Supplemental Digital Content is Available in the Text.
Keywords: Animal models, Chronic pain, Ketamine, Neuropathic pain, Pain models
Abstract
In humans, proof of long-term efficacy of ketamine treatment in neuropathic pain is lacking. To improve our understanding of ketamine behavior under various administration conditions, we performed a systematic review and meta-analyses of controlled studies on the efficacy of ketamine in mice and rats with a disease model of nerve injury on relief of allodynia. Searches in PubMed and EMBASE identified 31 unique studies. Four meta-analyses were conducted. The first analysis included 19 comparisons on a single ketamine dose and measurement of effect within 3 hours of dosing and showed an appreciable effect (standardized mean difference 1.6, 95% confidence interval 1.1-2.1). Subgroup analyses showed no effect of species, administration route, or dose. A single administration was insufficient to sustain relief of allodynia at 24 or 72 hours after dosing, as observed in our second analysis (7 comparisons) with similar effects in ketamine-treated and control animals. Chronic ketamine administration (9 comparisons) caused profound relief of allodynia when tested during ketamine exposure (effect size 5.1, 3.7-6.5). The final analysis (6 comparisons) showed that chronic administration caused a slow loss of relief of allodynia with 70% loss of effect 24 days after end of treatment. No subgroups analyses were possible in the last 3 meta-analyses due to small group sizes. These results indicate long-term ketamine anti-allodynic effects after chronic exposure (>3 days) but not after a single administration. Given several limitations, extrapolation of the animal data to the human condition is tenuous.
1. Introduction
Ketamine a versatile drug that, apart from its original indication as anesthetic agent (since 1970), is widely used since the 1990s at low (subanesthetic) dose in the treatment of pain.1,20 For example, in the treatment of acute (nociceptive) postoperative pain, there is ample evidence that ketamine is an effective analgesic and is opioid sparing.1,20 In chronic pain, particularly in the management of neuropathic pain refractory to treatment with more conventional medication such as antiepileptic and antidepressant drugs, ketamine is frequently administered as a last resort option.6,21 Theoretically, ketamine seems to be well suited to produce long-term relief of neuropathic pain symptoms, especially those symptoms that are related to central sensitization. Ketamine is a noncompetitive antagonist of the N-methyl-D-aspartate (NMDA) receptor,39 which plays an important role in the chronification and amplification of (neuropathic) pain.9,41 Ketamine reduces wind-up and temporal summation,9 which are surrogate measures of central sensitization. However, narrative and systematic reviews indicate that proof of efficacy of ketamine in neuropathic pain is absent or limited.2,6,20,21 It is not evident why ketamine efficacy is limited in human studies. We earlier suggested that efficacy of ketamine in chronic pain is related to treatment duration (ie, single dose vs multiple doses or continuous infusion), whereas other factors such as dose or etiology of neuropathic symptoms were considered less important.38 Finding options that improve our ability to effectively treat neuropathic pain with ketamine is important given the large number of patients who experience limited efficacy from conventional treatment modalities including opioids. To advance our understanding of the role of ketamine in the relief of neuropathic pain symptoms, we performed a systematic review and meta-analyses of animal studies that provide data on the efficacy of ketamine in the relief of allodynia. We focused on animal studies in which ketamine (racemic or S-ketamine) is administered systemically or through the intrathecal route to relieve neuropathic pain symptoms (allodynia) associated with nerve, spinal cord, or brain damage models. We performed 4 meta-analyses to determine the ketamine effect size after a single dose, shortly (<3 hours) and 24 to 72 hours after injection, and the effect of chronic dosing, during ketamine exposure and in the days after exposure. We further explore the possibility whether the information obtained from these animal studies may be helpful in designing human studies on the efficacy of ketamine in neuropathic pain.
2. Methods
2.1. Search strategy
In this review, we focus on the effect of ketamine on experimentally induced neuropathic pain symptoms related to surgically induced (partial) damage to nerves or spinal cord, lesions in the brain, postinfectious neuritis, and disease- or drug-induced neuropathy. The study protocol was prospectively registered on the PROSPERO Web site under registration number 20119 (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=201190) and performed according to published guidelines.7 We systematically searched 2 electronic literature databases (PubMed and EMBASE) to identify preclinical studies on racemic ketamine or S-ketamine treatment of allodynia induced by a variety of neuropathic pain models. Allodynia is defined as “pain due to a stimulus that does not normally provoke pain” by the International Association of the Study of Pain (https://www.iasp-pain.org/terminology?navItemNumber=576#Allodynia). The search strategy was developed in collaboration with information specialists of the Walaeus library of Leiden University Medical Center and the Systematic Review Center for Laboratory Animal Experimentation (SYRCLE) at Radboud University Medical Center (Nijmegen, the Netherlands). For our complete search strategy, see supplemental file 1 (available at http://links.lww.com/PAIN/B302). No publication date or language restrictions were applied.
The literature search was first performed on July 22, 2020. Thereafter, the search strategy was amended including a filter for animal studies,16 developed by SYRCLE, and an additional search in PubMed and Embase was performed on September 22, 2020. The 2 searches yielded similar outputs. Finally, a last search was performed on October 2, 2020, to search for more recently published studies (none were identified). After removal of duplicates, articles were first selected based on the title/abstract level and thereafter at the full-text level. We also checked relevant articles such as review articles for additional references. Inclusion criteria were as follows: (1) original nonhuman studies; (2) available full-text articles; (3) disease model (neuropathic pain) induced by one of the following methods: spared nerve injury, (full or partial) ligation of peripheral nerves (chronic nerve constriction injury [CCI]), spinal nerve ligation, spinal cord injury, plexus ablation, viral infections (postherpetic neuralgia), chemotherapeutics, streptozotocin administration, or central lesions; (4) systemic (intravenous, subcutaneous, intraperitoneal, or oral) or intrathecal administration of ketamine, either the racemic formulation or the S-enantiomer, to relief mechanical or heat/cold allodynia as measured by withdrawal responses to tactile or thermal stimuli; (5) ketamine is tested against control conditions (eg, saline or vehicle); (6) ketamine treatment was initiated after the neuropathic disease model was fully established; and (7) relief of allodynia (our primary end point) was quantitatively reported either in a table or in a graph. We included relief of mechanical allodynia in our analysis if reported (85% of studies), or relief of thermal allodynia as alternative. In case both were reported, we chose mechanical allodynia for inclusion in our analyses. Exclusion criteria included case reports, case series, review articles, conference abstracts, studies that tested preemptive ketamine, the combined administration of ketamine with another drug, ketamine as positive or negative control (eg, when administered at subeffective low dose or excessively high dose as stated in the text), ketamine administered directly into the brain, or ketamine tested as an anesthetic or analgesic for nerve damage surgery. Finally, studies with insufficient quantitative data to extract threshold values were excluded. Two reviewers independently performed the selection procedure (A.D. and M.v.V.). Differences in opinion were resolved by consensus and when needed a third reviewer (J.D.C.D.) was consulted.
2.2. Data extraction
The absolute values of the withdrawal thresholds after treatment were extracted with ketamine and control data obtained at identical time points. If no quantitative data were reported directly (eg, in text or tables), authors were contacted or the graphical data were measured using a digital ruler (A Ruler for Windows, https://a-ruler-for-windows.en.softonic.com/) with 2 reviewers performing an independent assessment of treatment effect (A.D. and E.L.A.v.D.). Standard errors of the mean were transformed into SDs. When the animal group size was reported as a range, a conservative approach was chosen and the lowest number of animals was used in the analysis. Articles that described multiple independent experiments in different groups of animals were included as independent comparisons. The variables that were extracted from the studies are presented in Table 1 and included the frequency of ketamine dosing (single, repetitive, and continuous), the delay between ketamine injection and neuropathic pain symptom measurement, and outcome measures.
Table 1.
Animal study characteristics.
| First author publication year | Animals | Treatment | Control | Admin. route | Dose | Dosing | Pain model | Outcome measure | Measurement delay since injection* | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Sex | Age (wk) | Weight (g) | |||||||||
| Studies on single ketamine administration (n = 21) | ||||||||||||
| Chaplan et al., 19972 | Rat (Sprague-Dawley) | Male | Ketamine | Saline | i.t. | 100 µg | Single | SNL | Paw withdrawal to a mechanical stimulus | 60 min | ||
| Christoph et al., 20063 | Rats (Sprague-Dawley) | Male | 170-310 | Ketamine | Saline | i.v. | 4.64 mg/kg | Single | CCI | Paw withdrawal to a cold stimulus | 15 min, 24 h | |
| Claudino et al., 20184 | Rat (Wistar) | Male | 200-220 | S-ketamine | Vehicle | i.n. | 1 mg/kg | Single | CION | Paw withdrawal to a mechanical stimulus | 30 min | |
| De Vry et al., 20048 | Rat (Wistar) | Male | 180-200 | Ketamine | Vehicle | i.p. | 20 mg/kg | Single | CCI | Paw withdrawal to a mechanical stimulus | 30 min | |
| Doncheva et al., 201910 | Rat (Wistar) | Male | 200-220 | Ketamine | Saline | i.p. | 50 mg/kg | Single | CCI | Paw withdrawal to a mechanical stimulus | 60 min | |
| Fang 201811 | Rat (Sprague-Dawley) | Male | 180-230 | Ketamine | Saline | i.p. | 10 mg/kg | Single | SNI | Paw withdrawal to a mechanical stimulus | 72 h | |
| Hama and Sagen, 201212 | Rat (Sprague-Dawley) | Male | 100-150 | Ketamine | Vehicle | i.t. | 100 µg | Single | SCI | Paw withdrawal to a mechanical stimulus | 90 min | |
| Humo et al., 202019 | Mouse (C75BL/6) | Male | 8 | Ketamine | Saline | i.p. | 15 mg/kg | Single | CCI | Paw withdrawal to a mechanical stimulus | 120 min, 24 h | |
| Kroin et al., 201922 | Mouse (D1) | Female | 20 | Ketamine | Saline | i.p. | 10 mg/kg | Single | SNI | Paw withdrawal to a mechanical stimulus | 60 min, 72 h | |
| Lim et al., 201325 | Mouse (ICR) | Male | 25-30 | Ketamine | Vehicle | i.t. | 100 µg | Single | CCI | Paw withdrawal to a mechanical stimulus | 90 min | |
| Mao† et al., 199327 | Rat (Sprague-Dawley) | Male | 400-500 | Ketamine | Saline | i.t. | 23.7 µg | Single | CCI | Paw withdrawal to radiant heat | 30 min | |
| M'Dahoma 201428 | Rat (Sprague-Dawley) | Male | 7-8 | 225-250 | Ketamine | Vehicle | i.p. | 50 mg/kg | Single | SCI | Paw withdrawal to a mechanical stimulus | 30 min |
| M'Dahoma et al., 201529 | Rat (Sprague-Dawley) | Male | 175-200 | Ketamine | Saline | i.p. | 50 mg/kg | Single | CCI | Paw withdrawal to a mechanical stimulus | 60 min | |
| Mehta et al., 201230 | Rat (Wistar) | Male | 180-250 | Ketamine | Control | i.p. | 25 mg/kg | Single | CCI | Paw licking or jump latency on hot plate | 45 min | |
| Mei et al., 201032 | Rat (Sprague-Dawley) | Male | 180-200 | S-ketamine | Saline | i.t. | 1000 µg/kg | Single | CCI | Paw withdrawal to a mechanical stimulus | 30 min, 72 h | |
| Pan et al., 201840 | Rat (Sprague-Dawley) | Male | 6-7 | 200-250 | Ketamine | Saline | i.p. | 10 mg/kg | Single | SNI | Paw withdrawal to a mechanical stimulus | 72 h |
| Qian et al., 199643 | Rat (Sprague-Dawley) | Male | 125-150 | Ketamine | Saline | i.p. | 50 mg/kg | Single | SNL | Paw withdrawal to a mechanical stimulus | 15 min | |
| Rodrigues-Filho et al., 200445 | Rat (Wistar) | Male | 250-300 | Ketamine | Saline | i.p. | 25 mg/kg | Single | BPA | Paw withdrawal to a mechanical stimulus | 30 min | |
| Sasaki et al., 200847 | Mouse (C57BL/6J) | Female | 6 | Ketamine | Vehicle | i.p. | 50 mg/kg | Single | PHN | Paw withdrawal to a mechanical stimulus | 60 min | |
| Truin et al., 201152 | Rat (Sprague-Dawley) | Male | 300-350 | Ketamine | Saline | i.t. | 200 µg | Single | CCI | Paw withdrawal to a mechanical stimulus | 45 min | |
| Wang et al., 201153 | Rat (Sprague-Dawley) | Male | 250-300 | Ketamine | Saline | i.p. | 10 mg/kg (1 h), 50 mg/kg (24 h) | Single | SNI | Paw withdrawal to a mechanical stimulus | 1 h, 24 h | |
| Multiple ketamine administrations or continuous ketamine infusion (n = 11) | ||||||||||||
| Hota et al., 200717 | Rat (Wistar) | Male | 200-250 | Ketamine | Control | Gavage | 2.5 mg/kg | 1 daily dose for 25 days | SNI | Paw licking latency on hot plate | 0 d | |
| Huang et al., 200418 | Rat (Sprague-Dawley) | Female | 200-250 | Ketamine | Saline | i.p. | 10 mg/kg | 6 days for 4 weeks | SNL | Paw withdrawal to a mechanical stimulus | 1 d | |
| Kwon et al., 201423 | Rat (Sprague-Dawley) | Male | 180-200 | Ketamine | Saline | s.c. | 40 mg/kg per day | 7-day infusion | SNL | Paw withdrawal to a mechanical stimulus | 0 d | |
| Mak et al., 201526 | Rat (Wistar) | Male | Ketamine | Control | s.c. | 20 mg/kg per day | 5-day infusion | STZ DN | Paw withdrawal to a mechanical stimulus | 14 d | ||
| Mao† et al., 199327 | Rat (Sprague-Dawley) | Male | 400-500 | Ketamine | Saline | i.t. | 23.7 µg | 4 daily doses for 3 days | SNL | Paw withdrawal to radiant heat | 7 days after last dose | |
| Mei‡ 2009a,c31 Mei 2009b,d |
Rat (Sprague-Dawley) | Male | 180-200 | S-ketamine | Saline | i.t. i.p. | 100 µg/kg 20 mg/kg | 1 dose/day for 3 or 7 days | SNL | Paw withdrawal to a mechanical stimulus | 0 d | |
| Mei et al., 2011a33 | Rat (Sprague-Dawley) | Male | 180-200 | S-ketamine | Saline | i.t. | 300 µg/kg | 1 dose/day for 3 days | SNL | Paw withdrawal to a mechanical stimulus | 0 d | |
| Mei et al., 2011b34 | Rat (Sprague-Dawley) | Male | 180-200 | S-ketamine | Saline | i.t. | 300 µg/kg | 1 dose/day for 3 days | SNL | Paw withdrawal to a mechanical stimulus | 0 d | |
| Salvat§ et al., 2018a46 Salvat 2018b |
Mouse (6J) | Male | 6 | Ketamine | Saline | i.p. | 15 mg/kg | 2 doses/day for 10 days (0-10 after CCI); 2 doses/day (25-35 days after CCI) | CCI | Paw withdrawal to a mechanical stimulus | 15 d and 34 d | |
| Swartjes 201150 | Rat (Sprague-Dawley) | Female | 9 | 230 | Ketamine | Saline | i.v. | 9 mg/kg per day | 5-day infusion | SNI | Paw withdrawal to a mechanical stimulus | 28 d |
| Swartjes 201351 | Mouse (C57BL/6) | Female | Ketamine | Vehicle | i.p. | 50 mg/kg | In week 1, every other day 1 dose, followed by weekly dosing in week 2-6 | SNI | Paw withdrawal to a mechanical stimulus | 0 d | ||
Ketamine is the racemic mixture, S-ketamine is the S-enantiomer of ketamine.
Time points included in the meta-analysis.
Mao et al.27 performed 2 independent comparisons: one comparing a single ketamine administration vs saline and one comparing single multiple administrations vs multiple saline administrations.
Mei et al.33 performed 4 independent comparisons: S-ketamine vs saline administered through the intrathecal route and S-ketamine vs saline administered intraperitoneally with 1 dose per day for 3 and for 7 days.
Salvat et al.46 performed 2 independent comparisons: one comparing ketamine administration on days 0 to 10 after surgery for CCI and one comparing ketamine administered on days 25 to 35 after surgery for CCI.
Neuropathic pain models: BPA, brachial plexus avulsion; CCI, chronic constriction injury; CION, constriction of infraorbital nerve; PHN, postherpetic neuralgia; Route of administration: i.p., intraperitoneal; i.t, intrathecal; i.v, intravenous; i.n, intranasal; s.c, subcutaneous; SNI, spared nerve injury; SNL, spinal nerve ligation; STZ DN, streptozotocin-induced diabetic neuropathy.
2.3. Risk of bias assessment
To get an indication of the quality of the animal studies, we determined their risk of bias (RoB) using the RoB tool developed by the SYRCLE and published by Hooijmans et al.15 After training by SYRCLE, 2 reviewers performed the risk assessment (J.D.C.D. and M.v.V.), and differences in opinion were resolved by consensus and when needed a third reviewer (A.D.) was consulted. A “yes” score indicates low RoB, a “no” score indicates high RoB, and a “?” score indicates unknown RoB. To overcome the problem of judging too many items as “unclear risk of bias” because reporting of experimental details on animals, methods, and materials is very poor in the pain preclinical literature,44 we added 3 items on reporting: reporting of any measure of randomization, reporting of any measure of blinding, and reporting of power calculation. For these 2 items, a “yes” score indicates “reported” and a “no” score indicates “unreported.”
2.4. Meta-analysis
We aimed at obtaining a general indication of the efficacy of ketamine in relieving neuropathic pain symptoms and therefore initially pooled all behavioral pain experiments regardless of etiology or methods used for measurement of pain symptom relief. We performed the following 4 exploratory meta-analyses:
(1) to determine the pooled peak effect size of a single administration of ketamine within 3 hours of its administration (the largest effect size observed within this time frame was used). We had set the time frame initially at within 2 hours of ketamine administration but amended the protocol during the course of data extraction (as published on the PROSPERO Web site);
(2) to determine the pooled peak effect size of a single administration of ketamine at 24 hours or later after its administration;
(3) to determine the pooled effect size of repetitive or continuous ketamine administration(s) with measurements made at the end of ketamine administration; and
(4) to determine the pooled effect size of chronic ketamine administration with measurements made in the days after ketamine administration had ended.
Prespecified subanalyses included the comparison of species (rats vs mice), neuropathic pain models, administration routes, use of mechanical threshold responses vs other outcomes, or doses.
The meta-analyses were performed using the Comprehensive Meta-Analysis software package version 3.0 for Windows (Biostat Inc., Englewood, NJ). The difference between treatments was used in the analysis (standardized mean difference). For each study, Hedges g and 95% confidence intervals were calculated and presented in a forest plot. Hedges g is preferable over Cohen d when sample sizes are small (n < 20). The data were analyzed using random effects models, assuming 2 sources of variance: within-study and between-study error. Subgroup comparisons were performed by mixed-effects analysis; subgroup comparisons were restricted to subgroups with at least 3 studies. We expected the variance to be comparable within subgroups; therefore, we assumed a common among-study variance across subgroups. Differences between subgroups should be interpreted with caution and should be used for constructing new hypotheses rather than for drawing conclusions. A sensitivity analysis was performed to assess the effect of a single study on the meta-analysis outcome by using the leave-one-out method. Heterogeneity was assessed by visual inspection of the forest plot and by measuring the degree of between-study inconsistency in the studies' results (I2). We constructed funnel plots and performed Egger and trim-and-fill analyses to search for evidence of publication bias in meta-analyses that included at least 15 independent comparisons in Stata (StataCorp. 2019. Stata Statistical Software: Release 16; College Station, TX). Because standardized mean differences may cause funnel plot distortion, we plotted the standardized mean differences against a sample size-based precision estimate (1/√n).54
Statistical significance was set at P < 0.05. For subgroup analyses, we adjusted our significance level according to the conservative Bonferroni method to account for multiple analyses (P *number of comparisons).
3. Results
3.1. Study selection and characteristics
The flowchart of studies identified in the search and the process of elimination is given in Figure 1. The search retrieved 324 studies from PubMed and 309 from EMBASE. After removal of duplicates, 473 studies were screened and 407 were discarded, mostly because other end points than neuropathic pain symptoms were measured or because ketamine was used as anesthetic or analgesic given during or after surgery. Sixty-six articles remained of which 2 could not be retrieved in full-text mode (1 Japanese and 1 Russian article). Sixty-four articles were read in full and carefully screened for eligibility. Thirty-two articles were excluded from the analysis for various reasons (Fig. 1). One additional article was post hoc excluded because ketamine and norketamine were administered in a crossover design, which may have influenced the relief of allodynia from ketamine. A total of 31 articles were included in the analyses (study characteristics of all articles are given in Table 1 and Fig. 2), of which 20 report on the effects of a single ketamine administration,2–4,8,10–12,19,22,25,28–30,32,40,43,45,47,52,53 10 articles on chronic ketamine,17,18,23,26,31,33,34,46,50,51 and one article on both a single and chronic administrations.27 In addition, 2 articles reported on multiple independent comparisons (2 in one study and 4 in another).31,46 This makes a total of 35 independent comparisons, 21 studying a single ketamine injection, and 14 repetitive daily administrations and one on a continuous infusion. Of the studies on a single injection, most (n = 19) measured the effect of ketamine shortly after injection (mean ± SD delay = 1 ± 0.7 hours), whereas 7 studies report on measurements at 24 or 72 hours after the single ketamine injection. Of the 15 studies that tested the chronic administration of ketamine over several days (median duration of treatment = 6 days, range 3-42 days), 9 measured treatment effect during the administration period, whereas 6 assessed the effect of the drug at least one day after the administration was terminated (median duration of treatment = 7.5 days, range 3-28 days; median test delay = 24 days, range 1-36 days). The characteristics of the individual studies (Table 1 and Fig. 2) indicate that most studies were performed in male rats (84%) after surgery for chronic nerve constriction injury (CCI) (35%), spinal nerve ligation injury (SNL) (23%), or spared nerve injury (23%) and after intraperitoneal (53%) or intrathecal (31%) ketamine administration. Mechanical withdrawal responses were tested in 87% of studies using manual von Frey filaments or similar techniques (eg, by electronic von Frey technique). The total number of animals included in the 31 studies that received ketamine was 331 with 333 control animals of which 270 and 274, respectively, were rats and the remaining were mice. The median number of animals per treatment group was 7 with range 4 to 12 in both ketamine and control groups. The majority of studies (71%) injected racemic ketamine, while the remainder injected the S-isomer.
Figure 1.
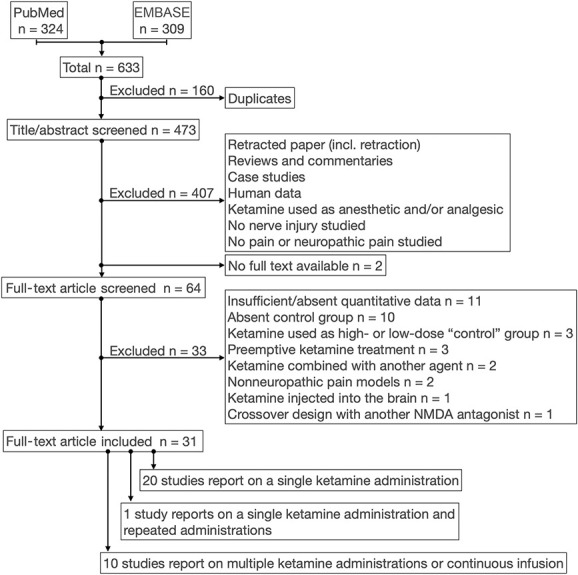
Flowchart of study search and selection process.
Figure 2.
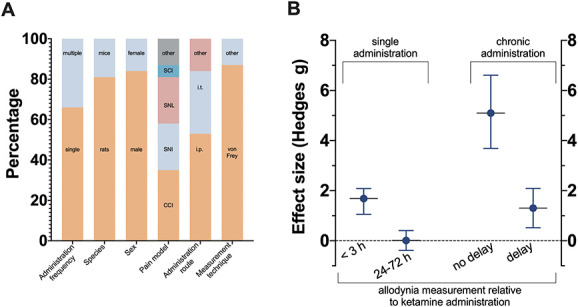
(A) Main characteristics of studies selected in the meta-analysis. Pain models: SCI, spinal cord injury; SNL, spinal nerve ligation; SNI, spared nerve injury; CCI, chronic nerve constriction injury; and “other” pain models were constriction of infraorbital nerve (n = 1), brachial plexus ablation (n = 1), postherpetic neuralgia (n = 1), and streptozotocin-induced neuropathy (n = 1). Administration route: i.t., intrathecal; i.p., intraperitoneal; and “other” routes were subcutaneous (n = 2), intranasal (n = 1), and intravenous (n = 2). Measurement techniques: “other” includes measuring withdrawal latency to cold and heat stimuli. (B) Summary of the 4 meta-analyses. No delay indicates that the measurement of neuropathic pain was performed during the period of ketamine administration. The data point of the single administration 24-72 h is without Fang et al. (Ref. 11).
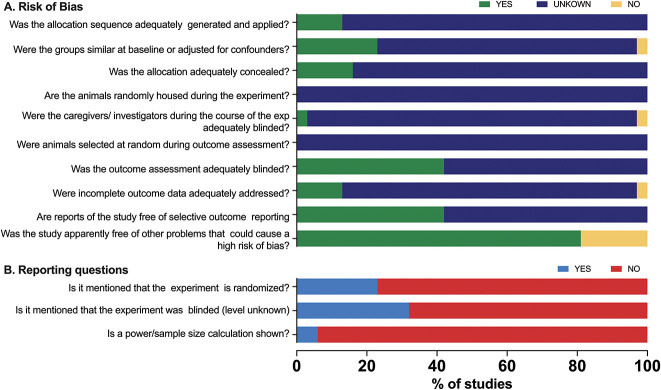
3.2. Risk of bias
The results of the RoB assessment is presented in Figure 3A. As a consequence of inadequate reporting of essential methodological details, the RoB was in most studies and most RoB domain scores as “unclear” due to poor reporting. This is further emphasized in the 3 reporting questions (Fig. 3B) that we added to the SYRCLE RoB tool on randomization (reported: yes/no), blinding (reported: yes/no), and power calculation (reported: yes/no) that showed that in 77%, 68%, and 94% of studies, respectively, these were not reported.
Figure 3.
(A) Risk of bias assessment of the included studies. (B) Reporting questions on randomization, blinding, and power calculation.
3.3. Meta-analysis 1: effect of a single ketamine dose and measurement within 3 hours of administration
The 19 comparisons (coming from 19 individual studies) that tested the ketamine effect within 3 hours after administration showed an appreciable effect size. The pooled effect size (Hedges g ± standard error) was 1.6 ± 0.3 with 95% confidence interval 1.1 to 2.1 (P = 0.00); Figures 2 and 4. Across all studies heterogeneity was large (I2 = 72%); the leave-one-out method did not point towards studies that dominated the outcome. Subgroup analyses indicated (Fig. 5) that there were no differences between species (rat: n = 15; 1.7 ± 0.3 [1.1-2.3], P = 0.00, I2 = 73% vs mouse: n = 4; 1.1 ± 0.4 [0.3-2.0], P = 0.007, I2 = 48%; group comparison P = 0.30), administration routes (i.p.: n = 10; 1.6 ± 0.4 [0.8-2.4], P = 0.00, I2 = 81% vs i.t.: n = 6; 1.5 ± 0.4 [0.8-2.2], P = 0.00, I2 = 46%; group comparison P = 0.26), or doses (systemic dose 5-25 mg/kg: n = 7, 1.5 ± 0.5 [0.4-2.5], P < 0.01, I2 = 83%; systemic dose 50 mg/kg: n = 5, 2.0 ± 0.5 (1.1-3.0), P = 0.00, I2 = 65%, and intrathecal dose 100–200 μg: n = 4, 1.4 ± 0.4 (0.6-2.1), P = 0.00, I2 = 27%, group comparison P = 0.55). Most studies were performed in animals with a disease model induced by CCI (n = 9) or SNL (n = 3). The effect direction was similar for all nociceptive assays with a rather large variability in the 3 studies performed using SNL (Fig. 5). Finally, 18 studies measured the mechanical withdrawal response, whereas the 3 others measured the withdrawal response to cold or heat (radiant heat or hot plate). The pooled effect size for studies that measured the mechanical withdrawal response was 1.7 ± 0.3 (1.1-2.3), P = 0.00, I2 = 73%.
Figure 4.
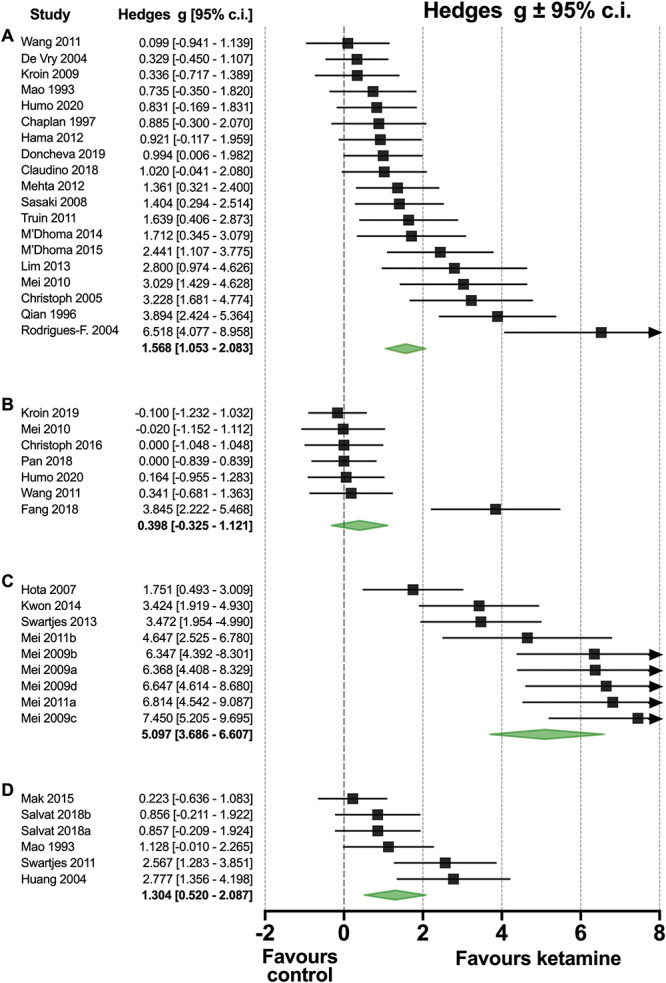
(A) Forest plot of the effect of a single ketamine injection on neuropathic pain measured within 3 hours of injection. (B) Forest plot of the effect of a single ketamine injection on neuropathic pain measured at 24 or 72 hours after injection. (C) Forest plot of the effect of repetitive ketamine injections or a continuous infusion on neuropathic pain, measured during ketamine administration. (D) Forest plot of the effect of repetitive ketamine injections or a continuous infusion on neuropathic pain, measured in the days after ketamine administration. The green diamonds are the group effect sizes ± 95% confidence intervals.
Figure 5.
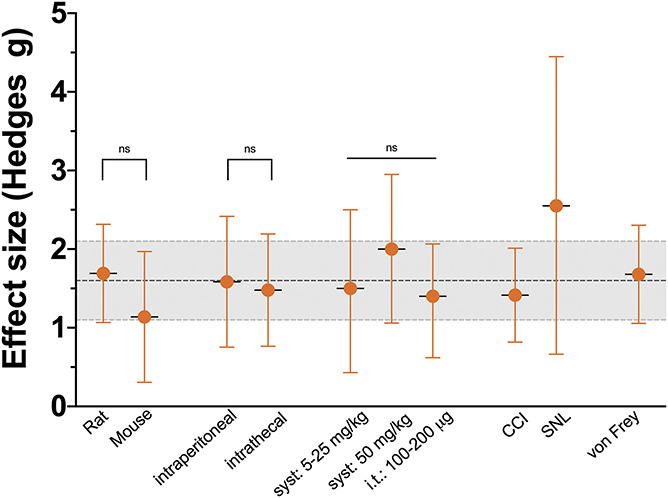
Subgroups of comparisons on the effect of a single administration of ketamine on relief of allodynia measured within 3 hours of administration. The data are pooled effect size (Hedges g) ± 95% confidence interval. The dotted line and gray area are the effect size of all the complete data set ± 95% confidence interval. CCI, chronic nerve constriction injury; i.t., intrathecal; SNL spinal nerve ligation injury.
3.3.1. Publication bias
No evidence was found for small study effects (P = 0.74) and missing studies (zero imputed; Fig. 6). However, the number of studies was small (n = 19), and therefore this result does not mean that there is no publication bias. We expect, based on previous systematic reviews of animal studies, that some studies are missing and the summary effect is somewhat overestimated.
Figure 6.
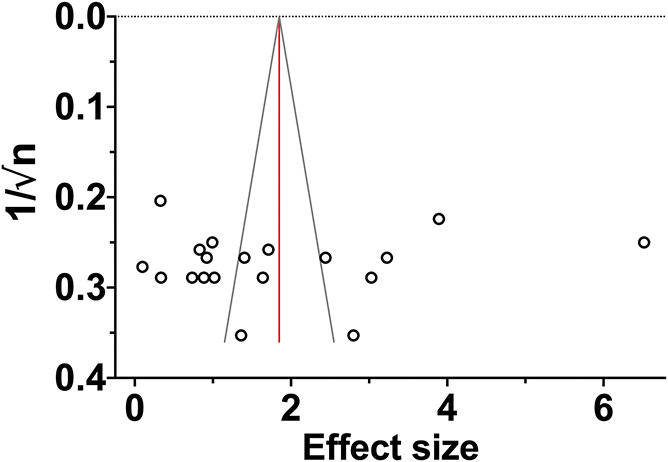
Funnel plot of meta-analysis 1 examining the ketamine effect within 3 hours of a single administration. Each symbol represents an independent comparison.
3.4. Meta-analysis 2: effect of a single ketamine dose and measurement at 24 hours or 72 hours after dosing
Seven comparisons from 7 studies tested the effect of ketamine 24 or 72 hours after administration with pooled effect size 0.4 ± 0.4 (−0.3 to 1.1), P = 0.28, I2 = 70.7%. One study (Fang et al.11) was considered an outlier and without that study the effect size was reduced to 0.010 ± 0.201 (−0.4 to 0.4), P = 0.96, I2 = 0% (Fig. 4B). The effect size at 24 hours (n = 3) was 0.1 ± 0.3 (−0.5 to 0.6), P = 0.80, I2 = 0%, and at 72 hours (n = 3, excl. Fang et al.11) −0.05 ± 0.28 (−0.6 to 0.5), P = 0.86, I2 = 0%. All predefined subgroups were too small to conduct reliable analyses.
3.5. Meta-analysis 3: effect of chronic ketamine and measurement during exposure
Nine comparisons (obtained from 6 studies) tested the ketamine effect within the period of drug administration and had a pooled effect size of 5.1 ± 0.7 (3.6-6.5), P = 0.00, I2 = 82% (Fig. 4C). In 8 comparisons, rats were tested with effect size 5.3 ± 0.8 (3.7-6.9) P = 0.00, I2 = 83%. All other subgroups were too small to conduct reliable analyses.
3.6. Meta-analysis 4: effect of chronic ketamine and measurement after exposure
Six comparisons (from 5 studies) that tested the effect of ketamine after treatment had a pooled effect size of 1.3 ± 0.4 (0.5-2.1), P = 0.001, I2 = 66% (Fig. 4D). In 4 comparisons, rats were tested (effect size 1.6 ± 0.6 [0.4-2.8], P = 0.01, I2 = 79%) and mice in the 2 remaining studies. All other subgroups were too small to conduct reliable analyses.
Comparison between analyses #3 and #4 showed that the delay in testing (respectively during and after ketamine exposure) resulted in a significant reduction in effect size of ketamine from 5.1 ± 0.7 to 1.3 ± 0.4 (P = 0.00).
4. Discussion
The analyses of ketamine studies performed in nerve-injured rodents demonstrate that a single ketamine administration has an appreciable effect on the relief of allodynia when tested within 3 hours after administration. This effect dissipated rapidly in the subsequent days. When chronically administered for at least 3 days, ketamine had a large effect when tested in the treatment period, an effect that diminished in the days after exposure.
In contrast to most analgesics, the effect of ketamine is not driven by its pharmacokinetics.19 There is evidence from human and animal data that the effect of ketamine persists at times when plasma ketamine concentrations (and metabolites) are low or undetectable. For example, in nerve-injured rats, Christoph et al.3 showed that ketamine inhibits the electrophysiological response of spinal dorsal horn wide-dynamic-range neurons with a time course consistent with the ketamine plasma half-life (t1/2 = 10-12 minutes), indicative of rapid receptor kinetics,39 whereas its anti-allodynic effects lasted >3 hours. In patients with chronic neuropathic pain, we observed differences in half-lives of the decay of ketamine-induced analgesia, depending on the duration of treatment. A short (2 hours) administration of intravenous S-ketamine in patients with complex regional pain syndrome type 1 resulted in effective analgesia (pain reduced from 6 to 0 on a 10-cm visual analogue scale) during treatment followed by a rapid return of pain with a half-life of 2 hours.48 In a similar patient population, a 100-hour infusion of esketamine resulted in the reduction of perceived pain from 7 to 2.5.5 On termination of treatment, pain intensity slowly returned to baseline levels with a half-life of 11 days. In both studies, ketamine and norketamine plasma concentration dropped below 100 ng/mL within minutes after the termination of infusion. Summarized, these data suggest that the ketamine efficacy in relieving neuropathic pain symptoms depends on the duration of administration and the timing of effect measurement. We believe that this behavior may be one of the main reasons why most human randomized controlled trials examining the efficacy of ketamine in relieving neuropathic pain yield negative results. One example is a recent trial that tested a single dose of 0.5 mg/kg ketamine in 20 patients with neuropathic pain from a variety of origins (related to surgery, radiculopathy, trauma, diabetes mellitus, or chemotherapy) and observed no relief of neuropathic pain at 5 weeks after treatment (primary outcome), although at 1 week a small effect was observed (secondary end point).42 To get insight in this matter, we sought help from animal studies that tested the effect of ketamine on relief of allodynia. The aim of our review is to explore whether ketamine efficacy is time dependent in terms of exposure and neuropathic symptom relief testing.
We first analyzed the data from 19 studies that examined the effect of a single systemic or intrathecal ketamine injection in a population of predominantly male rats after nerve injury and measured withdrawal responses to mechanical stimuli within 3 hours of ketamine administration. The effect of ketamine was appreciable (effect size 1.6 ± 0.3) albeit with high between-study heterogeneity. Subgroup analyses showed no differences between species, administration route, assay, or dose (Fig. 5). This, however, does not mean that there are no effects of these independent variables because the power of identifying the effects in our subgroup analyses is low. The immediate effect of a single ketamine dose is best explained by the blockade of afferent nociceptive input from injured nerves to central sites through antagonism of NMDA receptors and possibly involvement of other receptor systems (eg, opioid receptors).49 However, a single dose is insufficient to produce long-lasting effects as observed in our second analysis. Six of 7 comparisons included in the analysis showed allodynia in magnitude similar to control when tested at 24 or 72 hours after ketamine injection without heterogeneity. One outlier (Fang et al.11; Fig. 4) observed a rather large effect after 10 mg/kg ketamine in animals selected for their anhedonia susceptibility as a surrogate measure of depression. Possibly, the animal selection was the reason for the divergent effect relative to the other 6 studies.
We next determined the effect of chronic ketamine administration (treatment given for 3-42 days). Across these 9 comparisons a large effect size was detected with high between-study heterogeneity levels. In contrast to the effect of a single ketamine administration, the chronic treatment strategy caused a slow loss of the relief of allodynia. At 24 days after termination of ketamine administration (the median test delay period), allodynia had partially returned but, relative to control, the effect was still present and similar to the effect observed after a single administration. These data indicate that ketamine effect persists beyond the treatment exposure time and suggests that already after 3 days of treatment a long-term pharmacokinetic-independent effect is observed.5,39,48 Various mechanisms have been proposed for the persistent ketamine effect: (1) blocking NMDA channel function and changes in NMDA receptor phosphorylation and expression and consequently disrupting pathologic glutamatergic neurotransmission at spinal and/or central levels49; (2) an alteration of pain phenotype from changes in connectivity among the various pain-related brain centers37; and (3) a restorative anti-inflammatory effect at spinal and/or central levels from activation of several receptors system including the CD131 cytokine receptor.24,51
4.1. Limitations
To explore the certainty in the evidence, we assessed the domains of the GRADE approach for animal studies.13 First, heterogeneity among studies was high (I2 >50%), which can be expected and is related to the often exploratory nature of animal studies, and part of the heterogeneity is intentionally induced.13 To account for the expected heterogeneity, we analyzed the data with random effects models, and heterogeneity was explored by sensitivity and subgroup analyses. Meta-analyses allow the exploration of the causes for heterogeneity, which is not only informative but may also help in the design of future animal studies. An important observation was that there was consistency between the 2 species tested, which is a strength in our analyses.
Second, the number of animals and comparisons in the meta-analyses described in this review is relatively low. Consequently, the calculated effect estimates in the meta-analyses may be imprecise. In combination with the high between-study heterogeneity in meta-analyses of animal studies, the certainty in the calculated effect decreases. Fortunately, as recommended in meta-analyses of animal studies, we focus on direction of effects rather than on actual effect sizes.13,14 For the results of the subgroup analyses, the risk of imprecise results is even larger as subgroup analyses are often conducted on even smaller numbers of studies. Therefore, the results of subgroup analyses should be interpreted with caution and treated as hypothesis generating.
An additional issue is the indirectness of evidence from the studies compared with their original research question and translatability towards humans. Considering the first issue, the study of pain relief by ketamine was often a secondary or tertiary end point (with primary end points related to, eg, relief of depression or measurement of cytokines). Although this does not degrade the internal validity of the outcome of pain-related experiments, we cannot exclude an interfering effect of multiple end points measured in the same animals. Regarding the second item of indirectness, (1) the animals were all studied in the hours or days after surgery. In humans, patients with neuropathic pain are often treated with ketamine after years of suffering with often highly ineffective earlier treatments; (2) most animal models of allodynia do not represent the most common causes of neuropathic pain in humans, which also include genetic syndromes, surgery/trauma, infectious disease, metabolic syndromes, and degenerative disease; (3) several studies administered ketamine through the intrathecal route, which is not the pathway of choice in humans; (4) patients with neuropathic pain report many additional symptoms not studied in these animal models; and (5) the experiments were predominantly restricted to male animals, which ignores existing sex differences in pain perception and efficacy of pain treatment (including ketamine).36 These issues suggest that blind extrapolation of our findings towards humans is tenuous. To improve translation, further animal studies are needed that use randomized and blinded designs, are sufficiently powered, use multiple species beyond mice and rats, study males and females, and apply ketamine administration routes used in humans. Nevertheless, our results seem in correspondence with the sparse human data showing ketamine efficacy when administered over multiple days and lack of efficacy when administered only once.5,35,38,48
Finally, our RoB analysis revealed that essential details regarding the design and conduct of the included experiments are poorly reported. Consequently, the RoB could not be estimated for most studies. This is a serious concern because a lack of reporting methodological details will to some extend indicate neglected use of these methods to reduce bias, and this negatively impacts the ability to draw reliable conclusions based on the included experimental animal studies.
4.2. Conclusions
Ketamine efficacy in relief of allodynia in rodent models of neuropathic pain seems dependent on exposure time and timing of allodynia testing. Prolonged exposure (>3 days) results is long-term relief of allodynia. Given the limitations, extrapolation of the animal data to the human condition is tenuous.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B302.
Supplementary Material
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
Contributor Information
Monique van Velzen, Email: jack.d.c.dahan@gmail.com.
Jack D.C. Dahan, Email: e.l.a.van_dorp@lumc.nl.
Eveline L.A van Dorp, Email: jeffrey.mogil@mcgill.ca.
Jeffrey S. Mogil, Email: Carlijn.Hooijmans@radboudumc.nl.
Albert Dahan, Email: m.van_velzen@lumc.nl.
References
- [1].Bell RF, Kalso EA. Ketamine for pain management. Pain Rep 2018;3:e674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chaplan SR, Malmberg AB, Yaksh TL. Effect of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther 1997;280:829–38. [PubMed] [Google Scholar]
- [3].Christoph T, Schiene K, Engelberger W, Parsons CG, Chizh BA. The antiallodynic effect of NMDA antagonists in neuropathic pain outlasts the duration of the in vivo NMDA antagonism. Neuropharmacol 2006;51:12–7. [DOI] [PubMed] [Google Scholar]
- [4].Claudino R, Nones C, Araya E, Chicorro J. Analgesic effects of intranasal ketamine in rat models of facial pain. J Oral Facial Pain Headache 2018;32:238–46. [DOI] [PubMed] [Google Scholar]
- [5].Dahan A, Olofsen E, Sigtermans M, Noppers I, Niesters M, Aarts L, Bauer M, Sarton E. Population pharmacokinetic-pharmacodynamic modeling of ketamine-induced pain relief of chronic pain. Eur J Pain 2011;15:258–67. [DOI] [PubMed] [Google Scholar]
- [6].Dahan A, van Velzen M, Niesters M. Ketamine for neuropathic pain: a tiger that won't bite? Br J Anaesth 2020;125:e275–6. [DOI] [PubMed] [Google Scholar]
- [7].de Vries RBM, Hooijmans CR, Langendam MW, van Luijk J, Leenaars M, Ritskes-Hitinga M, Wever KE. A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evidence Based Preclin Med 2015;1:1–9(e00007). [Google Scholar]
- [8].De Vry J, Kuhl E, Franken-Kunkel P, Eckel G. Pharmacological characterization of the chronic constriction injury model of neuropathic pain. Eur J Pharmacol 2004;491:137–48. [DOI] [PubMed] [Google Scholar]
- [9].Dickenson AH. A cure for wind up: NMDA receptor antagonists as potential analgesics. Trends Pharmacol Sci 1990;11:307–9. [DOI] [PubMed] [Google Scholar]
- [10].Doncheva ND, Vasileva L, Saracheva K, Dimitrova D, Getova D. Study of antinociceptive effect of ketamine in acute and neuropathic pain models in rats. Adv Clin Exp Med 2019;28:573–9. [DOI] [PubMed] [Google Scholar]
- [11].Fang X, Zhan G, Zhang J, Xu H, Zhu B, Hu Y, Yang C, Luo A. Chronic neuropathic pain-related anhedonia in a rat model of spared nerve injury. Clin Psychopharmacol Neurosci 2019;17:189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hama A, Sagen J. Combinations of intrathecal gamma-amino-butyrate receptor agonists and N-methyl-d-aspartate receptor antagonists in rats with neuropathic spinal cord injury pain. Eur J Pharmacol 2012;683:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hooijmans CR, de Vries RBM, Ritskes-Hoitinga M, Rovers MM, Leeflang MM, IntHout J, Wever KE, Hooft L, de Beer H, Kuijpers T, Macleod MR, Sena ES, ter Riet G, Morgan RL, Thayer KA, Rooney AA, Guyatt GH, Schünemann HJ, Langendam MW. GRADE working group. Facilitating healthcare decisions by assessing the certainty in the evidence from preclinical animal studies. PLoS ONE 2018;13:e0187271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hooijmans CR, IntHout J, Ritskes-Hoitinga M, Rovers MM. Meta-analyses of animal studies: an introduction of a valuable instrument to further improve healthcare. ILAR J 2014;55:418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. Med Res Method 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hooijmans CR, Tillema A, Leenaars M, Ritskes-Hoitinga M. Enhancing search efficacy by means of a search filter for finding all studies on animal experimentation in PubMed. Lab Anim 2010;44:170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hota D, Bansal V, Pattanaik S. Evaluation of ketamine, nimodipine, gabapentin and imipramine in partial sciatic nerve transection model of neuropathic pain in rat: an experimental study. Meth Find Exp Clin Pharmacol 2007;29:443–6. [DOI] [PubMed] [Google Scholar]
- [18].Huang C, Li HT, Shi YS, Han JS, Wan Y. Ketamine potentiates the effect of electroacupuncture on mechanical allodynia in a rat model of neuropathic pain. Neurosci Let 2004;368:327–31. [DOI] [PubMed] [Google Scholar]
- [19].Humo M, Ayazgök B, Becker LJ, Waltisperger E, Rantamäki T, Yalcin I. Ketamine induces rapid and sustained antidepressant-like effects in chronic pain induced depression: role of MAPK signaling pathways. Progr Neuropsychopharmacol Biol Psychiat 2020;100:109898. [DOI] [PubMed] [Google Scholar]
- [20].Jonkman K, Dahan A, van de Donk T, Aarts L, Niesters M, van Velzen M. Ketamine for pain. F1000Res 2017;6:1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kamp J, van Velzen M, Olofsen E, Boon M, Dahan A, Niesters M. Pharmacokinetic and pharmacodynamic considerations for NMDA-receptor antagonist ketamine in the treatment of chronic neuropathic pain: an update of the most recent literature. Exp Opin Drug Metab Toxicol 2019;15:1033–41. [DOI] [PubMed] [Google Scholar]
- [22].Kroin JS, Das V, Moric M, Buvanendran A. Efficacy of the ketamine metabolite (2R,6R)-hydroxynorketamine in mice models of pain. Reg Anesth Pain Med 2019;44:111–7. [DOI] [PubMed] [Google Scholar]
- [23].Kwon SY, Yeom JH, Joo JD. Ketamine reduces the induced spinal p38 MAPK and proinflammatory cytokines in a neuropathic rats. Kor J Anesthesiol 2014;66:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li Y, Shen R, Wen G, Ding R, Du A, Zhou J, Dong Z, Ren X, Yao H, Zhao R, Zhang G, Lu Y, Wu X. Effects of ketamine on levels of inflammatory cytokines IK-6, Il-1β, and TNF-α in the hippocampus of mice following acute or chronic administration. Front Pharmacol 2017;8:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lim HS, Kim JM, Choi JG, Ko YK, Shin YS, Jeon BH, Park JB, Lee JH, Kim HW. Intrathecal ketamine and pregabalin at sub-effective doses synergistically reduces neuropathic pain without motor dysfunction in mice. Biol Pharm Bull 2013;36:125–30. [DOI] [PubMed] [Google Scholar]
- [26].Mak P, Broadbear JH, Kolosov A, Goodchild CS. Long-term antihyperalgesic and opioid-sparing effects of 5-day ketamine and morphine infusion (“burst ketamine”) in diabetic neuropathic rats. Pain Med 2015;16:1781–93. [DOI] [PubMed] [Google Scholar]
- [27].Mao J, Price DD, Hayes RL, Lu J, Mayer DJ, Frenk H. Intrathecal treatment with dextrorphan or ketamine potently reduces pain-related behaviors in a rat model of peripheral mononeuropathy. Brain Res 1993;605:164–8. [DOI] [PubMed] [Google Scholar]
- [28].M'Dahoma S, Bourgoin S, Kayser V, Bartélémy S, Chevarin C, Chali F, Orsal D, Hamon M. Spinal cord transection-induced allodynia in rats—behavioral, physiopathological and pharmacological characterization. PLoS One 2014;9:e102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].M'Dahoma S, Barthélemy S, Tromilin C, Jeanson T, Viguier F, Michot B, Pezet S, Hamon M, Bourgoin S. Respective pharmacological features of neuropathic-like pain evoked by intrathecal BDNF versus sciatic nerve ligation in rats. Eur Neuropsychopharmacol 2015;25:2118–30. [DOI] [PubMed] [Google Scholar]
- [30].Mehta AK, Halder S, Khanna N, Tandom OP, Singh UR, Sharma KK. Role of NMDA and opioid receptors in neuropathic pain induced by chronic constriction injury of sciatic nerve in rats. J Basic Clin Physiol Pharmacol 2012;23:49–55. [DOI] [PubMed] [Google Scholar]
- [31].Mei X, Wang W, Wang W, Li Y, Zhang H, Wu S, Li Y, Xu L. Inhibiting astrocyte activation: a novel analgesic mechanism of ketamine at the spinal level? J Neurochem 2009;109:1691–700. [DOI] [PubMed] [Google Scholar]
- [32].Mei XP, Wang W, Wang W, Li Y, Zhang H, Wu S, Li Y, Xu L. Combining ketamine with astrocytic inhibitor as a potential analgesic strategy for neuropathic pain—ketamine, astrocytic inhibitor and pain. Mol Pain 2010;6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mei XP, Zhang H, Wang W, Wei YY, Zhai MZ, Wang W, Xu LX, Li YQ. Inhibition of spinal astrocytic c-Jun N-terminal kinase (JNK) activation correlates with the analgesic effects of ketamine in neuropathic pain. J Neuroinflamm 2011;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mei XP, Zhou Y, Wang W, Tang J, Wang W, Zhang H, Xu LX, Li YQ. Ketamine depresses Toll-like receptor 3 signaling in spinal microglia in a rat model of neuropathic pain. Neurosignals 2011;19:44–53. [DOI] [PubMed] [Google Scholar]
- [35].Michelet D, Brasher C, Horlin AL, Bellon M, Julien-Marsollier F, Vacher T, Pontone S, Dahmani S. Ketamine chronic non-cancer pain: a meta-analysis and trial sequential analysis of randomized controlled trials. Eur J Pain 2018;22:632–46. [DOI] [PubMed] [Google Scholar]
- [36].Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci 2020;21:353–65. [DOI] [PubMed] [Google Scholar]
- [37].Niesters M, Khalili-Mahani N, Martini C, Aarts L, van Gerven J, van Buchem MA, Dahan A, Rombouts S. Effect of sub-anesthetic ketamine on intrinsic functional brain connectivity: a placebo-controlled functional magnetic resonance imaging study in healthy volunteers. Anesthesiology 2012;117:868–77. [DOI] [PubMed] [Google Scholar]
- [38].Noppers I, Niesters M, Aarts L, Smith T, Sarton E, Dahan A. Ketamine for treatment of chronic non-cancer pain (Review). Expert Opin Pharmacother 2010;11:2417–29. [DOI] [PubMed] [Google Scholar]
- [39].Orser B, Pennefather PS, MacDonald JF. Multiple mechanisms of ketamine blockade of N-methyl-D-aspartate receptors. Anesthesiology 1997;86:903–17. [DOI] [PubMed] [Google Scholar]
- [40].Pan W, Zhang GF, Li HH, Li MH, Zhou ZQ, Li KY, Yang JJ. Ketamine differentially restores diverse alterations of neuroligins in brain regions in a rat model of neuropathic pain-induced depression. NeuroRep 2018;29:863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-d-aspartate (NMDA) receptors in pain: a review. Anesth Analg 2003;97:1108–16. [DOI] [PubMed] [Google Scholar]
- [42].Pickering G, Pereira B, Morel VC, Corriger A, Giron F, Mercaillou F, Bidar-Beauvallot A, Chandeze E, Lambert C, Bernard L, Delage N. Ketamine and magnesium for refractory neuropathic pain: a randomized, double-blind, crossover trial. Anesthesiology 2020;133:154–64. [DOI] [PubMed] [Google Scholar]
- [43].Qian J, Brown SD, Carlton SM. Systemic ketamine attenuates nociceptive behaviors in a rat model of peripheral neuropathy. Brain Res 1996;715:51–62. [DOI] [PubMed] [Google Scholar]
- [44].Rice ASC, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I; Preclinical Pain Consortium, Mogil JS, Stöhr T. Animal models and the prediction of efficacy in clinical trials of analgesic drugs; a critical appraisal and call for uniform reporting standards. PAIN 2009;139:243–7. [DOI] [PubMed] [Google Scholar]
- [45].Rodrigues-Filho R, Campos MM, Ferreira J, Santos ARS, Bertelli JA, Calixto JB. Pharmacological characterization of the rat brachial plexus avulsion model of neuropathic pain. Brain Res 2004;1018:159–70. [DOI] [PubMed] [Google Scholar]
- [46].Salvat E, Yalcin I, Muller A, Barrot M. A comparison of early and late treatments on allodynia and its chronification in experimental neuropathic pain. Mol Pain 2018;14:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sasaki A, Serizawa K, Andoh T, Shiraki K, Takahata H, Kuraishi Y. Pharmacological differences between static and dynamic allodynia in mice with herpetic or postherpetic pain. J Pharmacol Sci 2008;108:266–73. [DOI] [PubMed] [Google Scholar]
- [48].Sigtermans M, Noppers I, Sarton E, Bauer M, Mooren R, Olofsen E, Dahan A. An observational study on the effect of S(+)-ketamine on chronic pain versus experimental acute pain in Complex Regional Pain Syndrome type 1 patients. Eur J Pain 2010;14:302–7. [DOI] [PubMed] [Google Scholar]
- [49].Sleigh J, Harvey M, Voss L, Denny B. Ketamine—more mechanisms than just NMDA blockade. Trends Anaesth Crit Care 2014;4:76–81. [Google Scholar]
- [50].Swartjes M, Morariu A, Niesters M, Aarts L, Dahan A. Non-selective and NR2B-selective NMDA receptor antagonists produce antinociception and long-term relief of allodynia in acute and neuropathic pain. Anesthesiology 2011;115:165–74. [DOI] [PubMed] [Google Scholar]
- [51].Swartjes M, Niesters M, Heij L, Dunne A, Aarts L, Cerami Hand C, Kim HS, Brines M, Cerami A, Dahan A. Ketamine does not produce relief of neuropathic pain in mice lacking the β-common receptor (CD131). PLoS One 2013;8:e71326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Truin M, Janssen SPM, van Kleef M, Joosten EAJ. Successful pain relief in non-responders to spinal cord stimulation: the combined use of ketamine and spinal cord stimulation. Eur J Pain 2011;15:1049.e1–9. [DOI] [PubMed] [Google Scholar]
- [53].Wang J, Goffer Y, Xu D, Tukey DS, Shamir DB, Eberle SE, Zou AH, Blanck TJJ, Ziff EB. A single subanesthetic dose of ketamine relieves depression-like behaviors induced by neuropathic pain in rats. Anesthesiology 2011;115:812–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zwetsloot PP, van der Naald M, Sena ES, Howells DW, IntHout J, de Groot JAH, Chamuleau SAJ, MacLeod MR, Wever KE. Standardized mean differences cause funnel plot distortion in publication bias assessments. eLife 2017;6:24260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B302.