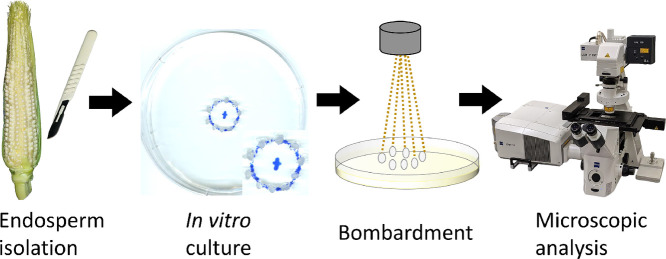
Abstract
Expressing transgenes in the endosperm of cereals by developing stably transformed lines is an expensive and labor-intensive process. An alternative that is less expensive and faster is to express the transgenes transiently. We describe here a detailed protocol to express transiently genes in maize aleurone cells by biolistic bombardment of in vitro cultured developing endosperms. Maize endosperms are isolated from kernels at 6–8 days after pollination and placed on culture medium plates for 1–2 days. Afterwards, the endosperms can be transfected with either a single gene or multiple transgenes simultaneously. Microparticles coated with the selected plasmids are delivered into the aleurone cells by biolistic bombardment. As a demonstration, we co-expressed two transgenes simultaneously, one tagged by GFP and the other tagged by mCherry. Our transfection efficiency is comparable to that obtained with Agrobacterium-mediated transformation, but requires a shorter time for gene expression after transfection. We provide optimized conditions and parameters for key steps in this procedure.
-
•
Small, non-binary plasmids can be used to drive expression of fluorescent proteins.
-
•
Optimized distribution of DNA-coated microparticles maximizes transfection of in vitro grown maize endosperms while minimizing cellular damage.
-
•
Transgene expression can be detected as early as one day after bombardment.
Keywords: In vitro endosperm culture, Biolistic bombardment, Co-expression of GFP and mCherry
Graphic abstract
Specifications Table
| Subject Area | Agricultural and Biological Sciences |
| More specific subject area | Transient gene expression in maize endosperm |
| Method name | Transient expression by biolistic bombardment in developing maize endosperm |
| Name and reference of original method | Plant material, In vitro endosperm culture, and aleurone bombardment pressure:Gruis, D. F., Guo, H., Selinger, D., Tian, Q., & Olsen, O. A. (2006). Surface position, not signaling from surrounding maternal tissues, specifies aleurone epidermal cell fate in maize. Plant Physiology, 141(3), 898–909.Gold particle preparations and bombardment chamber setup:Wang, K., & Frame, B. (2009). Biolistic gun-mediated maize genetic transformation. In Transgenic maize (pp. 29–45). Humana Press, Totowa, NJ.Whitham, S. A., Lincoln, L. M., Chowda‐Reddy, R. V., Dittman, J. D., O'Rourke, J. A., & Graham, M. A. (2016). Virus‐induced gene silencing and transient gene expression in soybean (Glycine max) using Bean pod mottle virus infectious clones. Current protocols in plant biology, 1(2), 263–283. |
| Resource availability | N.A. |
Introduction
The endosperm in cereals consists of starchy endosperm cells, which accumulate storage proteins and starch, the peripheral (epidermal) aleurone cells, which mobilize these storage compounds during germination, and transfer cells, which are in contact with the plant vascular system [1]. The maize endosperm is an important model for studying growth, development, and nutrient accumulation of cereal endosperms. However, it is expensive and time-consuming to obtain genetically modified maize lines for cellular and molecular analyses. As a result, methods for gene delivery directly into maize endosperm cells have been developed, including polyethylene glycol (PEG)-mediated DNA delivery of endosperm protoplasts [2,3], Agrobacterium tumefaciens-mediated transformation of maize endosperm [4], and biolistic bombardment of maize aleurone cells [5]. For the study of maize aleurone cells, these methods present advantages and disadvantages. PEG-mediated gene delivery into aleurone protoplasts involves the time-consuming and technically challenging process of dissecting aleurone tissue, optimizing protoplast culture conditions, and purifying aleurone protoplasts from contaminating starchy endosperm protoplasts. In addition, once removed from their cell wall and tissue context, the aleurone protoplasts may not faithfully follow the behavior of aleurone cells grown in planta. Agrobacterium-mediated transformation and biolistic bombardment rely on the in vitro culture of developing endosperms (6–8 days after pollination or DAP). Under the in vitro culture condition, both starchy endosperm and aleurone cells differentiate normally as their counterparts grown in intact kernels [4], [5], [6], which is a critical advantage to study cellular processes with a relevant developmental context. Once the endosperms are established on culture plates, gene delivery can be achieved by co-cultivation with Agrobacterium or bombardment with DNA-coated microparticles. Agrobacterium-mediated transformation is an unexpensive method since it does not require a specialized biolistic bombardment system or microparticles; however, the transgene must be cloned into a binary vector.
In this study, we present a protocol for gene delivery into developing maize aleurone cells by biolistic bombardment that incorporates optimized steps and modifications to earlier published methods [5,7,8]. We describe step-by-step how to a) set up maize endosperms for in vitro culture, b) prepare microparticles coated with plasmid DNA, and c) set up conditions for bombarding DNA-coated microparticles into maize aleurone cells. To validate our method, we co-expressed two endoplasmic reticulum (ER) markers, one tagged by mCherry and one tagged by GFP, in developing maize aleurone cells. We confirmed the expression of mCherry and GFP by fluorescence spectral analysis and calculated transfection efficiency. The transfection efficiency of our method is comparable to that of Agrobacterium-mediated methods [4], but it requires a shorter period of time for detectable gene expression (1 day for our methods, 4 days for Agrobacterium-mediated transformation).
Method details
Plant growth and in vitro culture of maize endosperm
Materials
-
1.
Maize Hi II F1 seeds.
-
2.
100 mm x 15 mm sterile Petri dishes.
-
3.
Murashige and Skoog (MS) Basal Salt Mixture (MS), powder (MilliporeSigma, M5524–50 L).
-
4.
Murashige Skoog (MS) powdered vitamin mixture (Fisher Scientific, 50,213,490).
-
5.
6-Benzylaminopurine (MilliporeSigma, B3408).
-
6.
Thiamine hydrochloride (MilliporeSigma, T1270).
-
7.
L-Asparagine (MilliporeSigma, A4159).
-
8.
Carbenicillin, Disodium Salt (Dot Scientific, DSC46000).
-
9.
Gelrite (RPI, G35020).
-
10.
Dimethyl sulfoxide (MilliporeSigma, D8418).
-
11.
Sucrose.
-
12.
Sterile and non-sterile Milli-Q water.
-
13.
1 M potassium hydroxide (made in Milli-Q water).
-
14.
Stainless-steel scalpel.
-
15.
Fine-tipped tweezers.
-
16.
70% ethanol.
Procedure
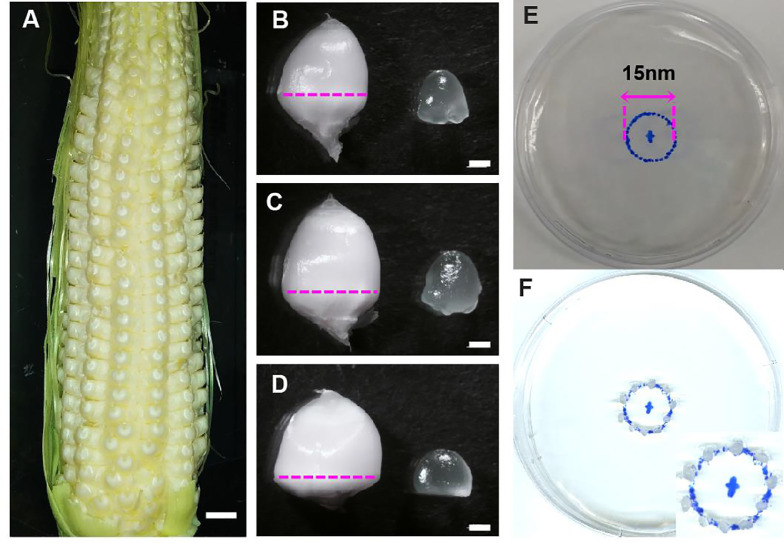
Maize Hi-II [9] F1 seeds can be grown and pollinated in a greenhouse all year long. The maize ears used for the demonstration in this study were collected from plants grown in the greenhouse of the Wisconsin Crop Innovation Center (University of Wisconsin, Madison) under a 16 h light/8 h darkness photoperiod, with supplemental lighting provided at an intensity of 330 μmol m − 2 s − 1 at ~1.7 m below the lights, and average temperatures of 28 °C during the light period and 21 °C during the dark period. The plants were self- or sib- pollinated after flowering. The maize ears were collected at 6–8 day DAP. Kernels at this developmental stage appear white and are ~3–5 mm in diameter (Fig. 1A-D).
Fig. 1.
Endosperm dissection from 8 DAP maize Hi-II ears and culture on solid medium. A) 8 DAP maize Hi-II ear. B-D) Maize kernels and endosperms collected from the top, middle, and bottom regions of the maize ear shown in A . E, F) Maize endosperms on solid medium for in vitro culture. Scale bar in A is 5 mm. Scale bars in B, C, D are 1 mm.
Culture plates should be prepared prior to kernel collection. The medium for endosperm in vitro culture system (EICS; [5,6]) consists of 4.3 g/l Murashige and Skoog Basal Salt, 0.5% v/v Murashige Skoog vitamins stock solution, 5 mg/l thiamine HCl, 400 mg/l Asn, 10 μg/L 6-benzylaminopurine, 15% sucrose with pH adjusted to 5.8 and 3 g/l gelrite for solid medium. We also supplemented the EICS medium with 500 μg/ml carbenicillin [4] to prevent bacterial contamination. We recommend making all the stock solutions as described in Table 1 and store them at −20 °C. Then, 250 ml of liquid and 250 ml solid media can be made as follows:
-
1.
Dissolve 2.15 g MS basal salt and 75 g sucrose in 400 ml Milli-Q water, add 1 ml of the 6-benzylaminopurine stock solution, and adjust the pH of the solution to 5.8 by slowly adding 1 M potassium hydroxide.
-
2.
Adjust the total volume of the solution to 500 ml and mix the solution well. To prepare solid medium, add 0.75 g gelrite and 250 ml of the well-mixed solution into a 500 mL glass bottle. Pour the rest 250 ml of the solution into another glass bottle, and autoclave for 20–30 min.
-
3.
Let the solutions cool down to approximately 50 °C and add the rest of the stock solutions listed in Table 1 to both bottles. Mix well the solid medium and pour it into sterile disposable plates (approximately 25 ml per plate). Store solid medium plates and the liquid medium in the dark at 4 °C.
Table 1.
Stock solutions for making the EICS culture medium supplemented with Carbenicillin.
| Chemical | Concentration | Solvent | Sterilization | Amount to add to 250ml EICS medium after autoclave |
|---|---|---|---|---|
| MS powdered vitamin | 1000x (as directed by the manufacturer) | Milli-Q water | filtration | 1.25 ml |
| 6-benzylaminopurine | 5 µg/ml | Dimethyl sulfoxide | Not required | \ |
| Thiamine HCl | 25 mg/ml | Milli-Q water | filtration | 50 µl |
| L-Asparagine (Asn) | 40 mg/ml | Milli-Q water | filtration | 2.5 ml |
| Carbenicillin, Disodium Salt | 250 mg/ml | Milli-Q water | filtration | 500 µl |
To prepare developing maize endosperms for in vitro culture, we usually place 10–15 excised endosperms on each EICS solid medium plate. We recommend preparing two or three plates for each transgene or combination of transgenes to be delivered. The dissection of endosperms from Hi-II ears at 6–8 DAP should be performed in a sterile laminar flow hood as follows:
-
1.
Take the EICS liquid medium and solid medium plates out of the fridge. Draw with a permanent marker a circle 15 mm in diameter at the center of each plate bottom part (Fig. 1E).
-
2.
Remove kernels from the ear with a scalpel. Sterilize kernels by submersion in 70% ethanol for approximately 2 min and rinse them with sterilized Milli-Q water. For the remaining the steps, all tools and culture medium need to be kept sterile. The scalpel and tweezers can be sterilized by spraying with 70% ethanol. Kernels should be dissected while submerged in sterile Milli-Q water to avoid dehydration.
-
3.
With the scalpel, cut away the bottom part of the kernels and expose the endosperm as shown in Fig. 1B-D. Use the fine-tipped tweezers to carefully remove the pericarp and release the endosperm. Endosperms at 6–8 DAP are small (~2–3 mm in diameter, Fig. 1B-D), semi-translucent, and fragile. It is critical to handle the dissected endosperms with care and avoid dehydration. Pour some sterilized liquid EICS medium in a sterile plastic petri dish and submerge the endosperms into the liquid medium right after dissection and before placing them onto the EICS solid plates.
-
4.
Place approximately 10–15 endosperms on each EICS solid medium plate with the apical part of the endosperm facing up and the basal part in contact with the medium (Fig. 1F). We recommend making small, shallow dents on the solid medium along the circle drawn on each plate bottom to keep the endosperms stably in position. Alternatively, endosperms can be placed in two concentric rings at each side of the drawn circle, to increase the number of transfected endosperms.
-
5.
After placing the endosperms onto EICS plates, place 100 µl sterilized Milli-Q water on top of them. Wrap the plates with parafilm and stack them vertically. Wrap the plate stack with aluminum foil and place it in a 28 °C growth chamber (70% humidity) for 1–2 days before bombardment.
Notes:
-
a.
After autoclaving, gelrite tend to crystalize quickly when under 50 °C. We recommend making EICS fresh solid medium for each use and pour into plates right after preparation.
-
b.
The center of the circle drawn on the plate bottom will later line up with the center of the gene gun. We do not place endosperm in the center of this circle because this is the area where the microparticles travel at highest speed and can cause excessive tissue damage.
Microparticle Preparation for Coating
Materials
-
1.
1.0 µm Gold Microparticles (Bio-rad, #1,652,263) or equivalent product (e.g., tungsten microparticles of similar size).
-
2.
Ethanol.
-
3.
Sterile Milli-Q water.
-
4.
Ultrasonic water bath.
-
5.
Tabletop centrifuge.
-
6.
Vortex mixer.
-
7.
Sterile 1.5 ml Eppendorf tubes.
Procedure
The purpose of this step is to sterilize the microparticles and aliquot them into small volumes. Tungsten particles are less expensive and more heterogenous in size and shape compared to gold particles. The disadvantage of using tungsten particles is that tungsten can degrade DNA over time and may have adverse effect on certain cells [10]. The disadvantage of using gold microparticles is that they tend to agglomerate in water and in high humidity condition. This problem can be solved by using an ultrasonic water bath during the preparation and coating of the microparticles. The procedure described below is adapted from [7] and [8]:
-
1.
Weigh 60 mg of 1.0 µm gold microparticles in a sterile 1.5 ml Eppendorf tube.
-
2.
In a sterile laminar flow hood, add 1 ml 100% ethanol at −20 °C into the tube with the particles. Place the tube in an ultrasonic water bath for 15 s. Tap the closed tube on the bench to gather all droplets to the tube bottom and let the tube sit until most of the gold particles have settled down to the bottom of the tube. This takes approximately 10–30 min at room temperature.
-
3.
Spin the tube in a tabletop centrifuge for 60 s at 5000x g and remove the ethanolic supernatant by pipetting. Make sure to keep the teardrop-shaped gold pellet undisturbed while pipetting.
-
4.
To rinse the gold microparticles, add 1 ml ice-cold, sterile Milli-Q water by dribbling the water down the side of the microcentrifuge tube. Resuspend the pellet by gently tapping the tube with a finger and let the gold particles settle down again.
-
5.
Spin the particles inside the tube in a tabletop centrifuge for 60 s at 5000x g and remove the water by pipetting. Repeat this rinsing step two more times.
-
6.
After removing the water from the final wash, suspend the gold particles in 1 ml sterile Milli-Q water.
-
7.
Place the tube in an ultrasonic water bath for 15 s. Immediately after, place the tube on a vortex mixer to keep the gold particle suspended in water during the subsequent aliquoting process.
-
8.
Pipette 50 µl (i.e., 3 mg) of suspended gold microparticles into sterile 1.5 ml tubes. This amount of microparticles is enough for six bombardments where ~8 µl or 480 µg of gold microparticles are used for each bombardment.
-
9.
Store gold microparticles aliquots at −20 °C.
Coating Gold Microparticles with Plasmid DNA
Materials
-
1.
Prepared gold microparticles or equivalent products (e.g., tungsten microparticles of similar size).
-
2.
Milli-Q water (sterile).
-
3.
1.5 ml Eppendorf tubes (sterile).
-
4.
15 ml falcon tubes.
-
5.
Plasmid(s) with selected transgene(s).
-
6.
Spermidine Free Base (RPI, S92150).
-
7.
Glycerol.
-
8.
Calcium chloride dihydrate.
-
9.
100% and 70% isopropanol.
-
10.
Anhydrous calcium chloride pellets.
-
11.
Tabletop centrifuge.
-
12.
Vortex mixer.
-
13.
Ultrasonic water bath.
-
14.
Macrocarriers (Bio-rad, 1,652,335).
-
15.
Stopping screens (Bio-rad, 1,652,336).
Procedure
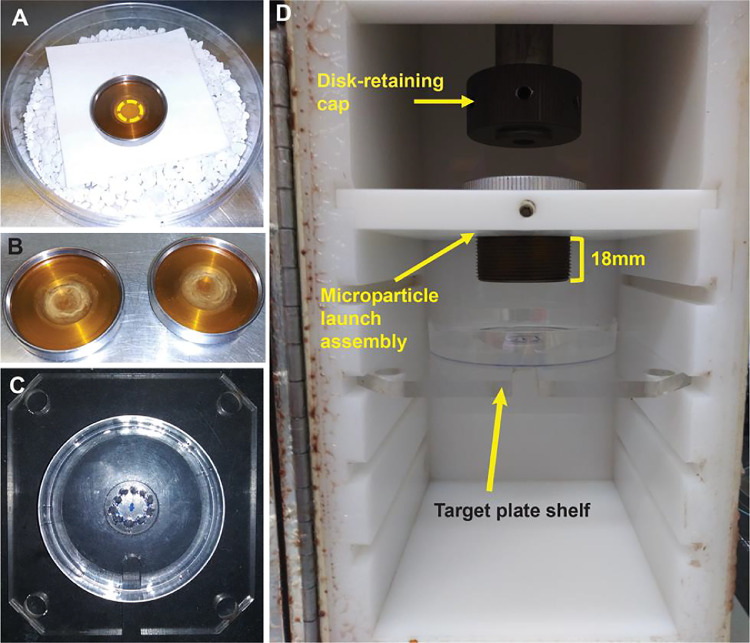
The following steps explain how to coat the gold microparticles with plasmid DNA for gene delivery. First, the gold microparticles will be coated with plasmid(s) to be used for transformation by mixing plasmid(s), prepared microparticles, and a spermidine master mix. Then, the coated microparticles will be washed and spread onto sterilized macrocarriers to dry in a drying chamber. When spreading the coated microcarriers onto macrocarriers, instead of spreading the particles evenly in the center of the macrocarriers as described in many protocols, we spread them in a circle around the edge of the central circle of the macrocarrier corresponding to the arrangement of the endosperms on the EICS plates (Fig. 2A-B). The procedure described below is adapted from [5,7], and [8]. As mentioned in the previous section, tungsten microparticles can be used to replace the gold microparticles but have a few potential drawbacks that should be taken into consideration (see Note 1 at the end of this section).
-
1.
Prepare 50% glycerol (v/v), 2.5 M CaCl2•2H2O, and 1 M spermidine using Milli-Q water. Sterilize the glycerol and CaCl2•2H2O solutions by autoclaving and sterilize the spermidine solution by filtration. Aliquot the solutions in sterile 1.5 ml Eppendorf tubes, and store them at −20 °C.
-
2.
Prepare 70% isopropanol (v/v) in Milli-Q water. Store 10 ml 70% and 10 ml 100% isopropanol in two separate 15 mL falcon tubes at −20 °C.
-
3.
Place one macrocarrier into each macrocarrier holder to be used for bombardment using a tweezer (Fig. 2A-B); avoid touching macrocarriers with bare hands. Wrap the assembled macrocarrier holders and stopping screens in aluminum foil and autoclave them.
-
4.
Add anhydrous calcium chloride pellets to sterile plastic petri dishes to cover the bottom of the plate and place a piece of filter paper on top before putting the lid back on (Fig. 2A). This is to create a drying chamber with low humidity for the coated microparticles to dry without agglomerating after being spread on the macrocarriers. Sterilize these drying chambers by placing them under an ultraviolet lamp for ~10 min.
-
5.
Isolate plasmids and resuspend them in Milli-Q water to reach a final concentration of 500 – 1000 ng/µl. For example, we use 1.5 µg the of the pRTL2 plasmids [11] per bombardment. The remaining steps should be carried out in a sterile laminar flow hood.
-
6.To make the spermidine master mix, first add 90 µl of sterile Milli-Q water to a tube containing 10 µl of 1 M spermidine to make 0.1 M spermidine. Then, to a new sterile Eppendorf tube add the following per each plasmid or plasmid combination for bombardment:
-
•50 µl of 50% glycerol.
-
•25 µl of 2.5 M CaCl2•2H2O.
-
•10 µl 0.1 M spermidine.
-
•
-
7.
Always prepare enough spermidine master mix for one extra bombardment than needed. Mix the master mix by pipetting the solution up and down and place the tube on ice until use. Discard any remaining spermidine.
-
8.
The resulting 85 µl of spermidine master mix can be used to coat enough amount of gold microparticles for two to three bombardments. We added 6 µg of plasmid DNA (3 µg of each plasmid encoding different fluorescent markers for two bombardments) into one sterile Eppendorf tube and added sterile Milli-Q water to make the total volume to be 10 µl.
-
9.
Thaw the prepared gold microparticle aliquots. Keep the tubes containing the plasmid DNA, gold microparticles, and spermidine master mix on ice.
-
10.
Place the tube containing the plasmid DNA on a vortex mixer at medium speed. Place the tube containing gold microparticles in an ultrasonic water bath for 10 s. Using a wide-bore 200 ml tip, pipette gold microparticles up and down to mix well and add 16 µl (for two bombardments) or 24 µl (for three bombardments) of gold microparticles to each tube containing plasmids. Continue vortexing for 2 min.
-
11.
Add 85 µl of the spermidine master mix to each tube, close tube lids, and then continue vortexing for another 10 min.
-
12.
Remove the tubes from the vortex mixer and let them sit on ice for 5 min. Centrifuge the tubes for 15 s at 5000x g, room temperature, to pellet the gold. Remove the supernatant by pipetting without disturbing the gold pellet.
-
13.
Add 85 µl of 70% isopropanol at −20 °C to wash the pellet by pipetting up and down a few times. Note that it is normal for the gold microparticles to agglomerate at this step.
-
14.
Centrifuge the tubes for 15 s at 5000x g, room temperature, to pellet down the gold microparticles. Remove the supernatant by pipetting.
-
15.
Add 85 µl of 100% isopropanol at −20 °C to the pellet. Do not mix the pellet and the isopropanol.
-
16.
Centrifuge the tubes for 15 s at 5000x g, room temperature, to pellet the gold microparticles. Remove the supernatant by pipetting.
-
17.
Resuspend the pellets in 18 µl (for two bombardments) or 26 µl (for three bombardments) of 100% isopropanol by pipetting up and down a few times and close the tubes immediately to avoid evaporation. Place the tubes in an ultrasonic water bath for 10 s.
-
18.
Spray tweezer with 70% ethanol and left it on a sterile surface to air dry. Use the tweezer to place one sterilized macrocarrier holder into a drying chamber.
-
19.
Pipette up and down to mix well the gold microparticles using wide-bore tips, and spread 8.5 µl of the gold suspension around the edge of the central circle of the macrocarrier (Fig. 2A). Close the petri dish immediately to avoid agglomeration of gold microparticles and wait until the isopropanol evaporates completely (Fig. 2B).
-
20.
Use the tweezer to place the macrocarrier holders with dry, coated gold microparticles into a labeled (plasmid name) sterile plastic petri dish. The macrocarriers should be used within 2 h after coating.
Fig. 2.
Loading of coated gold particles onto macrocarriers for bombardment in a PDS-1000/He Biolistic Particle Delivery System. A) Macrocarrier with coated gold microparticles in a drying chamber. The yellow line shows the area where the coated particles should be applied. B) Macrocarriers with dried DNA-coated microparticles. C) Position of endosperm culture plate on the target plate shelf of the biolistic bombardment system. D) setup of the bombardment chamber.
Notes:
-
1.
Tungsten microparticles are an alternative to gold microparticles due to their lower cost and comparable performance under most transformation scenarios. However, several potential problems with Tungsten microparticles have been reported, including toxicity in some tissues, nicking and degradation of DNA adhered to microparticles, acidification of aqueous environments, and microparticles oxidation overtime which potentially reduces DNA binding capacity [10,12].
-
2.
To spread the coated gold microparticles evenly on the macrocarrier (Fig. 2A, 2B), it is best to move the wide-bore pipette tip in a circular motion around the center of the macrocarrier (Fig. 2A) while slowly dispensing the microparticles.
Endosperm Bombardment
Materials
-
1.
Maize Hi-II endosperms after 1 to 2 days in vitro culture.
-
2.
Assembled macrocarrier holders with DNA-coated microparticles.
-
3.
Tweezer.
-
4.
PDS-1000/He Biolistic Particle Delivery System (Bio-rad, 1,652,257).
-
5.
Sterilized stopping screens (Bio-rad, 1,652,336).
-
6.
650 psi rupture disks (Bio-rad, 1,652,327) or 1100 psi rupture disks (Bio-rad, 1,652,329).
-
7.
70% isopropanol.
-
8.
70% ethanol.
-
9.
Paper wipes.
Procedure
We use a PDS-1000/He Biolistic Particle Delivery System [13] from Bio-Rad for gene delivery. Detailed instructions for the use of this system can be found in the corresponding manual (https://www.bio-rad.com/webroot/web/pdf/lsr/literature/10000070900.pdf, Bio-Rad document number 10,000,070,900). Here we will focus key settings and parameters used for the bombardment of developing maize endosperms:
-
1.
Adjust the brass nest until it is 18 mm below the microparticle launch assembly (Fig. 2D).
-
2.
Clean the inside of the bombardment chamber by spraying with 70% ethanol. Dry the chamber with paper wipes.
-
3.
Open the helium tank valve, turn on the vacuum pump, and turn on the PDS-1000/He system.
-
4.
Use the tweezer to pick up a rupture disk, dip it briefly in 70% isopropanol, and place it in the disk-retaining cap (Fig. 2D). Screw the disk-retaining cap tightly to the end of gas acceleration tube. Use the tweezer to load the macrocarrier holder and stopping screen into the microparticle launch assembly. Place the microparticle launch assembly in the chamber (Fig. 2D).
-
5.
Remove the lid and place the plate with in vitro cultured endosperms in the center of the target plate shelf (Fig. 2C). Place the target plate shelf into the L2 slot (i.e., 6 cm from the microparticle launch assembly) as shown in Fig. 2D.
-
6.
Close the chamber door. Evacuate chamber and hold vacuum at 23–25 in Hg (11.3–12.3 psi). Press the fire button until the rupture disk is bursted.
-
7.
Release vacuum from chamber, remove the plate, and put the lid back onto the plate.
-
8.
Unload macrocarrier holder, stopping screens, and rupture disk and repeat steps 4–7 for another bombardment.
-
9.
In a sterile laminar flow hood, apply 100 µl of sterilized Milli-Q water onto the endosperms to keep them moist. Wrap the plates with parafilm and stack them vertically. Wrap the stack with aluminum foil and place them in an incubator at 28 °C (70% humidity). Gene expression can be detected after culturing the bombarded endosperms overnight.
Compared to a previous protocol for gene delivery in vitro grown endosperms [5], our protocol has some key advantages:
-
1.
Instead of using large binary plasmids [5], we drive gene expression from pRTL2, a small (~4 kb), high-copy vector, using the CaMV 35S promoter.
-
2.
We have used a larger amount of plasmid DNA (3 µg) than in previous protocols (200 ng, [5]), achieving a relatively high transfection efficiency with fluorescent signal detectable from day 1 to day 4 after bombardment (results discussed in detail in the next section).
-
3.
We have found that 650–1100 psi pressure range is adequate for endosperm bombardment. The use of 1100 psi rupture disks results in slightly higher although not statistically significant transfection efficiency compared to 650 psi rupture disks used previously [5], without causing excessive cell lesions (results discussed in detail in the next section). Having a wider range of bombardment pressures provides more flexibility in designing biolistic bombardment strategies for in vitro-cultured endosperms.
Method validation
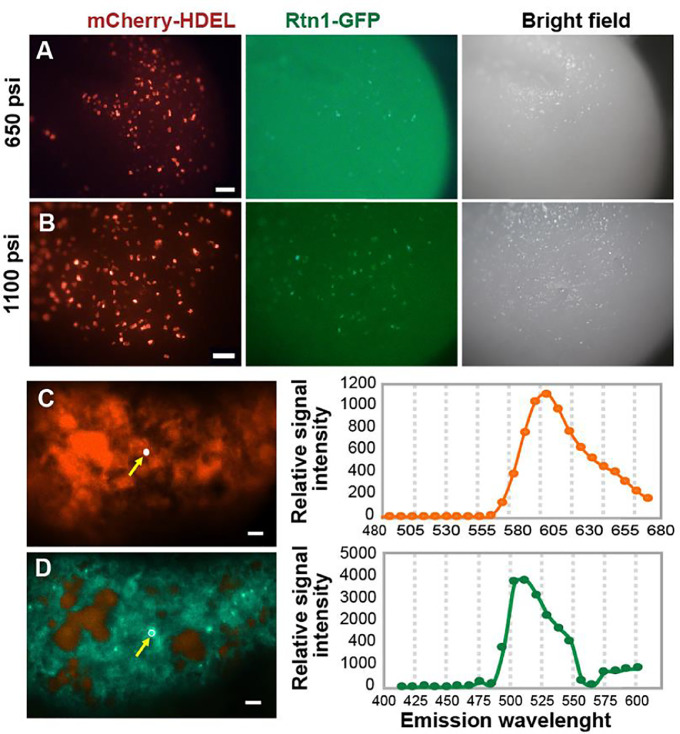
To demonstrate the efficiency of our optimized protocol for gene delivery to maize Hi-II endosperm cells, we co-expressed HDEL-mCherry [14], a soluble ER lumen marker, and Rtn1-GFP [14], an ER membrane protein (Fig. 3A-B). We collected maize Hi-II ears at 8 DAP, cultured excised endosperms in vitro for 2 days, and bombarded them with gold microparticles coated with pRTL2 plasmids containing the 35S::HDEL-mCherry and 35S::Rtn1-GFP expression cassettes and using 650 psi and 1100 psi rupture disks. We monitored the expression of the two fluorescent reporters from day 1 to day 4 after bombardment. To measure transfection efficiency using either 650 psi or 1100 psi rupture disks, we bombarded six plates with 10 endosperms each. When coating the gold microparticles, we mixed 6 µg of DNA (3 µg of each 35S::HDEL-mCherry and 35S::Rtn1-GFP plasmids), 16 µl (i.e., 960 µg) of gold microparticles, and 85 µl of the spermidine master mix for two bombardments. We did the coating for a total of 3 times to obtaint 6 macrocarriers with coated microparticles for bombardment. Three EICS plates were bombarded using 650 psi rupture disks while the other three using 1100 psi rupture disks.
Fig. 3.
Imaging of endosperms transfected with 35S::HDEL-mCherry and 35S::Rtn1-GFP. A, B) Endosperms bombarded using either 650 psi (A) or 1100 psi (B) rupture disks and imaged under a fluorescence dissecting microscope.? Rupture disk excited by light generated by a mercury lamp passing a DS-Red filter and a GFP filter, respectively. C,D) Confocal imaging and fluorescence spectral analysis of an endosperm epidermal/developing aleurone cell with positive mCherry-HDEL (C) and Rtn1-GFP (D) signals. The fluorescence spectral data was collected from the area indicated by yellow arrow.
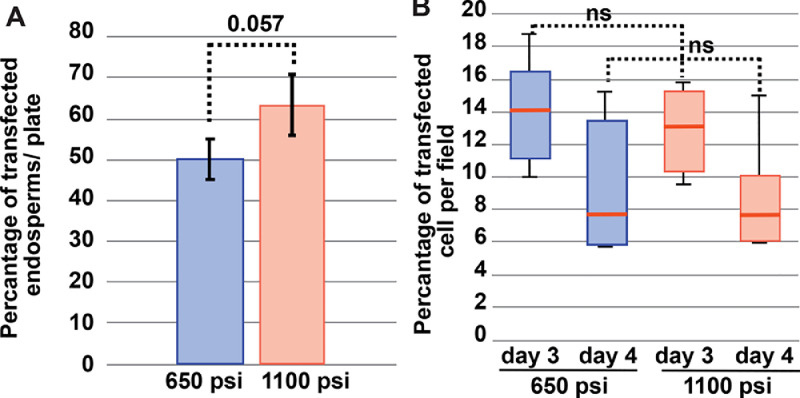
We were able to find developing aleurone cells co-expressing HDEL-mCherry and Rtn1-GFP in endosperms bombarded with 650 psi and 1100 psi rupture disks, respectively, without causing excessive tissue lesion or cell death (Fig. 3A-B). Since endosperm cells are highly autofluorescent, we confirmed the expression of HDEL-mCherry and Rtn1-GFP by confocal laser scanning microscopy and fluorescence spectral analysis (Fig. 3C-D). We observed fluorescent signal corresponding to the transfected reporters as early as 1 day and up to 4 days after bombardment. We then calculated the transfection efficiency as a) the percentage of endosperms with more than 20 transfected aleurone cells and b) the percentage of aleurone cells transformed per field of view using. There was a slight but not statistically significant increase in transfection efficiency with 1100 psi compared to 650 psi rupture disks (50–80% transfected endosperms per plate with 1100 psi compared to 40–60% with 650 psi rupture disks, p-value = 0.057; Fig. 4A). To calculate the percentage of transfected cells expressing the fluorescent reporters, we prepared fresh thin slices of endosperm containing aleurone cells and imaged them using a 40x objective under a confocal laser scanning microscope (Fig. 4B). We found that for both conditions (650 psi and 1100 psi rupture disks), the transfection efficiency was similar, reaching between 12 and 15% of aleurone cells 3 days after bombardment with a slight decline to 7–12% at 4 days after bombardment. This transfection rates are comparable to those reported for Agrobacterium-mediated transformation, where approximately 15% of aleurone cells expressed fluorescent reporters by 4 days after co-cultivation [4]. Therefore, we concluded that using a range of 650 to 1100 psi is adequate for biolistic bombardment of in vitro-cultured endosperm.
Fig. 4.
Transfection efficiency. A) Percentage of endosperms expressing mCherry-HDEL per plate at 1 day after bombardment. Three plates with 10 endosperms each were bombarded using either 650 psi and 1100 psi rupture disks. B) Percentage of endosperm epidermal/ aleurone cells expressing mCherry-HDEL per field as imaged in a confocal laser scanning microscope with a 40x objective. Five to seven images of similar magnification as size were collected from two samples of four different endosperms; 66 – 160 cells were captured per field. Data were compared using a student's t-test.
Declaration of Competing Interest
[MANDATORY – Delete as appropriate]
X The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We would like to acknowledge the support and assistance of the Wisconsin Crop Innovation Center (WCIC; University of Wisconsin, Madison), the Kabbage Lab (University of Wisconsin, Madison), and the Newcomb Imaging Center (NIC; University of Wisconsin, Madison). We want to give special thanks to Mark Thompson and Phoebe Blackman who manage the greenhouse at WCIC for consistently pollinating and providing maize ears to us. We also want to give special thanks to Dr. Mehdi Kabbage and Dr. Mitchell Roth for generously sharing the biolistic bombardment system with us. We would also like to thank Dr. Sarah Swanson from the NIC to have assisted us with imaging the transfected endosperms and provided us the image of the confocal microscope used in the graphic abstract.
Funding
This work was supported by National Science Foundation grants IOS-1840687 to M.S.O, the United States Department of Agriculture; National Institute of Food and Agriculture Hatch Act Formula Fund WIS01791 to M.S.O., and funds from the University of Wisconsin; Department of Botany to X.D. The funding sources only provided monetary support for this project and were not involved in the design of the study, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
References
- 1.Olsen O.A. The modular control of cereal endosperm development. Trends Plant Sci. 2020;25(3):279–290. doi: 10.1016/j.tplants.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Gallie D.R., Young T.E. The regulation of gene expression in transformed maize aleurone and endosperm protoplasts. Analysis of promoter activity, intron enhancement, and mRNA untranslated regions on expression. Plant Physiol. 1994;106(3):929–939. doi: 10.1104/pp.106.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu Y. Optimization of isolation and transfection conditions of maize endosperm protoplasts. Plant Methods. 2020;16:96. doi: 10.1186/s13007-020-00636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reyes F.C. Agrobacterium tumefaciens-mediated transformation of maize endosperm as a tool to study endosperm cell biology. Plant Physiol. 2010;153(2):624–631. doi: 10.1104/pp.110.154930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gruis D. Surface Position, Not Signaling from Surrounding Maternal Tissues, Specifies Aleurone Epidermal Cell Fate in Maize. Plant Physiol. 2006;141(3):898. doi: 10.1104/pp.106.080945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsen O.-.A. A first step towards in vitro cultured cereals. J. Cereal Sci. 2021 doi: 10.1016/j.jcs.2021.103182. in press. [DOI] [Google Scholar]
- 7.Wang K., Frame B. Biolistic gun-mediated maize genetic transformation. Methods Mol. Biol. 2009;526:29–45. doi: 10.1007/978-1-59745-494-0_3. [DOI] [PubMed] [Google Scholar]
- 8.Whitham S.A. Virus-iinduced gene silencing and transient gene expression in soybean (Glycine max) using bean pod mottle virus infectious clones. Curr Protoc Plant Biol. 2016;1(2):263–283. doi: 10.1002/cppb.20012. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong C. Development and availability of germplasm with high type II culture response. Maize Genet. Coop. News Lett. 1991;65:92–93. [Google Scholar]
- 10.Russell J.A., Roy M.K., Sanford J.C. Physical trauma and tungsten toxicity reduce the efficiency of biolistic transformation. Plant Physiol. 1992;98(3):1050–1056. doi: 10.1104/pp.98.3.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Restrepo M.A., Freed D.D., Carrington J.C. Nuclear transport of plant potyviral proteins. Plant Cell. 1990;2(10):987–998. doi: 10.1105/tpc.2.10.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshimitsu Y. Improvement of DNA/metal particle adsorption in tungsten-based biolistic bombardment; alkaline pH is necessary for DNA adsorption and suppression of DNA degradation. J. Plant Biol. 2009;52(6):524–532. [Google Scholar]
- 13.Kikkert J.R. The Biolistic® PDS-1000/He device. Plant Cell Tissue Organ Cult. 1993;33(3):221–226. [Google Scholar]
- 14.Zhang X. Reticulon proteins modulate autophagy of the endoplasmic reticulum in maize endosperm. Elife. 2020;9 doi: 10.7554/eLife.51918. [DOI] [PMC free article] [PubMed] [Google Scholar]