Abstract
Some of the most important pathogens affecting wildlife are transmitted indirectly via the environment. Yet the environmental stages of pathogens are often poorly understood, relative to infection in the host, making this an important research frontier. Sarcoptic mange is a globally widespread disease caused by the parasitic mite Sarcoptes scabiei. The bare-nosed wombat (Vombatus ursinus) is particularly susceptible, and their solitary nature and overlapping use of burrows strongly indicate the importance of environmental transmission. However, due to the challenge of accessing and monitoring within wombat burrows, there has been limited research into their suitability for off-host mite survival and environmental transmission (i.e., to serve as a fomite). We created a model using published laboratory data to predict mite survival times based on temperature and humidity. We then implemented innovative technologies (ground-penetrating radar and a tele-operated robotic vehicle) to map and access wombat burrows to record temperature and relative humidity. We found that the stable conditions within burrows were conducive for off-host survival of S. scabiei, particularly in winter (estimated mite survival of 16.41 ± 0.34 days) and less so in warmer and drier months (summer estimated survival of 5.96 ± 0.37 days). We also compared two areas with higher and lower average mange prevalence in wombats (13.35% and 4.65%, respectively), finding estimated mite survival was slightly higher in the low prevalence area (10.10 and 12.12 days, respectively), contrary to our expectations, suggesting other factors are also important for population prevalence. Our study is the first to demonstrate the suitability of the bare-nosed wombat burrow for off-host mite survival and environmental transmission. Our findings have implications for understanding observed patterns of mange, disease dynamics and disease management for not only bare-nosed wombats, but also other burrow or den-obligate species exposed to S. scabiei via environmental transmission.
Keywords: Burrow, Common wombat, Sarcoptic mange, Scabies, Fomite, Pathogen persistence
Graphical abstract
Highlights
-
•
Wombat burrows are a source of environmental transmission of Sarcoptes scabiei.
-
•
We used ground-penetrating radar and a robotic vehicle to measure burrow conditions.
-
•
We estimate S. scabiei can survive 5.96–16.41 days within burrows depending on season.
-
•
Seasonal variation in environmental survival may influence disease dynamics in wombats.
1. Introduction
Environmental transmission is characterised by the indirect exposure of a susceptible host to a pathogen carrying fomite in the environment, rather than transmission from direct contact between individuals (Breban, 2013; Park, 2012). For some of the most important pathogens affecting wildlife (e.g., Batrachochytrium dendrobatidis, Pseudogymnoascus destructans, and Sarcoptes scabiei) environmental transmission can be a major source of exposure and can cause individual host mortality and population declines (Hoyt et al., 2015; Kilpatrick et al., 2010; Martin et al., 2018a; Pence and Ueckermann, 2002; Scheele et al., 2017; Tompkins et al., 2015). From a pathogen's perspective, the ability to survive off-host can support persistence and allow transmission between individuals that rarely come into direct contact (Hoyt et al., 2018; Satterfield et al., 2017). Thus, transmission has the potential to be independent of host density, exacerbating the impacts of the pathogen (De Castro and Bolker, 2004; Hoyt et al., 2015, 2020). However, one of the major challenges in developing a deeper insight into environmentally transmitted pathogens is understanding environmental factors that influence off-host pathogen survival. Much research focuses on the host, with comparably less focus on the environmental component (Alasaad et al., 2013; Escobar et al., 2021). Understanding the factors that influence the environmental transmission of pathogens is an essential component of improved interpretation of disease dynamics, and may also lead to improved disease management (Montecino-Latorre et al., 2019; Stevenson et al., 2013).
Sarcoptic mange (or scabies in humans) is caused by the epidermal burrowing parasitic mite, Sarcoptes scabiei. The pathogen affects almost 150 species worldwide, including humans, domestic animals, and wildlife (Bornstein et al., 2001; Escobar et al., 2021; Mörner, 1992; Niedringhaus et al., 2019a; Pence and Ueckermann, 2002). Sarcoptic mange is labelled as an ‘emerging infectious disease’ in some North American and Australian wildlife and is documented to have an expanding host range (Escobar et al., 2021; Tompkins et al., 2015). Infection (sometimes also called infestation) results in immunopathology characterised by a type I or type IV hypersensitivity reaction which leads to skin inflammation and other more severe clinical signs, including alopecia, hyperkeratosis, pruritus and emaciation (Bornstein et al., 2001; Pence and Ueckermann, 2002; Skerratt, 2003). Highly susceptible or immunocompromised hosts experience the most severe form of mange disease, crusted mange (type IV hypersensitivity), characterised by intense clinical signs, secondary infections, and mortality (Bornstein et al., 2001; Martin et al., 2018b; Pence et al., 1983). The parasite is a priority concern for a range of species around the globe including the bare-nosed wombat (Vombatus ursinus), northern chamois (Rupicapra rupicapra), American black bear (Ursus americanus), San Joaquin kit fox (Vulpes macrotis mutica) and red fox (Vulpes vulpes) (Astorga et al., 2018; Escobar et al., 2021; Tompkins et al., 2015). It is also a recognised Neglected Tropical Disease of humans (Vos et al., 2012; World Health Organisation (WHO)).
Sarcoptes scabiei can be transmitted both directly and indirectly (Pence and Ueckermann, 2002). Once mites become established on the host, transmission can occur through direct contact among host individuals. Larvae, nymphs and adult mites inhabit and roam on the surface of the skin of the infected individuals and, therefore, may transfer to an uninfected individual during close contact (Arlian and Vyszenski-Moher, 1988). S. scabiei mites can also survive for periods off-host (Arlian et al., 1984a; Mellanby et al., 1942). Laboratory trials have shown that in humid (97% relative humidity) and relatively cool (10ᵒC) conditions mites can survive off-host for up to 19 days, with lower survival outside of these conditions (Arlian et al., 1984a). These ideal humidity and temperature conditions sustain mite survival for the longest because they reduce desiccation and lower mite metabolic rates (Arlian, 1989; Arlian and Morgan, 2017; Davis and Moon, 1987). Once mites in the environment contact the skin of a new host individual, they recommence epidermal burrowing to feed and reproduce. Mites dislodged from a host for 36 h in laboratory conditions can reliably penetrate the epidermis of the host once reattached (Arlian et al., 1984a). There are also indications that S. scabiei mites can remain infective for at least one-half to two-thirds of their off-host survival time (Arlian, 1989; Arlian and Morgan, 2017). Additionally, S. scabiei mites exhibit host-seeking behaviours which can increase their chances of successful environmental transmission. Mites can sense the temperature and odour of hosts and can crawl up to 150 mm to attach themselves to a new host after being dislodged (Arlian et al., 1984b). The combination of these traits permits environmental transmission; however, there has been little investigation of the environmental conditions for S. scabiei survival in nature.
Environmental transmission is primarily documented in mange affected species that use underground retreat sites, such as burrows and dens, for example, kit foxes, black bears, foxes, and bare-nosed wombats (Loredo et al., 2020; Niedringhaus et al., 2019b; Skerratt, 2005; Soulsbury et al., 2007). Burrows and dens are expected to provide favourable conditions, buffered from outside climatic variation, where mites shed from infected hosts can survive (Loredo et al., 2020; Martin et al., 2019b; Skerratt, 2005). As wildlife use these sites repeatedly and for long periods, there is a higher chance of mites being shed in this location and increased opportunities for these mites to reattach when other individuals later use the same retreat site (Costello et al., 2006; Koopman et al., 1998; Martin et al., 2019a; Sommerer, 2014). Modelling of S. scabiei transmission in kit foxes identified the importance of within-den indirect transmission as a key factor that increased establishment, persistence and spread of S. scabiei (Montecino-Latorre et al., 2019). Investigation into the climatic conditions within dens suggested that kit fox dens have a suitable microclimate for an estimated average of 4.8 days off-host S. scabiei survival (Loredo et al., 2020). This is the only study to estimate mite survival in nature. The inaccessibility of underground retreat sites often hinders the monitoring of the factors that may influence S. scabiei transmission and consequently, this remains an understudied area for most mange affected species where environmental transmission is important.
The bare-nosed wombat (a.k.a common wombat) is highly susceptible to sarcoptic mange disease (Fraser et al., 2016; Skerratt, 2005). Mange is widespread across the geographic range of the bare-nosed wombat (Martin et al., 1998), persisting endemically with periodic epizootics causing localised population decline and extirpation events (Gray, 1937; Martin et al., 1998, 2018a; Skerratt, 2005). Indirect transmission through environmental exposure appears to be the dominant transmission mode due to their solitary social structure (wombats rarely come into direct contact in the field except for mating; see Martin et al., 2018a; Triggs, 2009). They are non-territorial and use a small number of ‘core’ burrows for rest, thermoregulation, and avoidance of predators, which they switch every 4–10 days (Evans, 2008; Martin et al., 2019b). Wombats engage in ‘asynchronous burrow sharing’, characterised by when one wombat departs a burrow another may begin using it with varying delays between occupancy, and thus individual wombats often overlap in their use of core burrows (Martin et al., 2019a; Skerratt et al., 2004). The bedding chamber in burrows is widely considered the site responsible for most S. scabiei transmission events (Martin et al., 2018a; Old et al., 2017). This is supported by logical lines of evidence that suggest that the microclimate within burrows may approximate those needed to maximise mite survival off the host (high humidity and cool temperatures) (Arlian and Morgan, 2017; Shimmin et al., 2002). Furthermore, mathematical modelling and observations of disease spread in nature support transmission in burrows as the mode of mange spread in wombats (Beeton et al., 2019; Martin et al., 2019b). However, owing to the challenge of accessing and sampling within wombat burrows, research within these environments is limited and largely based on assumptions.
The objective of this study is to investigate the potential of wombat burrows for environmental transmission of S. scabiei. We have four aims: (1) to create a statistical model describing how temperature and relative humidity influence mite survival as reported from laboratory trials (Arlian et al., 1984a); (2) to access and monitor environmental conditions within wombat burrows to estimate mite survival times and the factors associated with variance in this; (3) compare burrow conditions between areas of known higher and lower mange prevalence in wombats to evaluate if estimated mite survival times exhibit spatial variation; and (4) investigate seasonal variation in mite survival. As underground retreat sites are expected to have relatively stable conditions compared to the outside environment (Brown, 1984; Pike and Mitchell, 2013; Shimmin et al., 2002), we hypothesise that conditions within burrows will be conducive for environmental transmission of S. scabiei, providing empirical support that asynchronous burrow sharing is a likely mechanism of indirect transmission among wombats. In warmer and drier times of year we hypothesise a decrease in off-host mite survival as these environmental conditions are expected to be less favourable. Additionally, as environmental differences between regions likely impacts the suitability of burrows for off-host mite survival and subsequent environmental transmission of S. scabiei, we hypothesise that observed spatial heterogeneity of mange prevalence is related to variation in internal temperature and relative humidity conditions. Collectively this research will contribute to understanding the role of environmental transmission in disease dynamics overtime and has important implications for predicting the effectiveness of disease intervention strategies.
2. Methods
This study was approved by the University of Tasmania Animal Ethics Committee (Approval number A0018662) and the Department of Primary Industries, Parks, Water and Environment (Permit No. FA, 20110).
2.1. Study site description
This study was conducted at Musselroe Wind Farm, Cape Portland Tasmania (Fig. 1), which in addition to being a wind farm is also an active beef production farm. The site was selected due to endemic mange disease present in the wombat population and the prevalence of mange has been monitored by the Department of Primary Industries, Parks, Water and Environment (DPIPWE) since 2017, with an average prevalence of 4.65% in the east and 13.35% in the west (Driessen et al., 2021; Driessen et al., 2018, Fig. 1). The eastern area includes a higher amount of native vegetation and sand dunes in addition to areas of pasture. In comparison, the western area is mostly low-lying pasture with restricted amounts of native vegetation. Remnant native vegetation across the site includes Allocasuarina verticillata forest, coastal scrub, coastal grass and herbfield, coastal heathland, Acacia longfolia coastal scrub and Melaleuca ericifolia swamp forest (Driessen et al., 2018; Tasmanian Government, 2018). Mean annual rainfall at Cape Portland is 732 mm, with an average of between 50 and 77 mm falling each month, excluding February which receives an average of 34 mm (Bureau of Meterology, 2020). Average monthly rainfall is highest in July and August. Average temperatures range from 15.4 to 21.2 °C in February and 8.4–13.3 °C in July (Bureau of Meterology, 2020).
Fig. 1.
Location of study area at Musselroe Wind Farm, Cape Portland. Map of DPIPWE spotlight survey transect routes, red: west transect, blue: east transect. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.2. Environmental conditions within burrows (Aim 2)
To monitor environmental conditions within burrows we focused on the western area of the property due to the open, flat areas suitable for the use of ground-penetrating radar (GPR). We used GPR to locate and map burrow tunnels from above. GPR is a geophysical method that uses radar pulses to detect and image features in the subsurface (Davis and Annan, 1989). This method has previously been used to map the burrows of southern hairy-nosed wombats (Swinbourne et al., 2015). A Mala GX GPR system was used with a Mala 450 MHz HDR antenna mounted on a rough-terrain cart. Burrow entrances were first inspected to identify the most likely direction of the tunnel. We then pushed the GPR cart at a slow walking pace over the burrow on a line approximately perpendicular to the observed tunnel. GPR signal quality was generally good in the sandy, low-conductivity soils at Cape Portland and the tunnel was usually clearly identifiable as a diffraction on the GPR section. The tunnel position and depth were inferred from the location of the apex of the diffraction hyperbola. The path of the burrow was tracked by walking an irregular grid of GPR lines spaced 0.5–1 m apart and the identified location of the tunnels were marked immediately with flagging tape. Once the burrow was mapped to its terminus, the path of the tunnel was then measured from the burrow entrance. Burrows were selected based on signs of use (e.g., fresh digging, presence of scats, footprints, lack of overgrowth around burrow entrance) and suitable surrounding terrain (e.g., clear, flat ground appropriate for manoeuvring the GPR cart). Burrows that were found to be less than 3 m long were omitted as this project aimed to investigate burrows with bedding chambers where wombats are sleeping and mites are likely to be shed, rather than shorter burrow that wombats may only use as temporary retreat sites (McIlroy, 1973). Elevation at each burrow entrance was determined in Google Maps using GPS coordinates.
To record the temperature and relative humidity of burrows, we used a 50 mm auger to create a small borehole into the ceiling of the burrow at its terminus (last point at which the tunnel could be detected by the GPR). A 50 mm PVC pipe was then inserted to the level of the roof of the burrow chamber (Supplementary material 1). A crosspiece was placed through the pipe, level with the depth of the tunnel, to ensure the pipe was not protruding into the burrow chamber. A datalogger (iButton [Maxim Integrated, Hygrochron DS 1923]) was lowered on a piece of string through the pipe to record environmental conditions within each burrow. The PVC pipe was capped aboveground to prevent airflow or heat loss to the outside environment. A data logger was also placed at the entrance of each burrow for comparison. This logger was pegged on the wall of the burrow opening and placed under a cover to shield from solar radiation and rain. Each iButton was programmed to take readings every 10 min and was placed in each burrow for 24 h. After completion of monitoring the PVC pipe was removed and the borehole was refilled. Due to limited numbers of ibuttons and the stability of environmental conditions within burrows (see results), the ibuttons were removed and placed in different burrows within the site, each day. Environmental data were collected over three field trips during the Austral winter and early spring (July 30 – August 2, August 22–26, September 3–6). In total, thirty-three burrows were monitored in the western area of the study site.
2.3. Comparison of environmental conditions between areas of higher and lower mange prevalence (Aim 3)
We sampled the temperature and relative humidity within burrows from both the western and eastern areas of the property (Fig. 1), which vary in wombat mange prevalence. The locations of burrows that were sampled aligned with the observational area of the mange prevalence surveys (Fig. 1). The eastern area of the property has low mange prevalence (mean = 5%) and a higher density of wombats (mean = 42 wombats/km2) compared to the western area which has a higher prevalence of mange (mean = 13%) and lower wombat density (mean = 20 wombats/km2) (Supplementary material 2, Driessen et al., 2018). The eastern area of Musselroe Wind Farm is covered by larger amounts of vegetation and uneven terrain unsuitable for the use of GPR. Thus, we used a robotic vehicle (the ‘WomBot’) that became available during this research to access the burrows in both areas for this study aim (Supplementary material 3) (Ross et al., 2021). The WomBot is a small tele-operated robot system designed to explore and study wombat burrows (Ross et al., 2021). The vehicle has a tracked drive system, forward and rear cameras, and inbuilt temperature and humidity sensors (Supplementary material 3). Within-burrow air temperature and humidity were recorded either in an identified bedding chamber or as far as the WomBot could access if terrain was difficult (minimum of 3 m). At this location within the burrow, temperature and relative humidity measurements were recorded. Readings were taken after the WomBot had been in the sampling location for 5 min to allow the sensor to equilibrate (Supplementary material 4). As a backup, an iButton data logger was also attached to the vehicle to confirm consistency of readings between the differing equipment (Supplementary material 5). To ensure that there was no transfer of mites between burrows via equipment, the WomBot was cleaned and sterilised with household grade disinfectant between each burrow. A total of 18 burrows were sampled in the eastern area and 12 within the west. Sampling was conducted over a five-day trip in spring (October 15–19) and burrow monitoring was alternated between morning and afternoon in each area to control for variation in daily conditions. Sarcoptic mange prevalence data was collected over several surveys every year from 2017 to 2020 (Driessen et al., 2018, Driessen et al., 2021).
2.4. Seasonal variation in mite survival (Aim 4)
To evaluate seasonal differences in mite survival we combined information from the western region of the study site from the winter period (July 30 – August 2, August 22–26, September 3–6, n = 33) using the GPR technique and spring (October 15–19, n = 12) using the WomBot. A further 7 burrows from the western area of the property were surveyed on March 12th, 2021 (late summer) using the WomBot.
2.5. Analysis
Statistical analyses were conducted using the program R v4.0.2 (R Core Team, 2020). Packages used: tidyverse (Wickham et al., 2019), mgcv (Wood, 2011), leaflet (Cheng et al., 2019), reshape2 (Wickham, 2007), lme4 (Bates et al., 2015), lmerTest (Kuznetsova, 2017) and ggplot2 (Wickham, 2016).
2.6. Modelling estimated mite survival (Aim 1)
To create a model to estimate the amount of time mites could survive in the environment, we examined the relationship of temperature and humidity to mite survival from known laboratory data (Arlian et al., 1984a) and fitted a generalized additive model (GAM) with gaussian error distribution (see results). During preliminary analysis we also investigated the fit of other models (e.g., linear, Loess model and log linear from Loredo et al. (2020)). Owing to the other models’ poorer fit of the laboratory data within the range of our data sampling, the GAM was considered the most appropriate (Supplementary material 6). We then input our own field data into this model to estimate mite survival for each burrow that was monitored (Aims 2–4).
2.7. Temperature and humidity temporal profiles, estimating mite survival and analysis of other burrow characteristics (Aim 2)
After accounting for sensor error (see supplementary material 5) we evaluated the temporal stability of the wombat burrow microclimates. We used repeated measures ANOVAs, with burrow ID as the random effect. This analysis evaluated variation in temperature and relative humidity conditions over time to determine whether there were differences in these parameters between the burrow entrance and the burrow chamber. We implemented our temperature and relative humidity data into the GAM model with gaussian error distribution from Aim 1 to estimate mite survival. We examined the relationships between temperature and relative humidity, finding these variables to be sufficiently independent (Pearson's correlation < 0.7) for independent inclusion in the GAMs. Using a repeated measures ANOVA, we also tested the stability of mite survival predictions within the burrow versus at the burrow entrance over the 24-h sampling period (burrow ID as random effect). Because the temporal period of predicted mite survival exceeded hourly measurements of data collection, we also averaged predicted mite survival for each burrow and compared these averages using a Student's t-test. To investigate possible variation in burrow suitability for off-host mite survival, we evaluated the effects of burrow length, depth, elevation, and field trip number on the average predicted mite survival times. To do this we used a generalized linear model for length, depth and elevation, and a one-way ANOVA to test for the effect of field trip.
2.8. Contrasting mite survival between regions of higher and lower mange prevalence (Aim 3)
Point measurements of burrow temperature and humidity data from the WomBot from the east and west survey areas were implemented into the GAM with gaussian error distribution to predict mite survival. We used a mixed effects with gaussian error distribution model to test for differences between the east and west survey areas in estimated mite survival, while accounting for the effect of day of field trip (random effect). We used a one-way ANOVA to test for differences in elevation between burrows sampled from the east and west areas. We also evaluated the relationship of elevation and predicted mite survival using a linear regression model.
2.9. Investigating seasonal variation in mite survival (Aim 4)
Using our temperature and relative humidity measurements from winter, spring and summer we evaluated seasonal variation in mite survival. We used a one-way ANOVA to test for differences between predicted mite survival between data collected from winter, spring, and summer. As Driessen et al. (2021) found that there was no seasonal variation in sarcoptic mange prevalence at the same study site, we did not compare seasonal mite survival times and mange prevalence in this study.
3. Results
3.1. A statistical model describing how temperature and relative humidity influence mite survival (Aim 1)
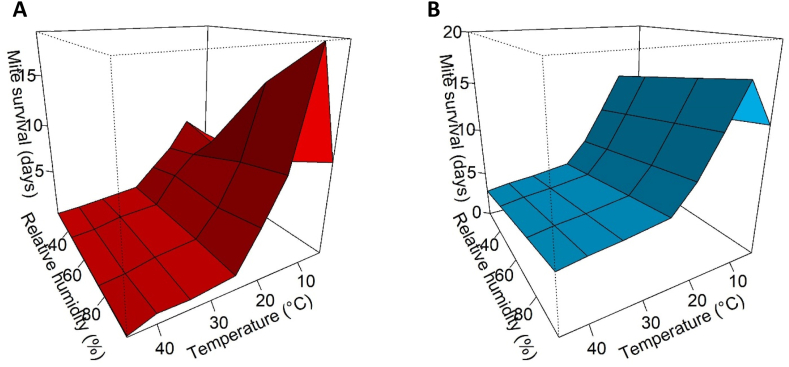
Laboratory data from Arlian et al. (1984a) demonstrates non-linear effects of temperature and relative humidity on survival of S. scabiei (Fig. 2). Accordingly, we fitted a GAM with gaussian error distribution and spline fit for each of these predictor variables. The GAM model fit was a reasonable approximation of the data (R2-adj = 0.834) and characterised the non-linear effects of temperature (F = 22.43, p < 0.001). Relative humidity was more linear owing to fewer values available for this variable (F = 11.22, p = 0.003). Nevertheless, this GAM was superior to other model formulations considered for the range of temperature and relative humidity conditions we observed in the field (see Aim 2–3 results and supplementary material 6).
Fig. 2.
3-Dimensional models of A) S. scabiei mite survival from laboratory data (Arlian et al., 1984a), and B) the estimated mite survival from GAM fit to the laboratory data (R2 = 0.834).
3.2. Environmental conditions within wombat burrows, mite survival times, and factors associated with variance in mite survival (Aim 2)
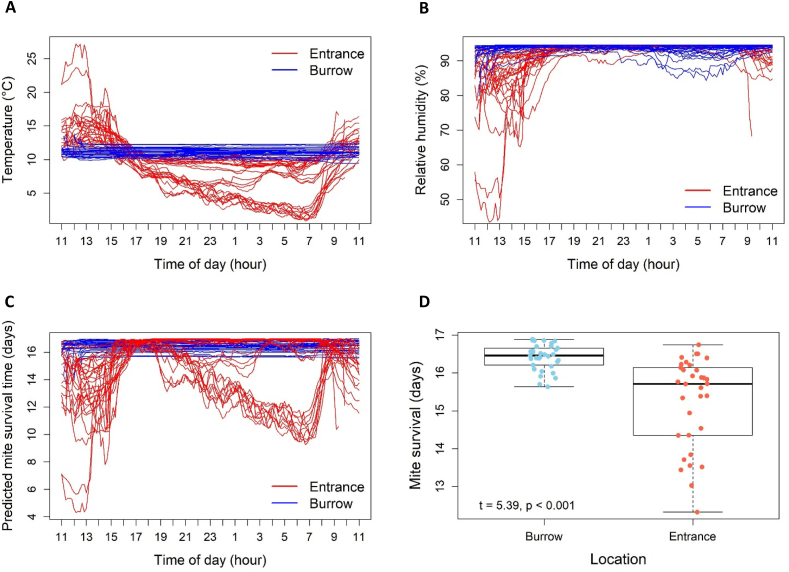
Air temperature within burrows was remarkedly stable during the period of study, although there was some variation among burrows (Fig. 3A). In contrast, air temperature at the burrow entrance varied substantially more over 24-h periods, with burrow entrances warmer during the day and cooler overnight (F = 865.50, p < 0.001; Fig. 3A). A similar pattern of more variation at the entrance was also observed for relative humidity (F = 451.40, p < 0.001; Fig. 3B). While mostly stable within burrows, some temporal variation was seen among some burrows.
Fig. 3.
Temporal profiles of A) air temperature, B) relative humidity, and C) estimated mite survival within wombat burrows and outside at the burrow entrances. D) Average estimated mite survival time at burrow entrances and within burrows (n = 33).
GAM predictions showed that off-host mite survival was greater and more stable within burrows than at burrow entrances over a 24-h period (F = 1592.0, p < 0.001; Fig. 3C). When averaging over a 24-h period, estimated mite survival was slightly higher within burrows (mean = 16.41 ± 0.37 days) than at the burrow entrance (mean = 15.23 ± 2.14 days) (Fig. 3D). There was no significant relationship between estimated mite survival within burrows and burrow length, depth, elevation, and field trip number (Fig. 4).
Fig. 4.
Relationship of burrow A) length, B) depth, C) elevation, and D) field trip number, to estimated mite survival time within burrow.
3.3. Mite survival between regions of high and low mange prevalence (Aim 3)
Comparison between burrows from both eastern (low mange prevalence) and western (high mange prevalence) areas was conducted in spring (a month after Aim 2 fieldwork was completed in winter). Temperatures and rainfall were higher during this time, and increased rain meant some low-lying areas could not be sampled due to burrow flooding. Data from the first two days of sampling (15/10/20 and 16/10/20) were omitted from analyses as adverse weather (rain) meant it was not possible to collect data from both the eastern and western areas on these days (Supplementary material 7). Therefore, 11 burrows from the east and 11 burrows from the west were used in analysis. Estimated mite survival time was slightly higher in the eastern area (mean = 12.12 ± 1.08 days) compared to the western area (mean = 10.10 ± 1.27 days, t = 24.13, p < 0.001; Fig. 5A). As this difference was contrary to our hypothesis and because burrows in the low-lying western area were more affected by flooding than the eastern area, we investigated the relationship between burrow elevation and mite survival. We found that elevation of accessible burrows in the eastern area (mean = 17 ± 8.46 m) was lower than the western area (mean = 32 ± 10.10 m, F(1,20) = 14.26, p = 0.001), and that estimated mite survival decreased as elevation of burrows increased (Fig. 5B).
Fig. 5.
A) Estimated mite survival within burrows across the eastern (low mange prevalence) and western (high mange prevalence) survey areas. B) The relationship between estimated mite survival and the elevation of burrows from the eastern and western areas.
3.4. Seasonal variation in mite survival (Aim 4)
Estimated mite survival was different between winter, spring, and summer (F = 938.4, p < 0.001). Predicted off-host survival was highest in winter (mean = 16.41 ± 0.37 days), followed by spring (mean = 10.10 ± 1.27 days), and finally late summer (March) with the lowest predicted mite survival (mean = 5.96 ± 0.37) (Fig. 6). Because our GAM model was a poorer fit to high temperature and low humidity conditions, we also calculated estimated late summer mite survival using a Loess fit (Supplementary material 6) which suggested a mean of 2.76 ± 0.52 days.
Fig. 6.
Seasonal variation in estimated mite survival within wombat burrows. Note: in winter, the burrows were sampled with a different method (data loggers) compared to the spring and summer (robotic vehicle, the WomBot).
4. Discussion
Environmental transmission is a key pathway of S. scabiei spread for a range of mange affected wildlife species (Kołodziej-Sobocińska et al., 2014; Montecino-Latorre et al., 2019; Niedringhaus et al., 2019b). However, environmental transmission of the parasite remains a critical knowledge gap (Alasaad et al., 2013; Martin et al., 2018a). Our study examined the potential of bare-nosed wombat burrows for environmental transmission of S. scabiei and is the first to demonstrate the suitability of the bare-nosed wombat burrow for off-host mite survival and environmental transmission. We found that temperature and relative humidity conditions within burrows were very stable, compared to outside of burrows. Our estimated average off-host mite survival of 16.41 (±0.34) days in Austral winter greatly exceeds the known frequency of wombat burrow switching (4–10 days) (Evans, 2008; Martin et al., 2019b). This supports our hypothesis that conditions within burrows are conducive for transmission of S. scabiei particularly in winter and less so in warmer and drier months (average estimated survival of 5.96 ± 0.37 days in summer depending on model choice). Our study provides another empirical line of evidence supporting asynchronous burrow sharing as a mechanism of indirect transmission among wombats. We also found estimated mite survival differed between areas of variable prevalence, but contrary to our hypothesis, higher mange prevalence did not appear to be connected to higher predicted mite survival and may indicate other factors (e.g., extent of burrow sharing) are important for local transmission. However, due to flooding events, we were not able to sample burrows in low-lying areas of the west, which our data suggests are conducive to mite survival (i.e., mite survival decreases as burrow elevation increases). As the eastern area has a higher elevation and lower mange prevalence, more representative sampling may have supported our hypothesis.
Knowledge of pathogen persistence in the environment is a crucial component of environmental transmission because it can influence host encounter rates with the pathogen (Blackburn et al., 2019; Fuller et al., 2012; Rohani et al., 2009). Laboratory studies of S. scabiei demonstrate non-linear effects of temperature, and to a lesser extent relative humidity, on off-host mite survival (Arlian et al., 1984a). This is unsurprising because temperature and humidity conditions can both promote and impede parasite survival and transmission based on physiological constraints (Mordecai et al., 2019). Consequently, we found that the use of a GAM was most appropriate for predicting mite survival, especially for the range of temperature and relative humidity conditions that we observed in the field during Austral winter and spring. In environments where higher temperatures and lower humidity are recorded the results suggest that an alternative model (e.g., Loess model) may also be appropriate (Supplementary material 6). Accordingly, for our summer temperature and relative humidity data we estimated mite survival of 2.76 ± 0.52 days with a Loess model which is 3.19 days less than estimated with the GAM model. Clearly model choice is important for estimates of mite survival in the environment. Previous estimates of S. scabiei off-host survival in the dens of kit foxes used a linear model with a logarithmic transformation of the data (Loredo et al., 2020). However, our results suggest that this model underestimates the peak of mite survival at optimal temperature and humidity conditions (Supplementary material 6). Therefore, estimates from Loredo et al. (2020) (1.97 days in summer and 7.38 days in winter) are likely conservative compared to our GAM model which predicts that conditions observed in kit fox dens can support off-host survival of 5.17 days in summer and 15.45 days in winter. Future study of the off-host survival of S. scabiei would benefit from additional laboratory data to better parameterise models, particularly at a wider range of relative humidities. Implementation of more complex model types, such as thermal performance curves, may also help refine estimates of environmental survival of S. scabiei. Additionally, advances in technology to sample mites from the environment, such as eDNA on soil and nesting material in burrows, could provide further empirical insights into environmental survival of mites.
Notwithstanding the importance of model choice, the stability of environmental conditions is important for fomite survival (Lindauer et al., 2020; Stevenson et al., 2013). We found that the temperature and relative humidity conditions within wombat burrows were different from those outside of burrows. Burrow conditions were also stable over a circadian cycle (mean = 11.12 °C ± 0.60, 93.76% RH ± 1.34). Unsurprisingly, outside of burrows there was greater variation in temperature and humidity during the day and overnight (mean = 9.49 °C ± 3.58, 92.21% RH ± 5.21). These findings are consistent with other research showing wombat burrows ameliorate surface conditions, providing an underground refuge relatively sheltered from external temperature extremes (Brown, 1984; Shimmin et al., 2002). This has important implications for the suitability of wombat burrows as an environmental reservoir for mange fomites because protection from fluctuating, and extremes of, environmental conditions is favourable for maintaining optimal conditions for pathogen persistence (Flory et al., 2012). Our comparison of entrance versus within burrow conditions was also conducted in winter, which is the season where there is likely the least difference between these two sites. Owing to the challenge of accessing the chambers within wombat burrows, our study is one of few to document the burrow microclimate of bare-nosed wombats beyond 3–4 m (Brown, 1984).
Understanding the duration of parasite off-host survival in the environment is an important part of predicting ongoing transmission, as it affects the likelihood of exposure and subsequent transmission to a new host. Arlian (1989) indicated that S. scabiei mites can remain infective for at least one-half to two-thirds of their survival time. Thus, our results (seasonal mite survival of 6–16 days) also support mite infectivity to exceed minimum burrow switching frequency (4–10 days) (Evans, 2008; Martin et al., 2019b). Consequently, our results provide an important line of evidence of environmental transmission for S. scabiei within wombat burrows and supports mathematical modelling of transmission via burrows (Beeton et al., 2019; Martin et al., 2019b). Understanding pathogen persistence in the environment and the role of environmental transmission is crucial for the implementation of effective management strategies (Astorga et al., 2018; Niedringhaus et al., 2019b). Estimates of off-host mite survival can be used in modelling to predict patterns of disease and the outcome and efficacy of different treatment strategies in the field. It is important to note that our results show high estimates of mite survival outside of the burrow during winter. However, the fluctuating climatic conditions observed outside burrows and other variables beyond the scope of this study (e.g., ultraviolet light, substrate type) would likely restrict off-host mite survival outside of burrows (Niedringhaus et al., 2019b). Furthermore, the probability of a wombat encountering the parasite in an open environment, relative to an enclosed burrow, is likely much lower.
The importance of varying factors on parasite survival in the environment is an essential consideration for predicting host exposure risk. We considered a range of potentially important factors in this study. We found estimated mite survival was not related to burrow length. Therefore, beyond 3 m, chambers within wombat burrows do not need to be a large distance from the burrow entrance for the internal environmental conditions to be favourable for off-host survival of S. scabiei. Consequently, both medium and major burrows (as characterised by McIlroy, 1973), where wombats are known to reside, have the potential to facilitate environmental transmission of mites. Similarly, burrow depth (between a range of 0.4–1 m) was not related to estimated mite survival, suggesting that both deep and shallow burrows can maintain a microclimate suitable for off-host survival. Burrow elevation also did not have an impact on predictions of mite survival time (within the western area only). However, it is important to note that elevation of burrows surveyed within the western area only ranged from 11 to 57 m and the importance of elevation on burrows conditions and mite survival likely changes when contrasting burrows from more elevated terrain and potentially during drier times of the year. Additionally, there was no difference in mite survival estimates between different sampling trips. Collectively these results support burrows as a relatively uniform environment for mite survival within seasons, with larger changes among seasons. Furthermore, other factors outside the scope of this study, such as soil type (e.g., clay versus sandy), may affect mite survival, as the ability of different soils to retain moisture within burrows could impact the rate of mite desiccation. As our study focused on low-lying, coastal areas modified for pasture production, future research could include a broader range of wombat habitats to improve our ability to predict the rate of environmental transmission at different sites.
Contrary to our hypothesis, this research showed that estimated mite survival was higher in the eastern area compared to the western area of the property. This is surprising as mange prevalence is higher (Driessen et al., 2021) and wombat density is lower in the western area (a.k.a., negative density dependence, Supplementary material 2). Furthermore, as elevation is lower across the western area of the property, we expected the lower-lying, wetter paddocks in the west to facilitate suitably cool and humid conditions for off-host mite survival (Supplementary material 2). Observed differences in estimated mite survival predicted from our model appear to be driven by lower temperature conditions observed in the eastern area compared to the western area (see the slope of GAM model fit). The higher vegetation cover around eastern burrows potentially maintains a cooler within burrow microclimate that is suitable for longer durations of off-host mite survival, however this requires further investigation (Song et al., 2013; Zheng et al., 1993). It is important to acknowledge that burrows in the eastern and western areas were sampled in spring, during a time following heavy rainfall, and consequently, monitoring was focused on hills instead of low-lying areas of the west, which could not be sampled due to flooding. Therefore, the elevation of burrows sampled was higher in the western area compared to the eastern area. Furthermore, lower mite survival was estimated in higher elevation burrows, that were predominantly from the west. This may explain, at least partially, the lower than expected estimates of mite survival in the western area, as burrows at higher elevations may not be able to maintain suitable conditions as external temperature begins to increase in spring. Additionally, other factors unrelated to mite survival may explain the differences between eastern and western areas, such as the extent of burrow sharing, which is thought to be a key factor driving transmission (Beeton et al., 2019; Martin et al., 2019a). Furthermore, the ratio of wombats per burrow, which was unavailable at the time of this study, may be a more important predictor of S. scabiei transmission than wombat density and is potentially an important future research direction. The precise location of mange affected wombats within each survey area was also unavailable, thus a finer scale comparison of burrow mite survival estimates with observations of mange was not possible and may be of interest in future research.
This study has potentially important implications for the design and implementation of disease management strategies. Specifically, the duration of mite persistence has implications for future disease management for bare-nosed wombats, in addition to other solitary den-using species, that are affected by mange (e.g., the southern hairy-nosed wombat and American black bear) (Niedringhaus et al., 2019b; Ruykys et al., 2009). It is important to know how long mites persist as this influences the risk and frequency of transmission to a susceptible host that may encounter the parasite. Our research may suggest the chances of locally eradicating S. scabiei are greatest during summer, when environmental conditions are most adverse for the mites. Targeted burrow management strategies, such as ventilation with hot air, chemical treatment, collapse, and incineration, have also been proposed to prevent transmission within underground retreat sites (Loredo et al., 2020; Montecino-Latorre et al., 2019). However, the feasibility of these strategies for wombats is likely limited due to the complexity of some major burrows and the amount of wombat burrows that would require management, in addition to significant ethical considerations (Martin et al., 2019b). Thus, the use of parasiticides on affected hosts is likely still the most appropriate disease management approach (Martin et al., 2019b).
This research was only possible through the use of innovative methodologies to address the long-standing challenge of accessing the bedding chambers of wombat burrows. The use of GPR to detect and map wildlife burrows is a relatively recent application of the technology (Cortez et al., 2013; Stott, 1996; Swinbourne et al., 2015). GPR successfully detected and mapped burrows at our study site, which consisted primarily of sandy soils. However, this technology may have limited use in other areas with more conductive soils (e.g., clay), vegetated sites and rough terrain (Swinbourne et al., 2015). Using data loggers allowed us to show the relative invariance of temperature and relative humidity within burrows over 24 h periods. Therefore, confirming that point measurements can be used down burrows to provide a measurement representative of any hour of the day. Accordingly, we were able to use a remote-controlled robotic vehicle (the WomBot) to sample a greater number of burrows in a short period (Ross et al., 2021). This was a truly unique aspect of this research. The WomBot was able to navigate most burrows and reach bedding chambers, providing a major advance for researching these underground environments. The technology did still have some limitations navigating the complex terrain underground. Future adaptations to the technology (e.g., wider tracks and greater clearance of undercarriage) may be of value.
Although not a primary aim of this study, the methodologies used also allowed us to make several observations of the hidden world of wombat burrows. The structure of wombat burrows can be complex. Consistent with previous records, we observed many burrows which had several off-branching tunnels and multiple chambers (McIlroy, 1973). Most burrows had only one entrance but burrow networks with two or three entrances were also observed. Burrow length varied but the longest burrow mapped with GPR in this study was 17.8 m, not including branching tunnels and a second exit that connected to the same network. As documented by McIlroy (1973), we found that wombat burrows often make abrupt turns. Contrary to reports by Triggs (2009), we found the presence of fresh wombat scats within tunnels and burrow chambers, although not frequently. Interestingly, we also found wombats sleeping short distances (<4 m) from burrows entrances in tunnels that clearly continued further, and not necessarily sleeping in an identifiable chamber. Four of these encounters were in late afternoon when this may be expected as wombats move closer to the entrance later in the day to doze before they are ready to leave the burrow (Triggs, 2009). However, four different wombats were observed sleeping near (2–4 m) burrow entrances before midday, when wombats are expected to be deeper within burrows (Evans, 2008; Triggs, 2009). We also observed several wombats sleeping only on sand in otherwise empty chambers, although we did observe bedding material within some chambers and one wombat sleeping on a large pile of bracken and grass. This may have interesting implications for mite survival in bedding material versus on substrate and may highlight an area for future research involving the sampling of mites from within bedding chambers.
5. Conclusion
Our study addressed a gap in current research regarding the environmental transmission of S. scabiei and is the first to produce estimates of off-host mite survival in bare-nosed wombat burrows across seasons. The results show that the microclimate within burrows was stable with cooler temperatures and higher humidities than outside; conditions more favourable for off-host S. scabiei survival. Our research suggests mite persistence within burrows matches or exceeds the known frequency of wombat burrow switching, thus, supporting burrows as the site of environmental transmission of S. scabiei. Between seasons mite survival was estimated to vary significantly (6–16 days) suggesting summer is the most adverse time of year for mites in the environment. We also found that estimated mite survival did not vary based on burrow characteristics during winter sampling (i.e., length, depth, elevation), providing further evidence for the relatively uniform environment within burrows within seasons. Contrary to our hypothesis, we found slightly higher estimated mite survival in an area of low mange prevalence, this is probably because burrows conducive to mite survival in the higher prevalence area were not accessible at the time of sampling. It is important to recognise a range of factors may influence mange prevalence in addition to mite survival time. Our research highlights several directions for future research; 1) further laboratory investigation of mite survival at a wider range of humidity and temperature readings to incorporate into future models, 2) further collection of seasonal data to determine how this may drive differences in burrow suitability over different areas at more of times of the year, 3) comparison with other sites that have variable disease dynamics (e.g., epidemic outbreak and mite free), 4) investigation of the risk of infection to other sympatric species that use wombat burrows and may encounter mites, and 5) development of methods to directly sample mites from within wombat burrows. Finally, while our study focuses on sarcoptic mange disease and the bare-nosed wombat, findings may be transferrable to other species and disease systems and contribute more broadly to bridging knowledge gaps of environmental transmission of pathogens in wildlife.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
The authors acknowledge the aboriginal custodians on whose traditional lands these surveys were conducted. We thank Chris Burridge and Geoff While for feedback and assessment of an early draft, and also the helpful suggestions of two anonymous reviewers. This work was supported by an Australian Research Council Linkage Project grant (LP180101251), the Harris Estate Charitable Foundation through Equity Trustees Limited, and an anonymous donor passionate about wombats to S. Carver, and the Tasmanian Government Honours Scholarship in Wildlife Conservation awarded to E. Browne by the Department of Primary Industries, Parks, Water and Environment.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2021.08.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alasaad S., Rossi L., Heukelbach J., Pérez J.M., Hamarsheh O., Otiende M., Zhu X.-Q. The neglected navigating web of the incomprehensibly emerging and re-emerging Sarcoptes mite. Infect. Genet. Evol. 2013;17:253–259. doi: 10.1016/j.meegid.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Arlian L.G. Biology, host relations, and epidemiology of Sarcoptes scabiei. Annu. Rev. Entomol. 1989;34:139–161. doi: 10.1146/annurev.en.34.010189.001035. [DOI] [PubMed] [Google Scholar]
- Arlian L.G., Morgan M.S. A review of Sarcoptes scabiei: past, present and future. Parasites Vectors. 2017;10:297. doi: 10.1186/s13071-017-2234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlian L.G., Runyan R.A., Achar B.S., Estes S.A. Survival and infestivity of Sarcoptes scabiei var. canis and var. hominis. J. Am. Acad. Dermatol. 1984;11:210–215. doi: 10.1016/s0190-9622(84)70151-4. [DOI] [PubMed] [Google Scholar]
- Arlian L.G., Runyan R.A., Sorlie L.B., Estes S.A. Host-seeking behavior of Sarcoptes scabiei. J. Am. Acad. Dermatol. 1984;11:594–598. doi: 10.1016/s0190-9622(84)70212-x. [DOI] [PubMed] [Google Scholar]
- Arlian L.G., Vyszenski-Moher D.L. Life cycle of Sarcoptes scabiei var. canis. J. Parasitol. 1988;74:427–430. [PubMed] [Google Scholar]
- Astorga F., Carver S., Almberg E.S., Sousa G.R., Wingfield K., Niedringhaus K.D., Van Wick P., Rossi L., Xie Y., Cross P., Angelone S., Gortazar C., Escobar L.E. International meeting on sarcoptic mange in wildlife, June 2018, Blacksburg, Virginia, USA. Parasites Vectors. 2018;11:449. doi: 10.1186/s13071-018-3015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Maechler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Software. 2015;67:1–48. [Google Scholar]
- Beeton N.J., Carver S., Forbes L.K. A model for the treatment of environmentally transmitted sarcoptic mange in bare-nosed wombats (Vombatus ursinus) J. Theor. Biol. 2019;462:466–474. doi: 10.1016/j.jtbi.2018.11.033. [DOI] [PubMed] [Google Scholar]
- Blackburn J.K., Ganz H.H., Ponciano J.M., Turner W.C., Ryan S.J., Kamath P., Cizauskas C., Kausrud K., Holt R.D., Stenseth N.C., Getz W.M. Modeling R0 for pathogens with environmental transmission: animal movements, pathogen populations, and local infectious zones. Int. J. Environ. Res. Publ. Health. 2019;16:954. doi: 10.3390/ijerph16060954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein S., Mörner T., Samuel W.M. Sarcoptes scabiei and sarcoptic mange. In: Samuel W.M., Pybus M.J., Kocan A.A., editors. Parasitic Diseases of Wild Mammals. Iowa State University Press; Ames, Iowa: 2001. pp. 107–119. [Google Scholar]
- Breban R. Role of environmental persistence in pathogen transmission: a mathematical modeling approach. J. Math. Biol. 2013;66:535–546. doi: 10.1007/s00285-012-0520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G.D. Thermoregulation in the common wombat (Vombatus ursinus) in an alpine environment. In: Hales J.R.S., editor. Thermal Physiology. Raven Press; New York: 1984. pp. 331–334. [Google Scholar]
- Bureau of Meterology . Commonwealth of Australia; 2020. Daily Rainfall - Rushy Lagoon (Cape Portland Road) [Google Scholar]
- Cheng J., Karambelkar B., Xie Y. 2019. Leaflet: Create Interactive Web Maps with the JavaScript 'Leaflet' Library. R package version 2.0.3. [Google Scholar]
- Cortez J.D., Henke S.E., Redeker E., Fulbright T.E., Riddle R., Young J. Demonstration of ground-penetrating radar as a useful tool for assessing pocket gopher burrows. Wildl. Soc. Bull. 2013;37:428–432. [Google Scholar]
- Costello C.M., Quigley K.S., Jones D.E., Inman R.M., Inman K.H. Observations of a denning-related dermatitis in American black bears. Ursus. 2006;17:186–190. [Google Scholar]
- Davis D.P., Moon R.D. Survival of Sarcoptes scabiei (De Geer) stored in three media at three temperatures. J. Parasitol. 1987;73:661–662. [PubMed] [Google Scholar]
- Davis J.L., Annan A.P. Ground-Penetrating Radar for high-resolution mapping of soil and rock stratigraphy. Geophys. Prospect. 1989;37:531–551. [Google Scholar]
- De Castro F., Bolker B. Mechanisms of disease-induced extinction. Ecol. Lett. 2004;8:117–126. [Google Scholar]
- Driessen M.M., Dewar E., Carver S., Gales R. Conservation status of common wombats in Tasmania I: incidence of mange and its significance. Pac. Conserv. Biol. 2021 doi: 10.1071/PC21007. [DOI] [Google Scholar]
- Driessen M.M., Gales R., Visoiu M., Dewar E., Hehn K. Nature Conservation Report 18/7. Department of Primary Industries, Parks, Water and Environment. 2018. Prevalence of wombat mange at Cape Portland, northeast Tasmania. (Hobart) [Google Scholar]
- Escobar L.E., Carver S., Almberg E.S., Sousa G.R., Niedringhaus K.D., Yabsley M., Wick P.V., Domineguez E., Rossi L., Xie Y., Cross P., Angelone S., Gortázar C., Gakuya F., Astorga F. Sarcoptic mange: an emerging panzootic in wildlife. Transbound. Emerg. Dis. 2021;1–16 doi: 10.1111/tbed.14082. [DOI] [PubMed] [Google Scholar]
- Evans M.C. Home range, burrow-use and activity patterns in common wombats (Vombatus ursinus) Wildl. Res. 2008;35:455–462. [Google Scholar]
- Flory A.R., Kumar S., Stohlgren T.J., Cryan P.M. Environmental conditions associated with bat white-nose syndrome mortality in the north-eastern United States. J. Appl. Ecol. 2012:680–689. [Google Scholar]
- Fraser T.A., Charleston M., Martin A., Polkinghorne A., Carver S. The emergence of sarcoptic mange in Australian wildlife: an unresolved debate. Parasites Vectors. 2016;9:316. doi: 10.1186/s13071-016-1578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller E., Elderd B.D., Dwyer G. Pathogen persistence in the environment and insect-baculovirus interactions: dsease-density thresholds, epidemic burnout, and insect outbreaks. Am. Nat. 2012;179:E70–E96. doi: 10.1086/664488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D.F. Sarcoptic mange affecting wild fauna in New South Wales. Aust. Vet. J. 1937;13:154–155. [Google Scholar]
- Hoyt J.R., Langwig K.E., Okoniewski J., Frick W.F., Stone W.B., Kilpatrick A.M. Long-term persistence of Pseudogymnoascus destructans, the causative agent of white-nose syndrome, in the absence of bats. EcoHealth. 2015;12:330–333. doi: 10.1007/s10393-014-0981-4. [DOI] [PubMed] [Google Scholar]
- Hoyt J.R., Langwig K.E., Sun K., Parise K.L., Li A., Wang Y., Huang X., Worledge L., Miller H., White J.P., Kaarakka H.M., Redell J.A., Görföl T., Boldogh S.A., Fukui D., Sakuyama M., Yachimori S., Sato A., Dalannast M., Jargalsaikhan A., Batbayar N., Yovel Y., Amichai E., Natradze I., Frick W.F., Foster J.T., Feng J., Kilpatrick A.M. Environmental reservoir dynamics predict global infection patterns and population impacts for the fungal disease white-nose syndrome. PNAS USA. 2020;117:7255–7262. doi: 10.1073/pnas.1914794117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt J.R., Langwig K.E., White J.P., Kaarakka H.M., Redell J.A., Kurta A., DePue J.E., Scullon W.H., Parise K.L., Foster J.T., Frick W.F., Kilpatrick A.M. Cryptic connections illuminate pathogen transmission within community networks. Nature. 2018;563:710–713. doi: 10.1038/s41586-018-0720-z. [DOI] [PubMed] [Google Scholar]
- Kilpatrick A.M., Briggs C.J., Daszak P. The ecology and impact of chytridiomycosis: an emerging disease of amphibians. Trends Ecol. Evol. 2010;25:109–118. doi: 10.1016/j.tree.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Kołodziej-Sobocińska M., Zalewski A., Kowalczyk R. Sarcoptic mange vulnerability in carnivores of the Białowieża Primeval Forest, Poland: underlying determinant factors. Ecol. Res. 2014;29:237–244. [Google Scholar]
- Koopman M.E., Scrivner J.H., Kato T.T. Patterns of den use by San Joaquin kit foxes. J. Wildl. Manag. 1998;62:373–379. [Google Scholar]
- Kuznetsova A., Brockhoff P.B., Christensen R.H.B. lmerTest package: tests in linear mixed effects models. J. Stat. Software. 2017;82:1–26. [Google Scholar]
- Lindauer A.L., Maier P.A., Voyles J. Daily fluctuating temperatures decrease growth and reproduction rate of a lethal amphibian fungal pathogen in culture. BMC Ecol. 2020;20:18. doi: 10.1186/s12898-020-00286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loredo A.I., Rudd J.L., Foley J.E., Clifford D.L., Cypher B.L. Climatic suitability of San Joaquin kit fox (Vulpes macrotis mutica) dens for sarcoptic mange (Sarcoptes scabiei) transmission. J. Wildl. Dis. 2020;56:126. [PubMed] [Google Scholar]
- Martin A.M., Burridge C.P., Ingram J., Fraser T.A., Carver S., Flory L. Invasive pathogen drives host population collapse: effects of a travelling wave of sarcoptic mange on bare-nosed wombats. J. Appl. Ecol. 2018;55:331–341. [Google Scholar]
- Martin A.M., Fraser T.A., Lesku J.A., Simpson K., Roberts G.L., Garvey J., Polkinghorne A., Burridge C.P., Carver S. The cascading pathogenic consequences of Sarcoptes scabiei infection that manifest in host disease. R. Soc. Open. Sci. 2018;5 doi: 10.1098/rsos.180018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A.M., Ricardo H., Tompros A., Fraser T.A., Polkinghorne A., Carver S. Burrows with resources have greater visitation and may enhance mange transmission among wombats. Aust. Mammal. 2019;41:287–290. [Google Scholar]
- Martin A.M., Richards S.A., Fraser T.A., Polkinghorne A., Burridge C.P., Carver S. Population‐scale treatment informs solutions for control of environmentally transmitted wildlife disease. J. Appl. Ecol. 2019;56:2363–2375. [Google Scholar]
- Martin R.W., Handasyde K.A., Skerratt L.F. Current distribution of sarcoptic mange in wombats. Aust. Vet. J. 1998;76:411–414. doi: 10.1111/j.1751-0813.1998.tb12391.x. [DOI] [PubMed] [Google Scholar]
- McIlroy J.C. Australian National University; 1973. Aspects of the Ecology of the Common Wombat Vombatus ursinus; p. 284. [Google Scholar]
- Mellanby K., Johnson C.G., Bartley W.C., Brown P. Experiments on the survival and behaviour of the itch mite, Sarcoptes scabiei DeG. var. hominis. Bull. Entomol. Res. 1942;33:267–271. [Google Scholar]
- Montecino-Latorre D., Cypher B.L., Rudd J.L., Clifford D.L., Mazet J.A.K., Foley J.E. Assessing the role of dens in the spread, establishment and persistence of sarcoptic mange in an endangered canid. Epidemics. 2019;27:28–40. doi: 10.1016/j.epidem.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Mordecai E.A., Caldwell J.M., Grossman M.K., Lippi C.A., Johnson L.R., Neira M., Rohr J.R., Ryan S.J., Savage V., Shocket M.S., Sippy R., Stewart Ibarra A.M., Thomas M.B., Villena O. Thermal biology of mosquito-borne disease. Ecol. Lett. 2019;22:1690–1708. doi: 10.1111/ele.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mörner T. Sarcoptic mange in Swedish wildlife. Revue scientifique et technique. 1992;11:1115–1121. [PubMed] [Google Scholar]
- Niedringhaus K.D., Brown J.D., Sweeley K.M., Yabsley M.J. A review of sarcoptic mange in North American wildlife. Int. J. Parasitol. Parasites Wildl. 2019;9:285–297. doi: 10.1016/j.ijppaw.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedringhaus K.D., Brown J.D., Ternent M.A., Peltier S.K., Yabsley M.J. Effects of temperature on the survival of Sarcoptes scabiei of black bear (Ursus americanus) origin. Parasitol. Res. 2019;118:2767–2772. doi: 10.1007/s00436-019-06387-7. [DOI] [PubMed] [Google Scholar]
- Old J.M., Sengupta C., Narayan E., Wolfenden J. Sarcoptic mange in wombats - a review and future research directions. Transbound. Emerg. Dis. 2017;65:399–407. doi: 10.1111/tbed.12770. [DOI] [PubMed] [Google Scholar]
- Park A.W. Infectious disease in animal metapopulations: the importance of environmental transmission. Eco. Evol. 2012;2:1398–1407. doi: 10.1002/ece3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence D.B., Windberg L.A., Pence B., Sprowls R. The epizootiology and pathology of sarcoptic mange in coyotes, Canis latrans, from South Texas. J. Parasitol. 1983;69:1100–1115. C. [PubMed] [Google Scholar]
- Pence D., Ueckermann E. Sarcoptic mange in wildlife. Rev. Sci. Tech. 2002;21:385–398. [PubMed] [Google Scholar]
- Pike D.A., Mitchell J.C. Burrow-dwelling ecosystem engineers provide thermal refugia throughout the landscape. Anim. Conserv. 2013;16:694–703. [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Rohani P., Breban R., Stallknecht D.E., Drake J.M. Environmental transmission of low pathogenicity avian influenza viruses and its implications for pathogen invasion. Proc. Natl. Acad. Sci. Unit. States Am. 2009;106:10365–10369. doi: 10.1073/pnas.0809026106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Carver S., Browne E., Thai B.S. WomBot - an exploratory robot for monitoring wombat burrows. SN Appl. Sci. 2021;3:647. [Google Scholar]
- Ruykys L., Taggart D.A., Breed W.G., Schultz D. Sarcoptic mange in southern hairy-nosed wombats (Lasiorhinus latifrons) distribution and prevalence in the Murraylands of South Australia. 2009;57:129. [Google Scholar]
- Satterfield D.A., Altizer S., Williams M.-K., Hall R.J. Environmental persistence influences infection dynamics for a butterfly pathogen. PloS One. 2017;12 doi: 10.1371/journal.pone.0169982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele B.C., Skerratt L.F., Grogan L.F., Hunter D.A., Clemann N., McFadden M., Newell D., Hoskin C.J., Gillespie G.R., Heard G.W., Brannelly L., Roberts A.A., Berger L. After the epidemic: ongoing declines, stabilizations and recoveries in amphibians afflicted by chytridiomycosis. Biol. Conserv. 2017;206:37–46. [Google Scholar]
- Shimmin G.A., Skinner J., Baudinette R.V. The warren architecture and environment of the southern hairy-nosed wombat (Lasiorhinus latifrons) J. Zool. 2002;258:469–477. [Google Scholar]
- Skerratt L.F. Cellular response in the dermis of common wombats (Vombatus ursinus) infected with Sarcoptes scabiei var. wombati. J. Wildl. Dis. 2003;39:193–202. doi: 10.7589/0090-3558-39.1.193. [DOI] [PubMed] [Google Scholar]
- Skerratt L.F. Sarcoptes scabiei: an important exotic pathogen of wombats. Microbiol. Aust. 2005;26:79–81. [Google Scholar]
- Skerratt L.F., Skerratt J.H., Banks S., Martin R., Handasyde K. Aspects of the ecology of common wombats (Vombatus ursinus) at high density on pastoral land in Victoria. Aust. J. Zool. 2004;52:303–330. [Google Scholar]
- Sommerer A.S. Department of Geography and Regional Planning. Indiana University of Pennsylvania; 2014. A Spatial Analysis of the Relationship between the Occurrence of Mange in Pennsylvania's Black Bear Population and Impervious Land Cover. [Google Scholar]
- Song Y., Zhou D., Zhang H., Li G., Jin Y., Li Q. Effects of vegetation height and density on soil temperature variations. Sci. Bull. 2013;58:907–912. [Google Scholar]
- Soulsbury C.D., Iossa G., Baker P.J., Cole N.C., Funk S.M., Harris S. The impact of sarcoptic mange Sarcoptes scabiei on the British fox Vulpes vulpes population. Mamm Rev. 2007;37:278–296. [Google Scholar]
- Stevenson L.A., Alford R.A., Bell S.C., Roznik E.A., Berger L., Pike D.A. Variation in thermal performance of a widespread pathogen, the Amphibian chytrid fungus Batrachochytrium dendrobatidis. PloS One. 2013;8 doi: 10.1371/journal.pone.0073830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott P. Ground-penetrating radar: a technique for investigating the burrow structures of fossorial vertebrates. Wildl. Res. 1996;23:519–530. [Google Scholar]
- Swinbourne M.J., Taggart D.A., Sparrow E., Hatch M., Ostendorf B. Ground penetrating radar as a non-invasive tool to better understand the population dynamics of a fossorial species: mapping the warrens of southern hairy-nosed wombats (Lasiorhinus latifrons) Wildl. Res. 2015;42:678–688. [Google Scholar]
- Tasmanian Government . Tasmanian Government; 2018. LISTmap - Land Information System Tasmania. [Google Scholar]
- Tompkins D.M., Carver S., Jones M.E., Krkošek M., Skerratt L.F. Emerging infectious diseases of wildlife: a critical perspective. Trends Parasitol. 2015;31:149–159. doi: 10.1016/j.pt.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Triggs B. CSIRO Publishing; Australia: 2009. Wombats. [Google Scholar]
- Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M., Shibuya K., Salomon J.A., Abdalla S., Aboyans V. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Who Scabies. http://www.who.int/lymphatic_filariasis/epidemiology/scabies/en/
- Wickham H. Reshaping data with the reshape package. J. Stat. Software. 2007;21:1–20. [Google Scholar]
- Wickham H. Springer-Verlag; New York: 2016. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- Wickham H., Averick M., Bryan J., Chang W., D'Agostino McGowan L., François R., Grolemund G., Hayes A., Henry L., Hester J. Welcome to the tidyverse. J. Open. Source Softw. 2019;4:1686. [Google Scholar]
- Wood S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. Roy. Stat. Soc. 2011;73:3–36. [Google Scholar]
- Zheng D., Hunt E.R., Running S.W. A daily soil temperature model based on air temperature and precipitation for continental applications. Clim. Res. 1993;2:183–191. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.