Highlights
-
•
Analyze and identify 4 levels of small molecule compounds in distillery wastewater.
-
•
Simple method for quantification of five antimicrobial compounds.
-
•
Column temperature affected the lactic and succinic acid chromatographs significantly.
Keywords: Liquid chromatography-tandem mass spectrometry, Qualitative analysis, Quantitative analysis, Distillery wastewater, Lactic acid, Succinic acid, Cinnamic acid, Phenyllactic acid, Methylmalonic acid, Acetophenone
Abstract
A high-resolution mass spectrometry (HR-MS) method was developed to analyze and identify small molecule compounds in distillery wastewater. According to identification confidence levels, 4 levels of compounds were identified. The five antimicrobial compounds (lactic acid, succinic acid, acetophenone, cinnamic acid, and phenyllactic acid), which shown in high concentrations, were at the highest level of confidence (level 1, confirmed structure). Thus, a rapid and sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was developed to simultaneously quantify these antimicrobial compounds. The analysis was performed in the selective reaction monitoring (SRM) mode via the electrospray ionization (ESI) source operating in the negative ionization mode. Linear calibration curves were obtained over the concentration range of 50–1000.0 ng/mL for succinic acid, acetophenone, cinnamic acid, phenyllactic acid, and 375–7500 ng/mL for lactic acid. Precision and recovery of the analytes were all satisfactory (relative standard deviation < 10%). The validated method was successfully applied to quantitative analysis of the five antimicrobial compounds in distillery wastewater.
-
•
Analyze and identify 4 levels of small molecule compounds in distillery wastewater.
-
•
Simple method for quantification of five antimicrobial compounds.
-
•
Column temperature affected the lactic and succinic acid chromatographs significantly.
Graphical Abstract
Specifications Table
| Subject Area: | Environmental Science |
| More specific subject area: | A dvanced mass spectrometric analysis for environmental and food safety, Analytical chemistry, Wastewater analysis |
| Method name: | Liquid chromatography-tandem mass spectrometry analysis for identification and quantification of antimicrobial compounds in distillery wastewater |
| Name and reference of original method: | E. L. Schymanski, J. Jeon, R. Gulde, K. Fenner, M. Ruff, H. P. Singer, J. Hollender, Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol., 48 (2014) 2097-2098 |
| Resource availability: | CompoundDiscoverer 2.1 (Thermo Scientific), mzCloud database (Thermo Scientific,http://www.mzcloud.org) |
*Method details
Introduction
Distillery wastewater could cause many environment issues due to its high generation amount and high concentration of organics and nutrients [1]. Therefore, it is important to develop methods to analyze the composition of distillery wastewater to support the improvement of resource recovery and treatment process of distillery wastewater. In this study, a high-resolution mass spectrometry (HR-MS) method was developed to analyze and identify small molecules compounds in distillery wastewater and 4 levels of compounds were identified. And an effective and rapid method has been developed for simultaneous determination of lactic acid, succinic acid, acetophenone, cinnamic acid and phenyllactic acid (the five identified major antimicrobial compounds) in the distillery wastewater using a simple one-step sample dilution preparation couple with UPLC-MS/MS.
Materials and reagents
Lactic acid, succinic acid, acetophenone, cinnamic acid and phenyllactic acid were purchased from the Sigma-Aldrich Company Ltd.
HPLC-grade formic acid and MS-grade methanol purchased from Merck (Darmstadt, Germany) were used for HPLC analysis and sample preparation.
Preparation of standard solution and distillery wastewater samples
Concentrated stock solutions of analytes were prepared by dissolving the appropriate amount of the standard samples in 50% methanol at a concentration of 1 mg/mL. And then it was further diluted with acetonitrile to form a series of working solutions used to prepare the calibration curve. All the solutions were stored at –20 °C.
A10 μl of the distillery wastewater sample was added with a 20 mL of 50% methanol solution was added. Then, the mixture was vortexed for 2 min and centrifugation at 13,000 rpm for 10 min at 4 °C. Subsequently, the supernatant liquor was transferred to centrifugation at 13,000 rpm for 5 min at 4 °C again, then the supernatant liquor was injected into the HPLC-MS/MS for analysis.
Identification of antimicrobial compounds by HR-MS
Analytical instrumentation
The LC–MS/MS system used was a Thermo Scientific Ultimate 3000 liquid phase system equipped with Q Exactive Orbitrap and an electrospray ionization source. A volume of 2 μl sample was injected to a Hypersil Gold C18 column (100 × 2.1 mm, 1.9 μm, Thermo Scientific) at 20 °C. The LC flow was set to 250 μl/min using H2O (0.1% formic acid) and methanol as eluents. The gradient elution started with 98% H2O for 2 min and was changed to 95% methanol over the course of 13 min, maintained for 3 min, then returned to 98% H2O within 0.1 min, and equilibrated for 1.9 min prior to the next injection. The heated electrospray ionization source had a capillary temperature of 350 °C.
Both positive and negative electrospray ionization were employed to obtain MS signals of analytes with spray voltages of +3.5 kV and -2.5 kV, respectively. Sheath gas flow rate, aux gas flow rate and sweep gas flow rate were set to 40, 10 and 0 (arbitrary units), respectively. Capillary temperature and aux gas heater temperature were set to 320 °C and 350 °C, respectively. The MS was set at full scan mode and acquire targeted first MS signals in at 70,000 fwhm and targeted MS/MS scan was set at a resolution of 175,00 fwhm with isolation width of 2.0 m/z. The instrument would automatically switch the positive and negative ion scanning mode and the scan mode was chosen as full MS scan-dd MS2 and acquire first MS signals at 70,000 fwhm and targeted MS/MS scan was set at a resolution of 175,00 fwhm with isolation width of 2.0 m/z. Meanwhile, the m/z scan range was 70–700.
Data processing
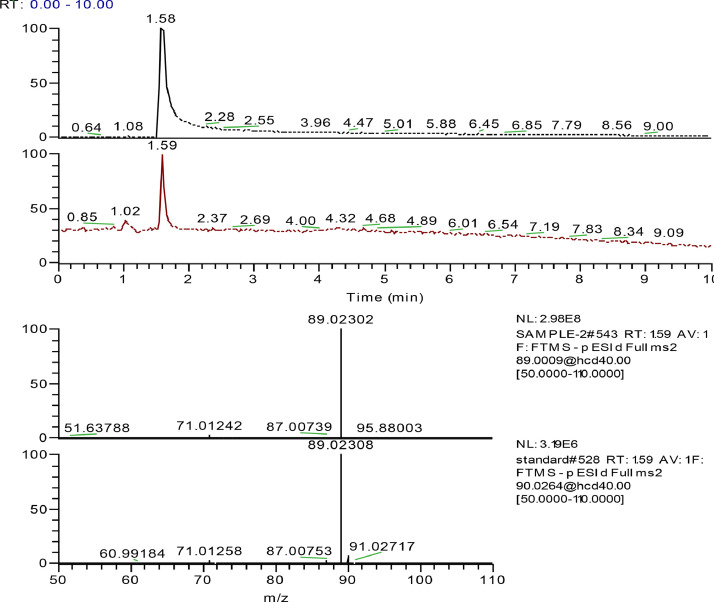
Peak detection and alignment of the LC−MS data were performed using Compound Discoverer 2.0 (Thermo Scientific) to obtain a peak list with peak areas, molecular weight, and retention time with the following settings: S/N threshold, 3; mass tolerance, 10 ppm; minimum peak intensity, 1 × 105. With the application of the software, a possible molecular formula fitting the exact mass and isotope patterns was calculated. Furthermore, the MS/MS fragments were compared to the mzCloud database. Fig. 1 and S1-S4 (in the supplementary materials) show how compounds were identified. As can be seen, the MS and, MS/MS information and retention time of the unknown compound were highly consistent with the reference substance.
Fig. 1.
The extract chromatogram and MS/MS of lactic acid in the sample (top) compared with reference standards (bottom).
According to Identification confidence levels reported by Schymanski et al. [2], 4 levels of unknown compound were classified inTable 2.
Table 1.
Gradient elution time program for mobile phase for qualitative analysis in LC-MS/MS.
| Time (min) | %A(0.1% formic acid) | %B (methanol) |
|---|---|---|
| 0 | 98 | 2 |
| 2 | 98 | 2 |
| 16 | 5 | 95 |
| 18 | 5 | 95 |
| 18.1 | 98 | 2 |
| 20 | 98 | 2 |
Table 2.
Identification confidence levels according to Schymanski et al. [13].
| Level | Identification confidence | Minimum data requirements |
|---|---|---|
| 1 | Confirmed structure by reference standard | MS, MS2, RT, reference Std. |
| 2 | Probable structure by library spectrum match | MS, MS2, library MS2 |
| 3 | Tentative candidates(s) | MS, MS2, Exp. data |
| 4 | Unequivocal molecular formula | MS isotope/adduct |
Table 3 showed the compounds contained in rice spirit distillery wastewater identified with four different confidence levels by HR-MS. Lactic acid, succinic acid, L-phenylalanine, caffeine, adenosine, D(+)-phenyllactic acid, DL-arginine, acetophenone and cinnamic acid were confirmed using the standard compounds. The MS, MS/MS and retention time compared with reference standards (lactic acid, succinic acid, acetophenone, cinnamic acid and phenyllactic acid) were shown in Fig. 1 and S1-4. Approximate 60 compounds were converged to level 2 in the identified top 100 most abundant compounds (based on peak area). Their MS/MS fragments were compared to the mzCloud database and had a direct matching. In Fig. S5, Υ-aminobutyric acid, L-glutamic acid, proline and D-(+)-pyroglutamic acid were chosen as representatives to show the MS2 spectrum comparison between the sample and mzCloud library. Fig. S6 was the chromatogram and ms2 spectrum of extract mass 132.1019, indicated the existence of leucine or isoleucine. In level 4, a possible molecular formula fitting the exact mass and isotope patterns was calculated.
Table 3.
Compounds contained in the rice spirit distillery wastewater identified with four different confidence levels by HR-MS (the top 100 most abundant compounds based on peak area).
| No. | Name | Formula | Molecular Weight | RT [min] | Area | Identification confidence levels |
|---|---|---|---|---|---|---|
| 1 | Lactic acid | C3 H6 O3 | 90.03169 | 1.58 | 3E+10 | 1 |
| 2 | Phenyllactic acid | C9H10O3 | 166.063 | 8.53 | 4E+09 | 1 |
| 3 | Succinic acid | C4 H6 O4 | 118.0266 | 2.85 | 1E+09 | 1 |
| 4 | Citraconic acid | C5 H6 O4 | 84.01995 | 1.85 | 1E+09 | 2 |
| 5 | L-Norleucine | C6 H13 N O2 | 131.0946 | 2.98 | 3E+08 | 3 |
| 6 | Cinnamic acid | C9H8O2 | 148.0524 | 8.53 | 2E+08 | 1 |
| 7 | Gluconic acid | C6 H12 O7 | 150.052 | 1.03 | 2E+08 | 2 |
| 8 | L-Phenylalanine | C9 H11 N O2 | 165.0789 | 5.39 | 2E+08 | 1 |
| 9 | Acetophenone | C8 H8 O | 120.0575 | 4.26 | 9E+07 | 1 |
| 10 | 6-Hydroxycaproic acid | C6 H12 O3 | 86.07209 | 8.16 | 7E+07 | 2 |
| 11 | D(+)-Phenyllactic acid | C9 H10 O3 | 120.0568 | 8.56 | 6E+07 | 2 |
| 12 | Υ-Aminobutyric acid (GABA) | C4 H9 N O2 | 103.0635 | 1.11 | 6E+07 | 2 |
| 13 | L-Leucine | C6 H13 N O2 | 131.0946 | 2.78 | 6E+07 | 3 |
| 14 | 2-Hydroxycinnamic acid | C9 H8 O3 | 164.0473 | 4.24 | 5E+07 | 4 |
| 15 | Adenine | C5 H5 N5 | 135.0544 | 2.26 | 5E+07 | 2 |
| 16 | DL-4-Hydroxyphenyllactic acid | C9 H10 O4 | 182.0574 | 7.07 | 4E+07 | 4 |
| 17 | trans-3-Indoleacrylic acid | C11 H9 N O2 | 187.0631 | 7.22 | 4E+07 | 2 |
| 18 | D-(+)-Proline | C5 H9 N O2 | 115.0634 | 1.16 | 3E+07 | 1 |
| 19 | D-(+)-Pyroglutamic Acid | C5 H7 N O3 | 129.0426 | 2.34 | 3E+07 | 2 |
| 20 | Guanine | C5 H5 N5 O | 151.0493 | 2.28 | 3E+07 | 2 |
| 21 | Methylmalonic acid | C4 H6 O4 | 118.0255 | 2.89 | 3E+07 | 2 |
| 22 | 2-Isopropylmalic acid | C7 H12 O5 | 116.0467 | 7.42 | 2E+07 | 4 |
| 23 | D-α-Hydroxyglutaric acid | C5 H8 O5 | 148.0363 | 1.99 | 2E+07 | 4 |
| 24 | Cyclo(leucylprolyl) | C11 H18 N2 O2 | 210.1365 | 8.42 | 1E+07 | 2 |
| 25 | Dimethyl succinate | C6 H10 O4 | 146.0579 | 7.42 | 1E+07 | 4 |
| 26 | Piceatannol | C14 H12 O4 | 244.0706 | 10.94 | 1E+07 | 2 |
| 27 | Glycyl-L-leucine | C8 H16 N2 O3 | 188.1159 | 6.11 | 1E+07 | 2 |
| 28 | L-(+)-Arginine | C6 H14 N4 O2 | 174.1114 | 1.04 | 1E+07 | 4 |
| 29 | Spermidine | C7 H19 N3 | 128.1311 | 0.92 | 1E+07 | 4 |
| 30 | L-(+)-Citrulline | C6 H13 N3 O3 | 158.0688 | 1.10 | 1E+07 | 2 |
| 31 | Cyclo(phenylalanyl-prolyl) | C14 H16 N2 O2 | 244.1209 | 8.90 | 1E+07 | 2 |
| 32 | Prolylleucine | C11 H20 N2 O3 | 456.2942 | 6.59 | 9E+06 | 2 |
| 33 | Cytosine | C4 H5 N3 O | 111.0433 | 1.25 | 9E+06 | 1 |
| 34 | DL-Lysine | C6 H14 N2 O2 | 146.1053 | 1.76 | 9E+06 | 2 |
| 35 | DL-Arginine | C6 H14 N4 O2 | 174.1114 | 1.57 | 9E+06 | 2 |
| 36 | (15Z)-9,12,13-Trihydroxy-15-octadecenoic acid | C18 H34 O5 | 330.241 | 11.56 | 8E+06 | 2 |
| 37 | 2-Hydroxyvaleric acid | C5 H10 O3 | 72.0564 | 6.08 | 8E+06 | 4 |
| 38 | Imidazolelactic acid | C6 H8 N2 O3 | 156.0531 | 1.33 | 7E+06 | 2 |
| 39 | Valylproline | C10 H18 N2 O3 | 214.1315 | 4.02 | 6E+06 | 4 |
| 40 | Hypoxanthine | C5 H4 N4 O | 136.0382 | 3.03 | 6E+06 | 2 |
| 41 | Ethyl oleate | C20 H38 O2 | 310.2865 | 14.57 | 6E+06 | 2 |
| 42 | Indole-3-lactic acid | C11 H11 N O3 | 205.0737 | 8.81 | 6E+06 | 2 |
| 43 | Histamine | C5 H9 N3 | 111.0797 | 0.98 | 6E+06 | 2 |
| 44 | DL-Homoserine | C4 H9 N O3 | 87.032 | 1.05 | 5E+06 | 4 |
| 45 | 3-Methylcrotonylglycine | C7 H11 N O3 | 157.0736 | 5.70 | 5E+06 | 4 |
| 46 | L(-)-Pipecolinic acid | C6 H11 N O2 | 129.0788 | 1.59 | 4E+06 | 2 |
| 47 | D-(-)-Mannitol | C6 H14 O6 | 182.0783 | 1.04 | 4E+06 | 2 |
| 48 | Caffeine | C8 H10 N4 O2 | 194.0802 | 7.89 | 4E+06 | 1 |
| 49 | trans-Cinnamic acid | C9 H8 O2 | 148.0515 | 8.55 | 4E+06 | 4 |
| 50 | L-Histidine | C6 H9 N3 O2 | 155.0691 | 1.00 | 4E+06 | 2 |
| 51 | Trigonelline | C7 H7 N O2 | 137.0475 | 1.20 | 4E+06 | 2 |
| 52 | L(+)-Ornithine | C5 H12 N2 O2 | 132.0897 | 0.98 | 4E+06 | 4 |
| 53 | Daidzein | C15 H10 O4 | 254.0577 | 10.32 | 4E+06 | 2 |
| 54 | D(+)-Phenyllactic acid | C9 H10 O3 | 166.0622 | 8.71 | 3E+06 | 2 |
| 55 | (2R)-2,3-Dihydroxypropanoic acid | C3 H6 O4 | 106.0254 | 1.15 | 3E+06 | 4 |
| 56 | α,α-Trehalose | C12 H22 O11 | 342.1163 | 1.09 | 3E+06 | 4 |
| 57 | 3-(2-Hydroxyethyl)indole | C10 H11 N O | 129.0578 | 9.22 | 3E+06 | 2 |
| 58 | Acetylcholine | C7 H15 N O2 | 145.11 | 1.49 | 3E+06 | 2 |
| 59 | DL-Malic acid | C4 H6 O5 | 134.0204 | 1.40 | 3E+06 | 2 |
| 60 | N-Acetylalanine | C5 H9 N O3 | 131.0575 | 2.98 | 3E+06 | 4 |
| 61 | 2-Hydroxy-4-methylthiobutanoic acid | C5 H10 O3 S | 150.0342 | 6.28 | 3E+06 | 2 |
| 62 | Uracil | C4 H4 N2 O2 | 112.0273 | 1.90 | 3E+06 | 2 |
| 63 | D-(-)-Quinic acid | C7 H12 O6 | 192.0627 | 1.16 | 3E+06 | 2 |
| 64 | Carnosine | C9 H14 N4 O3 | 226.1063 | 2.75 | 3E+06 | 2 |
| 65 | Crotetamide | C12 H22 N2 O2 | 226.1678 | 10.15 | 3E+06 | 4 |
| 66 | Uric acid | C5 H4 N4 O3 | 168.0278 | 3.06 | 2E+06 | 2 |
| 67 | Acetylarginine | C8 H16 N4 O3 | 216.122 | 2.16 | 2E+06 | 2 |
| 68 | L-(+)-Arginine | C6 H14 N4 O2 | 174.1114 | 1.26 | 2E+06 | 4 |
| 69 | L-Ergothioneine | C9 H15 N3 O2 S | 229.088 | 1.37 | 2E+06 | 4 |
| 70 | Spermine | C10 H26 N4 | 202.2156 | 0.90 | 2E+06 | 2 |
| 71 | N3,N4-Dimethyl-L-arginine | C8 H18 N4 O2 | 202.1426 | 1.63 | 2E+06 | 4 |
| 72 | Nicotinic acid | C6 H5 N O2 | 123.032 | 1.94 | 2E+06 | 2 |
| 73 | 3-Ureidopropionic acid | C4 H8 N2 O3 | 132.0525 | 1.01 | 2E+06 | 2 |
| 74 | 2-Aminooctanedioic acid | C8 H15 N O4 | 143.0941 | 5.38 | 2E+06 | 4 |
| 75 | Prolylglycine | C7 H12 N2 O3 | 172.0845 | 1.47 | 2E+06 | 2 |
| 76 | 9-Oxo-10(E),12(E)-octadecadienoic acid | C18 H30 O3 | 312.2296 | 11.56 | 2E+06 | 2 |
| 77 | β-D-Glucopyranuronic acid | C6 H10 O7 | 194.0419 | 1.06 | 2E+06 | 4 |
| 78 | 5-Hydroxymethyl-2-furaldehyde | C6 H6 O3 | 126.0317 | 5.49 | 2E+06 | 2 |
| 79 | Genistein | C15 H10 O5 | 270.0527 | 10.94 | 1E+06 | 2 |
| 80 | Gallic acid | C7 H6 O5 | 170.0208 | 5.07 | 1E+06 | 2 |
| 81 | 2-Hydroxyvaleric acid | C5 H10 O3 | 118.0619 | 6.24 | 1E+06 | 4 |
| 82 | 2-(Acetylamino)hexanoic acid | C8 H15 N O3 | 173.1047 | 8.31 | 1E+06 | 2 |
| 83 | 7-Methylguanine | C6 H7 N5 O | 165.0649 | 3.73 | 1E+06 | 2 |
| 84 | 2-Aminoadipic acid | C6 H11 N O4 | 161.0683 | 4.50 | 1E+06 | 4 |
| 85 | Syringic acid | C9 H10 O5 | 198.0523 | 8.34 | 1E+06 | 4 |
| 86 | Prolinamide | C5 H10 N2 O | 97.05283 | 1.13 | 9E+05 | 2 |
| 87 | Thymine | C5 H6 N2 O2 | 126.043 | 4.40 | 8E+05 | 4 |
| 88 | N-Acetyl-L-phenylalanine | C11 H13 N O3 | 207.0893 | 8.64 | 8E+05 | 4 |
| 89 | Ethyl palmitoleate | C18 H34 O2 | 282.2554 | 13.82 | 8E+05 | 2 |
| 90 | 3-Isopropylmalic acid | C7 H12 O5 | 176.0676 | 1.39 | 7E+05 | 4 |
| 91 | Pseudouridine | C9 H12 N2 O6 | 244.0693 | 1.98 | 7E+05 | 2 |
| 92 | Hydrolyzed fumonisin B1 | C22 H47 N O5 | 405.3446 | 17.48 | 6E+05 | 4 |
| 93 | Corchorifatty acid F | C18 H32 O5 | 328.2254 | 11.50 | 6E+05 | 2 |
| 94 | Methylsuccinic acid | C5 H8 O4 | 132.0412 | 5.66 | 5E+05 | 2 |
| 95 | D-(+)-Maltose | C12 H22 O11 | 364.0973 | 1.09 | 5E+05 | 2 |
| 96 | N-Acetyl-L-tyrosine | C11 H13 N O4 | 223.0843 | 7.16 | 4E+05 | 4 |
| 97 | Suberic acid | C8 H14 O4 | 174.0885 | 8.88 | 4E+05 | 2 |
| 98 | (2R)-2,3-Dihydroxypropanoic acid | C3 H6 O4 | 106.0255 | 19.99 | 4E+05 | 4 |
| 99 | Citroflex 4 | C18 H32 O7 | 360.2141 | 13.33 | 3E+05 | 2 |
| 100 | Glutaric acid | C5 H8 O4 | 132.0413 | 5.02 | 3E+05 | 2 |
Quantification of antimicrobial compounds by LC-MS-MS
Among the compounds detected by LC-MS-MS, five of them are reported with antimicrobial activity and had relatively high concentrations in distillery wastewater, which may affect the resource recovery process for distillery wastewater via microorganisms. They are lactic acid [3], succinic acid [4], cinnamic acid [5], phenyllactic acid [6], acetophenone [7]. Therefore, an effective and rapid quantification method has been developed for these compounds in this study.
Analytical instrumentation
The LC–MS/MS system consisted of a Thermo Scientific Ultimate 3000 liquid phase system and TSQ Endura triple quadrupole mass spectrometer with an electrospray ionization source. Chromatographic separation was achieved at 20°C on a Hypersil Gold C18 column (100 × 2.1 mm, 1.9 μm, Thermo Scientific) by gradient solution with 0–2 min, 98% mobile phase A;2–4 min, 98%→80% mobile phase A; 4–7 min, 80%→10% mobile phase A; 7–9 min, 10% mobile phase A;9.1–12 min, 98% mobile phase A, flowing at 0.25 mL/min. Eluent A was water containing 0.1% formic acid, and B was methanol. The injection volume was 2 μL.
To achieve better retention and separation of both hydrophilic and polar compounds, two chromatographic columns with different stationary phases (i.e. a HILIC column and a C18 column) were examined with various mobile phases and additives (i.e. formic acid, acetic acid and ammonium acetate). Additionally, gradients, flow rate and column temperatures (20–40 °C) were also explored. It was found that the chromatographs of lactic acid and succinic acid were significantly affected by the column temperatures. Based on the chromatograph of lactic acid and succinic acid under 20 °C and 30 °C (Fig. S7), 20 °C was selected as the column temperature to obtain a good peak shape.
The addition of ammonium acetate into formic acid water or acetic acid water as mobile phase significantly decreased peak responses while did not improve peak shapes simultaneously. Compared with acetic acid in water, formic acid in water as the mobile phase could narrow peak widths. Therefore, 0.1% formic acid in water was selected as one of the mobile phases. Though the two columns had similar performance in resolution, retention time and peak shape, Hypersil Gold C18 as chromatographic separation column was chosen rather than Syncronis Hilic column (for polar components) because the former one was more commonly used.
The mass spectrometer was operated in negative ion mode using SRM to detect the mass transitions. High purity nitrogen served as both nebulizing and drying gas. Compound-dependent parameters of the mass spectrometer were set as follows: spray voltage at 2500 V, capillary temperature at 320 °C, vaporizer temperature at 350 °C, sheath gas at 35 (Arb) and auxiliary gas at 10 (Arb). The parameters of SRM scan mode for each compound are shown in Table 4. Fig. 2 demonstrated typical chromatograms of the five analytes.
Table 4.
MS/MS transitions and parameters for the analyses of the analytes.
| Compounds | Polarity | Precursor (m/z) | Product (m/z) | Collision Energy (V) |
|---|---|---|---|---|
| Lactic acid | Negative | 89.3 | 43.502(71.248*) | 10.25 |
| Succinic acid | Negative | 117.23 | 73.262(99.111*) | 10.25 |
| Acetophenone | Negative | 119.23 | 101.183(117.097*) | 16.42 |
| Cinnamic acid | Negative | 147.09 | 62.276(103.151*) | 10.25 |
| Phenyllactic acid | Negative | 165.07 | 103.151(147.04*) | 10.25 |
Note: *qualitative ion.
Fig. 2.
Typical chromatograms of the five analytes in distillery wastewater sample.
Validation of the method
The developed method was validated based on the recommendations published by FDA (Food and Drug Administration) [8]. The calibration curve consisted of five concentration levels. The linear regression of the areas of the analyte peaks versus the concentration were weighted with weighing factor 1/x2 (where x = concentration). The concentrations of the analyte were determined by interpolation from the calibration curve. Concentration of the standard sample in solvents with a signal-to-noise ratio (S/N) of 3 times is defined as instrumental detection limit. As shown in Table 5, all the analytes showed good linearity with regression coefficients (R2) values above 0.9981 (R > 0.9990). Linear ranges and IDL of the analytes were also shown in Table 5. The calibration curves of the five analytes were shown in Fig. S8.
Table 5.
Linear range, R2 value and IDL of the analytes.
| Compounds | Linear range (ng/mL) | R2 | IDL (ng/mL) | Linear regression equation (Y, peak area; X, concentration) |
|---|---|---|---|---|
| Lactic acid | 375–7500 | 0.9985 | 25 | Y=61.991+3.7752*X |
| Succinic acid | 50–1000 | 0.990 | 25 | Y=-1108.29+65.2021*X |
| Acetophenone | 50–1000 | 0.9983 | 0.5 | Y=296.551+61.5398*X |
| Cinnamic acid | 50–1000 | 0.9991 | 10 | Y=73.5861+15.9079*X |
| Phenyllactic acid | 50–1000 | 0.9984 | 1 | Y=2286.28+1166.98*X |
Three levels (low, medium and high) of organic acids were added to distillery wastewater samples to determine the precision (relative standard deviation, RSD) and extraction recovery (relative error, RE). Each level contained five validation samples. The recovery values of the five analytes at three concentration levels were shown in Fig. 3 and Table S1. All the recoveries were between 95.89% and 116.39% (RSD% < 9.80) at the three concentration levels of the analytes. These results were with the acceptance criteria and indicated that the method was accurate, reliable, and reproducible. Meanwhile, the wastewater samples were pretreated simply through dilution and centrifugation. These results of recoveries indicate that there was no significant matrix effect.
Fig. 3.
Recoveries of the five analytes at three concentration levels..
Application
The established LC-MS/MS method was applied for determining the concentration of the five major antimicrobial compounds in distillery wastewater obtained from the rice spirit distillery located in Foshan city, Guangdong, Southern China. Table 6 was the quantitative analysis results of the five analytes in distillery wastewater.
Table 6.
Quantitative analysis results of the five analytes in the distillery wastewater.
| Lactic acid | Succinic acid | Acetophenone | Cinnamic acid | Phenyllactic acid | |
|---|---|---|---|---|---|
| Concentration (mg/L) | 10,011–17,498 | 210–325 | 42–63 | 56–143 | 43–58 |
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was financially supported by National Natural Science Foundation of China (No. 51708131), China Postdoctoral Science Foundation (No. 2016M590761), and College Students Innovation and Entrepreneurship Training Program of Guangdong Province (No. 201811845158).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.mex.2021.101470.
Additional information
Background information of this topic and method
Distillery wastewater could cause many environment issues such as eutrophication due to its high generation amount and high concentration of organics and nutrients [1]. The compounds contained in the distillery wastewater mainly come from the making process including pretreatment and hydrolysis of crops or fruits, fermentation, distillation and dehydration [9]. For effective treatment and resource recovery process of the distillery wastewater, it is necessary to identify the components in wastewater, especially the antimicrobial compounds that may affect the conventional biological treatment process and the resource recovery process for distillery wastewater via microorganisms such as microbial lipid (can be further converted to biodiesel) or biogas production from wastewater [10], [11], [12].
The increased availability and development of high resolution mass spectrometry (HR-MS) had dramatically improved the qualitative analysis of compounds in environmental (and other) samples. The elucidation of small molecules both parent compounds and their transformation products using HR-MS based non-target analysis is gaining in relevance in many fields (e.g. metabolomics, drug discovery, forensics) [13]. Therefore, a HR-MS analysis method for identification of small molecular compounds in distillery wastewater was developed in this study.
The quantitative analysis for high-concentration confirmed compounds (match the measured retention time and tandem mass spectrum with reference standards) are usually necessary for research purpose. In all the confirmed compounds, lactic acid, succinic acid, acetophenone, cinnamic acid and phenyllactic acid were closely related to our microbial contamination control mechanism research. At present, the main analysis methods of these organic acids are enzymatic method [14], gas chromatography (GC) [15,16], high performance liquid chromatography (HPLC) [17], [18], [19], ion-exclusion chromatography [20], liquid chromatography-tandem mass spectrometry analysis (LC-MS) [21], [22], [23] and so on. Enzymatic methods had a high limit of detection and GC required pre-treatment of derivatization. Though most organic acids could be detected by HPLC, the UV sensitivity is relatively low. LC-MS is widely used because of its high selectivity and sensitivity. Therefore, a LC-MS-MS method was developed for the quantification of the five antimicrobial compounds.
Appendix B. Supplementary materials
References
- 1.Ghosh Ray S., Ghangrekar M.M. Comprehensive review on treatment of high-strength distillery wastewater in advanced physico-chemical and biological degradation pathways. Int. J. Environ. Sci. Technol. 2019;16:527–546. [Google Scholar]
- 2.Schymanski E.L., Jeon J., Gulde R., Fenner K., Ruff M., Singer H.P., Hollender J. Identifying small molecules via high resolution mass spectrometry: communicating confidence. Environ. Sci. Technol. 2014;48:2097–2098. doi: 10.1021/es5002105. [DOI] [PubMed] [Google Scholar]
- 3.Moreira T.F.M., De Oliveira A., Da Silva T.B.V., Dos Santos A.R., Gonçalves O.H., Gonzalez R.D.S., Droval A.A., Leimann F.V. Hydrogels based on gelatin: effect of lactic and acetic acids on microstructural modifications, water absorption mechanisms and antibacterial activity. Lwt. 2019;103:69–77. [Google Scholar]
- 4.Kumar R., Chandar B., Parani M. Use of succinic & oxalic acid in reducing the dosage of colistin against New Delhi metallo-beta-lactamase-1 bacteria. Indian J. Med. Res. 2018;147:97–101. doi: 10.4103/ijmr.IJMR_1407_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narasimhan B., Belsare D., Pharande D., Mourya V., Dhake A. Esters, amides and substituted derivatives of cinnamic acid: synthesis, antimicrobial activity and QSAR investigations. Eur. J. Med. Chem. 2004;39:827–834. doi: 10.1016/j.ejmech.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Bustos A.Y., Font de G., Valdez C.L. Gerez Optimization of phenyllactic acid production by Pediococcus acidilactici CRL 1753. Application of the formulated bio-preserver culture in bread. Biol. Control. 2018;123:137–143. doi: 10.1016/j.biocontrol.2018.05.017. [DOI] [Google Scholar]
- 7.Gul H.I., Denizci A.A., Erciyas E. Antimicrobial evaluation of some Mannich bases of acetophenones and representative quaternary derivatives. Arzneimittel-Forschung/Drug Res. 2002;52:773–777. doi: 10.1055/s-0031-1299965. [DOI] [PubMed] [Google Scholar]
- 8.U. S. Food and Drug Administration. Reviewer Guidance, Validation of Chromatographic Methods. 1994. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/reviewer-guidance-validation-chromatographic-methods
- 9.Wilkie A.C., Riedesel K.J., Owens J.M. Stillage characterization and anaerobic treatment of ethanol stillage from conventional and cellulosic feedstocks. Biomass Bioenergy. 2000;19:63–102. [Google Scholar]
- 10.Ling J., Xu Y., Lu C., Hou W., Liu Q., Wang F., Du Q. Microbial contamination control mechanism in lipid production using distillery wastewater and oleaginous yeast – antimicrobial compounds in wastewater as a double-edged sword. J. Environ. Manag. 2021;291 doi: 10.1016/j.jenvman.2021.112672. [DOI] [PubMed] [Google Scholar]
- 11.Graham J., Peter B., Walker G.M., Wardlaw A., Campbell E. Chapter 43: Characterisation of the pot ale profile from a malt whisky distillery. In: Walker G.M., Goodall I., Fotheringham R., Murray D., editors. Distilled Spirits: Science and Sustainability. Nottingham University Press/The Institute of Brewing and Distilling; Nottingham, UK: 2012. https://rke.abertay.ac.uk/ws/portalfiles/portal/8489027/WalkerDistilledSpirits43.pdf Proceedings of the Worldwide Distilled Spirits Conference (Distilled Spirits Series; Vol. 4) [Google Scholar]
- 12.White J.S., Stewart K.L., Maskell D.L., Diallo A., Traub-Modinger J.E., Willoughby N.A. Characterization of Pot Ale from a Scottish malt whisky distillery and potential applications. ACS omega. 2020;5:6429–6440. doi: 10.1021/acsomega.9b04023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escher B.I., Fenner K. Recent advances in environmental risk assessment of transformation products. Environ. Sci. Technol. 2011;45:3835–3847. doi: 10.1021/es1030799. [DOI] [PubMed] [Google Scholar]
- 14.Barker S.B., Summerson W.H. The colorimetric determination of Lactic acid in biological material. J. Biol. Chem. 1941;138:535. [Google Scholar]
- 15.Zhang H.Y., Tan X.X., Kang K., Wang W., Lian K.Q., Kang W.J. Simultaneous determination of lactic acid and pyruvic acid in tissue and cell culture media by gas chromatography after in situ derivatization-ultrasound-assisted emulsification microextraction. Anal. Bioanal. Chem. 2019;411:787–795. doi: 10.1007/s00216-018-1502-z. [DOI] [PubMed] [Google Scholar]
- 16.Pechlivanis A., Chatziioannou A.C., Veskoukis A.S., Kouretas D., Mougios V., Theodoridis G.A. GC–MS analysis of blood for the metabonomic investigation of the effects of physical exercise and allopurinol administration on rats. J. Chromatogr. B. 2014;966:127–131. doi: 10.1016/j.jchromb.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Ewaschuk J.B., Naylor J.M., Barabash W.A., Zello G.A. High performance liquid chromatographic assay of lactic, pyruvic and acetic acids and lactic acid stereoisomers in calf feces, rumen fluid and urine. J. Chromatogr. B. 2004;8005:347–351. doi: 10.1016/j.jchromb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Kemmei T., Kodama S., Yamamoto A. Reversed phase liquid chromatographic determination of organic acids using on-line complexation with copper (II) ion. Anal. Chim. Acta. 2015;886:194–199. doi: 10.1016/j.aca.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Restuccia D., Spizzirri U.G., Puoci F. LC with evaporative light-scattering detection for quantitative analysis of organic acids in juices. Food Anal. Method. 2017;10:704–712. [Google Scholar]
- 20.Chen P., Nie L.H., Yao S.Z. Determination of lactic acid and pyruvic acid in serum and cerebrospinal fluid by ion-exclusion chromatography with a bulk acoustic wave detector. J Chromatogr. B. 1995;673:153–158. doi: 10.1016/0378-4347(95)00266-0. [DOI] [PubMed] [Google Scholar]
- 21.Claudio G., Cristian D.B., Paolo S. Validation and application of an ultrahigh-performance liquid chromatographic-Orbitrap mass spectrometric method for the simultaneous detection and quantification of volatile and non-volatile organic acids in human faecal samples. J Pharm. Biomed. Anal. 2017;141:46–51. doi: 10.1016/j.jpba.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Rune I.D.B., Nicolaj B.S., Sigurd H., Torsten T.N., Niels G., Hans E.B. A UPLC–MS/MS application for profiling of intermediary energy metabolites inmicrodialysis samples—a method for high-throughput. J Pharm. Biomed. Anal. 2010;53:983–990. doi: 10.1016/j.jpba.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Sandín-España P., Mateo-Miranda M., López-Goti C. Development of a rapid and direct method for the determination of organic acids in peach fruit using LC-ESI-MS. Food Chem. 2016;192:168–273. doi: 10.1016/j.foodchem.2015.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.