Palmitoylethanolamide is involved in the ictal phase of episodic migraine, probably representing an analgesic and anti-inflammatory response. The endocannabinoid system is not involved in the modulation of nitroglycerin-induced central sensitization.
Keywords: Headache, Pain, Endocannabinoid system, Central sensitization, Nitroglycerin, Human migraine models, Analgesic response
Abstract
Migraine pathophysiology has been suggested to include dysregulation of the endocannabinoid system (ES). We simultaneously evaluated plasma anandamide (AEA) and palmitoylethanolamide (PEA) levels and spinal sensitization in a validated human model of migraine based on systemic nitroglycerin (NTG) administration. Twenty-four subjects with episodic migraine (MIG) and 19 healthy controls (HC) underwent blood sampling and investigation of nociceptive withdrawal reflex thresholds (RTh: single-stimulus threshold; TST: temporal summation threshold) before and 30 (T30), 60 (T60), and 120 (T120) minutes after sublingual NTG administration (0.9 mg). At baseline, the MIG and HC groups were comparable for plasma AEA (P = 0.822) and PEA (P = 0.182) levels, and for RTh (P = 0.142) and TST values (P = 0.150). Anandamide levels increased after NTG administration (P = 0.022) in both groups, without differences between them (P = 0.779). By contrast, after NTG administration, PEA levels increased in the MIG group at T120 (P = 0.004), while remaining stable in the HC group. Nitroglycerin administration induced central sensitization in the MIG group, which was recorded as reductions in RTh (P = 0.046) at T30 and T120, and in TST (P = 0.001) at all time points. In the HC group, we observed increases in RTh (P = 0.001) and TST (P = 0.008), which suggest the occurrence of habituation. We found no significant correlations between the ES and neurophysiological parameters. Our findings suggest a role for PEA in the ictal phase of episodic migraine. The ES does not seem to be directly involved in the modulation of NTG-induced central sensitization, which suggests that the observed PEA increase and spinal sensitization are parallel, probably unrelated, phenomena.
1. Introduction
The endocannabinoid system (ES) includes several lipid mediators (so-called endocannabinoids), of which the best characterized are anandamide (AEA) and 2-arachidonoylglycerol (2-AG). Cannabinoid receptors 1 (CB1) and 2 (CB2) are the most extensively studied molecular targets of endocannabinoids, although the ES also includes lipid mediators, such as palmitoylethanolamide (PEA), which have other molecular targets.18 The ES has a physiological role in endogenous pain control mechanisms,12 and its dysregulation has been associated with migraine pathophysiology in preclinical and clinical studies.23 Both AEA and PEA are deactivated by fatty acid amide hydrolase (FAAH). In the nitroglycerin (NTG)-based animal migraine model, inhibition of FAAH activity, which leads to enhanced signaling mediated by AEA and PEA in peripheral and central nervous structures, reduces nocifensive behavior and neuronal activation in the trigeminal nucleus caudalis.25
Sarchielli et al.40 reported lower AEA and higher PEA levels in the cerebrospinal fluid of subjects with chronic migraine, supporting a central alteration of ES activity in this condition. Possible involvement of acute medication overuse in ES dysfunction was suggested by Perrotta et al.33, who reported a reduction in FAAH activity after overuse interruption; this reduction was associated with normalization of pain facilitation.
Literature data on the role of the ES in episodic migraine are very scarce. Cupini et al.13 reported increased FAAH and endocannabinoid membrane transporter activities in platelets of women with episodic migraine. In a PET study, subjects with episodic migraine showed interictal increased CB1 binding to specific brain areas implicated in nociceptive processing.44 By contrast, Gouveia-Figueira et al.20 failed to identify significant peripheral changes in levels of AEA and other fatty acid ethanolamides between subjects with episodic migraine and controls during the interictal phase.
Considering their physiological analgesic and anti-inflammatory functions, PEA and AEA may have positive effects on migraine attack recurrence and on sensitization processes.29,30,35 Central sensitization is a hallmark of migraine, and it can be recorded at spinal level through evaluation of the nociceptive withdrawal reflex (NWR).10,38 Nitroglycerin-induced migraine is also a validated human experimental model of the disease5,16 that mimics several of its components besides headache: premonitory symptoms, allodynia, activation of specific brainstem circuitry, and central sensitization.2,3,15,31 We previously observed spinal sensitization in migraine subjects exposed to NTG: the phenomenon was captured as a reduction of the temporal summation threshold of the NWR after NTG administration, and it was especially pronounced in subjects with episodic migraine and a positive migraine-induction test.34 The NWR also proved sensitive enough to differentiate between subjects with low- vs high-frequency episodic migraine, showing more severe derangement of nociceptive control in more severe migraine phenotypes.15
In this study, we aimed to investigate the role of AEA and PEA during the ictal phase of migraine in subjects with episodic migraine, who were administered NTG to experimentally induce a migraine attack under controlled conditions. We also examined the relationship between AEA and PEA plasma levels and NTG-induced spinal sensitization, as measured by the NWR.
2. Materials and methods
2.1. Subjects
The study sample comprised 26 subjects suffering from moderate-to high-frequency episodic migraine consecutively recruited among those attending the Headache Science Center at the IRCCS Mondino Foundation in Pavia (Italy).
The inclusion criteria were males or females aged 18 to 70 years; migraine without aura diagnosed according to the ICHD-3 criteria at least 1 year before enrollment27; and between 6 and 14 migraine days per month.
The exclusion criteria were a history of major psychiatric or other neurological conditions; a history of chronic migraine; a diagnosis of tension-type headache with a frequency > 2 headache days per month; interictal allodynia or hyperalgesia; Beck's Depression Inventory score > 17; clinically significant medical conditions; chronic pain conditions; alcohol and/or drug abuse; pregnancy or lactation; previous exposure to NTG administration, and contraindications to NTG administration (systolic blood pressure < 90 mmHg, hypovolemia, anemia, angle-closure glaucoma, known allergy or intolerance to NTG, and concomitant therapy with sildenafil).
Preventive therapy with one of the following drug classes was allowed, providing the dose and regimen had been stable for at least 2 months before enrollment: β-blockers, calcium channel blockers, and angiotensin II receptor antagonists. Other preventive drug classes were excluded because they could affect the pain threshold.38
All subjects signed a written informed consent document before enrollment. All underwent a full medical examination and an ECG on the day of recruitment.
The subjects were informed that NTG might induce headache or migraine in some individuals, but no information was given about its possible timing of onset or characteristics.
Twenty-five healthy subjects with no personal or family (first-degree relatives) history of neurological disorders were enrolled as a control group.
Two subjects with migraine and 3 healthy controls dropped out due to symptomatic hypotension after NTG administration. Three healthy subjects withdrew their consent before NTG administration (Fig. 1).
Figure 1.
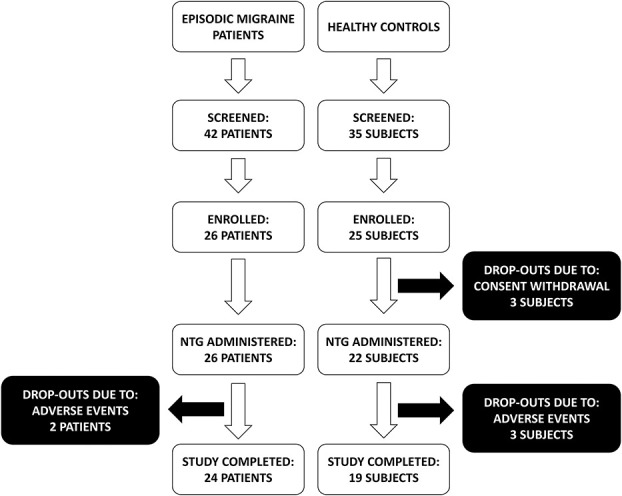
Flow diagram showing the phases of the study. NTG, nitroglycerin 0.9 mg sublingual.
The final data set thus refers to 24 subjects with migraine (MIG group—22 females, 33.0 ± 8.1 year old) and 19 healthy controls (HC group—15 females, 29.5 ± 9.3 year old).
The clinical and demographic features of the HC and MIG groups are shown in Table 1.
Table 1.
Clinical and demographic features of the study groups.
| MIG group | HC group | P | |
|---|---|---|---|
| N | 24 | 19 | — |
| Age (y) | 33.0 ± 8.1 | 29.5±9.3 | F1,41 1.773; P = 0.190 |
| Female sex | 22 (88.0%) | 15 (78.9%) | χ2 df 1 0.416; P = 0.416 |
| Preventive therapy | 15 (65.2%) | — | |
| Migraine duration (y) | 17.4 ± 9.7 | — | |
| Migraine days per month | 8.8 ± 2.0 | — | |
| Days with intake of acute drugs per month | 7.7 ± 1.9 | — |
MIG group: subjects with episodic migraine; HC group: healthy controls.
HC, healthy controls; MIG, migraine.
2.2. Experimental design
In this double-blind, parallel-group controlled study, subjects with migraine and healthy controls were administered NTG sublingually (0.9 mg—Acarpia Farmaceutici SRL, Italy) for the purpose of measuring changes in plasma levels of AEA and PEA and changes in nociceptive processing during an experimentally provoked migraine attack.
All participants arrived nonfasting at the clinic between 08:00 am and 08:30 am. All had been instructed to avoid coffee and tea on the morning of the study procedures. A neurologist from the Headache Science Center performed a full neurological examination, checked the inclusion and exclusion criteria, and, in the subjects with migraine, evaluated migraine frequency (by consulting their headache diaries) and verified that they were in an interictal phase (defined as no ongoing headache and no headache or acute medication intake in the previous 24 hours).
The subjects were then taken to a different room, where they were placed in the supine position. A venous catheter was inserted into the left or right antecubital vein for blood sampling, and they were allowed to rest for at least 30 minutes. Baseline blood pressure and heart rate measurements (GE Healthcare) were then taken, blood samples were collected, and the neurophysiological procedure was conducted. The operators performing the blood pressure monitoring, blood sampling, and neurophysiological evaluation were blind to the subject's clinical condition.
After collection of the baseline parameters, the subjects received NTG 0.9 mg. The monitoring of vital signs, blood sampling, and neurophysiological investigation were all repeated at 30 (T30), 60 (T60), and 120 (T120) minutes after NTG administration. The presence/absence of headache was ascertained every 10 minutes. If headache occurred, its features (location, pain intensity and type, and possible relationship to coughing and movement) and any associated symptoms (nausea, photophobia, and phonophobia) were recorded by an expert neurologist. The subjects were kept at the Center for an additional hour after the end of the experimental procedures, i.e., until 180 minutes post-NTG administration.
The NTG-based migraine induction test was recorded as “positive” (MIG+ group) in all subjects who developed a migraine-like attack that met the criteria for experimentally induced migraine headache in the 12-hour period after NTG administration.5 In fact, these criteria are a headache with onset 0 to 12 hours after NTG intake that fulfils ICHD-3 “C” and “D” criteria for migraine without aura, or that mimics usual migraine attacks and responds to a triptan. If a subject did not develop a headache meeting these criteria in the 12-hour period after NTG administration, the migraine-induction test was recorded as “negative” (MIG− group).
In the MIG+ subjects whose migraine-like attack occurred within the 180-minute observation period, blood sampling and neurophysiological evaluation were repeated at a further 2 time points: at onset of the migraine-like headache (T-MIG) and 1 hour later (T-MIG-1h). After T-MIG-1h, these MIG+ subjects were administered acute medications (nonsteroidal anti-inflammatory drugs, triptans, and/or antiemetics) according to their needs and preferences.
Upon discharge from the Center, all subjects were given a headache diary in which they were asked to record details of headache, associated features, and adverse events, if any, occurring during the 24 hours after the study procedures.
The study had local ethics committee approval (14/int/2016) and was conducted in accordance with the 1964 Declaration of Helsinki, as revised in 2008. This study was not preregistered; all the study procedures and analyses were performed as described in the protocol submitted to the ethics committee. The enrollment phase lasted from July 2016 to December 2019, and the study itself was conducted between April 2016 and January 2020.
2.3. Lipid extraction from human plasma
Lipids were extracted using a modified Bligh and Dyer method, described elsewhere.6 Briefly, 500 µL human plasma was added to 1 mL of methanol containing internal standards ([2H4]-AEA, [2H4]-PEA); lipids were extracted with CHCl3 (2 mL) and centrifuged for 15 minutes at 3000×rpm at 4°C. At the end of the process, the organic phase was transferred to glass vials. To increase the overall recovery, the aqueous fraction was extracted again with chloroform (1 mL) and 500 µL phosphate buffered saline. The 2 resulting organic phases were pooled, dried under a stream of N2, and the residues were dissolved in 2 mL chloroform.
These organic extracts were fractionated by Silica Gel G column chromatography (60-Å 230-400 mesh; Sigma-Aldrich, Milan, Italy). Anandamide and PEA were eluted from the silica column with 2 mL chloroform/methanol (9:1, vol/vol) and then with 2 mL chloroform/methanol (8:2, vol/vol). Eluates were evaporated under N2, and lipids were reconstituted in methanol/chloroform (75 μL; 9:1, vol/vol) and transferred into glass vials for liquid chromatography/mass spectrometry analyses.
As analytes are present endogenously in plasma, 2% bovine serum albumin in saline solution was used as surrogate matrix. Calibration standards were prepared by spiking the analytes in the surrogate matrix. Eight-point calibration curves (1-1000 nM) were prepared by serial dilution with saline solution containing 2% bovine serum albumin. Quality control (QC) samples were prepared at 2 different levels, ie, low QC (1 nM) and high QC (10 nM), using the same procedure described for the standard curve.
2.4. Nociceptive withdrawal reflex measurements
The NWR was recorded from the right lower limb according to a standardized procedure.15 It was recorded in the morning between 09:00 am and 11:00 am by the same blinded technician (V.G.). Subjects were tested in supine position with their ankle flexed to 90° and knee flexed to 130°. The stimulus was delivered to the sural nerve behind the lateral malleolus via a pair of Ag/AgCl surface electrodes. The electrical stimulation consisted of 5 squared consecutive pulses (1 ms, 200 Hz), randomly delivered every 30 to 60 seconds. Muscle electromyography (Synergy, Medelec, United Kingdom) was performed, obtaining recordings from the capitis brevis of the homolateral biceps femoris using a pair of Ag/AgCl surface electrodes. The thresholds were evaluated according to a staircase method (with stimulus intensity increasing in steps of 0.3 mA). The recording parameters were sweep duration 300 ms, sensitivity 20 mV, and filters from 3 to 3000 Hz. We recorded:
(1) Single-stimulus reflex threshold of the NWR (RTh): the lowest intensity (mA) able to elicit, consecutively, 3 muscle responses of at least 20 mV and 10 ms. At baseline, the evaluation was completed by recording the area under the curve (mV*ms) and the latency of the 3 muscle responses. At every time point, we recorded subjective pain perception rated on a 0- to 10-point visual analogue scale (VAS-RTh).
(2) Temporal summation threshold of the NWR (TST): a 2-Hz train of 5 electrical stimuli as described above was used for TST evaluation. Temporal summation threshold was defined as the lowest intensity (mA) able to elicit, consecutively, 3 muscle responses of at least 20 mV and 10 ms in the fourth and fifth sweeps. At every time point, we recorded the subjective pain perception of the first stimulus (VAS-TST-1) and fifth stimulus (VAS-TST-5) as rated on a 0- to 10-point visual analogue scale.
2.5. Statistical analysis
The sample size was calculated using Open Source Epidemiologic Statistics for Public Health software (www.openepi.com). The primary outcome of the study was to assess differences in the percentage changes in AEA levels between the MIG and HC groups 120 minutes after NTG administration. According to previous clinical data, we considered a 20% between-group difference in mean values (SD of 10%) to be meaningful.13 Moreover, because we preplanned 2 analyses (MIG vs HC, and MIG+ vs MIG−), we corrected the level of significance, according to the Bonferroni method, to P = 0.025. Therefore, the following parameters were used to calculate the sample size: confidence interval (2-sided): 97.5%; power: 80%; and ratio of sample size: 1. The calculation yielded a minimum suggested sample size of 42 participants.
The Statistical Package for the Social Sciences (SPSS) version 21.0 was used for the analysis. Skewness and kurtosis evaluation, and the z-test for skewness and kurtosis, all showed that the absolute values at baseline and the percentage variations at all time points were normally distributed.
Quantitative variables are presented as “mean ± SD”, whereas categorical variables are presented as “number (percentage)”. Between-groups comparisons were performed by a t test for independent samples, whereas categorical variables were compared using a χ2 test.
To analyze endocannabinoid levels and neurophysiological data, we performed a 2-factor analysis of variance (ANOVA) for repeated measures in which the factors were “GROUP” with 2 levels (MIG vs HC) and “TIME” with 4 levels (baseline, T30, T60, and T120). We also compared the MIG+ and MIG− subgroups using a 2-factor ANOVA for repeated measures, designed as follows: factor “HEADACHE” with 2 levels (MIG+ vs MIG−); and factor “TIME” with 4 levels (baseline, T30, T60, and T120). Post hoc analyses were performed only in the event of significant between-factor interactions.
In MIG+ subjects showing a positive migraine-induction test within the 180-minute observation period, we completed the analysis with a one-way ANOVA for repeated measures test to compare the additional time points, T-MIG and T-MIG-1h, with baseline. For all the presented analyses, P-values were corrected according to Bonferroni's method to account for the number of comparisons.
Finally, a correlation analysis between endocannabinoid levels and neurophysiological variables was performed using a Pearson Rho test.
The level of significance was set at α = 0.05.
3. Results
3.1. Clinical response to nitroglycerin administration
As expected from previous studies,9,37,41 NTG administration was found to induce 2 types of headache: a nonspecific headache, devoid of migraine features and characterized by early onset, mild intensity, and short duration, and a specific headache, i.e., a migraine-like headache meeting established criteria for experimentally induced migraine (Ashina et al., 2017).
The percentage of subjects developing a nonspecific headache was similar in the HC and MIG groups (HC 52.6%, MIG 58.3%; χ2 df1 0.140; P = 0.764) (Table 2). None of the HC group subjects developed a specific headache, whereas 16 of the 24 MIG group subjects (66.7%) did (MIG+ group).
Table 2.
Clinical response to nitroglycerin administration.
| MIG group | HC group | P | |
|---|---|---|---|
| Nonspecific headache | |||
| N | 14 (58.3%) | 10 (52.6%) | χ2df 1 0.140; P = 0.764 |
| Latency (min) | 15.4 ± 7.2 | 16.5 ± 7.8 | F1,22 0.137; P = 0.715 |
| Intensity | 2.1 ± 0.7 | 2.0 ± 0.9 | F1,22 0.167; P = 0.687 |
| Specific, migraine-like headache | |||
| N | 16 (66.7%) | 0 (0%) | — |
| Latency (min) | 97.8 ± 102.6 | — | — |
| Intensity | 6.8 ± 1.5 | — | — |
| Specific, migraine-like headache showing onset within 180-minute observation period | |||
| N | 13 (52.2%) | 0 (0%) | — |
| Latency (min) | 56.9 ± 49.7 | — | — |
| Intensity | 6.5 ± 1.6 | — | — |
MIG group: subjects with episodic migraine (n = 24); HC group: healthy controls (n = 19). Intensity was rated on a numerical rating scale graded from 0 (no pain) to 10 (excruciating pain).
HC, healthy controls; MIG, migraine; NTG, nitroglycerin.
In 13 of the 16 MIG+ subjects, the onset of the specific (migraine-like) headache occurred within the 180-minute in-hospital observation period, and the members of this subgroup were therefore also evaluated at 2 additional time points, T-MIG and T-MIG-1h (Table 2). The remaining 3 MIG+ subjects developed their migraine-like attack after hospital discharge, namely between 180 minutes and 12 hours from NTG administration. Eight of the MIG group subjects did not develop a migraine-like attack in the 12-hour period after NTG administration, and they formed the MIG− group.
No significant differences in clinical and demographic features were found between the MIG+ (92.3% females; 33.3 ± 6.2 years of age; migraine duration: 17.2 ± 5.8 years; 9.1 ± 1.7 monthly migraine days; 7.8 ± 1.9 days with acute drug intake per month, prevention therapy in 41.7%) and MIG− subjects (81.8% females; 32.7 ± 10.3 years of age; migraine duration: 17.4 ± 12.9 years; 8.5 ± 2.3 monthly migraine days; 7.5 ± 1.9 days with acute drug intake per month, preventive therapy in 27.3%) (P > 0.05 for all comparisons).
3.2. Baseline plasma levels of anandamide and palmitoylethanolamide
Baseline AEA and PEA levels were comparable between the MIG and HC groups (P = 0.822 for AEA, and P = 0.182 for PEA) (Table 3). The MIG+ and MIG− groups were also comparable for both AEA (P = 0.609) and PEA (P = 0.914) levels at baseline.
Table 3.
Baseline levels of AEA and PEA in the study groups.
| MIG group | HC group | P | |
|---|---|---|---|
| AEA (nmol/L) | 0.29 ± 0.16 | 0.30 ± 0.17 | F1,41 0.051; P = 0.822 |
| PEA (nmol/L) | 2.01 ± 1.47 | 2.74 ± 2.02 | F1,41 1.841; P = 0.182 |
MIG group: subjects with episodic migraine (n = 24); HC group: healthy controls (n = 19).
AEA, anandamide; HC, healthy controls; MIG, migraine; PEA, palmitoylethanolamide.
3.3. Baseline neurophysiological and psychophysical evaluation
At baseline, no significant differences in RTh (P = 0.142), latency of RTh (P = 0.587), area under the curve at RTh (P = 0.690), and TST (P = 0.150) were found between the MIG and HC groups. Psychophysical evaluations showed higher levels of subjective pain perception in the MIG than in the HC group subjects, as rated using VAS-RTh (P = 0.010), VAS-TST-1 (P = 0.001), and VAS-TST-5 (P = 0.001) (Table 4).
Table 4.
Baseline neurophysiological (nociceptive withdrawal reflex) parameters and psychophysical variables in the 2 study groups.
| MIG group | HC group | P | |
|---|---|---|---|
| RTh (mA) | 16.5 ± 6.3 | 13.7 ± 6.1 | F1,41 2.238; P = 0.142 |
| Latency (ms) | 107.7 ± 14.8 | 105.2 ± 15.2 | F1,41 0.299; P = 0.587 |
| Area (mV × ms) | 2255.1 ± 1066.6 | 2114.7 ± 1220.7 | F1,41 0.162; P = 0.690 |
| VAS-RTh | 6.2 ± 2.3 | 4.4 ± 1.7 | F 1,41 7.321; P = 0.010 |
| TST (mA) | 11.5 ± 5.4 | 9.3 ± 4.1 | F1,41 2.157; P = 0.150 |
| VAS-TST-1 | 5.4 ± 2.1 | 3.3 ± 1.3 | F 1,41 14.132; P = 0.001 |
| VAS-TST-5 | 5.9 ± 2.5 | 3.6 ± 1.4 | F 1,41 12.881; P = 0.001 |
MIG group: subjects with episodic migraine (n = 24); HC group: healthy controls (n = 19). RTh: single stimulus reflex threshold of the nociceptive withdrawal reflex (NWR); Area: area under the curve (mV*ms) of the NWR at RTh; VAS-RTh: pain perception as rated on a 0- to 10-point visual analogue scale at RTh; TST: temporal summation threshold of the NWR; VAS-TST-1 and VAS-TST-5: subjective pain perception of the first and fifth stimulus of the TST according to a 0- to 10-point visual analogue scale. In bold: statistically significant comparisons.
HC, healthy controls; MIG, migraine; RTh, reflex threshold; TST, temporal summation threshold; VAS, visual analogue scale.
No differences in any of the neurophysiological and psychophysical variables were observed between the MIG+ and MIG− subgroups.
3.4. Time course of plasma anandamide and palmitoylethanolamide levels after nitroglycerin administration in the migraine and healthy controls groups
Anandamide levels increased in all the subjects after NTG administration (TIME: P = 0.022), without differences emerging between the MIG and HC groups (GROUP: P = 0.779, and TIME x GROUP: P = 0.671). Compared with the baseline value, AEA was significantly increased at T30 (P = 0.019), T60 (P = 0.026), and T120 (P = 0.017) in both groups (Fig. 2).
Figure 2.
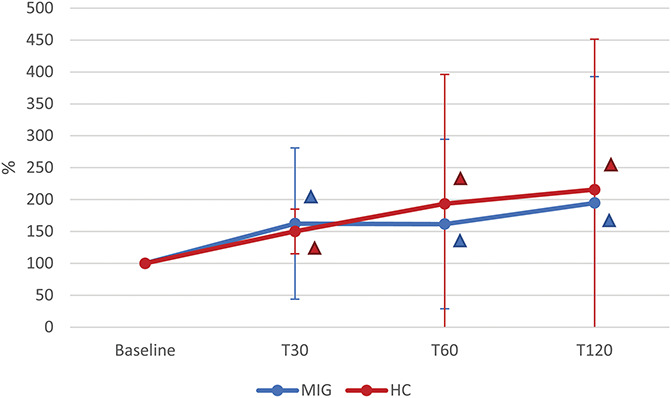
Time course of plasma AEA levels after NTG administration in MIG and HC groups. T30, T60, and T120: evaluations at 30, 60, and 120 minutes after nitroglycerin (NTG) administration (0.9 mg sublingual). MIG: subjects with episodic migraine (n = 24); HC: healthy controls (n = 19). Statistical analysis: 2-factor ANOVA for repeated measures (factor “GROUP” with 2 levels: MIG vs HC; factor “TIME” with 4 levels: Baseline, T30, T60, T120). TIME: P = 0.022; GROUP: P = 0.779; TIME × GROUP: P = 0.671. Δ = post hoc analysis: P < 0.050 vs Baseline (the color identifies the group). AEA, anandamide; ANOVA, analysis of variance.
Analysis of PEA levels revealed a significant TIME x GROUP interaction (P = 0.028). The post hoc analysis showed a significant increase in PEA in the MIG group (TIME: P = 0.007) at T120 (P = 0.004) when compared with baseline. Instead, plasma PEA levels in the HC group remained stable (P = 0.536) (Fig. 3).
Figure 3.
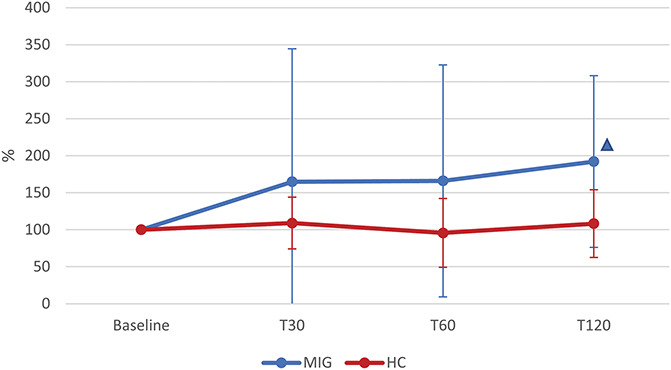
Time course of plasma PEA levels after NTG administration in MIG and HC groups. T30, T60, and T120: evaluations at 30, 60, and 120 minutes after nitroglycerin (NTG) administration (0.9 mg sublingual). MIG: subjects with episodic migraine (n = 24); HC: healthy controls (n = 19). Statistical analysis: 2-factor ANOVA for repeated measures (factor “GROUP” with 2 levels: MIG vs HC; factor “TIME” with 4 levels: Baseline, T30, T60, T120). TIME: P = 0.009; GROUP: P = 0.035; TIME × GROUP: P = 0.028. Δ = post hoc analysis: P < 0.050 vs Baseline (the color identifies the group). ANOVA, analysis of variance; PEA, palmitoylethanolamide.
3.5. Time course of plasma anandamide and palmitoylethanolamide after nitroglycerin administration in the MIG+ and MIG− groups
Comparison of the MIG+ group with the MIG− group showed no significant differences in plasma AEA and PEA levels (Figs. 4A and B).
Figure 4.
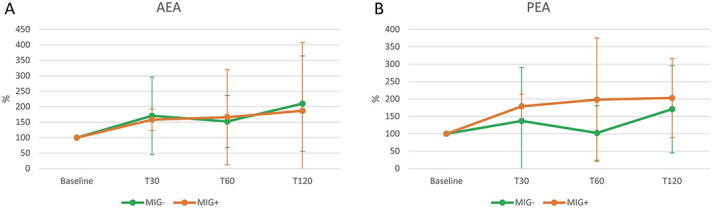
Time course of plasma AEA and PEA levels after NTG administration in MIG+ and MIG− subjects. T30, T60, and T120: evaluations at 30, 60, and 120 minutes after nitroglycerin (NTG) administration (0.9 mg sublingual). MIG+: subjects with episodic migraine and a positive migraine-induction test (n = 16). MIG−: subjects with episodic migraine and a negative migraine-induction test (n = 8); Statistical analysis: 2-factor ANOVA for repeated measures (factor “HEADACHE” with 2 levels: MIG+ vs MIG−; factor “TIME” with 4 levels: Baseline, T30, T60, T120): Plasma AEA levels (A): TIME: P = 0.169; HEADACHE: P = 0.909; TIME × HEADACHE: P = 0.872. Plasma PEA levels (B): TIME: P = 0.021; HEADACHE: P = 0.341; TIME × HEADACHE: P = 0.513. AEA, anandamide; ANOVA, analysis of variance; PEA, palmitoylethanolamide.
3.6. Neurophysiological changes induced by nitroglycerin administration in the migraine and healthy controls groups
Analysis of the RTh data revealed a significant TIME x GROUP interaction (P = 0.001): post hoc analysis showed significantly reduced RTh values in the MIG group (P = 0.046), specifically at T30 (P = 0.004) and at T120 (P = 0.015), when compared with baseline. By contrast, in the HC group, RTh increased significantly (P = 0.001) at all time points after NTG administration (T30, P = 0.025; T60, P = 0.001; T120, P = 0.001) (Fig. 5).
Figure 5.
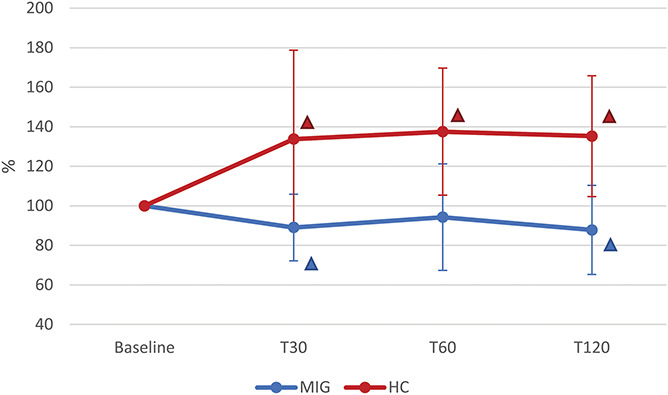
Changes in RTh induced by NTG administration in MIG and HC groups. T30, T60, and T120: evaluations at 30, 60, and 120 minutes after NTG administration (0.9 mg sublingual). MIG: subjects with episodic migraine (n = 24); HC: healthy controls (n = 19). Statistical analysis: 2-factor ANOVA for repeated measures (factor “GROUP” with 2 levels: MIG vs HC; factor “TIME” with 4 levels: Baseline, T30, T60, T120). TIME: P = 0.009; GROUP: P = 0.001; TIME × GROUP: P = 0.001. Δ = post hoc analysis: P < 0.050 vs Baseline (the color identifies the group). ANOVA, analysis of variance; NTG, nitroglycerin; RTh, reflex threshold.
A significant TIME × GROUP interaction (P = 0.001) was also found for TST: post hoc analysis showed significant reductions of this parameter in the MIG group (P = 0.001) at all time points (T30, P = 0.001; T60, P = 0.006; T120, P = 0.001) compared with the baseline value. By contrast, in the HC group, it was significantly increased (P = 0.008) at all time points (T30, P = 0.002; T60, P = 0.001; T120, P = 0.002) vs baseline (Fig. 6).
Figure 6.
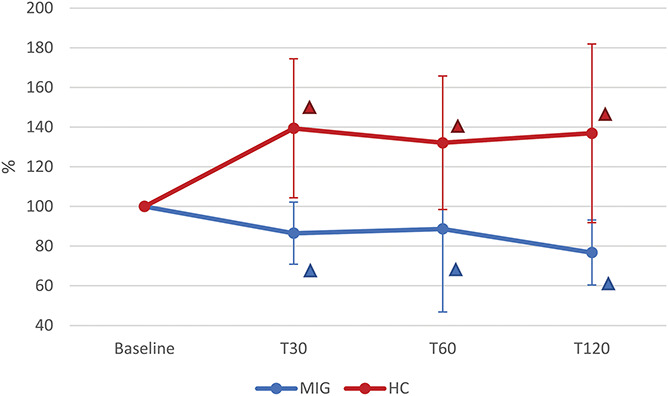
Changes in TST induced by NTG administration in MIG and HC groups. T30, T60, and T120: evaluations at 30, 60, and 120 minutes after NTG administration (0.9 mg sublingual). MIG: subjects with episodic migraine (n = 24); HC: healthy controls (n = 19). Statistical analysis: 2-factor ANOVA for repeated measures (factor “GROUP” with 2 levels: MIG vs HC; factor “TIME” with 4 levels: Baseline, T30, T60, T120). TIME: P = 0.125; GROUP: P = 0.001; TIME × GROUP: P = 0.001. Δ = post hoc analysis: P < 0.050 vs Baseline (the color identifies the group). ANOVA, analysis of variance; NTG, nitroglycerin; TST, temporal summation threshold.
3.7. Neurophysiological and psychophysical changes induced by nitroglycerin administration in the MIG+ and MIG− groups
Post-NTG changes in RTh did not differ significantly between the MIG+ and MIG− subjects (factor HEADACHE: P = 0.568, and TIME x HEADACHE interaction: P = 0.528) (Fig. 7A). Similarly, TST changes after NTG administration did not differ between the MIG+ and MIG− subjects (factor HEADACHE: P = 0.549, and TIME x HEADACHE interaction: P = 0.858) (Fig. 7B).
Figure 7.
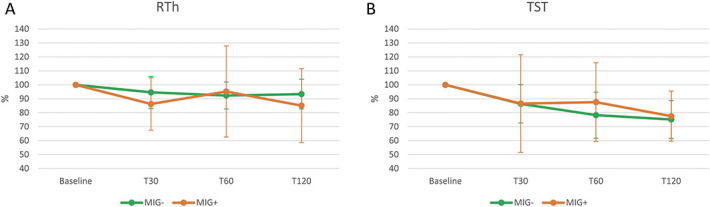
Changes in RTh and TST after NTG administration in MIG+ and MIG− subjects. T30, T60, and T120: evaluations at 30, 60, and 120 minutes after NTG administration (0.9 mg sublingual). MIG+: subjects with episodic migraine and a positive migraine-induction test (n = 16). MIG−: subjects with episodic migraine and a negative migraine-induction test (n = 8); Statistical analysis: 2-factor ANOVA for repeated measures (factor “HEADACHE” with 2 levels: MIG+ vs MIG−; factor “TIME” with 4 levels: Baseline, T30, T60, T120): RTh (A): TIME: P = 0.128; HEADACHE: P = 0.568; TIME × HEADACHE: P = 0.528. TST (B): TIME: P = 0.001; HEADACHE: P = 0.549; TIME × HEADACHE: P = 0.858. ANOVA, analysis of variance; NTG, nitroglycerin; RTh, reflex threshold; TST, temporal summation threshold.
3.8. Changes in plasma anandamide and palmitoylethanolamide levels and in neurophysiological variables at T-MIG and T-MIG-1h
As described in the methods sections, 13 of the 16 MIG+ subjects developed an NTG-induced migraine-like headache during the 180-minute observation period. In these subjects, blood sampling and NWR testing were repeated both at headache onset (T-MIG) and 1 hour later (T-MIG-1h).
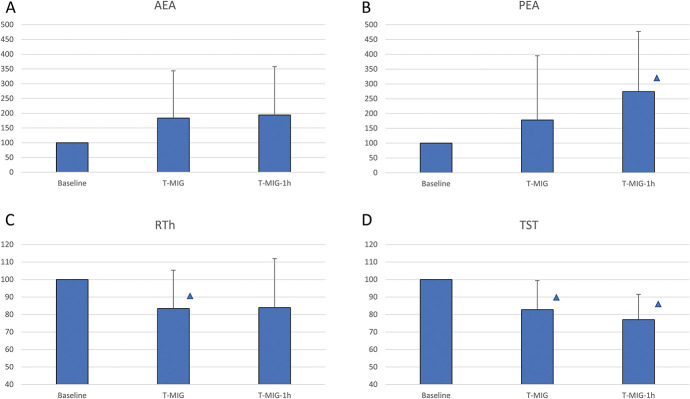
Plasma levels of AEA did not change either at the onset of the migraine-like attack or 1 hour later (TIME: P = 0.184). By contrast, plasma PEA increased significantly in these subjects (TIME: P = 0.035), specifically at T-MIG-1h (P = 0.028 vs baseline) (Figs. 8A and B).
Figure 8.
Changes in endocannabinoid levels and neurophysiological parameters recorded at migraine onset and 1 hour later in migraine subjects showing a positive response to the migraine-induction test within the 180-minute in-hospital observation period. Data from migraine subjects showing a positive migraine-induction test response within the 180-minute in-hospital observation period (MIG+ subgroup, n = 13). T-MIG: evaluation at migraine onset; T-MIG-1h: evaluation 1 hour after migraine onset. Statistical analysis: 1-factor ANOVA for repeated measures (factor “TIME” with 3 levels: Baseline, T-MIG, T-MIG-1h): AEA (A): TIME: P = 0.184. PEA (B): TIME: P = 0.035. RTh (C): TIME: P = 0.035. TST (D): TIME: P = 0.001. Δ = post hoc analysis: P < 0.050 vs Baseline. AEA, anandamide; ANOVA, analysis of variance; PEA, palmitoylethanolamide; RTh, reflex threshold.
Reflex threshold and TST both showed significant reductions at the additional time points (TIME: P = 0.035 and P = 0.001, respectively), although RTh was significantly lower only at T-MIG (P = 0.048 vs baseline), whereas TST was significantly reduced at both T-MIG and T-MIG-1h (P = 0.009 and P = 0.001, respectively) (Figs. 8C and D).
3.9. Correlation analysis
We did not find significant correlations between percentage changes in plasma AEA and PEA levels at any of the time points (T30: P = 0.190, T60: P = 0.286, T120: P = 0.728, T-MIG: P = 0.921, T-MIG-1h: P = 0.511). By contrast, the percentage changes in the RTh and TST values significantly correlated with each other (P = 0.001, at each time point).
No correlations emerged between plasma levels of AEA or PEA and neurophysiological variables at any time point.
4. Discussion
Although preclinical and clinical research has pointed to a dysregulation of the ES in migraine,23 the intricacies of this phenomenon remain largely unknown. We recently demonstrated an alteration in the expression of genes involved in endocannabinoid pathways, describing a gradient of migraine severity (from episodic to chronic).24 We have developed a working hypothesis based on the assumption that recurrent migraine attacks lead to neurogenic inflammation and subsequent release of proinflammatory cytokines, which in turn cause peripheral and central sensitization.19 In this context, ES dysregulation might contribute to nociceptive facilitation, which is the neurophysiological correlate of central sensitization.15
To test this hypothesis in acute migraine, we used the NTG model, a validated tool for studying the ictal phase of migraine under controlled experimental conditions.16 We simultaneously evaluated plasma AEA and PEA levels and 2 NWR thresholds (RTh and TST).
These were our main findings: (1) NTG induced a similar increase in plasma AEA levels in migraine subjects and healthy controls; (2) instead, NTG increased plasma PEA only in subjects with migraine; (3) neurophysiological evaluation confirmed that NTG induced a condition of spinal sensitization in migraine; and (4) no correlations were found between AEA or PEA levels and spinal sensitization, as measured using the NWR.
Our findings do not support a specific involvement of endogenous AEA release in the ictal phase of migraine, as shown by the comparable baseline plasma levels and similar increases after the NTG challenge in the migraine and healthy control groups. Thus, it seems reasonable to hypothesize that the post-NTG changes detected in both groups are related to the increased exogenous nitric oxide yielded by this nitrovasodilator. Notably, cross-talk between AEA and nitric oxide metabolism has previously been demonstrated in both pain and nonpain conditions.1,36,42 Although AEA was not found to play a role in ictal migraine, this does not exclude a dysregulation of the ES in the most severe form of migraine (chronic migraine), as suggested by the findings of Cupini et al. (2006, 2008) and Greco et al. (2020).13,14,25
Contrary to what was seen with AEA, plasma PEA was significantly increased in subjects with migraine 120 minutes after NTG exposure. This finding was confirmed in the migraine subjects who developed a specific migraine-like headache during the in-hospital observation period, and were tested at its onset and 1 hour later. The finding of increased plasma PEA both in MIG+ and in MIG− subjects, but not in controls, suggests that this response is not related to NTG-induced pain, but nevertheless reflects migraine-specific mechanisms. The data presented herein do not support involvement of AEA and PEA in the initiation of provoked migraine attacks, for several reasons: (1) their levels did not differ between migraine and healthy subjects at baseline; (2) the detection of increased plasma AEA levels both in migraine subjects and in healthy controls suggests that this increase is a nonspecific response to NTG, rather than a specific migraine-related mechanism; (3) the PEA increase occurred in the late phase of the NTG test (at 2 hours in the total migraine population, and at 1 hour after migraine onset in the MIG+ subgroup); and (4) the PEA increase was comparable between the MIG+ and MIG− groups, and thus also detected in migraine subjects who did not develop a migraine-like attack.
As for PEA, the pattern observed suggests that its release was triggered by NTG-induced neurovascular migraine-specific changes in a predisposed substrate, not by the pain phase itself. This interpretation supports the idea that PEA release is a compensatory response to the NTG challenge. Thus, PEA increase may have a role in the complex and still elusive events leading to spontaneous resolution of attacks. Additional investigations are needed to further explore this intriguing hypothesis.
Another possible explanation for the PEA increase is related to the enhanced L-arginine-NO metabolism observed during spontaneous migraine attacks, which leads to increased nitrite plasma levels within the first hour from onset.39 In this condition, S-nitrosylation of the peroxisome proliferator-activator receptor (a PEA receptor) might impair its function, thus causing an increase in PEA via a negative feedback mechanism.7
Anandamide and PEA are lipid messengers, synthetized by N-acylphosphatidylethanolamine-phospholipase D, and hydrolyzed by FAAH.26 Platelets from episodic migraine sufferers show increased FAAH activity together with increased AEA transporter.13 By contrast, chronic migraine subjects show decreased peripheral FAAH gene expression along with increased cerebrospinal fluid levels of PEA. These latter findings may be interpreted as a dynamic compensatory mechanism serving to keep AEA and PEA levels high when the organism is under stress, as in the case of a migraine attack.24,33,40 In line with this hypothesis, in the preclinical setting, we have previously shown that FAAH inhibitors, acting mainly through increased PEA and AEA levels, reduce neuronal activation in the trigeminal nucleus caudalis, as well as trigeminal hyperalgesia in the NTG animal migraine model.21,22 Convincing evidence indicates that PEA is cleaved not only by FAAH, but also by the cysteine hydrolase N-acyl-ethanolamine acid amidase.43 Palmitoylethanolamide levels were found to be increased and AEA levels decreased in the cerebrospinal fluid of subjects with chronic migraine, supporting the involvement of different biological pathways for the 2 molecules.40 Palmitoylethanolamide is a lipidic compound that physiologically exerts an anti-inflammatory and analgesic role in several inflammatory and chronic pain conditions.4,17 Its effects on PEA are mediated mainly via inhibition of proinflammatory cytokine release by mast cells and microglia.11,28 However, other mechanisms of action have been described, such as the “entourage” effect. Through this mechanism, PEA might indirectly enhance AEA effects via modulation of noncannabinoid receptors, as well as of the transient receptor potential vanilloid 1 receptor.45
Taken together, these observations suggest that PEA levels may have a role in the migraine ictal phase through compensatory analgesic and/or anti-inflammatory mechanisms. Evidence indicating a possible beneficial effect of PEA in the treatment of migraine has appeared in the literature.8,32 Our findings suggest that PEA supplements might, in the future, be tested in migraine prevention, as well as in the treatment of acute attacks, possibly also as an add-on. Specifically targeted studies would, of course, be needed to test their efficacy and tolerability. In the experimental field, more insight in this regard might be obtained by pretreating migraine subjects with PEA before an NTG challenge.
The neurophysiological findings of this study confirm the NTG-induced spinal sensitization (shown by a significant reduction of 2 NWR thresholds: RTh and TST) that we previously reported in subjects with migraine.15,34 Endocannabinoids are known to be involved in rapid modulation of synaptic transmission in the central nervous system. This effect is mediated by a retrograde signaling pathway that can locally influence synaptic excitability for a short period (a few seconds), thus modulating neural circuits, such as nociceptive signaling. In the present cohort, however, we found no correlations between biochemical and neurophysiological parameters, which suggests that endogenous release of AEA and PEA is not directly involved in the modulation of NTG-induced spinal sensitization. This finding was unexpected, given preclinical data showing that exogenous administration of AEA prevented NTG-induced activation and sensitization of the trigeminal system.29,30 A possible explanation for these apparently contrasting findings is that endocannabinoids may modulate migraine-related mechanisms upstream of NTG-induced effects, and not vice versa.
Finally, another somewhat unexpected finding concerned the habituation phenomenon of the NWR, which we observed in the healthy controls as a significant increase in NWR thresholds over time. This phenomenon was not detected in our previous studies, and we suggest that its manifestation in this study is most likely linked to the higher number of electrical stimulations delivered for RTh and TST testing.15,34 Interestingly, although the same stimulation paradigm was used in the migraine subjects and the controls, no habituation was shown by the former group, which is in keeping with a condition of neuronal hyperexcitability associated with spinal sensitization.
5. Limitations of the study
A cross-over, placebo-controlled study design would have added solidity to our findings. Our choice of a parallel-group, single-blind design, and careful selection of healthy controls without a personal or family history of migraine, was motivated by the dual need to ensure study feasibility and limit exposure of participants to a potentially unpleasant experimental procedure. In addition, this study design allowed us to compare the findings with previous results obtained by our group.
The participants were permitted to use preventive medication, and theoretically this, too, may have influenced our results. Although we excluded the drug classes that could have an impact on NWR parameters, and a stable dose and regimen was confirmed in the previous 2 months, we cannot completely rule out an influence of migraine-preventive treatment on our results.
Finally, on the basis of preclinical evidence, we focused solely on AEA and PEA. Thus, we cannot exclude the possibility that other lipids, such as 2-AG or oleoylethanolamide, or other mediators of endocannabinoid activity may play a role in ictal migraine.
6. Conclusions
The present findings suggest that PEA is involved in the ictal phase of migraine, probably in a compensatory analgesic and anti-inflammatory response to the neurovascular changes occurring during the early phase of the induced attack. Our study confirms the existence of a condition of spinal nociceptive facilitation in episodic migraine subjects during experimentally induced migraine attacks. The ES does not seem to be directly involved in the modulation of NTG-induced central sensitization, which suggests that the plasma PEA increase and the recorded spinal sensitization are simultaneous, but parallel phenomena.
Conflict of interest statement
G. Sances received honoraria for participation in advisory boards or for oral presentations from Eli-Lilly and Novartis. C. Tassorelli received honoraria for participation in advisory boards or for oral presentations from Allergan, ElectroCore, Eli-Lilly, Novartis, and Teva. C. Tassorelli has no ownership interest and does not own stocks of any pharmaceutical company. C. Tassorelli serves as Chief Section Editor of Frontiers in Neurology—Section Headache Medicine and Facial Pain and on the editorial board of The Journal of Headache and Pain. The remaining authors have no conflicts of interest to declare.
Acknowledgements
The authors are grateful to the Research Nurse Team for their valuable assistance in all the activities of the Headache Science Center of the IRCCS Mondino Foundation. They also thank Catherine Wrenn for her careful English language editing of the manuscript.
This study was funded by the Italian Ministry of Health (RF-2013-02355704).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
R. De Icco and R. Greco contributed equally to this work.
Contributor Information
Rosaria Greco, Email: rosaria.greco@mondino.it.
Chiara Demartini, Email: chiara.demartini@mondino.it.
Pietro Vergobbi, Email: pietro.vergobbi01@universitadipavia.it.
Annamaria Zanaboni, Email: annamaria.zanaboni@mondino.it.
Elena Tumelero, Email: elena.tumelero@mondino.it.
Angelo Reggiani, Email: angelo.reggiani@iit.it.
Natalia Realini, Email: natalia.realini@iit.it.
Grazia Sances, Email: grazia.sances@mondino.it.
Valentina Grillo, Email: valentina-grillo@mondino.it.
Marta Allena, Email: marta.allena@mondino.it.
Cristina Tassorelli, Email: cristina.tassorelli@mondino.it.
References
- [1].Abán CE, Accialini PL, Etcheverry T, Leguizamón GF, Martinez NA, Farina MG. Crosstalk between nitric oxide and endocannabinoid signaling pathways in normal and pathological placentation. Front Physiol 2018;9:1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Afridi SK, Matharu MS, Lee L, Kaube H, Friston KJ, Frackowiak RSJ, Goadsby PJ. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain 2005;128:932–9. [DOI] [PubMed] [Google Scholar]
- [3].Akerman S, Karsan N, Bose P, Hoffmann JR, Holland PR, Romero-Reyes M, Goadsby PJ. Nitroglycerine triggers triptan-responsive cranial allodynia and trigeminal neuronal hypersensitivity. Brain 2019;142:103–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Artukoglu BB, Beyer C, Zuloff-Shani A, Brener E, Bloch MH. Efficacy of palmitoylethanolamide for pain: a meta-analysis. Pain Physician 2017;20:353–62. [PubMed] [Google Scholar]
- [5].Ashina M, Hansen JM, Á Dunga BO, Olesen J. Human models of migraine-short-term pain for long-term gain. Nat Rev Neurol 2017;13:713–24. [DOI] [PubMed] [Google Scholar]
- [6].Astarita G, Piomelli D. Lipidomic analysis of endocannabinoid metabolism in biological samples. J Chromatogr B Anal Technol Biomed Life Sci 2009;877:2755–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cao Y, Gomes SA, Rangel EB, Paulino EC, Fonseca TL, Li J, Teixeira MB, Gouveia CH, Bianco AC, Kapiloff MS, Balkan W, Hare JM. S-nitrosoglutathione reductase-dependent PPARγ denitrosylation participates in MSC-derived adipogenesis and osteogenesis. J Clin Invest 2015;125:1679–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chirchiglia D, Cione E, Caroleo MC, Wang M, Di Mizio G, Faedda N, Giacolini T, Siviglia S, Guidetti V, Gallelli L. Effects of add-on ultramicronized N-palmitol ethanol amide in patients suffering of migraine with aura: a pilot study. Front Neurol 2018;9:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Christiansen I, Daugaard D, Thomsen LL, Olesen J. Glyceryl trinitrate induced headache in migraineurs - relation to attack frequency. Eur J Neurol 2000;7:405–11. [DOI] [PubMed] [Google Scholar]
- [10].Coppola G, Di Lorenzo C, Schoenen J, Pierelli F. Habituation and sensitization in primary headaches. J Headache Pain 2013;14:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Costa B, Comelli F, Bettoni I, Colleoni M, Giagnoni G. The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB1, TRPV1 and PPARγ receptors and neurotrophic factors. PAIN 2008;139:541–50. [DOI] [PubMed] [Google Scholar]
- [12].Cristino L, Bisogno T, Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol 2020;16:9–29. [DOI] [PubMed] [Google Scholar]
- [13].Cupini LM, Bari M, Battista N, Argirò G, Finazzi-Agrò A, Calabresi P, MacCarrone M. Biochemical changes in endocannabinoid system are expressed in platelets of female but not male migraineurs. Cephalalgia 2006;26:277–81. [DOI] [PubMed] [Google Scholar]
- [14].Cupini LM, Costa C, Sarchielli P, Bari M, Battista N, Eusebi P, Calabresi P, Maccarrone M. Degradation of endocannabinoids in chronic migraine and medication overuse headache. Neurobiol Dis 2008;30:186–9. [DOI] [PubMed] [Google Scholar]
- [15].De Icco R, Perrotta A, Grillo V, Cosentino G, Sances G, Sandrini G, Tassorelli C. Experimentally induced spinal nociceptive sensitization increases with migraine frequency: a single-blind controlled study. PAIN 2020;161:429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Demartini C, Greco R, Zanaboni AM, Sances G, De Icco R, Borsook D, Tassorelli C. Nitroglycerin as a comparative experimental model of migraine pain: from animal to human and back. Prog Neurobiol 2019;177:15–32. [DOI] [PubMed] [Google Scholar]
- [17].Di Cesare Mannelli L, Pacini A, Corti F, Boccella S, Luongo L, Esposito E, Cuzzocrea S, Maione S, Calignano A, Ghelardini C. Antineuropathic profile of N-Palmitoylethanolamine in a rat model of oxaliplatin-induced neurotoxicity. PLoS One 2015;10:e0128080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Di Marzo V, Wang J. The Endocannabinoidome. The world of endocannabinoids and related mediators. Amsterdam: Elsevier, 2015. [Google Scholar]
- [19].Edvinsson L, Haanes KA, Warfvinge K. Does inflammation have a role in migraine? Nat Rev Neurol 2019;15:483–90. [DOI] [PubMed] [Google Scholar]
- [20].Gouveia-Figueira S, Goldin K, Hashemian SA, Lindberg A, Persson M, Nording ML, Laurell K, Fowler CJ. Plasma levels of the endocannabinoid anandamide, related N-acylethanolamines and linoleic acid-derived oxylipins in patients with migraine. Prostaglandins Leukot Essent Fat Acids 2017;120:15–24. [DOI] [PubMed] [Google Scholar]
- [21].Greco R, Bandiera T, Mangione AS, Demartini C, Siani F, Nappi G, Sandrini G, Guijarro A, Armirotti A, Piomelli D, Tassorelli C. Effects of peripheral FAAH blockade on NTG-induced hyperalgesia—evaluation of URB937 in an animal model of migraine. Cephalalgia 2015;35:1065–76. [DOI] [PubMed] [Google Scholar]
- [22].Greco R, Demartini C, Zanaboni A, Casini I, De Icco R, Reggiani A, Misto A, Piomelli D, Tassorelli C. Characterization of the peripheral FAAH inhibitor, URB937, in animal models of acute and chronic migraine. Neurobiol Dis 2021;147:105157. [DOI] [PubMed] [Google Scholar]
- [23].Greco R, Demartini C, Zanaboni AM, Piomelli D, Tassorelli C. Endocannabinoid system and migraine pain: an update. Front Neurosci 2018;12:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Greco R, Demartini C, Zanaboni AM, Tumelero E, Icco RDe, Sances G, Allena M, Tassorelli C. Peripheral changes of endocannabinoid system components in episodic and chronic migraine patients: a pilot study. Cephalalgia 2021;41:185–96. [DOI] [PubMed] [Google Scholar]
- [25].Greco R, Demartini C, Zanaboni AM, Tumelero E, Reggiani A, Misto A, Piomelli D, Tassorelli C. FAAH inhibition as a preventive treatment for migraine: a pre-clinical study. Neurobiol Dis 2020;134:104624. [DOI] [PubMed] [Google Scholar]
- [26].Greco R, Gasperi V, Maccarrone M, Tassorelli C. The endocannabinoid system and migraine. Exp Neurol 2010;224:85–91. [DOI] [PubMed] [Google Scholar]
- [27].Headache Classification Committee of the International Headache Society. The international classification of headache disorders. 3rd ed. Cephalalgia 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- [28].Luongo L, Guida F, Boccella S, Bellini G, Gatta L, Rossi F, de Novellis V, Maione S. Palmitoylethanolamide reducesformalin-induced neuropathic-like behaviour through spinal glial/microglial phenotypical changes in mice. CNS Neurol Disord Drug Targets 2013;12:45–54. [DOI] [PubMed] [Google Scholar]
- [29].Nagy-Grócz G, Bohár Z, Fejes-Szabó A, Laborc KF, Spekker E, Tar L, Vécsei L, Párdutz Á. Nitroglycerin increases serotonin transporter expression in rat spinal cord but anandamide modulated this effect. J Chem Neuroanat 2017;85:13–20. [DOI] [PubMed] [Google Scholar]
- [30].Nagy-Grócz G, Tar L, Bohár Z, Fejes-Szabó A, Laborc KF, Spekker E, Vécsei L, Párdutz Á. The modulatory effect of anandamide on nitroglycerin-induced sensitization in the trigeminal system of the rat. Cephalalgia 2016;36:849–61. [DOI] [PubMed] [Google Scholar]
- [31].Onderwater GLJ, Dool J, Ferrari MD, Terwindt GM. Premonitory symptoms in glyceryl trinitrate triggered migraine attacks. PAIN 2020;161:2058–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Papetti L, Sforza G, Tullo G, Alaimo Di Loro P, Moavero R, Ursitti F, Ferilli MAN, Tarantino S, Vigevano F, Valeriani M. Tolerability of palmitoylethanolamide in a pediatric population suffering from migraine: a pilot study. Pain Res Manag 2020;2020:3938640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Perrotta A, Arce-Leal N, Tassorelli C, Gasperi V, Sances G, Blandini F, Serrao M, Bolla M, Pierelli F, Nappi G, MacCarrone M, Sandrini G. Acute reduction of anandamide-hydrolase (FAAH) activity is coupled with a reduction of nociceptive pathways facilitation in medication-overuse headache subjects after withdrawal treatment. Headache 2012;52:1350–61. [DOI] [PubMed] [Google Scholar]
- [34].Perrotta A, Serrao M, Tassorelli C, Arce-Leal N, Guaschino E, Sances G, Rossi P, Bartolo M, Pierelli F, Sandrini G, Nappi G. Oral nitric-oxide donor glyceryl-trinitrate induces sensitization in spinal cord pain processing in migraineurs: a double-blind, placebo-controlled, cross-over study. Eur J Pain 2011;15:482–90. [DOI] [PubMed] [Google Scholar]
- [35].Petrosino S, Di Marzo V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br J Pharmacol 2017;174:1349–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Romero TRL, Resende LC, Duarte IDG. The neuronal NO synthase participation in the peripheral antinociception mechanism induced by several analgesic drugs. Nitric Oxide Biol Chem 2011;25:431–5. [DOI] [PubMed] [Google Scholar]
- [37].Sances G, Tassorelli C, Pucci E, Ghiotto N, Sandrini G, Nappi G. Reliability of the nitroglycerin provocative test in the diagnosis of neurovascular headaches. Cephalalgia 2004;24:110–19. [DOI] [PubMed] [Google Scholar]
- [38].Sandrini G, Serrao M, Rossi P, Romaniello A, Cruccu G, Willer JC. The lower limb flexion reflex in humans. Prog Neurobiol 2005;77:353–95. [DOI] [PubMed] [Google Scholar]
- [39].Sarchielli P, Alberti A, Codini M, Floridi A, Gallai V. Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia 2000;20:907–18. [DOI] [PubMed] [Google Scholar]
- [40].Sarchielli P, Pini LA, Coppola F, Rossi C, Baldi A, Mancini ML, Calabresi P. Endocannabinoids in chronic migraine: CSF findings suggest a system failure. Neuropsychopharmacology 2007;32:1384–90. [DOI] [PubMed] [Google Scholar]
- [41].Sicuteri F, Del Bene E, Poggioni M, Bonazzi A. Unmasking latent dysnociception in healthy subjects. Headache J Head Face Pain 1987;27:180–5. [DOI] [PubMed] [Google Scholar]
- [42].Stefano GB, Esch T, Cadet P, Zhu W, Mantione K, Benson H. Endocannabinoids as autoregulatory signaling molecules: coupling to nitric oxide and a possible association with the relaxation response. Med Sci Monit 2003;9. Available at: https://pubmed.ncbi.nlm.nih.gov/12709683/. Accessed July 10, 2020. [PubMed] [Google Scholar]
- [43].Ueda N, Tsuboi K, Uyama T. N-acylethanolamine metabolism with special reference to N-acylethanolamine-hydrolyzing acid amidase (NAAA). Prog Lipid Res 2010;49:299–315. [DOI] [PubMed] [Google Scholar]
- [44].Van Der Schueren BJ, Van Laere K, Gérard N, Bormans G, De Hoon JN. Interictal type 1 cannabinoid receptor binding is increased in female migraine patients. Headache 2012;52:433–40. [DOI] [PubMed] [Google Scholar]
- [45].Vandevoorde S, Jonsson KO, Fowler CJ, Lambert DM. Modifications of the ethanolamine head in N-palmitoylethanolamine: synthesis and evaluation of new agents interfering with the metabolism of anandamide. J Med Chem 2003;46:1440–8. [DOI] [PubMed] [Google Scholar]