Key Points
Question
Can the availability of living donor donation in a liver transplant program overcome sex disparity in organ allocation?
Findings
In this cohort study of 1289 patients (830 men and 459 women) listed for liver transplant, presence of a living liver donor showed no statistically significant difference in the chances of liver transplant for men and women across the range of Model for End-stage Liver Disease incorporating sodium levels (MELD-Na ) scores.
Meaning
Access to living donor liver transplant may help rectify sex disparity on the liver transplant waiting list.
This cohort study compares availability of living liver donation in men and women on the transplant waiting list.
Abstract
Importance
The Model for End-stage Liver Disease (MELD)–based organ allocation system has significantly decreased mortality on the transplant waiting list for patients with end-stage liver disease. However, women have remained at a disadvantage with respect to access to deceased donor liver transplant (DDLT) even after introduction of the MELD score for organ allocation.
Objective
To determine whether availability of living donation in a transplant program can offset inequity in liver transplant (LT) allocation for women.
Design, Setting, and Participants
This cohort study retrospectively analyzed adult patients listed for LT at the University Health Network in Toronto, Ontario, Canada. Patients included had a potential living donor (pLD) at the moment of listing. This study was performed from November 13, 2012, to May 31, 2019. A total of 1289 listed patients (830 men; 459 women) were analyzed during the study period.
Main Outcomes and Measures
This study performed survival analysis and competing-risk analysis to delineate how access to livers from living donors was associated with events in women vs men on the transplant waiting list (LT, death, or dropout).
Results
Of 1289 included patients, 459 (35.6%) were women, and the mean (SD) age was 56.1 (10.0) years at assessment and listing. A total of 783 of 1289 listed patients underwent LT. Among those with no pLD at assessment, there was a higher median (range) Model for End-stage Liver Disease incorporating sodium levels (MELD-Na) score at listing (22 [6-50] vs 19 [6-50]; P < .001) and at LT (27 [6-49] vs 20 [6-52]; P < .001) in women receiving DDLT. Women were at a significant disadvantage without a pLD (hazard ratio [HR], 1.29; 95% CI, 1.04-1.60; P = .01); there was no difference in access to LT with availability of a pLD (HR, 0.93; 95% CI, 0.76,-1.14; P = .44). The instantaneous rate of receiving a transplant in men with a pLD was 1.39 times higher than men who did not have a pLD (HR, 1.39; 95% CI; P < .001) and the instantaneous rate of receiving a transplant in women with a pLD was 1.92 times higher than in women who did not (HR, 1.92; 95% CI, 1.51–2.44; P < .001). The HR was 1.38 times higher in women compared with men across the MELD-Na score strata (HR, 1.38; 95% CI, 1.03-1.84; P = .03) and 2.04 times higher when the MELD-Na score was less than 20 (HR, 2.04; 95% CI, 1.31-3.14; P = .001).
Conclusions and Relevance
These study findings suggest that women can overcome the complex problem of allocation inequity with access to livers from living donors. Women with access only to DDLT were much more unwell than men independent of liver disease at the time of listing, dropout, or LT. Therefore, the wider availability of living donation liver transplant would be helpful in addressing the sex disparity in access to LT in the current MELD-Na era.
Introduction
The Model for End-stage Liver Disease (MELD)–based allocation system for liver transplant (LT) was implemented to prioritize the sickest patients and improve equity in organ allocation.1 Although overall transplant wait list mortality has decreased in the post-MELD era, LT access for women remains compromised.2,3 A 2020 study on sex differences in allocation reported that women were 8.6% more likely to die while on the transplant waiting list and 14.4% less likely to receive an organ from a deceased donor compared with men, having accounted for geographic location, MELD score, and candidate anthropometric and liver measurements.4 Women are 2 times less likely than men to receive an LT overall, whether from a deceased or living donor.5 Therefore, sex disparities in LT have persisted following implementation of the MELD-based allocation system.6
This sex-based disparity is worse at higher MELD scores, while similar transplant rates have been reported at MELD scores less than 15.2 Lower muscle mass in women reduces serum creatinine levels, a major determinant of the MELD score, thereby underestimating the degree of kidney impairment. Women tend to have lower glomerular filtration rates pretransplant.7,8 However, adjustment for glomerular filtration rate still does not rectify sex-based disparity in organ allocation and wait list mortality.9,10 Differences in height also appear to be critical: candidates who are shorter than 165 cm are approximately 10% to 15% less likely to receive LT than taller patients.11 Size matching likely contributes, with most deceased donor organs coming from male donors.12,13 Small or split livers may be preferentially allocated to pediatric recipients, resulting in prolonged wait times and a higher risk of dropout for women. However, the adult recipient of a split liver is usually a small woman.12 Additionally, patients listed with exception points are most often men and undergo transplant at higher rates, representing an additional disadvantage to women.14,15 Given the complex combination of factors that results in this disparity, it has been difficult to devise a suitable solution.8,16
Access to living donor LT (LDLT) shortens the median waiting time and decreases risk of dropout or death on the transplant waiting list.17,18 However, to our knowledge, there are no studies showing the benefit of access to living donation in women compared with men.
In this study, we sought to address whether the availability of living donation in an LT program can overcome the sex-based disparity in access to transplant. As a program that performs a significant number of both LDLTs and deceased donor liver transplants (DDLT), we were in a unique position to be able to investigate these questions.
Methods
Study Design
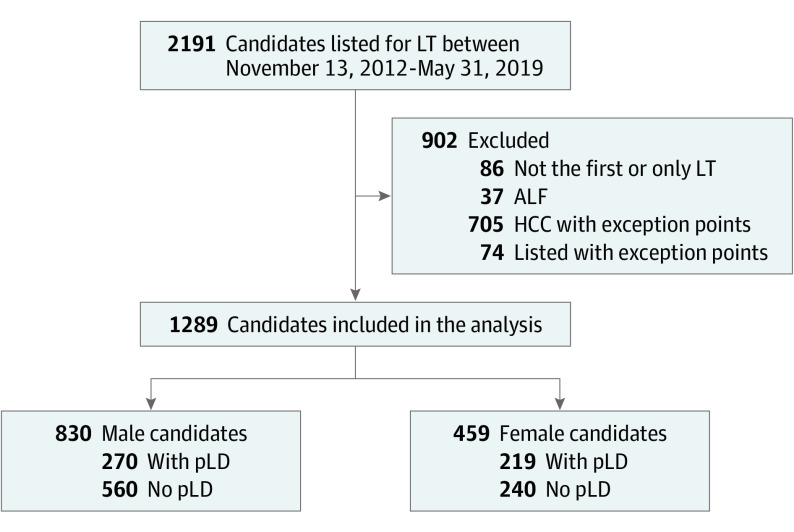
We included all adults listed for LT between November 13, 2012, and May 31, 2019, in the Multi-Organ Transplant Program at the University Health Network in Toronto, Ontario, Canada. Given our program’s transition to the MELD-Na system for listing on November 13, 2012 , we used this as the start date of our study (Figure 1). Candidates requiring exception points for listing, patients with fulminant liver failure, combined solid organ/multiorgan transplants, and those listed for retransplant were excluded.
Figure 1. Study Flowchart Showing Study Criteria and Subsequent Study Population.
ALF indicates acute liver failure; HCC, hepatocellular carcinoma; LT, liver transplant; pLD, potential living donor.
Patients were categorized into 2 groups: those having a potential living donor (pLD) at time of listing and those who did not (eAppendix 1 in the Supplement). A pLD was defined as an individual who (1) had submitted a medical history form and was evaluated as a living donor, (2) was found to be suitable for donation after the initial screening phase, and (3) had undergone imaging assessment.19,20 However, this does not mean the donor was ultimately deemed to be acceptable. Patients with a pLD could eventually receive LDLT, DDLT, or drop off from the list. Whenever a suitable deceased donor liver was available before complete evaluation of living donor or earlier to the proposed date of LDLT, the recipient underwent DDLT. Patients without a pLD either received DDLT or could drop off the list for various reasons. The study was approved by the Research Ethics Board of the University Health Network.
Events on the Waiting List
Listed candidates were monitored from the time of listing to LT, dropout from the waiting list, or death. Dropouts occurred because of patients being too sick for transplant, medical unsuitability, refusing LT, or improvement to the point of no longer requiring transplant.
Statistical Analysis
Descriptive statistics were provided for demographic and clinical variables. χ2 Tests or Fisher exact tests were used to compare categorical variables between men and women. Two-sample t tests or Wilcoxon tests were used to compare continuous variables between men and women. Kaplan-Meier product-limit curves for overall survival and cumulative incidence curves for time to transplant, which account for competing risk of death, were plotted for the overall sample, as well as stratified by sex and pLD status. Hazard ratio (HR) is the ratio of instantaneous risk of occurrence of event. The association of sex, MELD-Na score, and presence of pLD with overall survival and with time to transplant were assessed using the log-rank test and Gray test, respectively. Univariable and multivariable Cox proportional hazard regression models were conducted to examine associations between overall survival and variables of interest, such as sex, body mass index, height, age, presence of pLD at listing, blood type, and MELD-Na score. Univariable and multivariable Fine-Gray competing risk regression models were performed to examine associations between time to transplant and variables of interest, while accounting for effect of competing risk (death). Subgroup analyses were carried out by sex and by pLD status. The sex by pLD interaction within subgroups by MELD-Na range was also examined. Statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
Figure 1 details patients included in the study. Patient characteristics at time of listing are detailed in the Table. Of 1289 included patients, 459 (35.6%) were women, and the mean (SD) age was 56.1 (10.0) years. A total of 459 women and 830 men were listed for LT during the study period for decompensated cirrhosis of different etiologies. Women had a higher mean MELD-Na score at both listing and transplant.
Table. Demographic and Clinical Characteristics of Patients at the Time of Listing.
| Variable | No. (%) | P value | ||
|---|---|---|---|---|
| Total (N = 1289) | Women (n = 459) | Men (n = 830) | ||
| Age, mean (SD), y | 56.1 (10.0) | 55.7 (10.6) | 56.3 (9.7) | .71 |
| Height, mean (SD), cm | 169.5 (9.5) | 161.6 (7.3) | 173.9 (7.7) | .001 |
| Weight, mean (SD), kg | 79.7 (18.6) | 71.2 (16.4) | 84.3 (18.1) | .001 |
| BMI, mean (SD)a | 27.6 (5.6) | 27.3 (6.1) | 27.8 (5.3) | .02 |
| Etiology of liver disease | ||||
| Alcoholic liver disease | 288 (22) | 72 (16) | 216 (26) | <.001 |
| Hepatitis C | 277 (22) | 75 (16) | 202 (24) | <.001 |
| Nonalcoholic fatty liver disease | 204 (16) | 85 (19) | 119 (14) | .049 |
| Primary sclerosing cholangitis | 89 (7) | 31 (7) | 58 (7) | .87 |
| Hepatitis B | 65 (5) | 13 (3) | 52 (7) | .007 |
| Primary biliary cholangitis | 64 (5) | 58 (13) | 6 (1) | <.001 |
| Autoimmune hepatitis | 54 (4) | 39 (9) | 15(2) | <.001 |
| Cryptogenic/unknown | 41 (3) | 12 (3) | 29 (4) | .39 |
| Hepatocellular carcinoma (listed without exception points) | 100 (8) | 26 (6) | 74 (9) | .04 |
| Miscellaneous | 107 (8) | 48 (11) | 59 (5) | .09 |
| MELD-Na score at listing, mean (SD) | 20.3 (8.6) | 21.2 (8.3) | 19.8 (8.7) | .003 |
| With pLD | 19.9 (7.7) | 20.1 (8.0) | 19.6 (7.5) | .48 |
| Without pLD | 20.5 (9.0) | 22.1 (8.5) | 19.9 (9.2) | .006 |
| MELD-Na score at listing, median (range) | 19 (6-54) | 20 (6-54) | 19 (6-50) | .002 |
| With pLD | 19 (6-50) | 19 (6-50) | 19 (6-47) | .91 |
| Without pLD | 19 (6-54) | 22 (6-54) | 19 (6-50) | < .001 |
| Change in MELD-Na score, mean (SD) | 1.2 (5.8) | 1.3 (6) | 1.1 (5.6) | .24 |
| With pLD | 1.5 (5.9) | 1.1 (5.7) | 1.7 (6.0) | .09 |
| Without pLD | 1.0 (5.7) | 1.5 (6.3) | 0.7 (5.4) | .24 |
| MDRD eGFR, mean (range), mL/min/1.732 | 76.7 (29.4) | 69.2 (28.7) | 80.8 (28.8) | <001 |
| Blood group | ||||
| A | 493 (38) | 174 (38) | 319 (38) | .95 |
| AB | 77 (6) | 29 (6) | 48 (6) | |
| B | 179 (14) | 66 (14) | 113 (14) | |
| O | 540 (42) | 190 (41) | 350 (42) | |
| Type of transplant | ||||
| No transplant | 506 (39) | 188 (41) | 318 (38) | .001 |
| DDLT | 573 (44) | 172 (37) | 401 (48) | |
| LDLT | 210 (16) | 99 (22) | 111 (13) | |
| Time on the waiting list, median (range), mo | 5 (0-74) | 4 (0-74) | 5 (0-67) | .37 |
| Outcome | ||||
| Active listing | 90 (7) | 36 (8) | 54 (7) | .35 |
| Delisted | 197 (15) | 65 (14) | 132 (16) | |
| Death | 219 (17) | 87 (19) | 132 (16) | |
| Transplanted | 783 (61) | 271 (59) | 512 (62) | |
Abbreviations: BMI, body mass index; DDLT, deceased donor liver transplant; LDLT, living donor liver transplant; MDRD eGFR, Modification of Diet in Renal Disease estimated glomerular filtration rate; MELD-Na, Model for End-stage Liver Disease incorporating sodium levels; NA, not applicable; pLD, potential living donor.
Calculated as weight in kilograms divided by height in meters squared.
Time to Transplant, Dropout, and Death on the Waiting List
A total of 724 listed patients (55.7%) underwent LT by 1 year of listing, while the rest were either on the waiting list (302 [23.4%]) or dropped out (276 [21.4%]). The cumulative incidence of transplant among listed patients and those undergoing transplant based on time intervals is detailed in eTable 1 in the Supplement.
By the end of the study period, 783 patients had received LT (573 DDLT; 210 LDLT; eAppendix 2 in the Supplement), while 416 patients had dropped out (197 delisted; 219 deaths) and 90 patients were still active on the list. Delisted patients included patients too sick for LT (n = 122) and those with improved medical condition no longer warranting LT (n = 58).
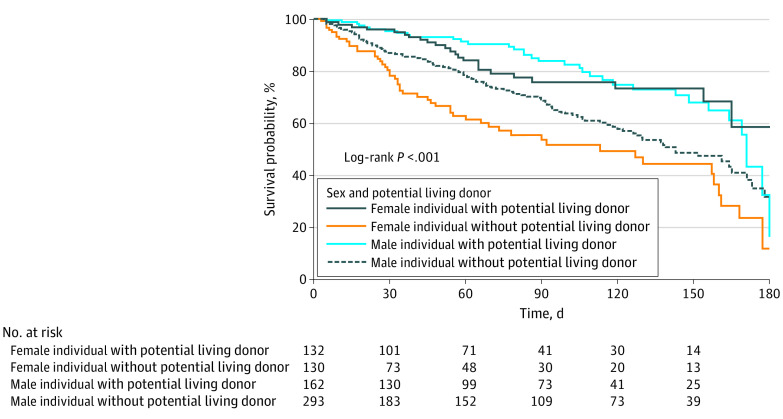
Although 489 patients had a pLD while on the wait list, 210 recipients (42.9%) had LDLT and 142 (29.0%) had DDLT. Among the 800 candidates who did not have a pLD during the waiting period, 431 (53.9%) received DDLT. A total of 309 of 800 (46.1%) failed to receive LT; 222 became too sick for LT, 56 had improved medical condition, and 31 dropped out because of reasons unrelated to underlying disease. A total of 107 patients with a pLD (28.0%) failed to receive LT: 89 became too sick for LT, 12 had improved medical condition, and 6 dropped out because of reasons unrelated to underlying disease. There was no difference in overall survival between men and women (women: 1-year overall survival estimate, 80.0% [76.4%-83.9%]; 95% CI, 5-year overall survival estimate, 67.5% [62.4%-73.1%]; 95% CI, men: 1-year overall survival estimate, 81.2% [78.5%-84.0%]; 95% CI, 5-year overall survival estimate, 68.1% [64.1%-72.3%]; 95% CI; P = .40) (eFigure 1 in the Supplement). Multivariable Cox proportional hazard regression analysis adjusting for time-dependent covariate MELD-Na, age, height, and blood type suggested having a pLD was associated with lower deaths or dropouts in both men and women in the first 6 months after listing (women: HR, 0.41; 95% CI, 0.25-0.68; P < .001; men: HR, 0.60; 95% CI, 0.40-0.91; P = .01) (Figure 2) and only women showed this benefit even at 15 months postlisting (women: HR, 0.52; 95% CI, 0.34-0.79; P = .002, men: HR, 0.81; 95% CI, 0.59-1.11; P = .19) For this analysis, we only accounted for dropouts owing to adverse events, thereby excluding 68 of 1289 patients removed from the list owing to improved medical condition (5.3%) (eTable 6 in the Supplement).
Figure 2. Kaplan-Meier Plot for Time to Death or Dropout 6 Months Stratified by Sex and Presence of Potential Living Donor.
Association of MELD-Na Score With Time to LT
With high MELD-Na scores (25 or higher) at listing, both men and women had higher and earlier chances of LT in our transplant program as compared with those with scores of less than 25 (MELD-Na score of 25 or greater vs less than 25: men: HR, 1.73; 95% CI, 1.39-2.17; P < .001; women: HR, 1.82; 95% CI, 1.39-2.39; P < .001) (eFigure 2A and B in the Supplement).
Compared with men, the instantaneous rate of LT in women was similar in both the high (25 or higher) and low (less than 25) MELD-Na score groups (high: HR, 1.09; 95% CI, 0.92-1.30; P = .32; low: HR, 1.04; 95% CI, 0.83-1.31; P = .71) (eFigure 2C in the Supplement). Among MELD-Na score strata (less than 20, 20 to 30, and greater than 30), both women and men benefited from having a pLD in the first 2 strata. Among those with a MELD-Na score less than 20, compared with those without a pLD, instantaneous rate of LT increased by 2.72 times for women with a pLD (HR, 2.72; 95%CI, 1.90-3.90; P < .001) and 1.34 times for men with a pLD (HR, 1.34; 95%CI, 1.05-1.71, P = .02). Among those with a MELD-Na score of 20 to 30, compared with those without a pLD, instantaneous rate of LT increased by 1.80 times for women with a pLD (HR, 1.80; 95% CI, 1.25-2.60; P = .02) and 1.65 times for men with a pLD (HR, 1.65; 95% CI, 1.25–2.16; P <.001).
Association of Having a pLD With Access to LT
The instantaneous rate of LT in patients with a pLD was significantly higher than in those without a pLD (HR, 1.54; 95% CI, 1.35-1.76; P < .001) (Figure 3A and B in the Supplement). In patients without a pLD, instantaneous rate of LT was significantly higher in men than in women (HR, 1.29; 95% CI, 1.04-1.60; P = .01) (Figure 3A), whereas this difference disappeared in those with a pLD (HR, 0.93; 95% CI, 0.76-1.14; P = .49) (Figure 3B). Presence of a pLD in women resulted in a significantly higher instantaneous rate of LT (HR, 1.89; 95%CI, 1.49-2.39; P < .001) (Figure 3C). This benefit was also seen in men (HR, 1.40; 95% CI, 1.18-1.66; P<.001) (Figure 3D). However, the finding was larger in women. The instantaneous rate of receiving a transplant in men with a pLD was 1.39 times higher than men who did not have a pLD (HR, 1.39; 95% CI, 1.18-1.65; P < .001) and the instantaneous rate of receiving a transplant in women with a pLD was 1.92 times higher than in women who did not (HR, 1.92; 95% CI, 1.51-2.44; P < .001). The HR was 38% higher in women as compared with men.
Figure 3. Cumulative incidence function (CIF) to LT in men and women without and with a pLD (A and B). CIF to LT in women (C) and men (D) with and without a pLD.
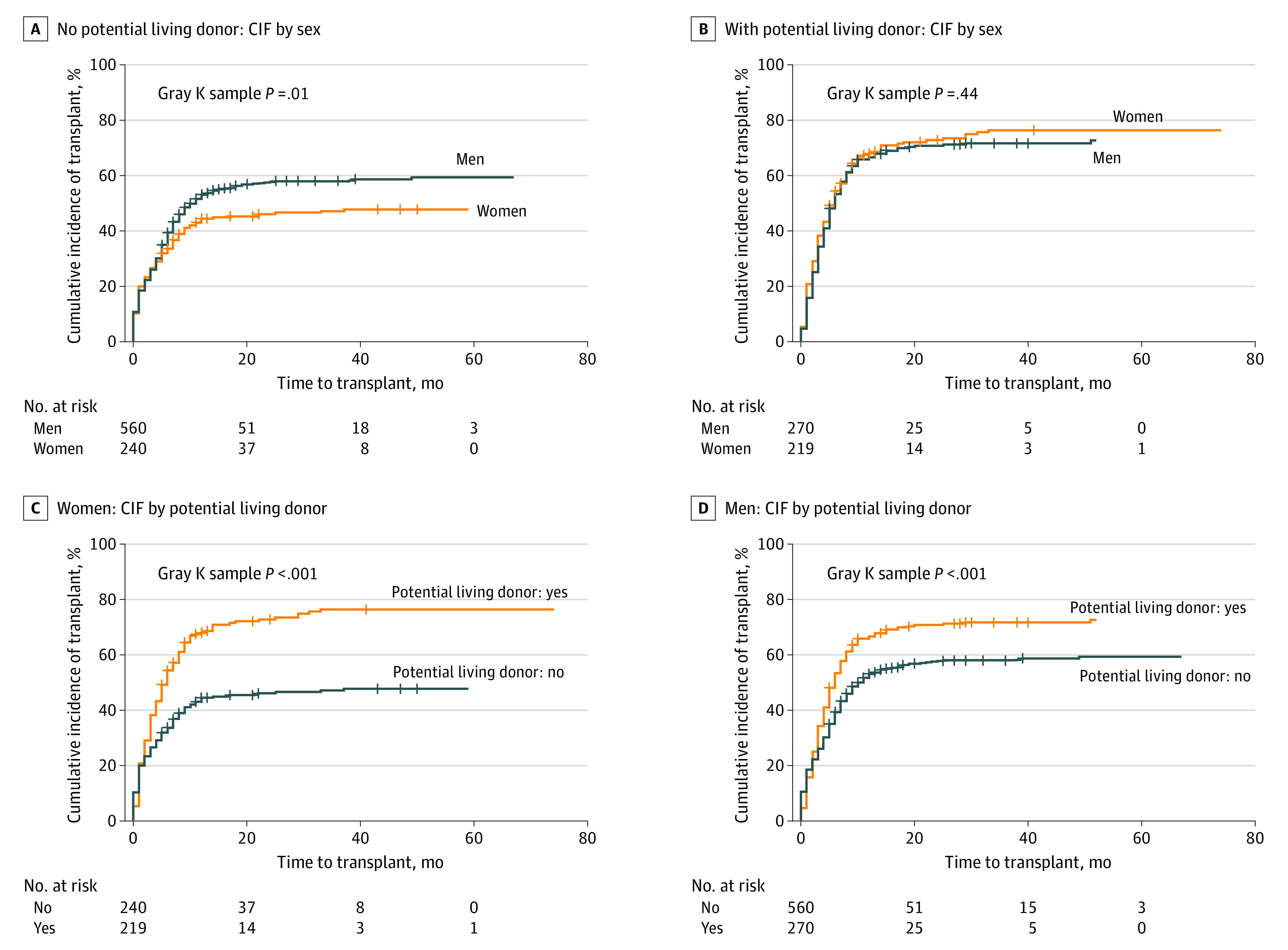
CIF indicates cumulative incidence function.
MELD-Na Score and Type of LT
In those with a pLD, the mean (SD) MELD-Na score was similar in men and women at the time of listing (19.6 [7.5] in men and 20.1 [1.8] in women; P = .91) and also at the time of LT (21.4 [9.4] in men and 21.2 [8.4] in women; P = .72).
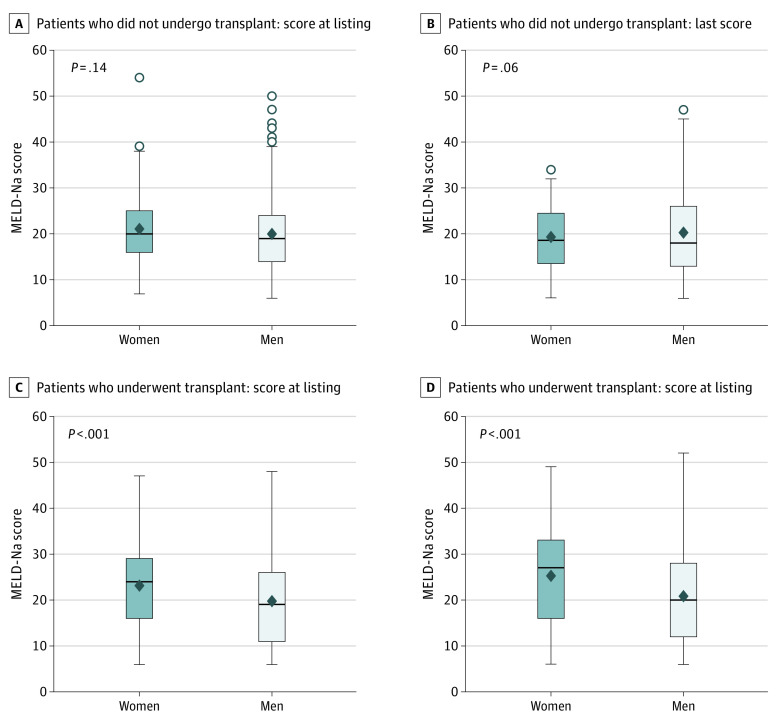
A similar analysis based on MELD-Na scores in the group with no pLD showed varied results (eTable 2 in the Supplement). At listing, women had a significantly higher median (range) MELD-Na score compared with men (22 [6-50] vs 19 [6-50]; P < .001). At time of LT, women had a significantly higher median (range) MELD-Na score than men at time of listing (27 [6-49] vs 20 [6-52]; P < .001). However, women who dropped out from wait list had a similar median (range) MELD-Na score as men at listing (21.1 [7-54] vs 19 [6-50]; P = .14) and also at time of dropout 20 [6-49] vs 18 [6-47]; P = .06) (Figure 4). Multivariable Cox proportional hazard model on overall survival for women with no pLD suggested that taller women were more likely to receive transplant (HR, 1.04; 95% CI, 1.01-1.07; P = .01) after adjusting for the effects of other variables of interest (eTable 3 in the Supplement).
Figure 4. MELD-Na Scores in Women and Men Listed for LT in the Group With No pLD.
Box plot shows that women had higher listing MELD-Na score and similar MELD-Na score at dropout. MELD-Na, Model for End-stage Liver Disease Sodium; pLD, potential living donor.
Univariable and multivariable Fine-Gray competing risk regression models were built to examine the associations of variables of interest with time to transplant (eTable 4 in the Supplement). Covariates considered were sex, baseline MELD-Na score, height, change in MELD-Na score, age at listing, pLD, blood type, and interaction between pLD and sex. The interaction between presence of a pLD and sex was examined but was not significant (HR, 1.34; 95% CI, 0.99-1.81; P = 0.05) (Figure 3C and D).
Subgroup analysis across different MELD-Na strata showed that when the MELD-Na score was less than 20, the interaction between sex and pLD with MELD-Na subgroup was significant, suggesting the magnitude of the beneficial effects of pLD was more pronounced in women than in men.21 In the group with MELD-Na score less than 20, the instantaneous rate of LT was higher among both men and women with a pLD compared with no pLD. Among women, the instantaneous rate of transplant for those with a pLD was 2.74 times higher than those who did not have a pLD (HR, 2.74; 95% CI,1.91-.92; P < .001). Among men, the instantaneous rate of transplant for those with a pLD was 1.34 times higher than those who did not have a pLD (HR, 1.34; 95% CI, 1.05–1.71; P = .02). The HR was 2.04 times higher in women compared with men. There was no difference in overall access to transplant between women and men in the MELD-Na strata between 21 to 30 and more than 30, owing to availability of LDLT (eTable 5 in the Supplement).
Discussion
The MELD scoring system for DDLT allocation was designed to prioritize patients on the transplant waiting list based on their severity of illness.1 Evaluation of the association of MELD score with organ allocation has revealed the persistence of significant sex disparity in access to LT.4,22 LDLT has been increasingly undertaken to meet the shortage of deceased organ transplants and has rapidly developed as a viable alternative to DDLT.23 Our center was well placed to examine how availability of LDLT affects access to transplant, given that we perform considerable volumes of both DDLT and LDLT. Our data clearly confirm the benefit of a pLD in lower deaths or dropouts in both women and men on the transplant waiting list.24 Additionally, we discovered that the presence of a living donor was important to improve women’s access to LT. Conversely, having access only to DDLT represented a significant disadvantage to women, and their shorter height resulted in lesser access to deceased donor organs.25
In our study, women needed to have significantly higher MELD-Na scores than men to qualify for a deceased donor organ, meaning that they had to be much sicker to have access to transplant. The higher MELD-Na score in women at listing could in part be because of late referral to the transplant center. However, among candidates opting for LDLT, there was no difference in MELD-Na scores at time of listing and LT. Interestingly, women dropped out from the list at MELD-Na scores that were similar to men. Taller women had better chances of early DDLT, consistent with previously published studies.11
The addition of points to women’s MELD scores has been proposed to help women qualify for a deceased donor organ.11,16 However, our study shows that this idea may be futile as women already had a higher MELD-Na score at listing and at LT.
Therefore, living donation can circumvent women’s disadvantaged access to LT for women in the current MELD era, despite higher MELD scores. Our study demonstrates that living donation should be more widely offered and encouraged as a means by which access to LT can be equalized between women and men with decompensated cirrhosis on the transplant wait list. There are complex factors that underpin the sex disparity in deceased donor organ allocation, making it difficult to devise a solution able to fully rectify this inequity. In fact, LDLT may be the most preferable strategy to address sex inequity in access to LT, as it best addresses the problem of donor/recipient size differences. This is especially true in women with a MELD-Na score less than 20. In general, women have a lower estimated liver volume that can be easily satisfied with LDLT. LDLT has been less widely offered in the Western world owing to health care resource allocation and surgical expertise being confined to high-volume centers.21 However, the number of centers that offer living donation in the US has encouragingly increased in the last 5 years. Another option to consider is the use of split livers when living donation is not feasible, which has been associated with reduced mortality in a 2020 study,26 but this is limited by the availability of suitable donor organs to split.
Limitations
Our study limitations included a lack of access to socioeconomic data, which may affect accessibility to DDLT. Additionally, we did not account for degree of ascites while calculating body mass index in patients with decompensated cirrhosis. Nonetheless, height was a critical factor in access to DDLT. Evaluation in an even larger sample size would further consolidate our findings. However, the questions we asked herein would not be feasible to examine in a larger database, such as the Scientific Registry of Transplant Recipients, because details such as whether a patient had a pLD and specific reasons for dropout are not available. Also, grouping LDLT recipients at individual centers to examine outcomes would not accurately reflect the natural setting of a program where both LDLT and DDLT are available and performed in large numbers.
Conclusions
Our article should provoke discussion on the persistent disparity in women’s access to deceased donor organs in the current MELD era, and the role of living donation to address this inequity while helping in early access to LT in men and women. Since multiple factors contribute to this disparity, we need to develop better policies to ensure more equitable access to organs. Our findings suggest that in this era of MELD-based allocation, LDLT can help in bridging the sex-based disparity in LT and hence should be particularly encouraged among women.
eAppendix 1. Living donor practice.
eTable 1. Results of quality assessment per study
eFigure1. Cumulative incidence to transplant of listed patients and overall survival in men and women and those with DDLT and LDLT
eFigure2. Cumulative incidence of LT in patients based on MELD-Na scores
eFigure 3. Cumulative incidence to LT and overall survival in patients with and without pLD
eTable 2. MELD-Na scores in no pLD group at various time from listing
eTable 3. Competing risk multivariable model for time to LT in women with no pLD
eTable 4. Competing risk multivariable model for time to LT
eTable 5. MELD-Na scores in group with no pLD
eTable 6. Multivariable Cox PH model for time to death or dropouts due to bad outcomes
eAppendix 2. Donor sex in DDLT and LDLT
References
- 1.Freeman RB Jr, Wiesner RH, Harper A, et al. ; UNOS/OPTN Liver Disease Severity Score, UNOS/OPTN Liver and Intestine, and UNOS/OPTN Pediatric Transplantation Committees . The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8(9):851-858. doi: 10.1053/jlts.2002.35927 [DOI] [PubMed] [Google Scholar]
- 2.Mathur AK, Schaubel DE, Gong Q, Guidinger MK, Merion RM. Sex-based disparities in liver transplant rates in the United States. Am J Transplant. 2011;11(7):1435-1443. doi: 10.1111/j.1600-6143.2011.03498.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai JC, Terrault NA, Vittinghoff E, Biggins SW. Height contributes to the gender difference in wait-list mortality under the MELD-based liver allocation system. Am J Transplant. 2010;10(12):2658-2664. doi: 10.1111/j.1600-6143.2010.03326.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locke JE, Shelton BA, Olthoff KM, et al. Quantifying sex-based disparities in liver allocation. JAMA Surg. 2020;155(7):e201129. doi: 10.1001/jamasurg.2020.1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryce CL, Angus DC, Arnold RM, et al. Sociodemographic differences in early access to liver transplantation services. Am J Transplant. 2009;9(9):2092-2101. doi: 10.1111/j.1600-6143.2009.02737.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarkar M, Watt KD, Terrault N, Berenguer M. Outcomes in liver transplantation: does sex matter? J Hepatol. 2015;62(4):946-955. doi: 10.1016/j.jhep.2014.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fussner LA, Charlton MR, Heimbach JK, et al. The impact of gender and NASH on chronic kidney disease before and after liver transplantation. Liver Int. 2014;34(8):1259-1266. doi: 10.1111/liv.12381 [DOI] [PubMed] [Google Scholar]
- 8.Cholongitas E, Marelli L, Kerry A, et al. Female liver transplant recipients with the same GFR as male recipients have lower MELD scores—a systematic bias. Am J Transplant. 2007;7(3):685-692. doi: 10.1111/j.1600-6143.2007.01666.x [DOI] [PubMed] [Google Scholar]
- 9.Sharma P, Schaubel DE, Messersmith EE, Guidinger MK, Merion RM. Factors that affect deceased donor liver transplantation rates in the United States in addition to the model for end-stage liver disease score. Liver Transpl. 2012;18(12):1456-1463. doi: 10.1002/lt.23548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers RP, Shaheen AA, Aspinall AI, Quinn RR, Burak KW. Gender, renal function, and outcomes on the liver transplant waiting list: assessment of revised MELD including estimated glomerular filtration rate. J Hepatol. 2011;54(3):462-470. doi: 10.1016/j.jhep.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 11.Allen AM, Heimbach JK, Larson JJ, et al. Reduced access to liver transplantation in women: role of height, MELD exception scores, and renal function underestimation. Transplantation. 2018;102(10):1710-1716. doi: 10.1097/TP.0000000000002196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mindikoglu AL, Emre SH, Magder LS. Impact of estimated liver volume and liver weight on gender disparity in liver transplantation. Liver Transpl. 2013;19(1):89-95. doi: 10.1002/lt.23553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rustgi VK, Marino G, Halpern MT, Johnson LB, Umana WO, Tolleris C. Role of gender and race mismatch and graft failure in patients undergoing liver transplantation. Liver Transpl. 2002;8(6):514-518. doi: 10.1053/jlts.2002.33457 [DOI] [PubMed] [Google Scholar]
- 14.Washburn K, Edwards E, Harper A, Freeman R. Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system. Am J Transplant. 2010;10(7):1643-1648. doi: 10.1111/j.1600-6143.2010.03127.x [DOI] [PubMed] [Google Scholar]
- 15.Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999-2008. Am J Transplant. 2010;10(4, pt 2):1003-1019. doi: 10.1111/j.1600-6143.2010.03037.x [DOI] [PubMed] [Google Scholar]
- 16.Huo SC, Huo TI, Lin HC, et al. Is the corrected-creatinine model for end-stage liver disease a feasible strategy to adjust gender difference in organ allocation for liver transplantation? Transplantation. 2007;84(11):1406-1412. doi: 10.1097/01.tp.0000282867.92367.d0 [DOI] [PubMed] [Google Scholar]
- 17.Shah SA, Levy GA, Greig PD, et al. Reduced mortality with right-lobe living donor compared to deceased-donor liver transplantation when analyzed from the time of listing. Am J Transplant. 2007;7(4):998-1002. doi: 10.1111/j.1600-6143.2006.01692. [DOI] [PubMed] [Google Scholar]
- 18.Berg CL, Gillespie BW, Merion RM, et al. ; A2ALL Study Group . Improvement in survival associated with adult-to-adult living donor liver transplantation. Gastroenterology. 2007;133(6):1806-1813. doi: 10.1053/j.gastro.2007.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sapisochin G, Goldaracena N, Laurence JM, Levy GA, Grant DR, Cattral MS. Right lobe living-donor hepatectomy-the Toronto approach, tips and tricks. Hepatobiliary Surg Nutr. 2016;5(2):118-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorgen A, Goldaracena N, Zhang W, et al. Surgical complications after right hepatectomy for live liver donation: largest single-center Western world experience. Semin Liver Dis. 2018;38(2):134-144. doi: 10.1055/s-0038-1636932 [DOI] [PubMed] [Google Scholar]
- 21.Kim PT, Testa G. Living donor liver transplantation in the USA. Hepatobiliary Surg Nutr. 2016;5(2):133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moylan CA, Brady CW, Johnson JL, Smith AD, Tuttle-Newhall JE, Muir AJ. Disparities in liver transplantation before and after introduction of the MELD score. JAMA. 2008;300(20):2371-2378. doi: 10.1001/jama.2008.720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermann HC, Klapp BF, Danzer G, Papachristou C. Gender-specific differences associated with living donor liver transplantation: a review study. Liver Transpl. 2010;16(3):375-386. doi: 10.1002/lt.22002 [DOI] [PubMed] [Google Scholar]
- 24.Berg CL, Merion RM, Shearon TH, et al. Liver transplant recipient survival benefit with living donation in the model for endstage liver disease allocation era. Hepatology. 2011;54(4):1313-1321. doi: 10.1002/hep.24494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathur AK, Schaubel DE, Zhang H, Guidinger MK, Merion RM. Disparities in liver transplantation: the association between donor quality and recipient race/ethnicity and sex. Transplantation. 2014;97(8):862-869. doi: 10.1097/01.tp.0000438634.44461.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowring M, Schwarz KB, Cameron AM, Segev DL, Mogul D. Oral abstracts #5 survival benefit of split liver transplantation for pediatric and adult candidates. Hepatology. 2020;72(S1):3A-4A. doi: 10.1002/hep.31578 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Living donor practice.
eTable 1. Results of quality assessment per study
eFigure1. Cumulative incidence to transplant of listed patients and overall survival in men and women and those with DDLT and LDLT
eFigure2. Cumulative incidence of LT in patients based on MELD-Na scores
eFigure 3. Cumulative incidence to LT and overall survival in patients with and without pLD
eTable 2. MELD-Na scores in no pLD group at various time from listing
eTable 3. Competing risk multivariable model for time to LT in women with no pLD
eTable 4. Competing risk multivariable model for time to LT
eTable 5. MELD-Na scores in group with no pLD
eTable 6. Multivariable Cox PH model for time to death or dropouts due to bad outcomes
eAppendix 2. Donor sex in DDLT and LDLT