Key Points
Question
Is statin therapy associated with atherosclerotic plaque progression as assessed across a range of density measurements by coronary computed tomography angiography?
Findings
In this cohort study assessing serial coronary computed tomography angiographic images of 2458 coronary lesions among 857 patients, untreated coronary lesions progressed in volume for all 6 compositional plaque types—low attenuation (−30 to 75 Hounsfield units [HU]), fibro-fatty (76-130 HU), fibrous (131-350 HU), low-density calcium (351-700 HU), high-density calcium (701-1000 HU), and 1K (>1000 HU) plaque—whereas statin therapy was associated with decreases in low-attenuation and fibro-fatty plaque and with greater progression of high-density calcium and 1K plaque. Statin therapy was not associated with a change in calcified plaque but with a transformation toward more dense calcium, which was associated with slower overall plaque progression.
Meaning
These results suggest an association of statin use with greater rates of transformation of coronary atherosclerosis toward high-density calcium, supporting the concept of reduced atherosclerotic risk with increased densification of calcium.
Abstract
Importance
The density of atherosclerotic plaque forms the basis for categorizing calcified and noncalcified morphology of plaques.
Objective
To assess whether alterations in plaque across a range of density measurements provide a more detailed understanding of atherosclerotic disease progression.
Design, Setting, and Participants
This cohort study enrolled 857 patients who underwent serial coronary computed tomography angiography 2 or more years apart and had quantitative measurements of coronary plaques throughout the entire coronary artery tree. The study was conducted from 2013 to 2016 at 13 sites in 7 countries.
Main Outcomes and Measures
The main outcome was progression of plaque composition of individual coronary plaques. Six plaque composition types were defined on a voxel-level basis according to the plaque attenuation (expressed in Hounsfield units [HU]): low attenuation (−30 to 75 HU), fibro-fatty (76-130 HU), fibrous (131-350 HU), low-density calcium (351-700 HU), high-density calcium (701-1000 HU), and 1K (>1000 HU). The progression rates of these 6 compositional plaque types were evaluated according to the interaction between statin use and baseline plaque volume, adjusted for risk factors and time interval between scans. Plaque progression was also examined based on baseline calcium density. Analysis was performed among lesions matched at baseline and follow-up. Data analyses were conducted from August 2019 through March 2020.
Results
In total, 2458 coronary lesions in 857 patients (mean [SD] age, 62.1 [8.7] years; 540 [63.0%] men; 548 [63.9%] received statin therapy) were included. Untreated coronary lesions increased in volume over time for all 6 compositional types. Statin therapy was associated with volume decreases in low-attenuation plaque (β, −0.02; 95% CI, −0.03 to −0.01; P = .001) and fibro-fatty plaque (β, −0.03; 95% CI, −0.04 to −0.02; P < .001) and greater progression of high-density calcium plaque (β, 0.02; 95% CI, 0.01-0.03; P < .001) and 1K plaque (β, 0.02; 95% CI, 0.01-0.03; P < .001). When analyses were restricted to lesions without low-attenuation plaque or fibro-fatty plaque at baseline, statin therapy was not associated with a change in overall calcified plaque volume (β, −0.03; 95% CI, −0.08 to 0.02; P = .24) but was associated with a transformation toward more dense calcium. Interaction analysis between baseline plaque volume and calcium density showed that more dense coronary calcium was associated with less plaque progression.
Conclusions and Relevance
The results suggest an association of statin use with greater rates of transformation of coronary atherosclerosis toward high-density calcium. A pattern of slower overall plaque progression was observed with increasing density. All findings support the concept of reduced atherosclerotic risk with increased densification of calcium.
This cohort study uses coronary computed tomography angiography to investigate whether statin therapy is associated with alterations in the volume or calcium density of 6 compositional atherosclerotic plaque types in patients with coronary artery disease.
Introduction
The atherosclerotic plaque burden in the coronary tree has been associated with increased risk of future major cardiovascular events.1 The larger the volume of atherosclerotic plaque, the higher the likelihood of plaque destabilization (rupture or erosion) leading to vascular thrombosis and occlusion of the coronary artery.2 Atherosclerotic features associated with ruptured plaques are large necrotic cores with an inflamed and thin fibrous cap.3 However, plaque features hypothesized to contribute to plaque stability are small necrotic cores that have been replaced by sheets of calcification.
Through the use of intravascular ultrasonography (IVUS) or coronary computed tomography angiography (CCTA), statin therapy has been associated with a decrease in lipid-rich plaque and an increase in calcification.4,5 Although a large plaque burden corroborates with high future risk, it may be possible that atherosclerosis that is predominantly calcified may portend reduced risk. Using coronary artery calcium scoring from non–contrast-enhanced computed tomography (CT), indeed, higher-density calcium was associated with lower major event risk.6 In line with this, members of our group previously showed lower rates of future acute coronary syndromes (ACSs) for 1K plaque (very dense calcium >1000 Hounsfield units [HU]).7
Calcium as assessed by CCTA is typically considered 1 compositional type, and its density is not considered separately. However, 2 prior studies indicate that a more nuanced approach refining density ranges further may be necessary.6,7 Low-density calcium may not portend the same low-risk status as higher-density calcium, such as 1K plaque.7 From findings of the serial Progression of Atherosclerotic Plaque Determined by Computed Tomographic Angiography Imaging (CCTA PARADIGM) study,8 we hypothesized that alterations in plaque over time—across a range of density measurements—provide a more detailed understanding of atherosclerotic disease progression. First, changes in density subgroups of noncalcified plaque and calcified plaque were examined according to statin use. Second, plaque progression over time was evaluated by baseline calcium density.
Methods
Patients
The PARADIGM study is a dynamic, multinational observational registry that included patients who underwent clinically indicated serial CCTA.8 From 13 sites in 7 countries, 2252 consecutive patients with suspected or known coronary artery disease undergoing serial CCTA 2 or more years apart were enrolled from 2013 to 2016 (eFigure 1 in the Supplement). For the present cohort study, 492 patients with noninterpretable CCTA findings on a 0.5-mm slice basis, 431 patients without lesions present both at baseline and follow-up, 237 patients initiating or stopping statin therapy after baseline CCTA, and 235 patients with unknown information regarding statin use at baseline or follow-up were excluded. Furthermore, to allow for longitudinal assessment of plaque volume changes over time, tandem lesions at baseline that were confluent at follow-up were also excluded. Statin therapy was defined as the use of statins at baseline and follow-up CCTA, whereas no statin use was defined as no statin use before both CCTA scans. In a sensitivity analysis, a third group—patients who started statin therapy after baseline CCTA—was compared with statin-naive patients at both scans, with results presented in eFigure 3, eTable 6, and eTable 7 in the Supplement. This group may be more similar in clinical risk profile with patients not using statins than patients who were receiving statins at both CT scans. The study protocol was approved by the institutional review boards of all participating centers. When required, patients provided written informed consent that was obtained in a manner consistent with the Common Rule requirements. No one received compensation or was offered any incentive for participating in this study.
CCTA Image Analysis
The CT scans were acquired in accordance with the Society of Cardiovascular Computed Tomography guidelines.9 Baseline and follow-up CCTA DICOM (Digital Imaging and Communications in Medicine) files from each patient were transferred to a core laboratory for blinded image analysis. Coronary plaque was evaluated on multiplanar and cross-sectional images, and readers with level III experience and blinded to clinical data performed quantitative plaque analysis using dedicated semiautomated software with manual adjustments (QAngio CT Research Edition version 2.1.9.1; Medis Medical Imaging Systems).
Plaque quantification methods have been previously described.5 In brief, all coronary arteries, including side branches 2 mm or more in diameter, were evaluated. Atherosclerosis was defined as any tissue 1 mm2 or more within or adjacent to the lumen that could be discriminated from surrounding lumen, epicardial fat, and pericardial tissue and was identified in 2 or more planes.1 Coronary lesions were quantified for plaque volumes, and volumes of several composition types were derived based on fixed HU thresholds, on a voxel-level basis: low-attenuation plaque (LAP; −30 to 75 HU), fibro-fatty plaque (76-130 HU), fibrous plaque (131-350 HU), low-density calcium (351-700 HU), high-density calcium (701-1000 HU), and 1K plaque (>1000 HU). Ranges for LAP were defined according to histologic comparisons with CCTA.10 For longitudinal comparisons, coronary lesions at baseline and follow-up were coregistered using fiduciary landmarks, such as the distance from the ostium of the artery or side branches, as previously described.5 Changes in per-lesion plaque volumes (by composition) were calculated by subtracting volumes at baseline from follow-up. Core laboratory interobserver and intraobserver variability for plaque volume and the several composition types were excellent (all ≥0.95).5 Spotty calcification, a commonly used high-risk plaque feature, was defined as calcification less than 3 mm surrounded by noncalcified plaque. Compositional plaque volumes were provided according to the presence of spotty calcification.
Outcomes
Analysis was performed on a per-lesion level. First, progression or regression of LAP, fibro-fatty, fibrous, low-density calcium, high-density calcium, and 1K plaque according to statin use were evaluated by the interaction term between baseline plaque volume and statin use. Absolute changes in plaque volume over time are dependent on baseline plaque volume11; hence, larger progression and regression rates by statin use may be expected in larger baseline lesions. Analyses were also performed stratified by the median CT interval. Second, to isolate the association between statin use and coronary calcium density, the interactions between baseline plaque volume and statin use were examined among coronary lesions without LAP or fibro-fatty plaque. Third, the association of baseline calcium density with the progression of total plaque volume was evaluated by the interaction between baseline plaque volume and percentage of calcium being low-density, high-density, or 1K.
Statistical Analysis
Continuous data are presented as mean (SD) values, regardless of distribution, for uniformity of presentation. The t test or the Mann-Whitney test was used for comparison of continuous data, and the χ2 test was used for categorical data, as appropriate. Associations between statin use and change in plaque volume were assessed using linear mixed models with random intercept to account for the within-patient clustering of coronary lesions. Interactions between statin use and baseline plaque volume were adjusted for the 2 main effects, age, sex, diabetes, hypertension, smoking status, body mass index, and the CT interval, based on clinical judgment. Positive interaction terms between baseline plaque volume and statin use may be interpreted as statin use associated with a larger increase of the outcome when the baseline plaque volume is larger, and vice versa. For visual interpretation of the interaction terms, the estimated changes from the statistical models are plotted according to baseline plaque volume. Analyses were performed from August 2019 through March 2020 using SPSS software, version 25 (IBM Corp). A 2-sided value of P < .05 was considered statistically significant.
Results
Patients
Of 2252 patients, 857 (mean [SD] age, 62.1 [8.7] years; 540 [63.0%] men) were included in the study. Inclusion and exclusion criteria are shown in eFigure 1 in the Supplement. Compared with 309 patients who did not receive statin therapy, 548 patients who received statins were older (mean [SD] age, 61.1 [8.7] vs 62.6 [8.7] years), more often men (181 [58.6%] vs 359 [65.5%]), and presented more frequently with diabetes (62 [20.1%] vs 161 [29.4%]) and hypertension (156 [50.5%] vs 350 [64.1%]) (Table 1). Statin use was associated with lower mean (SD) low-density lipoprotein cholesterol levels at baseline (107 [40] mg/dL vs 113 [29] mg/dL; P = .03) and at follow-up (88 [31] mg/dL vs 110 [30] mg/dL; P < .001) (to convert milligrams per deciliter to millimoles per liter, multiply by 0.0259).
Table 1. Baseline Characteristics of Patients.
| Characteristic | Statin use, No. (%) of patients | P value | |
|---|---|---|---|
| Yes (n = 548) | No (n = 309) | ||
| Demographic characteristics | |||
| Age, mean (SD), y | 62.6 (8.7) | 61.1 (8.7) | .01 |
| Male | 359 (65.5) | 181 (58.6) | .04 |
| BMI, mean (SD) | 25.6 (3.4) | 25.7 (3.3) | .56 |
| CT interval, y | |||
| 2-4 | 362 (66.1) | 223 (72.2) | .15 |
| 4-6 | 141 (25.3) | 62 (20.1) | |
| >6 | 45 (8.2) | 24 (7.8) | |
| Location | |||
| Korea | 376 (68.6) | 245 (79.2) | .22 |
| Canada | 17 (3.1) | 10 (3.2) | |
| Europe | 145 (26.5) | 45 (14.6) | |
| Brazil | 10 (1.8) | 9 (2.9) | |
| Risk factors | |||
| Diabetes | 161 (29.4) | 62 (20.1) | .003 |
| Hypertension | 350 (63.9) | 156 (50.5) | <.001 |
| Family history for CAD | 135 (24.6) | 67 (21.7) | .33 |
| Currently smoking | 100 (18.3) | 54 (17.5) | .78 |
| Medication use at baseline | |||
| Aspirin | 359 (65.5) | 101 (32.7) | <.001 |
| ACE inhibitor or ARB | 230 (42.0) | 79 (25.6) | <.001 |
| β-Blocker | 237 (43.3) | 63 (20.5) | <.001 |
| Lipid profile at baseline | |||
| Cholesterol, mean (SD), mg/dL | |||
| Total | 180 (44) | 186 (35) | .05 |
| LDL | 107 (40) | 113 (29) | .03 |
| HDL | 49 (13) | 50 (13) | .72 |
| Lipid profile at follow-up | |||
| Cholesterol, mean (SD), mg/dL | |||
| Total | 159 (37) | 181 (34) | <.001 |
| LDL | 88 (31) | 110 (30) | <.001 |
| HDL | 49 (13) | 49 (12) | .51 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CAD, coronary artery disease; CT, computed tomography; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
SI conversion factors: To convert total cholesterol, LDL, or HDL to millimoles per liter, multiply by 0.0259.
Baseline Compositional Plaque Volumes
A total of 2458 lesions were evaluated, of which 1658 (67.5%) were among patients receiving statin therapy. Mean compositional plaque volumes of the lesions are presented in eFigure 2 in the Supplement. At baseline and follow-up, lesions in patients treated with statins vs patients who were not treated with statins had a lower volume of LAP and higher volumes of all 3 calcium subtypes (eFigure 2 in the Supplement). Compared with nonspotty calcification lesions, spotty calcification lesions were composed of greater noncalcified plaque components and low-density calcium, but not more high-density calcium or 1K plaque, (eTable 1 in the Supplement). With increasing density of plaque calcification, the proportion of LAP, fibro-fatty plaque, and fibrous plaque decreased (eTable 2 in the Supplement).
Progression of Plaque Volume in Patients With or Without Statin Therapy
Table 2 gives the adjusted interaction terms between baseline plaque volume and statin use for the change in LAP, fibro-fatty plaque, fibrous plaque, low-density calcium, high-density calcium, and 1K plaque. Changes in the volume of the 6 plaque components with or without statin treatment are shown in Figure 1. Statin therapy was associated with larger decreases in LAP (β, −0.02; 95% CI, −0.03 to −0.01; P = .001 for interaction) and fibro-fatty plaque (β, −0.03; 95% CI, −0.04 to −0.02; P < .001 for interaction) volumes compared with no statin therapy. Without statin therapy, LAP and fibro-fatty plaque volumes progressed with larger baseline plaque volumes. No significant interaction between baseline plaque volume and statin therapy was observed for changes in fibrous plaque (β, −0.01; 95% CI, −0.03 to 0.02; P = .58 for interaction) and for low-density calcium volume (β, −0.01; 95% CI, −0.03 to 0.003; P = .11 for interaction). Statin therapy was associated with a larger increase in high-density calcium volume (β, 0.02; 95% CI, 0.01-0.03; P < .001 for interaction) and 1K plaque volume (β, 0.02; 95% CI, 0.01-0.03; P < .001 for interaction) compared with no statin therapy. When stratifying the analyses by the median CT interval (3.2 years), significant associations of larger reductions in LAP and fibro-fatty plaque volumes and larger increases in high-density calcium and 1K plaque were observed only in lesions rescanned in the longer CT-interval cohort (eTable 3 in the Supplement).
Table 2. Baseline Plaque Volume and Statin Use Associated With Change in Compositional Volumetric Plaque.
| Modela | Change in compositional volumetric plaque | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low-attenuation plaque | Fibro-fatty plaque | Fibrous plaque | Low-density calcium | High-density calcium | 1K plaque | |||||||
| Adjusted β coefficient (95% CI)b | P value | Adjusted β coefficient (95% CI)b | P value | Adjusted β coefficient (95% CI)b | P value | Adjusted β coefficient (95% CI)b | P value | Adjusted β coefficient (95% CI)b | P value | Adjusted β coefficient (95% CI)b | P value | |
| Plaque volume at baseline, mm3 | 0.003 (−0.006 to 0.012) | .47 | 0.014 (0.003 to 0.026) | .01 | 0.01 (−0.01 to 0.04) | .19 | 0.10 (0.09 to 0.12) | <.001 | 0.04 (0.03 to 0.05) | <.001 | 0.025 (0.017 to 0.033) | <.001 |
| Statin use, No. | −0.27 (−1.26 to 0.72) | .60 | 0.09 (−1.15 to 1.33) | .89 | −2.51 (−4.79 to −0.24) | .03 | 0.31 (−1.20 to 1.81) | .69 | 0.34 (−0.42 to 1.09) | .38 | 0.03 (−0.71 to 0.77) | .94 |
| Plaque volume at baseline × statin use | −0.02 (−0.03 to −0.01) | .001 | −0.03 (−0.04 to −0.02) | <.001 | −0.01 (−0.03 to 0.02) | .58 | −0.01 (−0.03 to 0.003) | .11 | 0.02 (0.01 to 0.03) | <.001 | 0.02 (0.01 to 0.03) | <.001 |
Generalized linear mixed models with random intercept.
Adjusted for age, sex, diabetes, hypertension, smoking status, body mass index, and computed tomography interval.
Figure 1. Compositional Plaque Changes According to Baseline Plaque Volume and Statin Use.
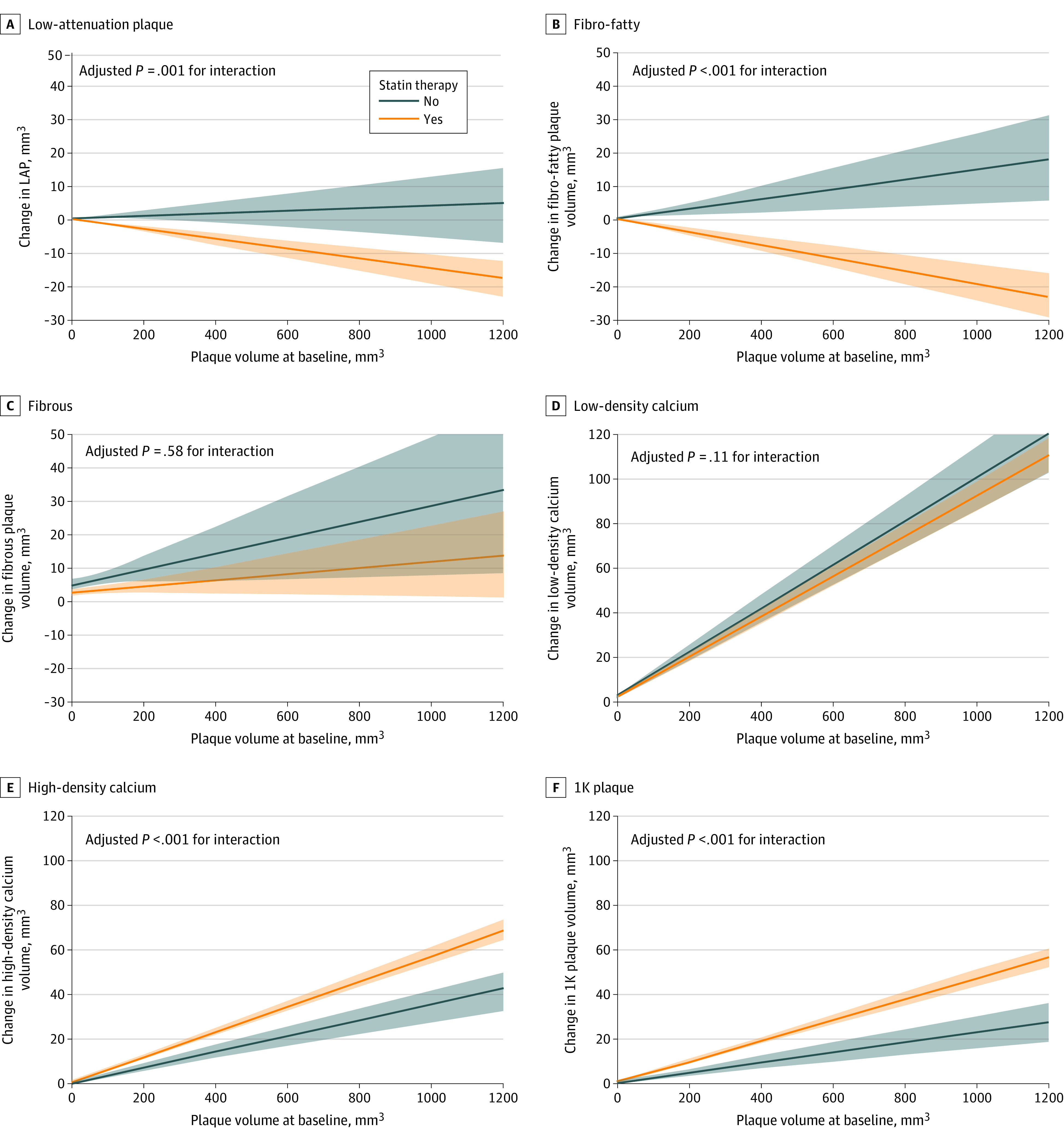
Estimated changes in low-attenuation plaque (LAP), fibro-fatty plaque, fibrous plaque, low-density calcium, high-density calcium, and 1K plaque are presented according to baseline plaque volume and statin use. The estimated changes (bold lines) and 95% CIs (shaded areas) are derived from a generalized linear model, including baseline plaque volume, statin use, and the interaction term. The P values for interaction are derived from Table 2. Without statin therapy, increasing trends for all noncalcified and calcified density subgroups are observed, whereas with statin therapy, significant decreases in LAP and fibro-fatty plaque and larger increases in high-density calcium and 1K plaque are observed.
Association of Statin Use With Calcium Density
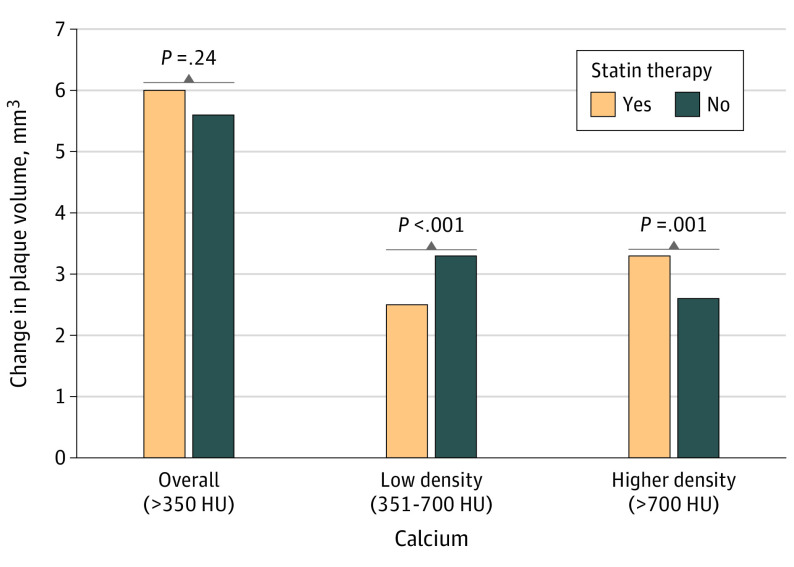
When restricting the analysis to 591 lesions—400 lesions (67.7%) among patients who received statin therapy—without LAP or fibro-fatty plaque, statin use was not associated with the change in overall calcium (>350 HU) volume (β, −0.03; 95% CI, −0.08 to 0.02; P = .24 for interaction) (eTable 4 in the Supplement). However, less progression of low-density calcium (351-700 HU) was observed with statin use (β, −0.08; 95% CI, −0.12 to −0.03; P < .001 for interaction), whereas the progression of higher-density calcification (>700 HU) was greater (β, 0.06; 95% CI, 0.02-0.09; P = .001 for interaction) with statin use (Figure 2). Examples of coronary plaques that evolve over time with or without statin therapy are shown in Figure 3.
Figure 2. Calcium Densification With Statin Therapy.
Data are restricted to lesions without low-attenuation and fibro-fatty plaque. Estimated changes in overall calcium, low-density calcium, and higher-density calcium according to statin therapy. Estimated changes in plaque volume are shown for the mean baseline plaque volume, the mean of other continuous variables, and the geometric mean of categorical variables. P values represent the interaction between plaque volume and statin use, adjusted for age, sex, diabetes, hypertension, smoking status, body mass index, and computed tomography interval, derived from linear mixed models. HU indicates Hounsfield units.
Figure 3. Coronary Atherosclerosis Progression With or Without Statin Therapy.
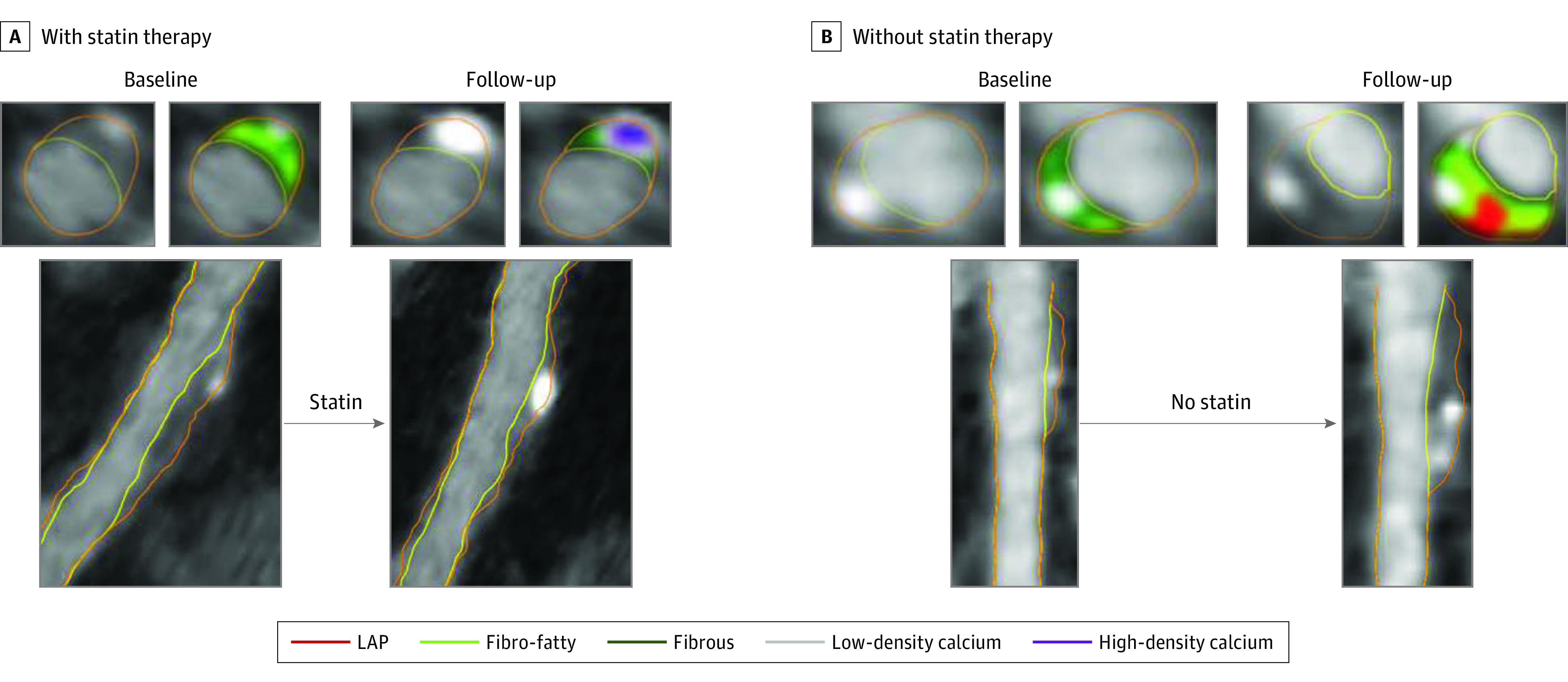
Progression of plaque over time in coronary lesions of patients treated with or without statins. For each of the 4 time points, 1 multiplanar reconstruction is provided with 2 cross-sectional views obtained at the same site at baseline and follow-up. A, Statin therapy is associated with the reduction of fibro-fatty (light green) plaque together with densification of the calcification (from low-density calcium [gray] to high-density calcium [purple]). B, Plaque expansion of both noncalcified and calcified plaque is observed. LAP indicates low-attenuation plaque.
Plaque Progression Rates by Calcium Density
Lesions with a higher proportion of calcium exhibited less overall plaque progression, either statin treated (β, −0.003; 95% CI, −0.004 to −0.002; P < .001) or not (β, −0.003; 95% CI, −0.005 to −0.002; P < .001) (eTable 5 in the Supplement). For patients who received statin therapy, a higher proportion of both high-density calcium (β, −0.005; 95% CI, −0.007 to −0.003; P < .001) and 1K plaque (β, −0.007; 95% CI, −0.009 to −0.005; P < .001) were associated with less plaque progression (P < .001 for interaction). For patients not using statins, only 1K plaque was associated with less plaque progression (β, −0.008; 95% CI, −0.013 to −0.002; P = .01 for interaction). When restricted to coronary lesions with high-density calcium or 1K plaque, 1K plaque was associated with the lowest plaque progression (β, −0.006; 95% CI, −0.009 to 0.003; P < .001 for interaction with statin; and β, −0.007; 95% CI, −0.015 to 0.001; P = .07 for interaction without statin).
Patients Starting Statin Therapy After Baseline CCTA
In the sensitivity analysis of patients who started receiving statin therapy after their baseline CCTA, baseline age, sex, and prevalence of diabetes or hypertension were comparable to patients not receiving statin therapy (eTable 6 in the Supplement). Low-density lipoprotein cholesterol levels were higher at baseline but lower at follow-up in patients who started statin therapy. Baseline plaque volumes of low- and high-density calcium were higher for patients who started statin therapy (eTable 7 in the Supplement). Newly initiated statin therapy was associated with larger reductions in LAP (β, −0.03; 95% CI, −0.05 to −0.01; P < .001 for interaction) and fibro-fatty plaque (β, −0.05; 95% CI, −0.07 to −0.03; P < .001 for interaction), and greater progression of the 3 calcium subtypes (β, 0.03; 95% CI, 0.01-0.05; P = .001 for interaction for low-density calcium; β, 0.02; 95% CI, 0.01-0.03; P < .001 for interaction for high-density calcium; and β, 0.02; 95% CI, 0.01-0.03; P < .001 for interaction for for 1K plaque) (eFigure 3 in the Supplement). When the analysis was restricted to lesions without LAP or fibro-fatty plaque, the association between statin therapy and low-density calcium progression was not significant (β, 0.02; 95% CI, −0.05 to 0.08; P = .62 for interaction), whereas greater progression was observed for calcium density higher than 700 HU (β, 0.30; 95% CI, 0.25-0.35; P < .001 for interaction) (eFigure 4 in the Supplement).
Discussion
Our analysis provides insight into the magnitude and directionality of coronary atherosclerotic disease progression, including observations following intercurrent preventive therapy. We observed a natural trend toward progression of both noncalcified and calcified plaque of atherosclerosis. This trend appeared to be modified in association with statin therapy to a decrease in LAP and fibro-fatty plaque volumes along with larger increases of high-density calcium and 1K plaque compared with plaques in patients not treated with statins. These findings appear to be associated with a seesaw effect, whereby plaque transforms toward higher-density calcification. Specifically, statin therapy was not associated with overall calcified plaque progression but with a transformation toward denser calcium. Finally, distinct differences in the overall plaque progression rates were observed according to calcium density, with the slowest progression for lesions with the densest calcium (1K plaque).
Natural History of Plaque Transformation Over Time
Serial CCTA and IVUS studies have shown a natural trend for coronary atherosclerosis to progress over time.4,5,12,13 Puri et al4 evaluated 224 patients not treated with statins undergoing serial IVUS 18 months apart and observed an increase in total atheroma volume. This also included a significant increase in calcified plaque, which was evaluated by a calcification index based on the number of slices with calcium and calcification arc. Similar findings of increased noncalcified and calcified plaque progression without statin therapy have been reported with serial CCTA.5,12,14,15 These observations reflect the natural disease progression of atherosclerosis. When plaques evolve, macrophages and smooth muscles cells within the necrotic core die, resulting in release of free calcium and phosphate that will crystalize and form microcalcifications. Microcalcifications coalesce to speckled fragments that may further evolve into larger sheets of dense calcium, which are detected by CCTA as dense calcified plaque.16
The present study extends prior findings by members of our group15 by examining atherosclerosis progression according to the volume of noncalcified and calcified plaques and the density of calcified plaque. Lee et al15 showed that the coronary artery calcium score progressed in patients using statins associated with a reduction in noncalcified plaque, whereas both calcified and noncalcified plaques progressed in patients not treated with statins.
Without statins, all subgroups of noncalcified and calcified plaques were associated with increasing trends for progression in our study. With the use of statins, the volumes of LAP and fibro-fatty plaque decreased, whereas the volumes of high-density calcium and 1K calcium increased. In addition, statins were not associated with an increase in calcified plaque volume, but an apparent transformation of calcium toward higher density.
Recent literature has highlighted the importance of the association between coronary calcium density and altered patterns of disease risk.6,7 Criqui et al6 examined individuals from the Multi-Ethnic Study of Atherosclerosis (MESA) and observed that higher calcium density on non–contrast-enhanced CT images was associated with reduced risk for future major vascular events. Members of our group previously showed that very dense calcium from CCTA images (1K plaque) was associated with a reduced risk for ACS.7
The contradictory behavior of low-density and higher-density calcium may be reflective of different stages in the calcification cascade. Microcalcifications (0.5-15 μm) represent the earliest form of calcification and are commonly observed in the deeper area of necrotic core, as the results of dying smooth muscle cells and macrophages.17,18 Microcalcifications coalesce into larger masses over time and form speckles and fragments of calcifications, and later, dense sheets. Owing to resolution issues, microcalcifications cannot be reliably detected with CT, but larger calcifications will be detected with increasing attenuation (Hounsfield units). Our results suggest that low-density calcium represents calcification early in the evolutionary cascade toward stable dense calcific sheets. Plaques with low-density calcium only (lacking higher-density calcium) were predominantly noncalcified, whereas 1K plaques were predominantly calcified. Our observation of a reduced volume of low-density calcium with statin therapy may be explained by a concomitant reduction in surrounding necrotic core and coalescing calcium fragments into larger masses, resulting in a shift toward a greater volume of higher-attenuation calcium. Explanations for accelerated calcification with statins remain speculative, but recent research suggests that this acceleration may be partly due to modulation of the macrophage Rac1-interleukin 1β signaling axis.19
The present study builds on prior analyses by members of our group concerning calcium density groups and risk for future ACS.7,20 In that study,7 189 patients who experienced ACS vs 189 patients who did not experience ACS after baseline CCTA imaging were matched based on age, sex, cardiovascular risk factors, and coronary disease severity. Although the mean (SD) low-density calcium was similar between groups (57.1 [73.6] mm3 vs 66.8 [99.1] mm3; P = .61), patients with ACS had less 1K plaque (mean [SD], 3.9 [8.3] mm3 vs 9.4 [23.2] mm3; P = .02). In addition, baseline CCTA culprit precursor lesions had less 1K plaque than the respective control lesions.
The findings of the present study provide insight into the compositional changes associated with statins, which may (partially) explain the effects on major vascular event reduction and may have implications for interpretation of plaque progression on serial CCTA. Volumetric progression of calcium higher than 700 HU may have protective implications, but this hypothesis requires further study. As a corollary, the prognostic power of coronary artery calcium and coronary artery calcium progression may lie not in its correlation of stable calcification with underlying plaque volume, but in its direct visualization of low-density calcium that is dynamically generated despite statin use and calcium densification.
Three prior trials—2 that randomized patients to groups with different statin intensity treatment regimens and 1 placebo-controlled trial—did not find differences in calcified volume or Agatston score progression on serial non–contrast-enhanced CT scanning.21,22,23 In light of the present study findings, there can be numerous explanations for those negative trial results. Despite a similar increase in the volume of calcified plaque, the calcium density may have increased in the groups with the highest-intensity statin regimens, but this was not evaluated. Furthermore, the potential of statins to reduce new plaque formation may have been offset by accelerated calcification of noncalcified plaque, eventually resulting in a null effect. Indeed, low-density lipoprotein cholesterol levels were effectively lowered in the most intensive treatment groups. The present study aimed to limit this latter effect by examining interactions of statin use with baseline plaque, given that the largest changes associated with statin therapy may be expected within the largest plaques.
The sensitivity analysis included patients who started receiving statins after baseline CCTA, and the results were confirmatory to the main study findings. The patients were more comparable in clinical risk profile to the statin nonusers than to patients using statins at the time of both CT scans.
Plaque Progression by Calcium Density
Calcified plaque was associated with slower plaque progression, similar to findings of serial IVUS,24 with the slowest progression for 1K plaque. Lesions with 1K plaque had little noncalcified plaque, similar to end-stage fibrocalcific plaques, which may explain the absence of plaque expansion in these lesions.
Spotty calcification (considered a high-risk plaque feature that has been associated with future ACS) was characterized by large volumes of noncalcified plaque and low-density calcification, but not by high-density calcification or 1K plaque. This less favorable plaque phenotype may explain its value in risk assessment but also highlights that any independent prognostic value of spotty calcification needs more study.25
Limitations
The observational design of the study represents a limitation, including nonrandomized assignment of statin therapy. This resulted in differences in clinical risk profile and baseline plaque volume and was adjusted for in multivariable models, but unknown confounders may still exist. In addition, causality between statin use and compositional plaque changes cannot be inferred. Follow-up CCTA was performed only when clinically indicated and not, ideally, performed systematically as was done by Smit et al.14 This approach introduced selection bias because patients with more rapid plaque progression eventually experiencing an event may not have been indicated for follow-up CCTA, whereas other patients who were clinically stable were not referred for follow-up CCTA. Hence, generalizability to higher-risk and lower-risk patients is not possible. The CT interval between baseline CT and follow-up CT was not standardized. Chronic total occlusions and lesions that had coalesced at follow-up were not evaluated, which may further limit generalizability. The prognostic implications of compositional plaque changes were not investigated because of the small number of hard event points; however, these implications should be further studied. Furthermore, fixed Hounsfield unit thresholds were applied, and the luminal contrast attenuation or the kilovolts provided have been known to influence the attenuation values of plaque compositional subtypes.
Conclusions
The present study provides insight into the magnitude and directionality of coronary atherosclerotic disease progression, including reductions in LAP and fibro-fatty plaque along with concomitant increases in high-density calcium and 1K plaque associated with statin use. These findings may be associated with a seesaw effect, whereby plaque transforms toward a higher-density atherosclerotic plaque. Specifically, statin therapy was not associated with overall progression but with a transformation toward denser calcium. Finally, distinct differences in overall plaque progression rates were observed by calcium density, with the slowest progression for lesions with the most dense calcium (1K plaque).
eFigure 1. Flowchart
eFigure 2. Plaque Volumes by Compositional Type at Baseline and Follow-up CCTA
eFigure 3. Compositional Plaque Changes According to Baseline Plaque Volume in Patients Begun on Statin Treatment After Baseline CCTA
eFigure 4: Calcium Densification With Statin Therapy
eTable 1. Atherosclerotic Profile per Coronary Lesions at Baseline
eTable 2. Plaque Composition Proportions According the Presence of Low-Density, High-Density Calcium, or 1K Plaque
eTable 3. Interaction Baseline Plaque Volume and Statin With Compositional Plaque Change, Stratified by the Median CT Interval
eTable 4. Statin Use and Progression of Calcium Density Subgroups, Restricted to Lesions Without Necrotic Core or Fibro-Fatty Plaque
eTable 5. Plaque Progression According to Calcium and Calcium Density Subgroups
eTable 6. Baseline Characteristics of Patients Who Began on Statins After Baseline CCTA and Patients not on Statins
eTable 7. Baseline Plaque Volumes According to Newly Started Statin and No Statin Use
References
- 1.Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50(12):1161-1170. doi: 10.1016/j.jacc.2007.03.067 [DOI] [PubMed] [Google Scholar]
- 2.Arbab-Zadeh A, Fuster V. The risk continuum of atherosclerosis and its implications for defining CHD by coronary angiography. J Am Coll Cardiol. 2016;68(22):2467-2478. doi: 10.1016/j.jacc.2016.08.069 [DOI] [PubMed] [Google Scholar]
- 3.Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336(18):1276-1282. doi: 10.1056/NEJM199705013361802 [DOI] [PubMed] [Google Scholar]
- 4.Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65(13):1273-1282. doi: 10.1016/j.jacc.2015.01.036 [DOI] [PubMed] [Google Scholar]
- 5.Lee SE, Chang HJ, Sung JM, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11(10):1475-1484. doi: 10.1016/j.jcmg.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 6.Criqui MH, Denenberg JO, Ix JH, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. 2014;311(3):271-278. doi: 10.1001/jama.2013.282535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Rosendael AR, Narula J, Lin FY, et al. Association of high-density calcified 1K plaque with risk of acute coronary syndrome. JAMA Cardiol. 2020;5(3):282-290. doi: 10.1001/jamacardio.2019.5315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SE, Chang HJ, Rizvi A, et al. Rationale and design of the Progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography IMaging (PARADIGM) registry: a comprehensive exploration of plaque progression and its impact on clinical outcomes from a multicenter serial coronary computed tomographic angiography study. Am Heart J. 2016;182:72-79. doi: 10.1016/j.ahj.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 9.Abbara S, Blanke P, Maroules CD, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: a report of the society of Cardiovascular Computed Tomography Guidelines Committee: endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr. 2016;10(6):435-449. doi: 10.1016/j.jcct.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 10.Han D, Torii S, Yahagi K, et al. Quantitative measurement of lipid rich plaque by coronary computed tomography angiography: a correlation of histology in sudden cardiac death. Atherosclerosis. 2018;275:426-433. doi: 10.1016/j.atherosclerosis.2018.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puri R, Nissen SE, Ballantyne CM, et al. Factors underlying regression of coronary atheroma with potent statin therapy. Eur Heart J. 2013;34(24):1818-1825. doi: 10.1093/eurheartj/eht084 [DOI] [PubMed] [Google Scholar]
- 12.Zeb I, Li D, Nasir K, et al. Effect of statin treatment on coronary plaque progression—a serial coronary CT angiography study. Atherosclerosis. 2013;231(2):198-204. doi: 10.1016/j.atherosclerosis.2013.08.019 [DOI] [PubMed] [Google Scholar]
- 13.Budoff MJ, Ellenberg SS, Lewis CE, et al. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA. 2017;317(7):708-716. doi: 10.1001/jama.2016.21043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smit JM, van Rosendael AR, El Mahdiui M, et al. Impact of clinical characteristics and statins on coronary plaque progression by serial computed tomography angiography. Circ Cardiovasc Imaging. 2020;13(3):e009750. doi: 10.1161/CIRCIMAGING.119.009750 [DOI] [PubMed] [Google Scholar]
- 15.Lee SE, Sung JM, Andreini D, et al. Differential association between the progression of coronary artery calcium score and coronary plaque volume progression according to statins: the Progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography Imaging (PARADIGM) study. Eur Heart J Cardiovasc Imaging. 2019;20(11):1307-1314. doi: 10.1093/ehjci/jez022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otsuka F, Joner M, Prati F, Virmani R, Narula J. Clinical classification of plaque morphology in coronary disease. Nat Rev Cardiol. 2014;11(7):379-389. doi: 10.1038/nrcardio.2014.62 [DOI] [PubMed] [Google Scholar]
- 17.Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11(1):127-142. doi: 10.1016/j.jcmg.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 18.Nakahara T, Dweck MR, Narula N, Pisapia D, Narula J, Strauss HW. Coronary artery calcification: from mechanism to molecular imaging. JACC Cardiovasc Imaging. 2017;10(5):582-593. doi: 10.1016/j.jcmg.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 19.Healy A, Berus JM, Christensen JL, et al. Statins disrupt macrophage Rac1 regulation leading to increased atherosclerotic plaque calcification. Arterioscler Thromb Vasc Biol. 2020;40(3):714-732. doi: 10.1161/ATVBAHA.119.313832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang HJ, Lin FY, Lee SE, et al. Coronary atherosclerotic precursors of acute coronary syndromes. J Am Coll Cardiol. 2018;71(22):2511-2522. doi: 10.1016/j.jacc.2018.02.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raggi P, Davidson M, Callister TQ, et al. Aggressive versus moderate lipid-lowering therapy in hypercholesterolemic postmenopausal women: Beyond Endorsed Lipid Lowering with EBT Scanning (BELLES). Circulation. 2005;112(4):563-571. doi: 10.1161/CIRCULATIONAHA.104.512681 [DOI] [PubMed] [Google Scholar]
- 22.Schmermund A, Achenbach S, Budde T, et al. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, double-blind trial. Circulation. 2006;113(3):427-437. doi: 10.1161/CIRCULATIONAHA.105.568147 [DOI] [PubMed] [Google Scholar]
- 23.Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol. 2005;46(1):166-172. doi: 10.1016/j.jacc.2005.02.089 [DOI] [PubMed] [Google Scholar]
- 24.Nicholls SJ, Tuzcu EM, Wolski K, et al. Coronary artery calcification and changes in atheroma burden in response to established medical therapies. J Am Coll Cardiol. 2007;49(2):263-270. doi: 10.1016/j.jacc.2006.10.038 [DOI] [PubMed] [Google Scholar]
- 25.Williams MC, Moss AJ, Dweck M, et al. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-HEART Study. J Am Coll Cardiol. 2019;73(3):291-301. doi: 10.1016/j.jacc.2018.10.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flowchart
eFigure 2. Plaque Volumes by Compositional Type at Baseline and Follow-up CCTA
eFigure 3. Compositional Plaque Changes According to Baseline Plaque Volume in Patients Begun on Statin Treatment After Baseline CCTA
eFigure 4: Calcium Densification With Statin Therapy
eTable 1. Atherosclerotic Profile per Coronary Lesions at Baseline
eTable 2. Plaque Composition Proportions According the Presence of Low-Density, High-Density Calcium, or 1K Plaque
eTable 3. Interaction Baseline Plaque Volume and Statin With Compositional Plaque Change, Stratified by the Median CT Interval
eTable 4. Statin Use and Progression of Calcium Density Subgroups, Restricted to Lesions Without Necrotic Core or Fibro-Fatty Plaque
eTable 5. Plaque Progression According to Calcium and Calcium Density Subgroups
eTable 6. Baseline Characteristics of Patients Who Began on Statins After Baseline CCTA and Patients not on Statins
eTable 7. Baseline Plaque Volumes According to Newly Started Statin and No Statin Use