufacturer's instructions. Identical DNA amounts of the corresponding empty vectors were used as controls in all transfection experiments. Luciferase activities of the transfectants were measured 24 h after transfection using a Luciferase Reporter Gene Assay kit (Roche) according to the manufacturer's instructions.
2.9. Prostaglandin E2 (PGE2) ELISA
For measurement of the PGE2 concentrations in COX-2-overexpressing chondrocytes, the supernatant was collected. For each assay, 100 μL of the supernatant was added to a well of the 96-well ELISA plate from the Human PGE2 Express EIA kit (batch no. 0473922, Cayman Chem, USA) and sequentially incubated with detection antibodies and chromogenic substrate according to the manufacturer's instructions. The optical density was read at 420 nm. The PGE2 concentration was determined based on a standard curve that was generated using the serially diluted reference samples provided in the PGE2 ELISA kit and is presented as pg/mL.
2.10. Cytotoxicity assayed by lactate dehydrogenase leakage
Lactate dehydrogenase (LDH) leakage from cells was measured to quantify cytotoxicity using a cytotoxicity detection kit (Roche, Germany). HACs were seeded in 24-well plates (5 × 104 cells/well). After drug treatment, the supernatants and cells of the cultures were collected for assay. According to the manufacturer's guidelines for the detection kit, the cells were lysed with 1% Triton X-100, and cell lysates and supernatants were assayed in 96-well plates. Briefly, 100 μL of catalyst solution was added to each assay well for 20 min. Absorbance was measured with an ELISA reader with a 490-nm filter. LDH leakage from HACs was calculated according to the following formula: LDH leakage = supernatant/(supernatant + cell).
2.11. Apoptotic cell measurement with TUNEL staining
We measured cell death using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining method and the In Situ Cell Death Detection Kit with TMR red (Roche, Germany). Following the manufacturer's guidelines, cells were fixed with 4% paraformaldehyde in PBS and were permeabilized by incubation in a permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate) for 2 min on ice. The TUNEL reaction mixture containing terminal deoxynucleotidyl transferase and rhodamine (the labeling dye) was added to the slides and incubated at 37 °C in a humidified chamber in the dark for 60 min. The reaction was stopped by a blocking buffer (0.1% Triton X-100/0.5% BSA in PBS). The cells nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI), and only dead cells were stained red by rhodamine. The slides were observed under a fluorescence microscope (Nikon TE300) with an excitation wavelength of 580 nm for rhodamine and 365 nm for DAPI. Stained cells were counted in 5 microscopic fields on each slide. Data were analyzed using Image-Pro Plus analytical software (Media Cybernetics, Silver Spring, MD). The death rate of chondrocytes was defined as the ratio of red-stained cells (dead cells) to blue-stained cells (total cells).
2.12. Cell hypertrophy detected by flow cytometry
HACs were resuspended and fixed with 4% paraformaldehyde in PBS and were incubated at room temperature for 10 min. After centrifugation, the cells were resuspended in ice-cold PBS. For analysis of cell size, cells from each individual experiment were treated with propidium iodide to stain the cell nuclei (1 μg/mL final concentration) and analyzed on a FACSCalibur flow cytometer (EPICS Elite; Coulter, Hialeah, FL, USA). Data were analyzed by Winmidi software (EPICS Elite) using propidium fluorescence to exclude cell debris and forward light scatter to evaluate the cell size.
2.13. Statistical analysis
Data are presented as the mean and SEM of the results from 4 samples in the in vitro study and 6 samples in the in vivo study. All experiments were repeated at least 3 times. Statistical significance was evaluated using one-way analysis of variance, and multiple comparisons were performed using Scheffe's test. P values less than 0.05 were considered significant.
3. Results
3.1. Celecoxib suppresses GAG loss in OA articular cartilage
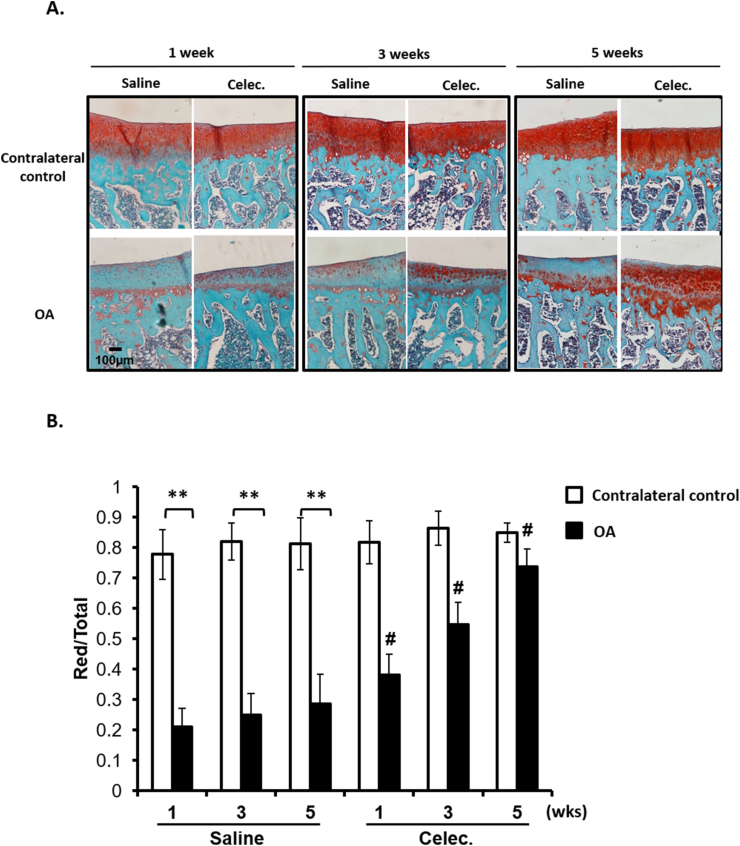
To identify the effects of COX-2 inhibitors on the articular cartilage of OA rats, we used the COX-2 inhibitor celecoxib for an in vivo study. Papain-induced OA rats were randomly divided into two groups: OA + Saline and OA + celecoxib (OA + Celec). First, we evaluated the effects of celecoxib on the proteoglycan content in normal and osteoarthritic articular cartilage. The results showed that GAG content significantly decreased in the OA + Saline group compared with that of the contralateral control at 1, 3 and 5 weeks (Fig. 1). GAG content in osteoarthritic cartilage in the OA + Celec group was significantly higher than that in the OA + Saline group, but no difference in the normal cartilage or growth plate (data not shown) was observed between the OA + Saline and OA + Celec groups (Fig. 1). The findings indicate that celecoxib has beneficial effects on proteoglycan homeostasis during OA progression and has no adverse effects on normal cartilage in vivo.
Figure 1.
Celecoxib reverses glycosaminoglycan (GAG) content (A) Safranin O staining analysis of GAG content in articular cartilage of tibia sections in the OA + Saline and OA + Celec groups. The upper panel shows the contralateral control, and the lower panel shows the osteoarthritic articular cartilage (scale bars: 100 μm) (B) Comparison of the ratio of the Safranin O-stained area to total area (red/total) among all groups after 1, 3, and 5 weeks of celecoxib treatment. Each column shows the mean and SEM of 6 samples. Data were evaluated by one-way analysis of variance (ANOVA), and multiple comparisons were performed using Scheffe's test. ∗, P < 0.05; ∗∗, P < 0.01 compared to the control culture. #, P < 0.05 compared to the OA + Saline group.
3.2. Celecoxib attenuates type X collagen and MMP13 in OA articular cartilage
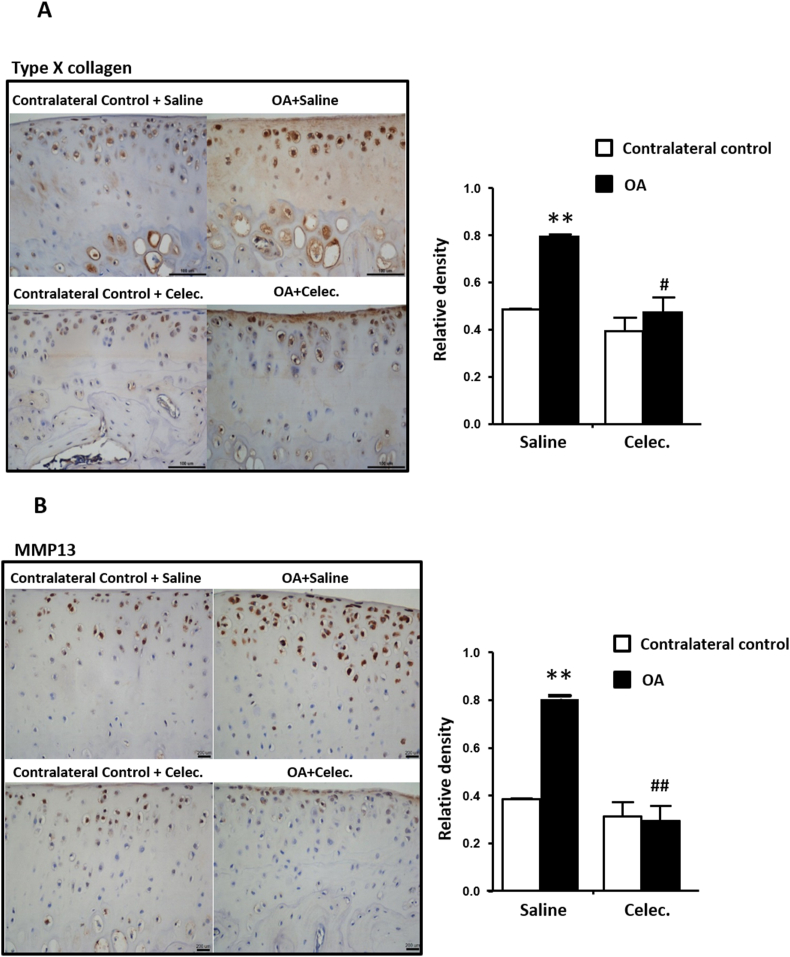
Type X collagen and MMP13 are markers of cartilage degeneration and hypertrophic chondrocytes [10]. The relative densities of type X collagen and MMP13 were significantly increased in OA articular cartilage compared with the contralateral control. Conversely, type X collagen and MMP13 were significantly decreased in osteoarthritic cartilage in the OA + Celec group compared with the OA + Saline group at 1, 3 and 5 weeks (only the 5th week data are shown) (Fig. 2A and B). Overall, celecoxib significantly inhibited type X collagen and MMP13 expression in OA articular cartilage.
Figure 2.
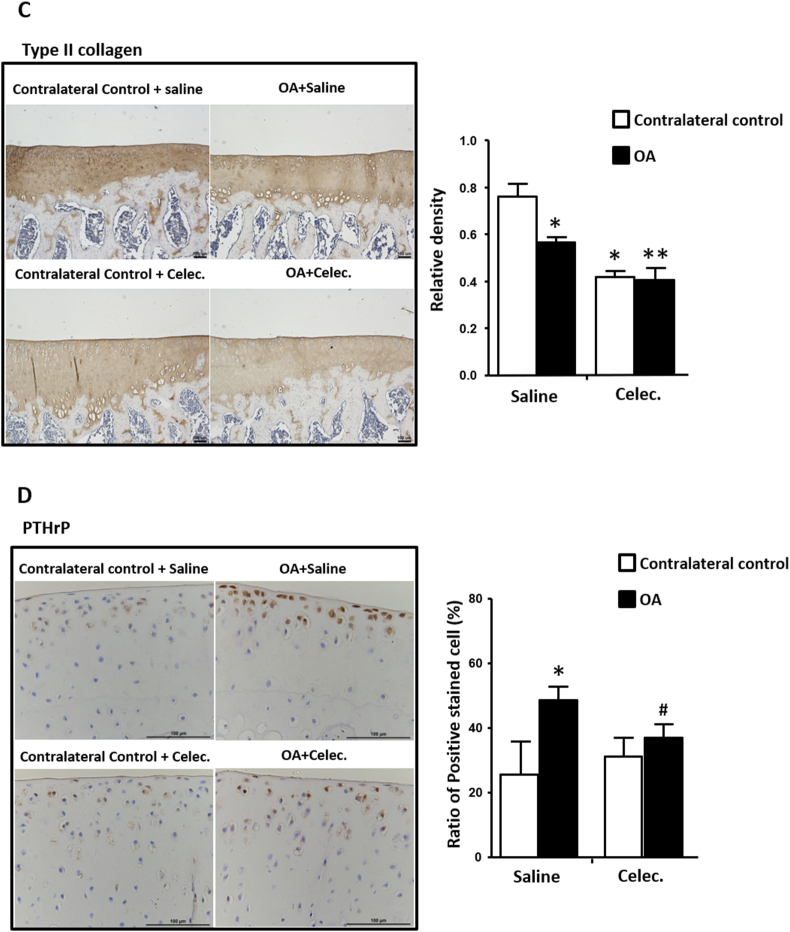
Celecoxib reduces type X collagen, MMP13 and PTHrP levels and inhibits type II collagen expression in osteoarthritic articular cartilage (A) Immunohistochemical analysis of type X collagen (scale bars: 100 μm) (B) MMP13 (scale bars: 200 μm) (C) type II collagen, and (D) PTHrP (scale bars: 100 μm) in articular cartilage of tibia sections in the OA + Saline and OA + Celec groups. The histogram illustrates the quantification of the positively stained type X collagen and the relative density of MMP13, type II collagen and PTHrP expression in articular cartilage. Each column represents the mean ± SEM of six samples. Data from each group were compared with those of the OA contralateral control group and were evaluated by one-way ANOVA and Scheffe's test. ∗, P < 0.05; ∗∗, P < 0.01; the OA + Celec groups compared to the OA + Saline groups #, P < 0.05; ##, P < 0.01.
3.3. Celecoxib reduces type II collagen and PTHrP in OA articular cartilage
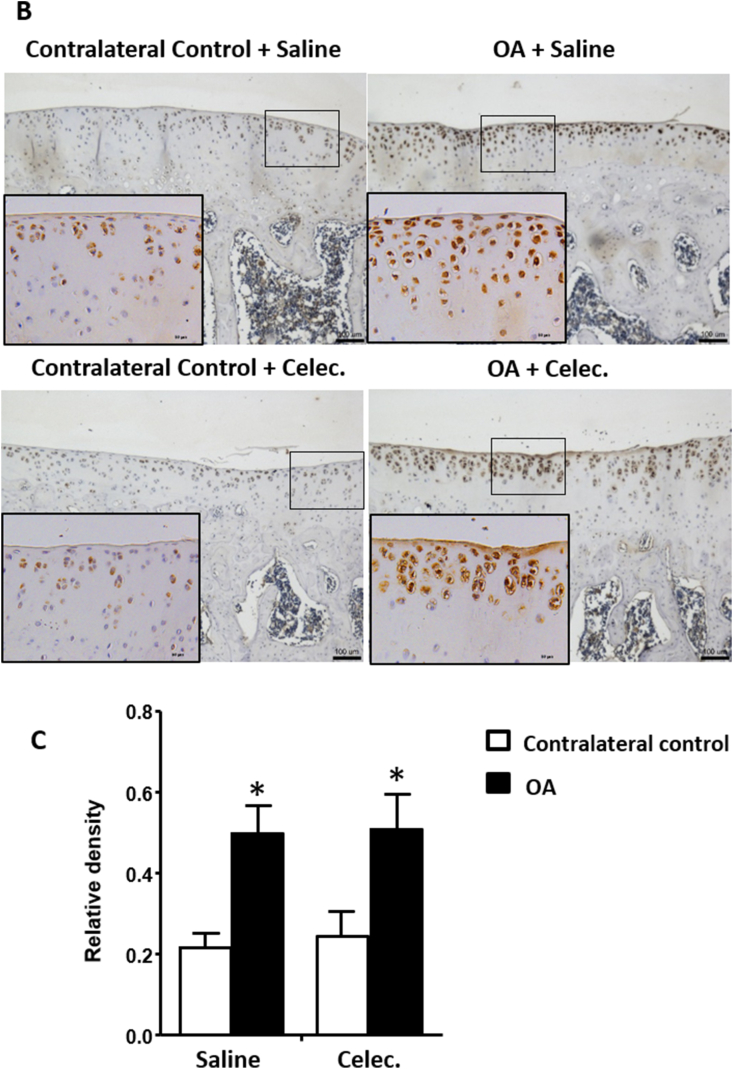
Type II collagen can be used to assess degenerative characteristics in OA cartilage, and the relative density of type II collagen was significantly reduced in OA articular cartilage compared with the contralateral control at the 5th week of observation. However, in the OA + Celec group, both normal and osteoarthritic articular cartilage showed significantly suppressed type II collagen expression compared with that of the contralateral control of the OA + Saline group (Fig. 2C). The results showed that type II collagen was decreased during OA progression and that celecoxib suppressed type II collagen in both OA and normal cartilage. Several studies have demonstrated that PTHrP has antihypertrophic and OA-protective effects [29]. Therefore, the effects of celecoxib on PTHrP expression during OA progression were evaluated. The results showed that PTHrP expression was significantly increased in osteoarthritic cartilage compared with the contralateral control in the OA + Saline group, whereas PTHrP in osteoarthritic cartilage was significantly suppressed in the OA + Celec group compared with the OA + Saline group (Fig. 2D). Therefore, celecoxib inhibition of COX-2 activity simultaneous downregulated PTHrP expression in OA cartilage.
3.4. COX-2 is elevated in OA articular cartilage
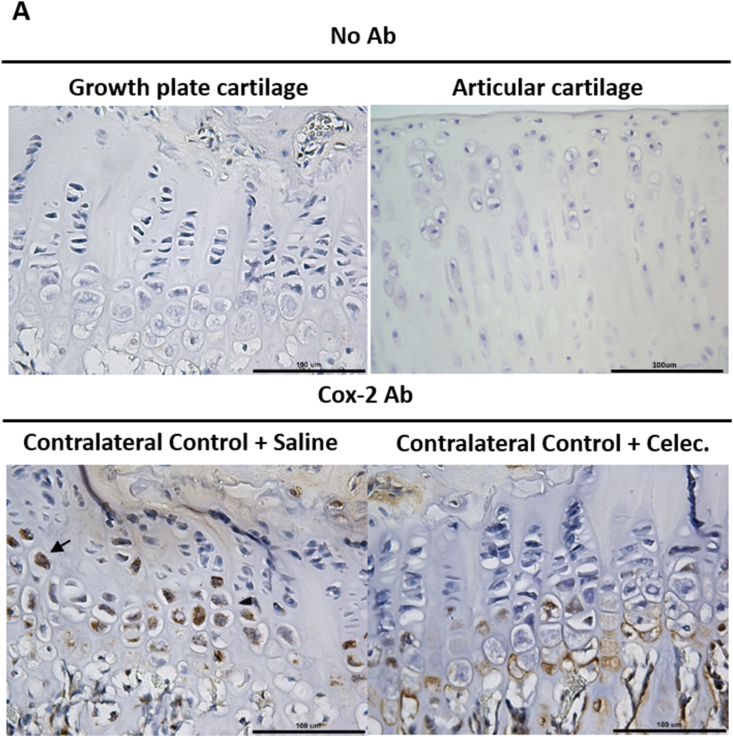
The effects of celecoxib on COX-2 expression in articular cartilage and growth plate cartilage were evaluated. The results showed that COX-2 was mainly located in the hypertrophy zone of the growth plate and the superficial zone of articular cartilage (Fig. 3). The cellular localization of COX-2 in growth plate cartilage was clearly different between the OA + Saline and OA + Celec groups. COX-2 was located in the nucleus in the contralateral control in the OA + Saline group but localized in the cell membrane in the OA + Celec group (Fig. 3A). Additionally, COX-2 levels increased significantly in osteoarthritic cartilage compared with normal cartilage (contralateral control) in both the OA + Saline and OA + Celec groups, but no difference was observed between the OA + Saline and OA + Celec groups (Fig. 3B and C). These results are consistent with the function of celecoxib in inhibiting COX-2 enzymatic activity but not COX-2 protein expression. COX-2 expression was very low in the superficial layer and hypertrophic chondrocytes of the deep zone (calcified zone) of normal articular cartilage. The results demonstrate that COX-2 is highly expressed in hypertrophic growth plate chondrocytes and in the superficial layer of OA articular cartilage. Overall, PTHrP, type X collagen and MMP13 were mainly expressed in the superficial layer of articular cartilage and correlated with the COX-2 expression profile.
Figure 3.
COX-2 expression increases in hypertrophic growth plate cartilage and osteoarthritic articular cartilage. Immunohistochemical analysis of COX-2 (brown color) expression in the growth plate and articular cartilage of the tibia sections at 5 weeks in the OA and OA + Celec groups (A) COX-2 is strongly expressed in the nuclei of hypertrophic chondrocytes but not in resting or proliferative chondrocytes in growth plate cartilage (bottom left panel). The right panel shows that COX-2 was mainly expressed near the cell membrane of hypertrophic chondrocytes (scale bar: 100 μm). The upper panel shows the no antibody control (B) High COX-2 expression was detected in osteoarthritic articular cartilage of tibial sections (upper and lower right panels) at the 5-week time points in the OA + Saline and OA + Celec groups but not in the contralateral control (upper and lower left panel) (scale bar: 100 μm). The bottom shows higher magnification of the boxed area (scale bar: 50 μm) (C) Histogram illustrating the relative density of COX-2 expression in articular cartilage. Each column represents the mean ± SEM of six samples. Data from each group were compared with those of the OA contralateral control group and were evaluated by one-way ANOVA and Scheffe's test. ∗, P < 0.05.
COX-2 inhibitors at therapeutic concentrations simultaneously suppress Aggrecan, Col2a1, PTHrP and Col10a1 expression without cytotoxic effects.
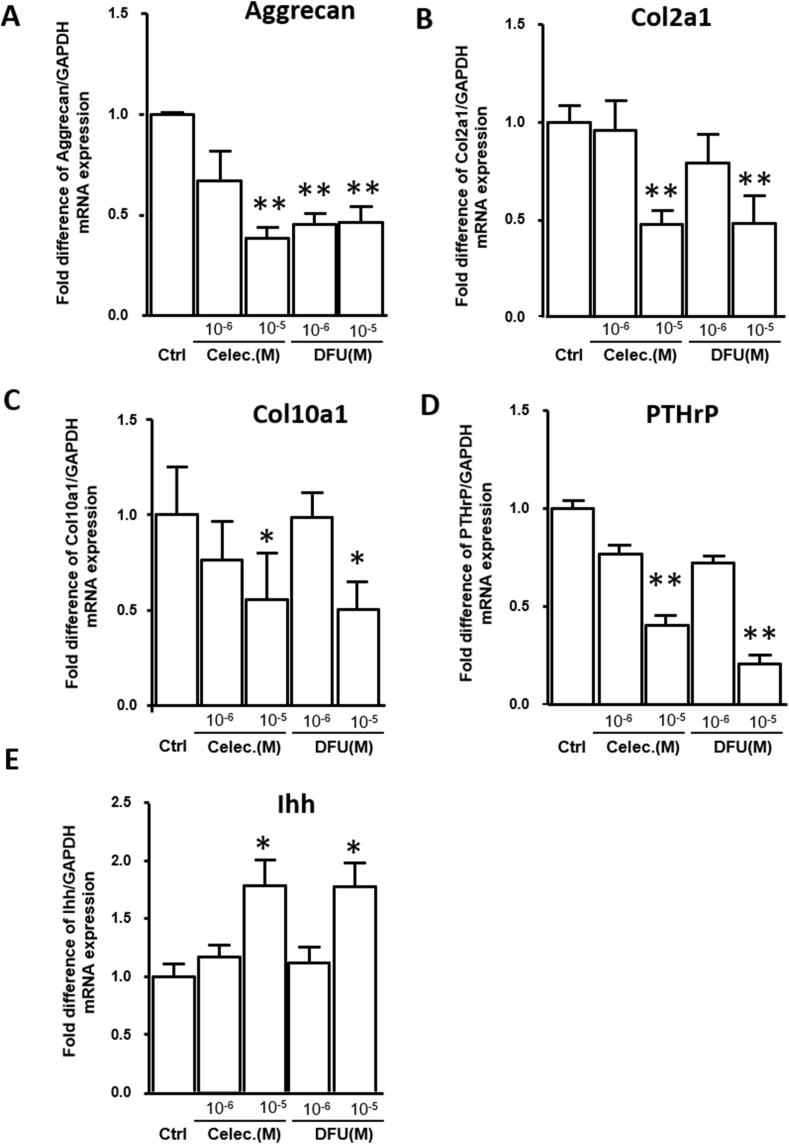
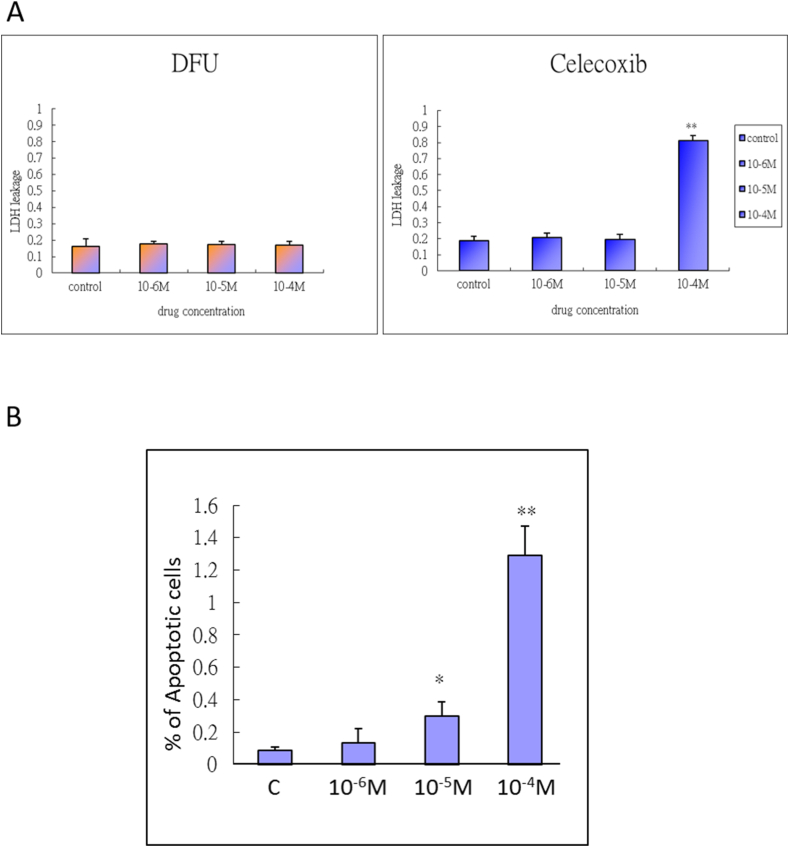
To verify the results of the in vivo celecoxib study, we further used the COX-2 inhibitors celecoxib and DFU to assess the expression of chondrogenic and terminal differentiation-related genes. We used an alginate bead culture of HACs that mimicked the cartilage phenotype and treated the cells with COX-2 inhibitors at a dose that ranged from 10−6 to 10−5 M, which was estimated from the therapeutic and maximum plasma concentrations. After 7 days of treatment, qRT-PCR was performed to analyze Aggrecan, Col2a1, Col10a1, PTHrP and Ihh mRNA expression. Inhibition of COX-2 significantly inhibited Aggrecan, Col2a1, Col10a1, and PTHrP mRNA expression but increased Ihh expression in the 10−5 M celecoxib and DFU groups compared with the control group (Fig. 4). Next, we evaluated the cytotoxicity and apoptotic effects of the COX-2-specific inhibitors in a dose-dependent manner with treatment for 48 h. LDH leakage from chondrocytes was significantly elevated to 80% in cells treated with 10−4 M celecoxib but not in cells treated with DFU. The results showed high cytotoxic effects of celecoxib at 10−4 M but not at 10−6 and 10−5 M therapeutic doses (Fig. 5A). Cell apoptosis was increased in a dose-dependent manner following 48 h of celecoxib treatment, and doses from 10−5 to 10−4 M showed significant apoptotic effects (Fig. 5B). These results indicate that only a high concentration of COX-2 inhibitor caused chondrocyte death, while a therapeutic concentration of 10−5 M had mild apoptotic effects.
Figure 4.
Inhibition of COX-2 activity by celecoxib and DFU influences downstream targets in cultured HACs (A) Aggrecan (B) Col2a1 (C) Col10a1 (D) PTHrP, and (E) Ihh mRNA expression in vitro in cells treated with celecoxib and DFU (10−6–10−5 M) for 7 days. GAPDH mRNA was used as the internal control. Each column represents the mean ± SEM of four replicate cultures. Data from each group were compared with those of the control group and were evaluated by one-way ANOVA and Scheffe's test. ∗, P < 0.05; ∗∗, P < 0.01 compared to the control culture.
Figure 5.
The cytotoxic and apoptotic effects of COX-2 inhibitors on human articular chondrocytes (A) Cultures were treated with DFU and celecoxib (10-4 M, 10-5 M, or 10-6 M) for 48 h and then harvested to quantify cytotoxicity. Each column represents the lactate dehydrogenase (LDH) leakage mean ± SEM of four replicate cultures. Data from the individual drug treatment experiments were evaluated by one-way ANOVA. ∗∗, P < 0.01 compared to the control culture (B) Cultures were treated with celecoxib (10-4 M, 10-5 M, or 10-6 M) for 48 h and then harvested to measure cell apoptosis. The nuclei of apoptotic cells were positively stained by TUNEL and were DNA counterstained by DAPI. Each column represents the % of apoptotic cells as the mean ± SEM of four replicate cultures. Data from each group were compared with those from the control cultures and were evaluated by one-way ANOVA. ∗, P < 0.05; ∗∗, P < 0.01 compared to the control culture.
3.5. Transient COX-2 overexpression does not cause cell death
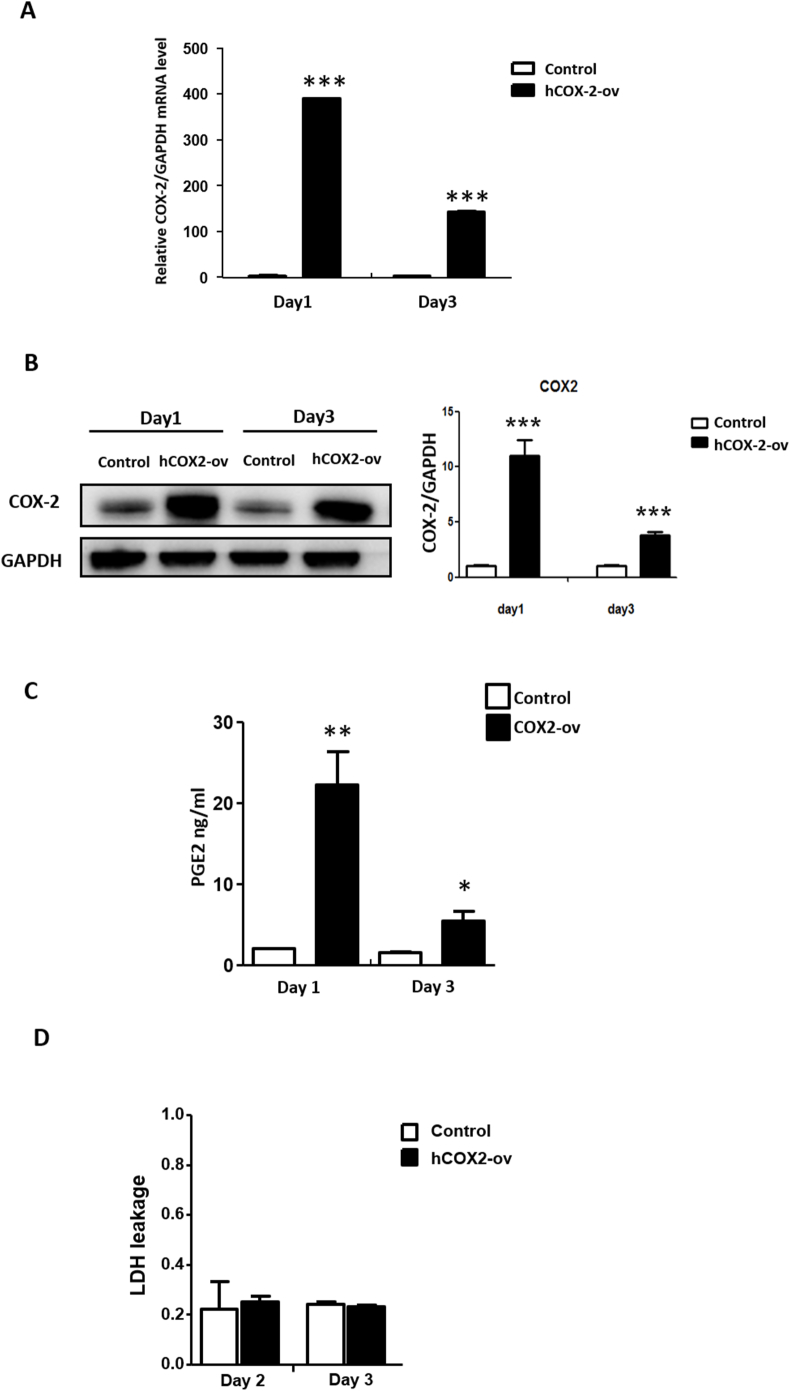
To assess the cellular effects of COX-2 on chondrocytes, we transiently transfected HACs with the pcDNA3.1-COX-2 (Ptgst2 cDNA) vector. First, we measured the COX-2 mRNA and protein levels and enzymatic activity after transfection of HACs with the COX-2 overexpression plasmid for 1 and 3 days. The results showed that the COX-2 overexpression group exhibited increased Ptgs2 mRNA expression, COX-2 protein level (Fig. 6A and B) and PGE2 concentration in the culture medium compared with the control group (Fig. 6C). In addition, based on an LDH leakage assay at day 2 and day 3, COX-2 overexpression in HACs did not cause cell death compared with the control (Fig. 6D).
Figure 6.
Transient overexpression of COX-2 in HACs (A) qRT-PCR analysis of COX2 mRNA expression was determined following transfection with pcDNA3-COX-2 and control pcDNA3-empty vector for 1 and 3 days. GAPDH mRNA was used as the internal control. ∗∗∗, P < 0.001 compared to the control pcDNA3-empty vector (B) Western blot analysis of COX-2 protein levels digitally detected and normalized to GAPDH levels as a loading control (left panel). Each bar represents the mean ± SEM of three replicate cultures (right panel). Data from each group were compared with those of the control cultures and were evaluated by one-way ANOVA. ∗∗, P < 0.01 compared to the control vector (C) Human PGE2 concentrations in the supernatant of COX-2-overexpressing chondrocytes measured using an ELISA kit. Each bar represents the mean ± SEM of three replicate cultures. Data from each group were compared with those of the control cultures and were evaluated by one-way ANOVA. ∗, P < 0.05; ∗∗, P < 0.01 compared to the control vector (D) LDH leakage from cells was measured to quantify the cytotoxicity after transfection with pcDNA3.1-COX-2 and control pcDNA3.1-empty vector for 2 and 3 days. Each column represents the mean ± SEM of six replicate cultures.
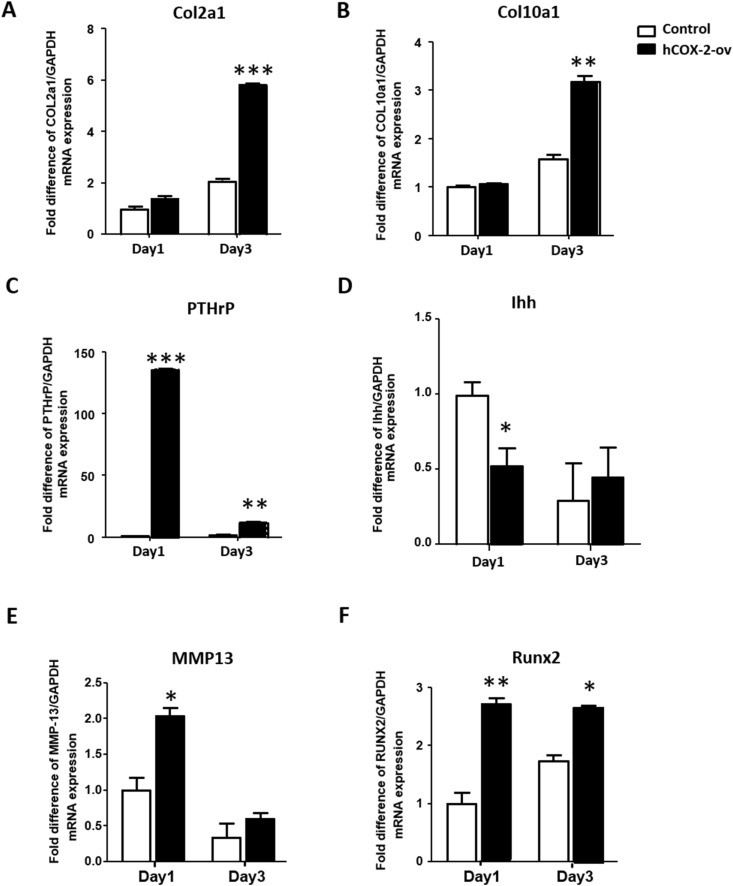
3.6. COX-2 promotes Col2a1, Col10a1, PTHrP, MMP13 and Runx2 expression
The downstream target genes of COX-2, including Col2a1, Col10a1, PTHrP, Ihh, MMP13, and Runx2, were assessed. COX-2 overexpression in HACs significantly increased the Col2a1 and Col10a1 transcript levels at day 3 (Fig. 7A and B) and increased the PTHrP, MMP13 and Runx2 transcript levels from day 1 to day 3 (Fig. 7C, E, F). Conversely, COX-2 overexpression in HACs significantly suppressed Ihh transcription at day 1 (Fig. 7D). Among these genes, PTHrP showed the greatest change, 136.45-fold on day 1 and 12.68-fold on day 3, compared with the control. In contrast with the results obtained with COX-2 inhibitors, COX-2 simultaneously promoted the expression of chondrogenic and hypertrophy-related genes.
Figure 7.
Overexpression of COX-2 increases Col2a1, Col10a1, PTHrP, MMP13 and Runx2 transcription (A) Col2a1 (B) Col10a1 (C) PTHrP (D) Ihh (E) MMP13, and (F) Runx2 mRNA expression was measured in vitro in cells transfected with pcDNA3.1-COX-2 and the control pcDNA3-empty vector for 1 and 3 days. GAPDH mRNA was used as the internal control. Each column represents the mean ± SEM of four replicate cultures. Data from each group were compared with those of the control group and were evaluated by one-way ANOVA and Scheffe's test. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 compared to the control culture.
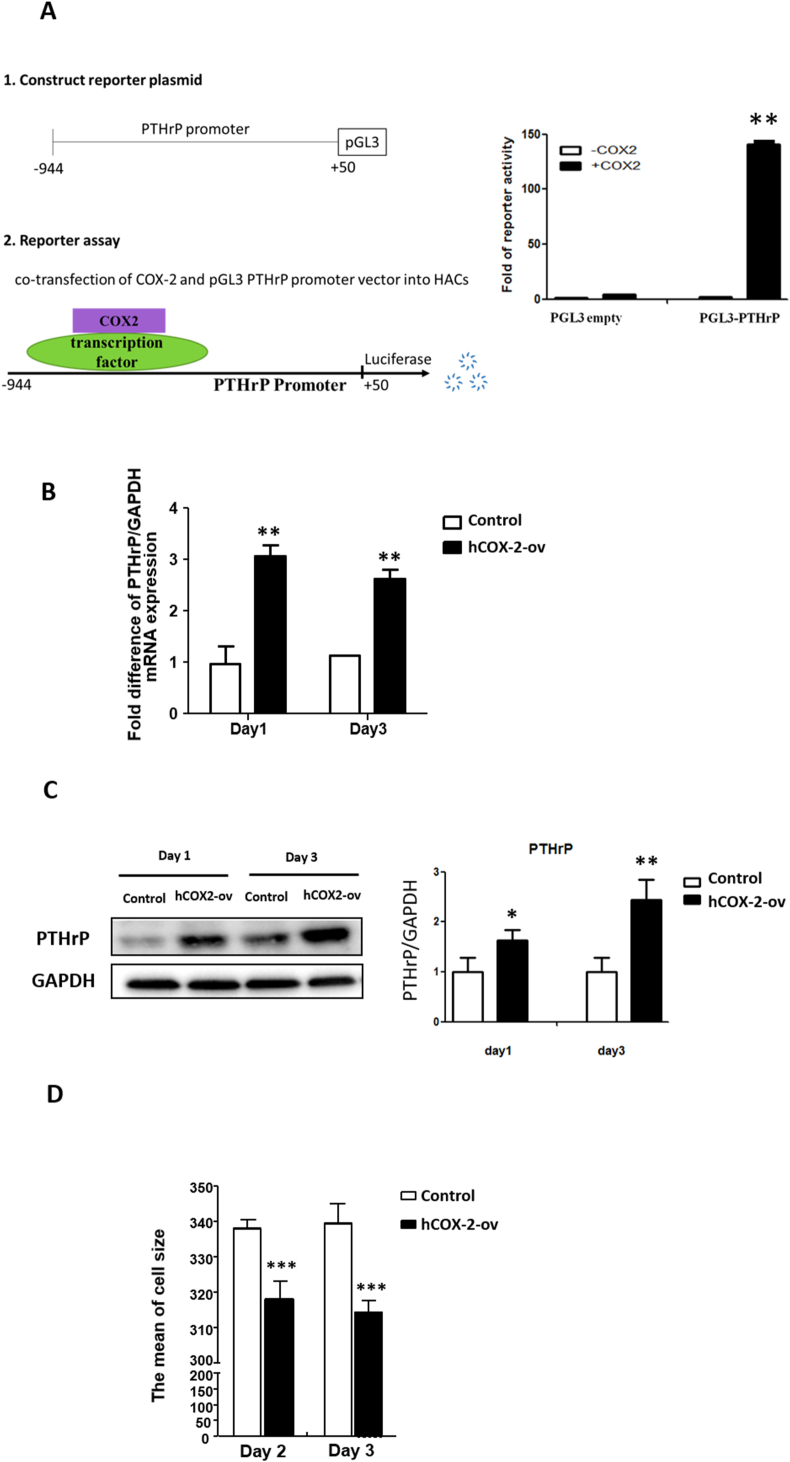
3.7. COX-2 plays a regulatory role in promoting PTHrP expression and reducing cell size
On days 1 and 3, COX-2 overexpression in HACs significantly increased the PTHrP mRNA and protein levels compared with those in the control cells (Fig. 8B and C). To further demonstrate that COX-2 might be a transcriptional cofactor that regulates PTHrP expression, a reporter assay was performed. The PTHrP promoter region was cloned into a reporter vector to assess whether COX-2 enhances PTHrP promoter activity. The results showed that transient transfection with COX-2 significantly increased reporter activity in HACs (Fig. 8A), suggesting that COX-2 plays a transcriptional regulatory role in PTHrP expression. Because COX-2 promoted PTHrP expression but suppressed Ihh expression (Fig. 7C D), we reasonably suggest that COX-2 reduced HAC hypertrophy through PTHrP-Ihh signaling. Flow cytometry was used to analyze the size of COX-2-overexpressing HACs. As expected, the COX-2-overexpressing HACs had a significantly reduced cell size compared with the control cells (Fig. 8D).
Figure 8.
COX-2 promotes PTHrP expression by increasing PTHrP promoter activity and suppressing cell hypertrophy (A) Putative COX-2-binding motifs in the PTHrP reporter. The 5′-flanking region of the human PTHrP gene was subcloned into the pGL3 basic reporter. COX-2 activates PTHrP reporter activity in HACs. pcDNA3.1-COX-2 or pcDNA3.1-empty was cotransfected with the PTHrP (−944 to +50) reporter. The data are presented as the mean ± SE of relative luciferase activity from three independent experiments performed in duplicate. ∗∗, P < 0.01 compared to the pGL3-empty vector (B) qRT-PCR analysis of PTHrP mRNA expression was performed following transfection with pcDNA3-COX-2 and control pcDNA3-empty vector for 1 and 3 days. GAPDH mRNA was used as the internal control. ∗, P < 0.05 compared to the control pcDNA3-empty vector (C) Western blot analysis of PTHrP protein levels digitally detected and normalized to GAPDH levels as a loading control (left panel). Each bar represents the mean ± SEM of three replicate cultures (right panel). Data from the pcDNA3.1-COX-2 vector (hCOX2-ov) group were compared with those of the control pcDNA3.1-empty vector (control vector) group and were evaluated by one-way ANOVA. ∗∗, P < 0.01 compared to the control vector (D) Flow cytometry evaluating the cell size after transfection of cells with pcDNA3.1-COX-2 and control pcDNA3.1-empty vector for 2 and 3 days. Representative results of the mean cell size are shown in the flow cytometry analysis (left panel). Each column represents the mean ± SEM of six replicate cultures. ∗∗∗, P < 0.001 compared to the control culture.
4. Discussion
In the present study, we found that COX-2 and PTHrP levels were elevated in OA articular chondrocytes, while systemic inhibition of COX-2 activity reduced the elevated PTHrP level. In normal HACs, COX-2 overexpression strongly increased PTHrP transcriptional activity, mRNA expression and protein level and decreased the size of HACs. A previous report from Gu et al. indicated that COX-2 not only possesses membrane-binding enzyme properties but also acts as a transcriptional cofactor [20]. In this study, overexpressed COX-2 was found to also potentially act as a transcriptional cofactor to increase the transcriptional activity of PTHrP and other genes. Based on this finding, we suggest that COX-2 may play a physiological role in upregulating PTHrP expression in articular chondrocytes and play a pathophysiological role in slowing terminal differentiation of articular chondrocytes by upregulating PTHrP at the early stage of OA progression.
In addition to the influence of COX-2 on PTHrP, we found that COX-2 overexpression in HACs simultaneously upregulates the expression of chondrogenic genes, aggrecan and collagen type II and several degeneration-related genes, namely, type X collagen, Runx2 and MMP13, but not Ihh. COX-2 expression was reportedly increased after chondrogenic differentiation, and Col2a1, Col10a1 and Runx2 gene expression was also simultaneously induced in ATDC5 cells, which are endochondral progenitors that can be induced to hypertrophic chondrocytes [21,34]. COX-2 was also found to be a Col10a1 interacting factor that shows crosstalk with the BMP-2 pathway and is required for chondrocytic hypertrophy [20,21]. Accordingly, these reports indicate that COX-2 may be involved in the process of terminal differentiation of chondrocytes residing in the long bone growth plate. On the other hand, articular chondrocytes normally maintain a permanent chondrogenic status and do not undergo terminal differentiation. During OA progression, articular chondrocytes undergo terminal differentiation, and degeneration occurs [[11], [12], [13]]. During the terminal differentiation of either epiphyseal or articular chondrocytes, PTHrP plays a role in maintaining chondrogenic function and survival of chondrocytes, while Ihh plays the opposite role. The ratio of PTHrP to Ihh has been proposed as an index of the initiation or inhibition of chondrocyte terminal differentiation. In this study, we found that COX-2 overexpression in normal articular chondrocytes strongly increases PTHrP expression but does not affect Ihh. This result suggests that COX-2 may function to maintain chondrocyte function and prevent terminal differentiation/degeneration through PTHrP. On the other hand, in the in vivo study, we found COX-2 and PTHrP were coexpressed in articular cartilage and especially predominant in osteoarthritic cartilage. Based on our in vitro study, the increased PTHrP may be regulated by COX-2. Previous studies have indicated that mechanical stress causes expression of COX-2 or PTHrP [35,36]. It is possible that the mechanical stress on articular cartilage induces COX-2 expression and subsequently upregulates PTHrP expression. However, these reports did not indicate whether the increased COX-2 induces subsequent cytokine release or inflammatory responses.
Taken together, the findings of this study and of previous studies indicate that the role of COX-2 in articular chondrocytes is more favorable for maintaining the chondrogenic status, unlike that in chondrocytes of epiphyseal growth plates, in which COX-2 favors terminal differentiation/bone development. More importantly, our animal study showed that COX-2, PTHrP and collagen type X expression is concomitantly elevated in OA cartilage. This indicates that the upregulated COX-2 may be involved in mechanisms directing the “go or no-go” signals determining terminal differentiation of articular chondrocytes. The key switch mechanism may rely on the amount of elevated PTHrP. If the PTHrP level is sufficient to maintain chondrogenesis, then normal cartilage is maintained; however, if it is not, chondrocytes undergo terminal differentiation, and OA develops.
In contrast to the effect of COX-2 overexpression in HACs, we also found that treatment of HACs with COX-2 inhibitors downregulated aggrecan, PTHrP, Col2a1 and Col10a1 mRNA expression but not Ihh expression. These results confirmed our findings concerning the influence of COX-2 on HACs. In the animal study, systemic treatment with a COX-2 inhibitor (celecoxib) reduced the collagen type II level in normal cartilage, and this effect was consistent with the findings in HACs in vitro. However, we found that the COX-2 inhibitor did not change GAG deposition, PTHrP, collagen type X, and MMP13 levels in non-osteoarthritic cartilage. A possible reason for this inconsistent effect of celecoxib between the in vitro and in vivo studies may be that the basal PTHrP, collagen type X and MMP13 protein levels in normal cartilage are extremely low. Therefore, the suppressive effect of celecoxib on these marker proteins may not be detectable. Most previous studies on the effect of COX-2 inhibitors on normal articular cartilage indicated that celecoxib showed no significant effect on normal articular cartilage or chondrocytes in vitro [37,38]. These results are similar to our findings, implying that celecoxib may not have harmful effect on normal articular cartilage. On the other hand, in previous studies of cultured OA cartilage, celecoxib reduced the disease-induced harmful effects on chondrocytic survival or matrix formation [[39], [40], [41]]. The beneficial effect of celecoxib in OA has also been reported in animal studies and human subjects and mainly results from its anti-inflammatory effect [42,43]. In addition, no chondroprotective effects of celecoxib were observed in an experimentally induced OA model [44,45]. However, some reports have indicated that celecoxib has chondroprotective properties and can potentially slow OA progression in human [46]. Accordingly, the disease-modifying effects of celecoxib on OA are contradictory. In this study, celecoxib upregulated GAG levels and downregulated MMP13 and Col10a1 levels but reduced PTHrP and type II collagen levels in osteoarthritic cartilage. These results indicate that celecoxib may have minor disease-modifying effects, but the suppression of COX-2 activity and the subsequently decreasing PTHrP level may be the reason that it cannot suppress the progression of OA.
In this study, we chose the papain-induced OA model that has been used to study the efficacy of OA drugs on inflammation or pain [47]. The benefit of this OA model is that it is an easy, short-term, and reproducible animal model. Moreover, this method only damaged articular cartilages and did not affect the subchondral bone. Although COX-2 inhibitors were reported to suppress bone formation and angiogenesis [48], the influence of celecoxib on articular cartilage found in this study may not be related to its effect on either bone volume or angiogenesis at subchondral bone. However, proof of concept on only one OA animal model is still a limitation of this study. The pathophysiology of post-traumatic OA (PTOA) or age-related OA differs from chemically induced OA, and researchers should also investigate the role COX-2 in OA with different causes in the future.
In this study, we provide more information regarding the ability of celecoxib to reverse the effect of COX-2 on terminal differentiation/degeneration of articular cartilage. More extensive research is needed to further clarify the role of COX-2 in articular cartilage using a chondrocyte-specific COX-2 knockout mouse model and investigate the role of COX-2 in OA progression.
Funding sources
This study was supported by grants from the National Science Council (NSC 98-2628-B-037-001-MY3), the Ministry of Science and Technology of Taiwan (MOST 104-2314-B-037-032-MY3), and Kaohsiung Medical University (KMU-TP104B09, KMU-DK105009, KMU-TC108A02-0, KMU-TC109A02-0). The study sponsors did not have any roles in the study design, in the collection, analysis and interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
The authors would like to thank Prof. Tsung-Lin Cheng (Kaohsiung Medical University Department of Physiology) for supplying the pGL3-basic vector and Orthopaedic Research Center (ORC) for technical assistance with this study.
References
- 1.Lee A.S., Ellman M.B., Yan D., Kroin J.S., Cole B.J., van Wijnen A.J. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013;527(2):440–447. doi: 10.1016/j.gene.2013.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crofford L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther. 2013;15(Suppl 3):S2. doi: 10.1186/ar4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanza F.L., Chan F.K., Quigley E.M. Practice parameters committee of the American college of G. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104(3):728–738. doi: 10.1038/ajg.2009.115. [DOI] [PubMed] [Google Scholar]
- 4.Laine L., White W.B., Rostom A., Hochberg M. COX-2 selective inhibitors in the treatment of osteoarthritis. Semin Arthritis Rheum. 2008;38(3):165–187. doi: 10.1016/j.semarthrit.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Nakata K., Hanai T., Take Y., Osada T., Tsuchiya T., Shima D. Disease-modifying effects of COX-2 selective inhibitors and non-selective NSAIDs in osteoarthritis: a systematic review. Osteoarthritis Cartilage. 2018;26(10):1263–1273. doi: 10.1016/j.joca.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Goldring M.B. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther Adv Musculoskelet Dis. 2012;4(4):269–285. doi: 10.1177/1759720X12448454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cucchiarini M., de Girolamo L., Filardo G., Oliveira J.M., Orth P., Pape D. Basic science of osteoarthritis. J Exp Orthop. 2016;3(1):22. doi: 10.1186/s40634-016-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troeberg L., Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 2012;1824(1):133–145. doi: 10.1016/j.bbapap.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M., Sampson E.R., Jin H., Li J., Ke Q.H., Im H.J. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15(1):R5. doi: 10.1186/ar4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Kraan P.M., van den Berg W.B. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage. 2012;20(3):223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Dreier R. Hypertrophic differentiation of chondrocytes in osteoarthritis: the developmental aspect of degenerative joint disorders. Arthritis Res Ther. 2010;12(5):216. doi: 10.1186/ar3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckwalter J.A., Martin J.A. Osteoarthritis. Adv Drug Deliv Rev. 2006;58(2):150–167. doi: 10.1016/j.addr.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Philipot D., Guerit D., Platano D., Chuchana P., Olivotto E., Espinoza F. p16INK4a and its regulator miR-24 link senescence and chondrocyte terminal differentiation-associated matrix remodeling in osteoarthritis. Arthritis Res Ther. 2014;16(1):R58. doi: 10.1186/ar4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang J.K., Chang L.H., Hung S.H., Wu S.C., Lee H.Y., Lin Y.S. Parathyroid hormone 1-34 inhibits terminal differentiation of human articular chondrocytes and osteoarthritis progression in rats. Arthritis Rheum. 2009;60(10):3049–3060. doi: 10.1002/art.24843. [DOI] [PubMed] [Google Scholar]
- 15.Chen C.H., Ho M.L., Chang L.H., Kang L., Lin Y.S., Lin S.Y. Parathyroid hormone-(1-34) ameliorated knee osteoarthritis in rats via autophagy. J Appl Physiol. 1985;124(5):1177–1185. doi: 10.1152/japplphysiol.00871.2017. 2018. [DOI] [PubMed] [Google Scholar]
- 16.Ripmeester E.G.J., Timur U.T., Caron M.M.J., Welting T.J.M. Recent insights into the contribution of the changing hypertrophic chondrocyte phenotype in the development and progression of osteoarthritis. Front Bioeng Biotechnol. 2018;6:18. doi: 10.3389/fbioe.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C. COX-2's new role in inflammation. Nat Chem Biol. 2010;6(6):401–402. doi: 10.1038/nchembio.375. [DOI] [PubMed] [Google Scholar]
- 18.Martel-Pelletier J., Pelletier J.P., Fahmi H. Cyclooxygenase-2 and prostaglandins in articular tissues. Semin Arthritis Rheum. 2003;33(3):155–167. doi: 10.1016/s0049-0172(03)00134-3. [DOI] [PubMed] [Google Scholar]
- 19.Sokolove J., Lepus C.M. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu J., Lu Y., Li F., Qiao L., Wang Q., Li N. Identification and characterization of the novel Col10a1 regulatory mechanism during chondrocyte hypertrophic differentiation. Cell Death Dis. 2014;5:e1469. doi: 10.1038/cddis.2014.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welting T.J., Caron M.M., Emans P.J., Janssen M.P., Sanen K., Coolsen M.M. Inhibition of cyclooxygenase-2 impacts chondrocyte hypertrophic differentiation during endochondral ossification. Eur Cell Mater. 2011;22:420–436. doi: 10.22203/ecm.v022a31. discussion 36-7. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi T., Chung U.I., Schipani E., Starbuck M., Karsenty G., Katagiri T. PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development. 2002;129(12):2977–2986. doi: 10.1242/dev.129.12.2977. [DOI] [PubMed] [Google Scholar]
- 23.van Donkelaar C.C., Huiskes R. The PTHrP-Ihh feedback loop in the embryonic growth plate allows PTHrP to control hypertrophy and Ihh to regulate proliferation. Biomech Model Mechanobiol. 2007;6(1–2):55–62. doi: 10.1007/s10237-006-0035-0. [DOI] [PubMed] [Google Scholar]
- 24.Duan Z.X., Huang P., Tu C., Liu Q., Li S.Q., Long Z.L. MicroRNA-15a-5p regulates the development of osteoarthritis by targeting PTHrP in chondrocytes. BioMed Res Int. 2019;2019:3904923. doi: 10.1155/2019/3904923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terkeltaub R., Lotz M., Johnson K., Deng D., Hashimoto S., Goldring M.B. Parathyroid hormone-related proteins is abundant in osteoarthritic cartilage, and the parathyroid hormone-related protein 1-173 isoform is selectively induced by transforming growth factor beta in articular chondrocytes and suppresses generation of extracellular inorganic pyrophosphate. Arthritis Rheum. 1998;41(12):2152–2164. doi: 10.1002/1529-0131(199812)41:12<2152::AID-ART10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 26.Okano K., Tsukazaki T., Ohtsuru A., Osaki M., Yonekura A., Iwasaki K. Expression of parathyroid hormone-related peptide in human osteoarthritis. J Orthop Res. 1997;15(2):175–180. doi: 10.1002/jor.1100150204. [DOI] [PubMed] [Google Scholar]
- 27.Guilak F., Ratcliffe A., Lane N., Rosenwasser M.P., Mow V.C. Mechanical and biochemical changes in the superficial zone of articular cartilage in canine experimental osteoarthritis. J Orthop Res. 1994;12(4):474–484. doi: 10.1002/jor.1100120404. [DOI] [PubMed] [Google Scholar]
- 28.Jiang J., Leong N.L., Mung J.C., Hidaka C., Lu H.H. Interaction between zonal populations of articular chondrocytes suppresses chondrocyte mineralization and this process is mediated by PTHrP. Osteoarthritis Cartilage. 2008;16(1):70–82. doi: 10.1016/j.joca.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W., Chen J., Zhang S., Ouyang H.W. Inhibitory function of parathyroid hormone-related protein on chondrocyte hypertrophy: the implication for articular cartilage repair. Arthritis Res Ther. 2012;14(4):221. doi: 10.1186/ar4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhosale A.M., Richardson J.B. Articular cartilage: structure, injuries and review of management. Br Med Bull. 2008;87:77–95. doi: 10.1093/bmb/ldn025. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez C., Mateus M.M., Defresne M.P., Crielaard J.M., Reginster J.Y., Henrotin Y.E. Metabolism of human articular chondrocytes cultured in alginate beads. Longterm effects of interleukin 1beta and nonsteroidal antiinflammatory drugs. J Rheumatol. 2002;29(4):772–782. [PubMed] [Google Scholar]
- 32.Morrison T.B., Weis J.J., Wittwer C.T. Quantification of low-copy transcripts by continuous SYBR Green I monitoring during amplification. Biotechniques. 1998;24(6):954–958. 60, 62. [PubMed] [Google Scholar]
- 33.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [eng] [DOI] [PubMed] [Google Scholar]
- 34.Caron M.M., Emans P.J., Sanen K., Surtel D.A., Cremers A., Ophelders D. The role of prostaglandins and COX-enzymes in chondrogenic differentiation of ATDC5 progenitor cells. PloS One. 2016;11(4) doi: 10.1371/journal.pone.0153162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gosset M., Berenbaum F., Levy A., Pigenet A., Thirion S., Cavadias S. Mechanical stress and prostaglandin E2 synthesis in cartilage. Biorheology. 2008;45(3–4):301–320. [PubMed] [Google Scholar]
- 36.Tanaka N., Ohno S., Honda K., Tanimoto K., Doi T., Ohno-Nakahara M. Cyclic mechanical strain regulates the PTHrP expression in cultured chondrocytes via activation of the Ca2+ channel. J Dent Res. 2005;84(1):64–68. doi: 10.1177/154405910508400111. [DOI] [PubMed] [Google Scholar]
- 37.Mastbergen S.C., Lafeber F.P., Bijlsma J.W. Selective COX-2 inhibition prevents proinflammatory cytokine-induced cartilage damage. Rheumatology. 2002;41(7):801–808. doi: 10.1093/rheumatology/41.7.801. [DOI] [PubMed] [Google Scholar]
- 38.Mastbergen S.C., Bijlsma J.W., Lafeber F.P. Selective COX-2 inhibition is favorable to human early and late-stage osteoarthritic cartilage: a human in vitro study. Osteoarthritis Cartilage. 2005;13(6):519–526. doi: 10.1016/j.joca.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Jeffrey J.E., Aspden R.M. Cyclooxygenase inhibition lowers prostaglandin E2 release from articular cartilage and reduces apoptosis but not proteoglycan degradation following an impact load in vitro. Arthritis Res Ther. 2007;9(6):R129. doi: 10.1186/ar2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ou Y., Tan C., An H., Jiang D., Quan Z., Tang K. Selective COX-2 inhibitor ameliorates osteoarthritis by repressing apoptosis of chondrocyte. Med Sci Mon Int Med J Exp Clin Res. 2012;18(6):BR247–B252. doi: 10.12659/MSM.882901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Hajjaji H., Marcelis A., Devogelaer J.P., Manicourt D.H. Celecoxib has a positive effect on the overall metabolism of hyaluronan and proteoglycans in human osteoarthritic cartilage. J Rheumatol. 2003;30(11):2444–2451. [PubMed] [Google Scholar]
- 42.de Boer T.N., Huisman A.M., Polak A.A., Niehoff A.G., van Rinsum A.C., Saris D. The chondroprotective effect of selective COX-2 inhibition in osteoarthritis: ex vivo evaluation of human cartilage tissue after in vivo treatment. Osteoarthritis Cartilage. 2009;17(4):482–488. doi: 10.1016/j.joca.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Cho H., Walker A., Williams J., Hasty K.A. Study of osteoarthritis treatment with anti-inflammatory drugs: cyclooxygenase-2 inhibitor and steroids. BioMed Res Int. 2015;2015:595273. doi: 10.1155/2015/595273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huh J.E., Baek Y.H., Kim Y.J., Lee J.D., Choi D.Y., Park D.S. Protective effects of butanol fraction from Betula platyphyla var. japonica on cartilage alterations in a rabbit collagenase-induced osteoarthritis. J Ethnopharmacol. 2009;123(3):515–521. doi: 10.1016/j.jep.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 45.Mastbergen S.C., Marijnissen A.C., Vianen M.E., Zoer B., van Roermund P.M., Bijlsma J.W. Inhibition of COX-2 by celecoxib in the canine groove model of osteoarthritis. Rheumatology. 2006;45(4):405–413. doi: 10.1093/rheumatology/kei187. [DOI] [PubMed] [Google Scholar]
- 46.Zweers M.C., de Boer T.N., van Roon J., Bijlsma J.W., Lafeber F.P., Mastbergen S.C. Celecoxib: considerations regarding its potential disease-modifying properties in osteoarthritis. Arthritis Res Ther. 2011;13(5):239. doi: 10.1186/ar3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuyinu E.L., Narayanan G., Nair L.S., Laurencin C.T. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg Res. 2016;11:19. doi: 10.1186/s13018-016-0346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klenke F.M., Gebhard M.M., Ewerbeck V., Abdollahi A., Huber P.E., Sckell A. The selective Cox-2 inhibitor Celecoxib suppresses angiogenesis and growth of secondary bone tumors: an intravital microscopy study in mice. BMC Canc. 2006;6:9. doi: 10.1186/1471-2407-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]