Abstract
Culture-based microdilution and disk diffusion tests are two commonly used reference methods for determining the susceptibility of causative bacteria to antibiotics. However, these methods are slow and laborious. Automated antimicrobial susceptibility test (AST) instruments are extensively used in clinical microbiology labs, replacing manual methods to perform gold standard microdilution or disk diffusion methods. These automated instruments require the use of isolated bacteria grown in pure culture against a fixed antimicrobial panel, and the susceptibility tests are based on measuring bacterial growth or turbidity changes over a range of pre-determined antimicrobial conditions. As a result, these automated technologies remain inherently inflexible to frequent adjustment of minimum inhibitory concentrations published by the Clinical and Laboratory Standards Institute and are limited by the detection methods that consumables were designed for. Here, we present a stripwell that is compatible with the 96-well format of most lab automation systems to provide a streamlined workflow to inoculate microorganisms for a customized or routine AST. The main goal of this method of stripwell preparation with various antibiotic conditions is to enable the utility of lab automation for phenotypic antibiotic response assays to address the reproducibility issues due to manual operation.
• A standardized and scalable solution from inoculation to antimicrobial incubation
• Microplates in stripwell format offer the advantage of greater flexibility in clinical microbiology and diagnostics
• Customized antimicrobials and dilution ranges tailored to unique specifications for research and development
Keywords: 1 × 8 stripwell, 96-well plate format, Customized antimicrobials, Customized dilution ranges, Scalable antibiotic incubation, 96-well standard heating block
Abbreviations: AST, antimicrobial susceptibility testing; CLSI, Clinical and Laboratory Standards Institute; MIC, minimum inhibitory concentration; DEPC, Diethyl pyrocarbonate
Graphical abstract

Specifications table
| Subject Area | Immunology and Microbiology |
| More specific subject area | Antimicrobial susceptibility testing |
| Method name | Microdilution with customized antimicrobial incubation stripwells compatible with 96-well format |
| Name and reference of original method | Chen CH, Lu, Y, Sin MLY, Mach KE, Zhang DD, Gau V, Liao JC, Wong PK. Rapid antimicrobial susceptibility testing using high surface-to-volume ratio microchannels. Anal Chem. 2010; 82(3): 1012. Mach KE, Mohan R, Baron EJ, Shih M-C, Gau V, Wong PK, Liao JC. A biosensor platform for rapid antimicrobial susceptibility testing directly from clinical samples. J Urol. 2011; 185(1): 148–153. Altobelli E, Mohan R, Mach KE, Sin MLY, Anikst V, Buscarini M, Wong PK, Gau V, Banaei N, Liao JC. Integrated biosensor assay for rapid uropathogen identification and phenotypic antimicrobial susceptibility testing. Eur Urol Focus. 2017; 3(2,3): 293–299. doi: 10.1016/j.euf.2015.12.010 |
| Resource availability | All resources including reagent, consumables and materials necessary to reproduce the method are described in the Methods details. |
Introduction
Although broth microdilution has been a longstanding method for antimicrobial susceptibility testing (AST), advancements in AST automation have proved to alleviate some of the hands-on time of traditional AST methods. However, even these automated platforms remain limited by their detection methods and inherent inflexibility to changing minimum inhibitory concentrations (MIC). Herein, we describe a method for categorizing microbial growth inhibition using a 1 × 8 antibiotic stripwell that is easily customizable and adaptable to most lab automation systems, as well as changing MICs. The described stripwell, when paired with our electrochemical biosensor assay, relies on the basic principles of the broth microdilution test but offers several advantages over the microdilution method, namely a much shorter antibiotic exposure time and quantifiable method for detecting 16S rRNA. According to Clinical and Laboratory Standards Institute guidelines, the microdilution method requires a 16–20 h antibiotic exposure period followed by a visual check for turbidity of bacterial inoculum to determine the minimum inhibitory concentration [1]. Our method requires 3 h of antibiotic exposure, which is also easily adjustable to shorter or longer times depending on the microbial load tested but was set to 3 h for this validation. Following antibiotic exposure, microbial growth inhibition is quantified using an electrochemical sensor assay as previously described rather than a visual check which is subject to variation between operators [2].
Method overview
This method of stripwell preparation utilizes three antibiotics commonly prescribed for complicated urinary tract infections and sepsis: ciprofloxacin (fluoroquinolone class), gentamicin (aminoglycoside class), and meropenem (carbapenem class) [3]. Here we detail the procedure for preparing two types of antibiotic stripwells: AST and one-drug. The configurations for these stripwells are shown in Fig. 1A. AST stripwells contain all three antibiotics with two concentrations for ciprofloxacin and gentamicin and three concentrations for meropenem. One-drug stripwells use only one antibiotic and include a broader range of seven concentrations of two-fold dilutions in order to determine the antimicrobial concentration needed to inhibit microbial growth. Additionally, both types of stripwells contain one well without antibiotics to be used as a growth control (GC).
Fig. 1.
Configuration of (A) ciprofloxacin, (B) gentamicin, and (C) meropenem one-drug, and (D) AST stripwells.
Antibiotics in their original manufactured form are used to make antibiotic stock reagents, which are then used to make stripwell reagents for stripwell preparation. Antibiotic stripwell reagents are dried onto flat-bottom medical grade polystyrene 8-well strips at selected concentrations such that resuspension of these reagents with 100 µL of bacterial inoculum results in the final working concentrations for each antibiotic [4]. It is necessary to prepare the antibiotic stock reagents and stripwell reagents immediately prior to stripwell production; any leftover antibiotic stock reagents and stripwell reagents must be properly discarded.
Materials
-
•
Ciprofloxacin (hydrochloride), Cat. No. 14,286, Cayman Chemical Company, Ann Arbor, MI.
-
•
Gentamicin Solution, Cat. No. G1272, Sigma-Aldrich, St. Louis, MO.
-
•
Meropenem (hydrate), Cat. No. 16068, Cayman Chemical Company, Ann Arbor, MI.
-
•
Tween 20, Cat. No. 9005–64–5, Fisher Scientific, Waltham, MA.
-
•
EIA/RIA 8-well strips, 96-well format, Cat. No. 2592, Corning, Corning, NY.
-
•
OmniPur Water, DEPC treated, Cat. No. 9601-OP, Sigma-Aldrich, St. Louis, MO.
-
•
Ktdorns food coloring kit, Shenzhen Sichuangyi Technology, Munich, Germany.
Ciprofloxacin reagent preparation
Steps 1–2 detail the procedure for preparing ciprofloxacin reagent to be used in AST stripwells.
-
1.
Dissolve 25 mg of ciprofloxacin powder into 25 mL of DEPC water to make 1 mg/mL ciprofloxacin stock solution.
-
2.
Dilute the stock solution by adding 80 µL of that solution to 8 mL of DEPC water with 0.1% Tween 20, resulting in a 10 µg/mL concentration. Add 8 µL of Ktdorns Royal Blue food coloring to this solution.
NOTE: The food coloring is added as an identifier of which antibiotic is in use and serves no other function in the performance of the stripwell.
If preparing one-drug stripwells, perform the steps outlined below.
-
1.
Dissolve 25 mg of ciprofloxacin powder into 25 mL of DEPC water to make 1 mg/mL ciprofloxacin stock solution.
-
2.
Dilute the stock solution by adding 160 µL of that solution to 3.84 mL of DEPC water with 1% Tween, or 3.84 µL of Tween 20, resulting in a 40 µg/mL concentration. Add 4 µL of Ktdorns Royal Blue food coloring to this solution.
-
3.
Dilute the 40 µg/mL solution by adding 0.5 mL of that to 3.5 mL of DEPC with 1% Tween, or 3.5 µL of Tween 20, resulting in a 5 µg/mL concentration. Add 4 µL of Ktdorns Royal Blue food coloring to this solution.
Gentamicin reagent preparation
Step 1 below details the procedure for preparing gentamicin reagent to be used in AST stripwells. If preparing one-drug stripwells, the additional step 2 will need to be performed. Because one-drug stripwells require a wider range of antibiotic conditions, two antibiotic solutions at different concentrations must be used to cover the entire spectrum.
-
1.
Dilute the stock gentamicin solution by adding 32 µL of this solution to 8 mL of DEPC water with 0.1% Tween 20, resulting in a concentration of 40 µg/mL. The certificate of analysis for the gentamicin stock solution must be checked for the working concentration, which may vary from the nominal 10 mg/mL. If the concentration is not 10 mg/mL, the amount of stock added to 8 mL of DEPC water with 0.1% Tween will need to be adjusted accordingly. Add 8 µL of Ktdorns Sunset Yellow food coloring to this solution.
-
2.
Dilute the 40 µg/mL solution by adding 0.5 mL of that into 3.5 mL of DEPC water with 1% Tween, or 3.5 µL of Tween 20, resulting in a 5 µg/mL concentration. Add 4 µL of Ktdorns Sunset Yellow food coloring to this solution.
Meropenem reagent preparation
The following steps detail the procedure for preparing meropenem reagent to be used in AST and one-drug stripwells. Both configurations require two separate meropenem solutions to cover the range of conditions tested.
-
1.
Dissolve 5 mg of stock meropenem hydrate powder in 35 mL of DEPC water with 0.1% Tween 20, or 35 µL Tween 20, resulting in a concentration of 125 µg/mL. Add 35 µL of Ktdorns Sunset Red food coloring to this solution. Meropenem is prepared at 125 μg/mL but this concentration is treated as 80 μg/mL when dispensing to account for the decrease in effective concentration observed during the drying process.
-
2.
Add 0.25 mL of this solution to 3.75 mL of DEPC water with 0.1% Tween 20, resulting in a 5 µg/mL concentration. Add 4 µL of Ktdorns Sunset Red food coloring to this solution.
One-drug stripwells
The one-drug stripwell contains 7 concentrations for only one antibiotic. The first well of each stripwell is left with no antibiotic to be used as a growth control during testing. Ciprofloxacin one-drug stripwells are prepared at the following concentrations: 0.25, 0.5, 1, 2, 4, 8, and 16 µg/mL. Gentamicin one-drug stripwells are prepared at the following concentrations: 0.25, 0.5, 1, 2, 4, 8, and 16 µg/mL. Meropenem one-drug stripwells are prepared at the following concentrations: 0.25, 0.5, 1, 2, 4, 8, and 16 µg/mL.
-
1.
Dispense volumes to each well as described in Table 1.
-
2.
Once reagents are added to stripwells, dry the stripwells at 37 °C for 12 h. Stripwells that are not used immediately following the drying period should be stored in vacuum-sealed bags with desiccant at 4 °C. These drying and storage methods apply to all stripwell types.
Table 1.
Volumes of reagent added to each well for preparation of one-drug antibiotic stripwells.
| Well | Ciprofloxacin one-drug |
Gentamicin one-drug |
Meropenem one-drug |
|||
|---|---|---|---|---|---|---|
| Ciprofloxacin reagent | Volume (µL) | Gentamicin reagent | Volume (µL) | Meropenem reagent | Volume (µL) | |
| 1 | N/A | 0 | N/A | 0 | N/A | 0 |
| 2 | 5 µg/mL | 5 | 5 µg/mL | 5 | 5 µg/mL | 5 |
| 3 | 5 µg/mL | 10 | 5 µg/mL | 10 | 5 µg/mL | 10 |
| 4 | 5 µg/mL | 20 | 5 µg/mL | 20 | 5 µg/mL | 20 |
| 5 | 5 µg/mL | 40 | 5 µg/mL | 40 | 5 µg/mL | 40 |
| 6 | 40 µg/mL | 10 | 40 µg/mL | 10 | 80 µg/mL | 5 |
| 7 | 40 µg/mL | 20 | 40 µg/mL | 20 | 80 µg/mL | 10 |
| 8 | 40 µg/mL | 40 | 40 µg/mL | 40 | 80 µg/mL | 20 |
AST stripwell preparation
The AST stripwell contains all three antibiotics and includes the following concentrations for each antibiotic: 1 and 4 µg/mL ciprofloxacin, 2 and 8 µg/mL gentamicin, and 1, 4, and 16 µg/mL meropenem. Antibiotic solutions are added to their corresponding wells at the following volumes (Table 2). The first well is left with no antibiotic to be used as a growth control during testing.
Table 2.
Volumes of reagent added to each well for preparation of AST stripwells.
| Well | Antibiotic reagent | Volume (µL) | Resulting concentration (µg/mL) |
|---|---|---|---|
| 1 | N/A | 0 | 0 |
| 2 | Meropenem (5 µg/mL) | 20 | 1 |
| 3 | Ciprofloxacin | 10 | 1 |
| 4 | Gentamicin | 5 | 2 |
| 5 | Meropenem (80 µg/mL) | 5 | 4 |
| 6 | Ciprofloxacin | 40 | 4 |
| 7 | Gentamicin | 20 | 8 |
| 8 | Meropenem (80 µg/mL) | 20 | 16 |
Method validation
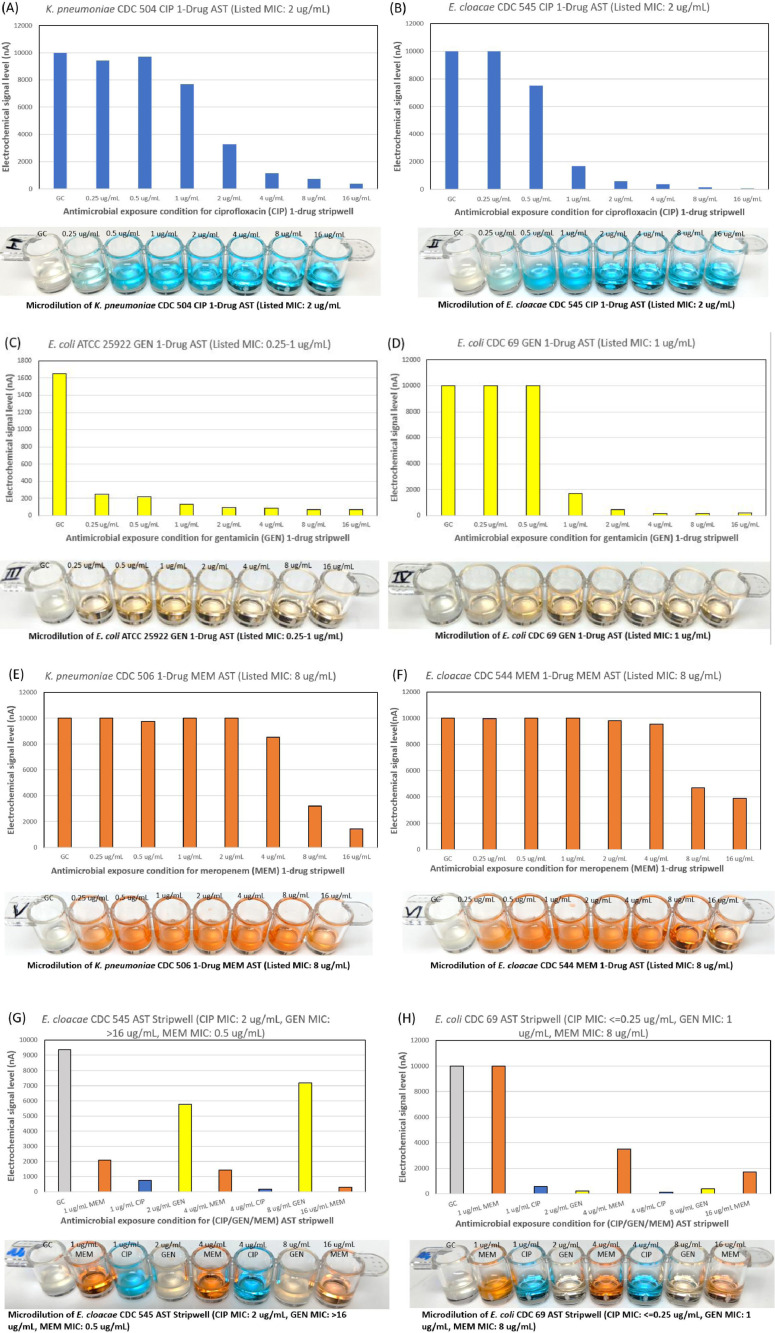
To verify that our stripwells were functionalized with the desired antibiotic concentrations, we performed the broth microdilution reference method as described in CLSI M07 [5]. Strains from the CDC AR Bank with different minimum inhibitory concentrations for each antibiotic were suspended in Mueller-Hinton II broth and diluted to a concentration of 5 × 105 CFU/mL for testing. Stripwells were inoculated with bacterial samples (100 µL per well) and incubated for 16–20 h at 37 °C. After this incubation period, the stripwells were visually checked for microbial growth inhibition. Wells with turbid inoculum indicated microbial growth, suggesting that the antibiotic condition for that particular well was ineffective against the bacteria. Wells with clear inoculum indicated microbial growth inhibition, suggesting the antibiotic condition for that well was effective against the bacteria. The concentration of the first well to exhibit complete growth inhibition was compared to the strain's minimum inhibitory concentration as listed on the CDC AR Bank website. Fig. 2 below shows the microdilution results for AST and one-drug stripwells, as well as the results of an electrochemical biosensor assay quantifying 16S rRNA content of the inoculum in each well after the 3 h antimicrobial exposure.
Fig. 2.
Representative results for (A, B) ciprofloxacin, (C, D) gentamicin, (E, F) meropenem one-drug stripwells, and (G, H) AST stripwells with 3 h antimicrobial exposure with a corresponding microdilution result with 18–24 h antimicrobial exposure as a reference.
The signal level generated (y-axis) reflects the amount of 16S rRNA present in the well after antibiotic exposure. Higher signal levels indicate higher growth, while lower signal levels indicate lower microbial growth, or inhibition. These signal levels may be interpreted in a manner similar to the turbidity of the wells in the microdilution method. For example, in Fig. 2D, the first three wells including the GC and two lower gentamicin concentrations all showed high signal level at 10,000 nA, suggesting that these two lower gentamicin concentrations were ineffective against this strain. The next well, which contained 1 µg/mL, showed a lower signal level just below 2000 nA. This large difference in signal level between these wells suggests that 1 µg/mL is the minimum gentamicin concentration needed to inhibit this strain. The same trend was observed for its corresponding microdilution. The first three wells were turbid while the rest of the wells were clear, also suggesting that 1 µg/mL (fourth well) is the minimum inhibitory concentration of this strain. The stripwell exhibited comparable performance in both the microdilution and electrochemical biosensor assay, each of which had very different exposure times (16–20 h for microdilution and 3 h for assay).
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Jade Chen, Michael Tomasek, and Vincent Gau are employed by GeneFluidics. As a non-academic, commercial company, the employer and funder provided support in the form of salaries for authors [J.C., M.T., V.G.], but did not have any additional role in the protocol design, data collection and decision to publish.
References
- 1.CLSI . Clinical Laboratory Standards Institute; Wayne, PA: 2009. Methods For Dilution Antimicrobial Susceptibility Tests For Bacteria That Grow Aerobically; Approved Standard-Eighth Edition. CLSI Document M07-A8. [Google Scholar]
- 2.J. Chen, M. Tomasek, E. Navarro, V. Gau, Method for molecular quantification of 16S rRNA with multiplexed-electrochemical
- 3.Tamma P.D., Cosgrove S.E., Maragakis L.L. Combination therapy for treatment of infections with gram-negative bacteria. Clin. Microbiol. Rev. 2012;25:450–470. doi: 10.1128/CMR.05041-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altobelli E., Mohan R., Mach K.E., Sin M.L.Y., Anikst V., Buscarini M., Wong P.K., Gau V., Banaei N., Liao J.C. Integrated biosensor assay for rapid uropathogen identification and phenotypic antimicrobial susceptibility testing. Eur. Urol. Focus. 2017;3(2–3):293–299. doi: 10.1016/j.euf.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CLSI . Clinical Laboratory Standards Institute; Wayne, PA: 2009. Methods For Dilution Antimicrobial Susceptibility Tests For Bacteria That Grow Aerobically; Approved Standard-Eighth Edition. CLSI Document M07-A8. [Google Scholar]